Abstract
Background
Gait and balance disorders in advanced Parkinson's disease (aPD) heavily impact the disease burden. In this prospective observational open‐label study, our aim was to evaluate the effectiveness of levodopa/carbidopa intestinal gel (LCIG) infusion on balance and gait over a long‐term follow‐up.
Methods
The motor status of 15 aPD patients with balance and gait symptoms was assessed with UPDRS (I–IV) and H&Y at baseline in OFF and ON conditions, and after 52 weeks of LCIG infusion. Berg Balance Scale (BBS), Tinetti Gait & Balance Score (TS), Gait and Falls Questionnaire (G&F‐Q), FOG Questionnaire (FOG‐Q), and New FOG Questionnaire (NFOG‐Q) were used to specifically test balance and gait.
Results
UPDRS, H&Y, BBS, TS, G&F‐Q, FOG‐Q, NFOG‐Q improved significantly. All FOG types benefited from LCIG.
Conclusions
Our preliminary data show the beneficial effect of LCIG therapy not only on FOG, but also on gait and balance. Results need to be confirmed in larger cohort studies.
Keywords: axial symptoms, FOG, freezing of gait, levodopa/carbidopa intestinal gel infusion, Parkinson's disease
Introduction
Gait and balance disorders in Parkinson's disease (PD) impact heavily on fall risk, quality of life (QoL), and healthcare costs. Although numerous treatments have been trialed, they are still difficult to treat with conventional therapies. Some benefits from levodopa‐carbidopa intestinal gel (LGIC) infusion have been reported,1, 2, 3 however there has yet to be a prospective study that takes into consideration LCIG treatment specifically for these axial symptoms.
We aimed to evaluate the effectiveness of LCIG on balance and gait disorders in a longitudinal design, with particular attention to the freezing of gait (FOG).
Methods
Patient Selection
We conducted an observational open‐label study enrolling patients who were screened as candidates for LCIG therapy according to the criteria described elsewhere.2 Only those who reported the feeling of feet glued to the floor, or unsteadiness while walking or standing, or any other gait and/or balance complaints were included in this sample. Among the 15 patients recruited, 13 were identified as “definite freezers,” because they fulfilled the Snijders et al.4 criteria.
Evaluation of Motor Features
All data were collected at baseline, before percutaneous endoscopic gastrojejunostomy (PEGJ) implant, in OFF condition as well as in ON oral antiparkinsonian therapy, and at 52 weeks after starting LCIG treatment, with the following outcomes.
Motor Symptoms and Disease Severity
Motor symptoms and disease severity were measured using the Unified Parkinson's Disease Rating Scale‐section III (UPDRS‐III) and IV (UPDRS‐IV) and Hoehn and Yahr stages (H&Y).
Gait and Balance
Gait and balance were measured using the Gait and Falls Questionnaire (G&F‐Q), Freezing of Gait Questionnaire (FOG‐Q), New Freezing of Gait Questionnaire (NFOG‐Q), Tinetti Gait & Balance Score (TS), and Berg Balance Scale (BBS). FOG was classified according to Espay et al.5 into four categories: (1) “OFF FOG” in case of FOG reduction or complete disappearance after levodopa intake; (2) “pseudo‐ON FOG” during suboptimally controlled ON phase; (3) “ON FOG” as induced by dopaminergic stimulation; and (4) “unresponsive (or resistant) FOG” as not influenced by levodopa administration.
Patients were labeled as “frequent fallers” when a history of at least five falls over the previous three months was reported.6 The term “shuffling steps” was used to describe patients' gait when their feet were not clearly raised from the ground during the swing phase.
Direct clinical observation of gait detected FOG subtypes and triggers, such as start hesitation and FOG while turning or reaching a target or passing through a doorway. In each clinical condition (OFF, ON oral therapy, ON LCIG) patients were videotaped while carrying out the same protocol: they were asked to get up from a chair (without armrests), walk along a corridor for 15 meters crossing a narrow passage, turn 360 degrees on place to the left and 540 degrees on place to the right, return to the starting point, and sit.
Non‐Motor Symptoms and Cognitive Assessment
UPDRS‐I, UPDRS‐II, Mini‐Mental State Examination (MMSE), and Frontal Assessment Battery (FAB) evaluated cognition, whereas Non‐motor Symptom Scale (NMSS) assessed non‐motor symptoms. QoL was measured with the short version of Parkinson's Disease Questionnaire (PDQ8).
LCIG Infusion Protocol
In all individuals, LCIG infusion was set to run throughout diurnal hours only, from awakening to bedtime.
Statistical Analysis
Pre‐ and post‐data were analyzed using Wilcoxon Signed‐rank Test (using SPSS v22) with statistical significance set at P ≤ 0.05.
Results
The sample consisted of 15 nonconsecutive advanced PD (mean H&Y 3.40 ± 0.51) patients, whose mean age was 68.87 (± 5.72) years, mean disease duration 19.07 (± 8.02) years and with slightly more males than females (9:6). The proportion of tremor dominant and akinetorigid phenotypes were comparable (8:7; Supporting Table S1).
A statistically significant improvement (P < 0.05) in all the items of UPDRS‐III was documented between baseline and follow‐up, except for the UPDRS‐III item‐28 (describing posture) showing a downward trend (P > 0.05). Also, NMSS declined significantly (P < 0.05).
All patients suffered from gait disorders that interfered with balance and 13/15 were recognized as “definite freezers.”4 According to the four FOG types,5 6/15 displayed OFF FOG, 6/15 pseudo‐ON FOG, and 1/15 ON FOG, but none had any resistant FOG. Table 1 summarizes the clinometric findings collected before PEGJ implant (in OFF and ON conditions) as well as 52 weeks later, during LCIG stable infusion.
Table 1.
Performances at baseline and at 52‐week follow‐up during LCIG infusion.
| Baseline ‐ OFF state | Baseline ‐ ON oral therapy | 52 weeks ‐ ON duodopa | P value (oral therapy vs duodopa) | |
|---|---|---|---|---|
| UPDRS III (total score) | 45.53 (17.45) | 26.60 (11.26) | 19.73 (11.25) | < 0.001 |
| UPDRS III ‐ Item 27 | 2.40 (1.72) | 1.27 (1.16) | 0.73 (0.96) | < 0.05 |
| UPDRS III ‐ Item 28 | 2.47 (0.83) | 1.80 (0.77) | 1.47 (0.83) | NS |
| UPDRS III ‐ Item 29 | 2.47 (1.25) | 1.27 (0.70) | 0.80 (0.77) | < 0.05 |
| UPDRS III ‐ Item 30 | 2.13 (1.30) | 1.27 (0.78) | 0.80 (0.77) | < 0.05 |
| UPDRS III ‐ Item 31 | 2.60 (1.06) | 1.53 (0.64) | 1.07 (0.59) | < 0.05 |
| BBS | 19.47 (12.89) | 34.33 (9.56) | 41.00 (8.77) | < 0.0001 |
| TS | 9.93 (8.05) | 18.20 (5.91) | 22.73 (4.07) | < 0.001 |
| TS ‐ Balance | 5.53 (5.22) | 10.60 (3.88) | 12.73 (3.21) | < 0.001 |
| TS ‐ Gait | 4.40 (3.42) | 7.60 (2.24) | 10.14 (1.21) | <0.001 |
| Baseline ‐ ON oral therapy | 52 weeks ‐ ON duodopa | P value (oral therapy vs duodopa) | |
|---|---|---|---|
| UPDRS I | 2.00 (2.17) | 2.07 (2.02) | NS |
| UPDRS II | 15.40 (4.22) | 13.00 (4.36) | < 0.05 |
| UPDRS IV | 8.33 (3.99) | 5.73 (2.56) | < 0.01 |
| H&Y | 3.40 (0.51) | 2.43 (0.26) | < 0.0001 |
| G&F‐Q | 32.60 (10.59) | 19.13 (10.80) | < 0.001 |
| FOG‐Q | 17.07 (4.53) | 11.00 (5.17) | < 0.001 |
| NFOG‐Q | 22.80 (4.77) | 13.40 (7.22) | < 0.001 |
| NMSS | 77.73 (37.27) | 62.67 (39.84) | < 0.05 |
| PDQ8 | 49.79 (16.85) | 32.29 (17.14) | < 0.001 |
| MMSE | 26.34 (2.63) | 25.37 (2.39) | NS |
| FAB | 14.47 (2.75) | 14.49 (2.75) | NS |
Baseline evaluations were performed in both OFF at early morning (after >10 hours wash‐out for levodopa, 24 hours for dopamine agonists) and ON oral therapy, defined as the best motor condition reached with 125% LEDD of the usual morning best oral medical treatment.
At baseline, none of the patients were affected by cognitive decline except for one with mild‐to‐moderate cognitive impairment detected during the enrolment phase (MMSE = 20.3; FAB = 7.3). In 14/15, cognitive performances remained unchanged at the follow‐up, even in the aforementioned patient. Only one converted to mild cognitive impairment (MMSE: baseline 24.3, follow‐up 22.3; FAB: baseline 15.3, follow‐up 14.4).
Infusion ran throughout the daytime, for a mean of 14.80 (± 1.24) hours. None of the patients used nocturnal infusion.
Overall, a little reduction in the mean levodopa equivalent daily dose (LEDD) was detected, namely from 1477.09 (± 468.82 mg/die) at baseline to 1422.20 (± 353.25 mg/die) at 52‐week follow‐up.
A further analysis was performed in order to find out the possible impact of LEDD modification on FOG outcome. It was found that 4/6 patients suffering from FOG during OFF at baseline benefited from LEDD reduction from 1479.11 (± 5 29.66) to 1240.50 (± 465.72) mg/die, whereas 2/6 from unchanged LEDD. With regard to those with pseudo‐ON FOG (6/15), LEDD was not significantly modified in three of them, while in two it rose from 930.00 (± 28.28) to 1180.50 (± 28.99) mg/die, and in one it was cut down from 1696.08 to 1364.00 mg/die. The sole ON freezer witnessed a progressive decline in LEDD (from 1176.80 to 970.00) with concomitant lower frequency and magnitude of FOG episodes (Fig. 1).
Figure 1.
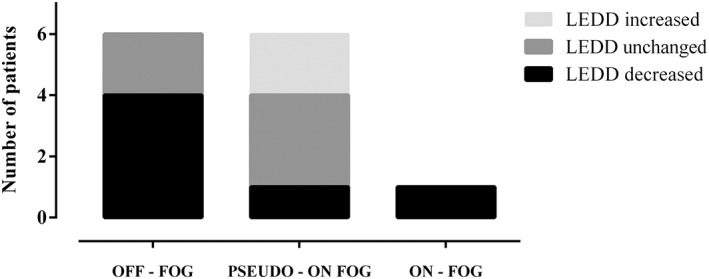
LEDD modification in distinct FOG types.
It was reduced in 6/13 FOG patients, mainly among those with OFF‐ FOG. LEDD was unmodified in other six, of who four were in the pseudo‐ON FOG group, and it was incremented in only two.
Discussion
The present study reported positive outcomes on balance and gait disorders of LCIG treatment. Moreover, the results on QoL, motor scores, and non‐motor symptoms are in line with previous research.2, 3 There was no effect on cognition (Table 1).
All patients showed a significant improvement in both balance and gait (BBS = +19%, TS = +25%, G&F‐Q = ‐ 41%, FOG‐Q = ‐ 36%, NFOG‐Q = ‐ 41%; P < 0.05); notably, FOG‐related scores followed the same trend regardless of the FOG type.
Previous research has been inconclusive about the effect of LCIG effectiveness on gait and balance. Case series have reported inconsistent results because they analyzed distinct types of FOG and variable infusional schemes.1, 7 Cossu et al.1 retrospectively described seven patients with ON FOG that disappeared during LCIG infusion: 4/7 were considered as pseudo‐ON freezers since FOG relief was obtained with LEDD increase after PEGJ implantation. Additionally, 24h‐LCIG infusion was effective in treating five PD unresponsive freezers, as reported by Chang et al.7 It should be noted that in both papers, the clinical scales and questionnaires used were different and therefore the results are not comparable. By contrast, the present study employed, for the first time to the best of our knowledge, clinical tools reviewed as “recommended” or “suggested” by a recent consensus on instrumental measurements of posture, gait, and balance.8
With regard to the LEDD modifications, we found that a significant proportion of patients with FOG in OFF condition improved despite a mild total LEDD lowering after PEGJ implant. Similarly but more surprisingly, in most of those suffering from pseudo‐ON FOG, total LEDD was not sizably changed. This differs from what was reported by Cossu and colleagues,1 where a slight total LEDD increase was required only in two pseudo‐ON freezers to reach an optimal control of cardinal motor symptoms as well as gait issues.
The positive outcomes achieved by the LEDD reduction may be in contradiction with PD pathophysiology; they could be interpreted as results of the more stable levodopa supply at the basal ganglia level. Likewise, this explanation seems to be also applicable to the sole ON freezer, who benefited from lower total LEDD to better control the FOG, without a negative impact on the overall motor and non‐motor performances.
To summarize, this study provides three main conclusions: (1) for the first time, data suggesting the effectiveness of LCIG on gait and balance disorders was collected longitudinally; (2) positive results on FOG were observed in all the three levodopa‐responsive FOG types; which (3) were probably due to the fine tailoring of LCIG daytime infusion and not to the overall LEDD administered. The latter was actually more frequently unmodified or decreased. Since the present study has several limitations (single center, small series, lack of control group), future larger trials should be conducted to refute or confirm these promising preliminary findings.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
V.R.: 1B, 1C, 2A, 2B, 2C, 3A, 3B
N.G‐A.: 1B, 1C
G.P.: 1B, 1C, 2B
E.C.: 1A, 1B, 1C
F.P.: 1A, 1B, 1C
M.S.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work, and patient consent was not required. We have read the Journal's position on issues involved in ethical publication and affirm this work is consistent with those guidelines. The authors have followed the standards as stated in the “Declaration of Helsinki”.
Funding Sources and Conflict of Interest: The authors declare no conflicts of interest relevant for this work. No funding was received for this article.
Financial disclosures from previous 12 months: The authors declare that there are no additional disclosures to report.
Supporting information
Supporting Table S1. Demographic and clinical features. Complete references about demographic and clinical characteristics of patients.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Cossu G, Ricchi V, Pilleri M, et al. Levodopa–carbidopa intrajejunal gel in advanced Parkinson disease with ‘on’ freezing of gait. Neurol Sci. 2015;36(9):1683–1686. [DOI] [PubMed] [Google Scholar]
- 2. Sensi M, Preda F, Trevisani L, et al. Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. JNeural Transm. 2014;121(6):633–642. [DOI] [PubMed] [Google Scholar]
- 3. Devos D. Patient profile, indications, efficacy and safety of duodenal levodopa infusion in advanced Parkinson's disease. Mov Disord. 2009;24(7):993–1000. [DOI] [PubMed] [Google Scholar]
- 4. Snijders AH, Haaxma CA, Hagen YJ, et al. Freezer or non‐freezer: Clinical assessment of freezing of gait. Parkinsonism Relat Disord; 2012;18(2):149–154. [DOI] [PubMed] [Google Scholar]
- 5. Espay AJ, Fasano A, van Nuenen BFL, et al. ‘On’ state freezing of gait in Parkinson disease: A paradoxical levodopa‐induced complication. Neurology. 2012;78(7):454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas AA, Rogers JM, Amick MM, Friedman JH. Falls and the falls efficacy scale in Parkinson's disease. JNeurol. 2010;257(7):1124–1128. [DOI] [PubMed] [Google Scholar]
- 7. Chang FCF, Tsui DS, Mahant N, et al. 24 h Levodopa–carbidopa intestinal gel may reduce falls and ‘unresponsive’ freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2015;21(3):317–320. [DOI] [PubMed] [Google Scholar]
- 8. Bloem BR, Marinus J, Almeida Q, et al. Measurement instruments to assess posture, gait, and balance in Parkinson's disease: critique and recommendations. Mov Disord. 2016;31(9):1342–1355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table S1. Demographic and clinical features. Complete references about demographic and clinical characteristics of patients.


