Abstract
Background
Dystonia is often associated with damage to basal ganglia (BG), but neuropathological assessments of these cases are infrequent.
Methods
A brain was assessed with possible post‐traumatic focal dystonia that appeared after an accident occurred during childhood.
Results
Tau pathology was found within putamen and globus pallidus of the right hemisphere, and chronic traumatic encephalopathy (CTE) was observed in the cortex of the left hemisphere. No diffuse axonal injury (DAI), β‐amyloid, ubiquitin, p62, or pTDP43 pathology was found.
Conclusions
Post‐traumatic dystonia could be associated with post‐traumatic tau pathology formation. However, more cases are necessary to establish causality. The tau lesions found in the BG of this patient did not fit within CTE criteria. We hypothesize that due to the anatomo‐histological characteristics of the BG, tau pathology associated with brain traumas produce histopathological patterns different from sulcal‐tau pathology, which is the only tau pathology distribution currently accepted as pathognomonic of CTE.
Keywords: basal ganglia, post‐traumatic dystonia, traumatic brain injury, tau pathology, CTE spectrum
Introduction
Dystonia represents the third most common movement disorder after essential tremor (ET) and Parkinson's disease (PD).1, 2 While numerous genetic causes of dystonia have been identified,3 post‐traumatic dystonia has also been reported.4, 5, 6, 7 Dystonia can be classified on different diagnostic axes8 and it is recognized as a disorder consisting of both motor and non‐motor components.9, 10 The clinical involvement of different parts of the body and the complex phenomenology of the dystonic movements depend on the specific somatotopic‐anatomical localization of those abnormally activated or damaged neurocircuits localized in different anatomical regions along the loops of highly interconnected neuroanatomical circuits, such as the basal ganglia (BG).11, 12, 13 Little is known about the specific neuropathologic features of genetically based,14, 15 idiopathic, or post‐traumatic dystonia.16, 17, 18, 19, 20, 21, 22, 23 However, different neurophysiologic and functional neuroimaging techniques have confirmed, among other regions,24 the abnormal involvement of the BG, specifically of the globus pallidus (GP) and putamen (Pu) in different types of dystonia.25, 26, 27 While genetic discoveries and neurophysiological imaging methods have certainly increased our knowledge on various pathophysiological and neuroanatomical aspects of dystonia, neuropathological investigations of this complex disorder are not common, precluding the possibility to pathologically confirm most of the findings observed in vivo in either genetic, idiopathic as well as post‐traumatic cases.
We report a detailed neuropathologic assessment of the brain of a patient with a history of possible post‐traumatic focal dystonia that appeared after a head trauma that occurred at age 12.
Material and Methods
Clinical History
The patient (male, Caucasian) died at 78 years after developing septic shock and respiratory failure of unknown causes. Medical records reported three episodes of falls with consequent facial bruises and balance instability during the week before death associated with episodes of bright red blood per rectum. A full‐body autopsy was performed to verify the final cause of death. No family history of dystonia, movement disorders, other neurological or psychiatric diseases, drug abuse, smoking, or alcoholism was reported.
Medical records reported a possible child‐onset post‐traumatic focal dystonia of the left hand associated with a tremor of the right hand. A diagnosis of possible post‐traumatic dystonia was described as occurring after a bicycle/motor vehicle head trauma at the age of 12 (1935). No loss of consciousness (LOC) or hospitalization was reported. Although skull fracture was reported, no radiological findings were available (x‐rays and other imaging methods were not available at the time). While the exact interval of time between head trauma and manifestation of dystonia was not reported, medical records (and as per his wife's recent confirmation) referred to a diagnosis of post‐traumatic focal dystonia since childhood as consequent of the bicycle/motor vehicle accident. Different physicians repeatedly confirmed the diagnosis of a possible childhood‐onset post‐traumatic dystonia during the following years. No diagnosis about the specific type of tremor was reported; however, the wife confirmed that the dystonic disorder and tremor appeared a few weeks after the bike accident and remained stable across the patient's entire life. Since the accident, the patient was able to control his hand tremor (but not the dystonia) by taking low daily doses of diazepam.
For more detail on the neuropathology assessment, as well as histology and immunohistochemistry methods, see Supporting Text S1.
Results
Upon microscopic evaluation of all available brain regions, arteriolosclerosis and signs of focal chronic ischemia were observed in the nucleus accumbens and the insular and cerebellar (vermis) cortex of the right hemisphere. An area of tissue rarefaction as per an old ischemic lesion in the Pu and GP area was also observed (Fig. 1A,B,C). Perl's stain (Fig. 1C) demonstrated iron deposition in the lenticular region as per a previous cerebrovascular accident. All examined brain regions were negative for all considered antibodies except for pTau‐positive lesions in neurons (pTau‐neurofibrillary tangles [pTau‐NFTs] and pTau‐neurites) and glial cells (pTau‐positive astroglial pathology), iba‐1 positive activated microglia and GFAP‐positive reactive astrocytes confined to the area between inferomedial of the Pu and inferolateral border of the GPe of the right hemisphere (Fig. 2). Moreover, focal GFAP‐astrogliosis was observed in the nucleus accumbens region and cerebellar (vermis) cortex, both lesions compatible with previous ischemic events (Fig. 3). In the left hemisphere, pTau‐NFTs and pTau‐neurites were found in the olfactory tract, hypothalamus, nucleus accumbens, hippocampus, entorhinal cortex, primary somatosensory cortex and somatosensory association cortex (BA3,1,2,5), and orbitofrontal cortex (BA11/12). pTau lesions in almost all examined regions were typical of AD‐like pathology (with a possible diagnosis of primary age‐related tauopathy [PART]),28 while only the pTau lesion in the orbitofrontal cortex (BA11/12) was pathognomonic for CTE (Fig. 4). Furthermore, reactive gliosis and myelin loss was observed in the left cerebellar hemisphere (Fig. 5).
Figure 1.
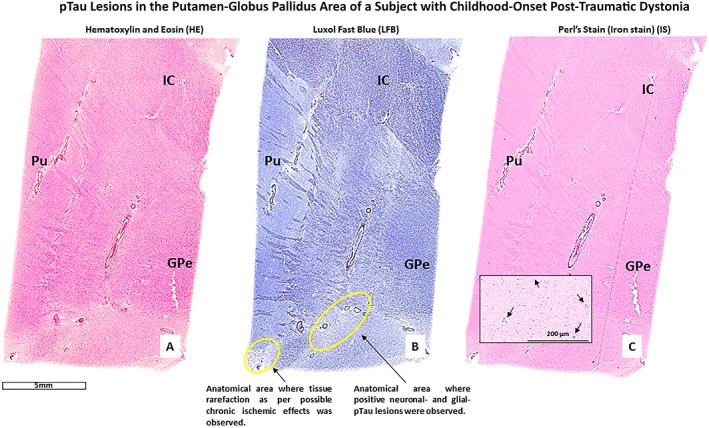
The figure shows histological sections from the right Putamen (Pu) ‐ Globus Pallidus external (GPe) area of a patient with possible child‐onset post‐traumatic focal dystonia. Part A, B, and C are images of three consecutive sections respectively stained with hematoxylin and Eosin (HE), Luxol Fast Blue (LFB), and iron stain (Perl's stain). Note: the presence of iron (hemosiderin) deposition (blue spots, part C) in the lenticular region of the right BG of this patient.
Figure 2.
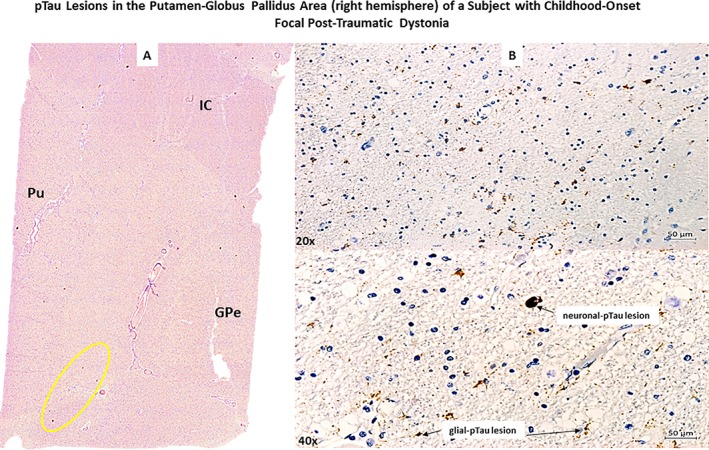
The figure (Part A) shows a section stained with AT8 antibody (anti‐phosphorylated‐tau antibody) of the right Putamen (Pu)‐Globus Pallidus external (GPe) area of a patient with possible child‐onset post‐traumatic focal dystonia. (Part B). Top and bottom images show neuronal‐ and glia‐tau lesions respectively at a higher progressive magnification (20x, 40x objectives).
Figure 3.
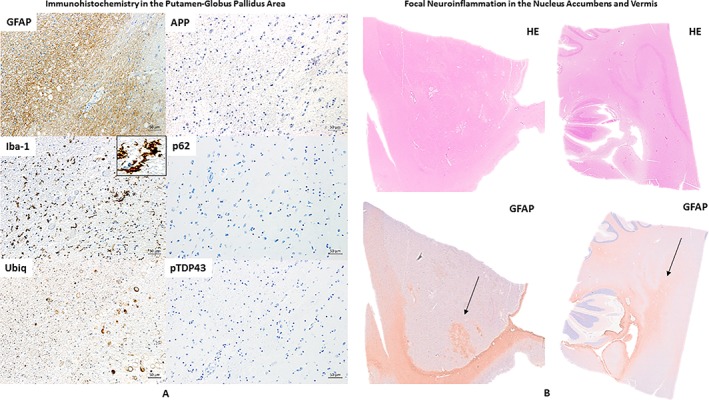
(A) Consecutive histological sections respectively immunostained for GFAP, iba‐1 (to note the microglia activation as shown by the black arrow), Ubiq, APP (negative), p62 (negative), and pTDP43 (negative) showing the same anatomical area (right Putamen (Pu)‐Globus Pallidus external (GPe). (B) The nucleus accumbens and cerebellar cortex (including vermis region) of the right hemisphere stained with HE and GFAP. To note the distinct areas of focal neuroinflammation (GFAP positive) in both regions (black arrow).
Figure 4.
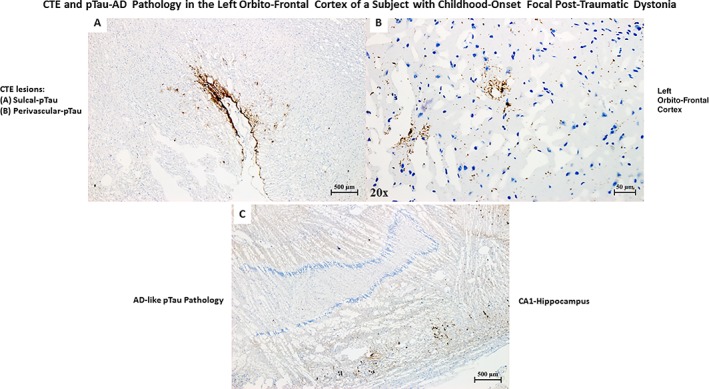
(A) pTau pathology (AT8 stain) pathognomonic of chronic traumatic encephalopathy (CTE) in the orbitofrontal frontal cortex of the left hemisphere of a patient with a single TBI occurred during childhood. (B) Perivascular pTau pathology, part of CTE pathology. (C)pTau pathology (AT8 stain) in the CA1 region of the left hippocampus typical of Alzheimer's disease (AD) pathology. The patient was never diagnosed with memory or other cognitive deficits until death. Based on the age of the patient, the presence of pTau pathology in the hippocampus is compatible with a diagnosis primary age‐related tauopathy (PART).
Figure 5.
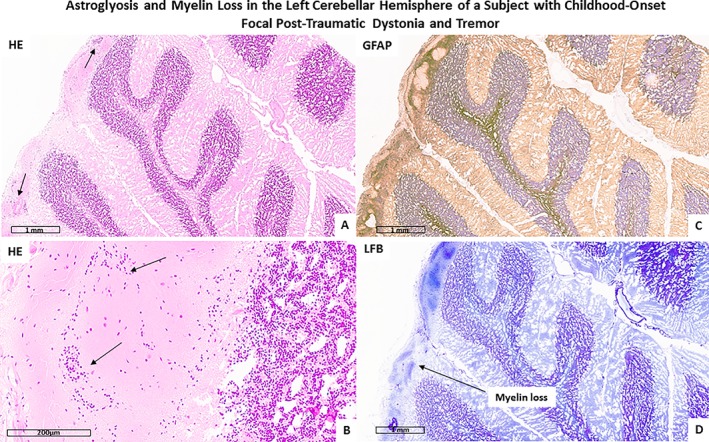
The figure shows reactive astrogliosis as evidenced by hematoxylin and eosin (HE; A and B) and GFAP (C) stain. Panel D shows cerebellar myelin loss as evidenced by Luxol Fast Blue (LFB) stain.
No β‐amyloid pathology or other types of pathology (e.g., intraneuronal inclusions positive for ubiq, p62, or pTDP43) was found in any examined region of both hemispheres. Corpora amylacea (CA) were observed across almost all examined regions of the brain.
Discussion
This neuropathologic investigation represented a rare opportunity to assess the brain of a patient with a history of possible post‐traumatic child‐onset focal dystonia and tremor. The neuropathological assessment showed focal positivity for pTau‐NFTs/neurites and pTau‐glial cells localized in the right BG at the junction between Pu and GPe. By contrast, on the left hemisphere, tau pathology was sufficient for a diagnosis of PART due to the age of the patient and the absence of a clinical diagnosis of MCI or AD. Also, an isolate CTE29, 30 lesion in the left orbital‐frontal cortex was also found. Intriguingly, while pTau pathology in the left orbitofrontal cortex corresponded to a pathognomonic CTE lesion, pTau pathology in the right BG did not fit with the current definition of CTE.31 The pTau pathology observed across all different areas of the left hemisphere (a marked pTau pathology asymmetry was observed between left vs. right hemisphere) was typical of AD pathology (pTau pathology spread at the deeper cortical layers). By contrast, the distribution of pTau pathology lesions in the neurons and glial cells of right BG in such a focal form in one hemisphere only (the right) and the CTE lesion (pTau pathology spread at the most superficial cortical layers) in the left orbitofrontal cortex are not typical of AD pathology and are not related to aging.
We hypothesize that the pTau lesions observed in the BG of the right cerebral hemisphere of this patient with a history of a single TBI and possible consequent TBI‐related clinical manifestation as a focal dystonic phenomenon could exemplify a new and specific histological distribution pattern of post‐traumatic pTau pathology due to the peculiar neuroanatomical characteristics of the BG. In fact, during human brain maturation, there is no gyrification of the BG (a process characteristic of the cerebral and cerebellar cortex during development), so BG‐pTau lesions cannot be histologically localized in the cortical sulci, which are the only specific neurohistological sites currently accepted to define a tau lesion as pathognomonic of CTE. Moreover, CTE, as currently defined, is usually associated with repetitive TBI32 (e.g., contact‐sport practice). However, it is under constant debate if CTE can also be associated to a single TBI.33
In pathophysiologic terms, the specific anatomical localization of pTau lesions (right Pu‐GPe area) fit well with the clinical manifestations of a focal dystonic phenomenon in the left hand.34 The tremor, based on limited clinical description, could only be hypothesized as a diagnosis of a cerebellar tremor, possibly associated with neuroinflammatory phenomena on the left cerebellar hemisphere. Furthermore, it is not possible to exclude that a genetic predisposition (either for dystonia or tremor or both) could have been associated with the formation of pTau pathology in those same cerebral regions associated with dystonia (e.g., BG). Importantly, we cannot exclude that micro‐cerebrovascular events linked to traumas could trigger the formation of pTau pathology. Indeed, acute and chronic brain ischemic phenomena have been showed to lead to tau phosphorylation.35, 36
Intriguingly, it seems that the pTau pathology in Pu and GPe did not spread along any other neuroanatomical circuits of the right hemisphere. In fact, the numerous other brain regions of the right hemisphere were negative for pTau pathology, including regions frequently associated with aging‐related tau‐NFTs, such as entorhinal cortex or CA1 of the hippocampus (usually found positive in PART, for example),28 which were actually found to be positive in the opposite hemisphere (the left). Moreover, the absence of α‐syn lesions (e.g., Lewy bodies), p62 and pTDP43 intraneuronal inclusions, CD68 intraparenchymal macrophages activation, and APP‐positive axonal damage (i.e., axonal diffuse injury, DAI), excluded the co‐occurrence of other neurodegenerative processes, recent ischemic events, or acute TBI in the BG or other regions of the brain.
Future studies need to confirm the possible association between post‐traumatic focal or segmental dystonia and accumulation of neuronal and glial pTau pathology in those brain regions particularly vulnerable to TBI, such as the BG, which are part of complex somatotopically organized neuronal circuits that can be differently affected across different types of movement disorders. Future investigations should also consider the specific physicomechanical forces and sequence of events occurring in each type of TBI (e.g., impact‐TBI vs. blast‐TBI) as well as their interactions with genetic and environmental modifying factors.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
D.I.: 1A, 1B, 1C
P.L.: 1C
M.H.: 3B
D.P.: 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work. The next‐of‐kin of this patient gave signed consent. The authors confirm that have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Financial Disclosures and Conflicts of Interest: This study was supported by the Brain Tissue Repository and Neuropathology Core, CNRM award #308049‐4.01‐60855.
Financial Disclosures for previous 12 months: None.
Supporting information
Supporting information may be found in the online version of this article.
Supporting Text S1. This file describes the procedures performed for the gross inspection of the brain and the methods used of a detailed neuropathological and immunohistochemistry assessment. Also included is a description of the procedures performed for the micro sectioning of each tissue block dissected from both the hemisphere of this brain and the type of antibodies used for the assessment of this rare case of child‐onset post‐traumatic focal dystonia.
Acknowledgments
The opinions expressed herein are those of the authors and not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUSH), the Department of Defense (DOD) or the United States Army, Navy, Air Force, or any other US federal agency.
We thank the family that consented for the brain donation of their family member for the better understanding of TBI and its possible consequences. We also thank Mrs. Nichelle Gray for her technical work, Mr. Harold Kramer Anderson for his writing assistance and editing skills, and Mrs. Stacey Gentile for her administrative support. Human tissue was obtained from the NIH NeuroBioBank at the University of Maryland, Baltimore, MD. We are also thankful to all personnel of the University of Maryland Brain and Tissue Bank (UMBTB), and especially to Dr. Zielke MD and John Cottrell, MS, PA, ASCP for their assistance.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. [DOI] [PubMed] [Google Scholar]
- 2. Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta‐analysis. Mov Disord. 2012;27:1789–1796. [DOI] [PubMed] [Google Scholar]
- 3. Lohmann K, Klein C. Update on the Genetics of Dystonia. Curr Neurol Neurosci Rep. 2017;17:26. [DOI] [PubMed] [Google Scholar]
- 4. Koller WC, Wong GF, Lang A. Posttraumatic movement disorders: a review. Mov Disord. 1989;4:20–36. [DOI] [PubMed] [Google Scholar]
- 5. Jankovic J. Post‐traumatic movement disorders: central and peripheral mechanisms. Neurology. 1994;44:2006–2014. [DOI] [PubMed] [Google Scholar]
- 6. Lee MS, Rinne JO, Ceballos‐Baumann A, Thompson PD, Marsden CD. Dystonia after head trauma. Neurology. 1994;44:1374–1378. [DOI] [PubMed] [Google Scholar]
- 7. Krauss JK. Movement disorders secondary to craniocerebral trauma. Handb Clin Neurol. 2015;128:475–496. [DOI] [PubMed] [Google Scholar]
- 8. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zurowski M, McDonald WM, Fox S, Marsh L. Psychiatric comorbidities in dystonia: emerging concepts. Mov Disord. 2013;28:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conte A, Berardelli I, Ferrazzano G, Pasquini M, Berardelli A, Fabbrini G. Non‐motor symptoms in patients with adult‐onset focal dystonia: sensory and psychiatric disturbances. Parkinsonism Relat Disord. 2016;22 Suppl 1:S111‐114. [DOI] [PubMed] [Google Scholar]
- 11. Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol. 2004;115:2542–2557. [DOI] [PubMed] [Google Scholar]
- 12. Alam M, Sanghera MK, Schwabe K, et al. Globus pallidus internus neuronal activity: a comparative study of linear and non‐linear features in patients with dystonia or Parkinson's disease. JNeural Transm (Vienna). 2016;123:231–240. [DOI] [PubMed] [Google Scholar]
- 13. Kumbhare D, Holloway KL, Baron MS. Parkinsonism and dystonia are differentially induced by modulation of different territories in the basal ganglia. Neuroscience. 2017;353:42‐57. [DOI] [PubMed] [Google Scholar]
- 14. Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paudel R, Hardy J, Revesz T, Holton JL, Houlden H. Review: genetics and neuropathology of primary pure dystonia. Neuropathol Appl Neurobiol. 2012;38:520–534. [DOI] [PubMed] [Google Scholar]
- 16. LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. [DOI] [PubMed] [Google Scholar]
- 17. Kostić VS, Stojanović‐Svetel M, Kacar A. Symptomatic dystonias associated with structural brain lesions: report of 16 cases. Can J Neurol Sci. 1996;23:53–56. [DOI] [PubMed] [Google Scholar]
- 18. Münchau A, Mathen D, Cox T, Quinn NP, Marsden CD, Bhatia KP. Unilateral lesions of the globus pallidus: report of four patients presenting with focal or segmental dystonia. JNeurol Neurosurg Psychiatry. 2000;69:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol. 2013;12:597–608. [DOI] [PubMed] [Google Scholar]
- 20. Defebvre L, Krystkowiak P. Movement disorders and stroke. Rev Neurol (Paris). 2016;172:483–487. [DOI] [PubMed] [Google Scholar]
- 21. Monbaliu E, de Cock P, Ortibus E, Heyrman L, Klingels K, Feys H. Clinical patterns of dystonia and choreoathetosis in participants with dyskinetic cerebral palsy. Dev Med Child Neurol. 2016;58:138–144. [DOI] [PubMed] [Google Scholar]
- 22. Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iacono D, Geraci‐Erck M, Peng H, Rabin ML, Kurlan R. Reduced number of pigmented neurons in the substantia nigra of dystonia patients? Findings from extensive neuropathologic, immunohistochemistry, and quantitative analyses. Tremor Other Hyperkinet Mov (NY). 2015;5. doi: 10.7916/D8T72G9G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khooshnoodi MA, Factor SA, Jinnah HA. Secondary blepharospasm associated with structural lesions of the brain. JNeurol Sci. 2013;331:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asanuma K, Carbon‐Correll M, Eidelberg D. Neuroimaging in human dystonia. JMed Invest. 2005;52 Suppl:272‐279. [DOI] [PubMed] [Google Scholar]
- 26. Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional, and molecular imaging of the brain in primary focal dystonia‐‐a review. Neuroimage. 2011;56:1011–1020. [DOI] [PubMed] [Google Scholar]
- 27. Obermann M, Yaldizli O, de Greiff A, et al. Increased basal‐ganglia activation performing a non‐dystonia‐related task in focal dystonia. Eur J Neurol. 2008;15:831–838. [DOI] [PubMed] [Google Scholar]
- 28. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age‐related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iacono D, Shively SB, Edlow BL, Perl DP. Chronic traumatic encephalopathy: known causes, unknown effects. Phys Med Rehabil Clin N Am. 2017;28:301–321. [DOI] [PubMed] [Google Scholar]
- 31. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9(4):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long‐term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011;3(10 Suppl 2):S460–467. [DOI] [PubMed] [Google Scholar]
- 34. Liuzzi D, Gigante AF, Leo A, Defazio G. The anatomical basis of upper limb dystonia: lesson from secondary cases. Neurol Sci. 2016;37:1393–1398. [DOI] [PubMed] [Google Scholar]
- 35. Song B, Ao Q, Wang Z, et al. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen Res. 2013;8:3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li JF, Wang Z, Sun QJ, Du YF. Expression of Tau protein in rats with cognitive dysfunction induced by cerebral hypoperfusion. Int J Clin Exp Med. 2015;8:19682–19688. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information may be found in the online version of this article.
Supporting Text S1. This file describes the procedures performed for the gross inspection of the brain and the methods used of a detailed neuropathological and immunohistochemistry assessment. Also included is a description of the procedures performed for the micro sectioning of each tissue block dissected from both the hemisphere of this brain and the type of antibodies used for the assessment of this rare case of child‐onset post‐traumatic focal dystonia.


