Abstract
Rationale: Rural residence is associated with poor outcomes in several chronic diseases. The association between rural residence and chronic obstructive pulmonary disease (COPD) exacerbations remains unclear.
Objectives: In this work, we sought to determine the independent association between rural residence and COPD-related outcomes, including COPD exacerbations, airflow obstruction, and symptom burden.
Methods: A total of 1,684 SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) participants with forced expiratory volume in 1 second/forced vital capacity < 0.70 had geocoding-defined rural-urban residence status determined (N = 204 rural and N = 1,480 urban). Univariate and multivariate logistic and negative binomial regressions were performed to assess the independent association between rurality and COPD outcomes, including exacerbations, lung function, and symptom burden. The primary exposure of interest was rural residence, determined by geocoding of the home address to the block level at the time of study enrollment. Additional covariates of interest included demographic and clinical characteristics, occupation, and occupational exposures. The primary outcome measures were exacerbations determined over a 1-year course after enrollment by quarterly telephone calls and at an annual research clinic visit. The odds ratio (OR) and incidence rate ratio (IRR) of exacerbations that required treatment with medications, including steroids or antibiotics (total exacerbations), and exacerbations leading to hospitalization (severe exacerbations) were determined after adjusting for relevant covariates.
Results: Rural residence was independently associated with a 70% increase in the odds of total exacerbations (OR, 1.70 [95% confidence interval (CI), 1.13–2.56]; P = 0.012) and a 46% higher incidence rate of total exacerbations (IRR 1.46 [95% CI, 1.02–2.10]; P = 0.039). There was no association between rural residence and severe exacerbations. Agricultural occupation was independently associated with increased odds and incidence of total and severe exacerbations. Inclusion of agricultural occupation in the analysis attenuated the association between rural residence and the odds and incidence rate of total exacerbations (OR, 1.52 [95% CI, 1.00–2.32]; P = 0.05 and IRR 1.39 [95% CI, 0.97–1.99]; P = 0.07). There was no difference in symptoms or airflow obstruction between rural and urban participants.
Conclusions: Rural residence is independently associated with increased odds and incidence of total, but not severe, COPD exacerbations. These associations are not fully explained by agriculture-related exposures, highlighting the need for future research into potential mechanisms of the increased risk of COPD exacerbations in the rural population.
Keywords: chronic obstructive pulmonary disease, exacerbation, rural health
Chronic obstructive pulmonary disease (COPD) is a common condition worldwide, with a prevalence estimated at 10% (1, 2). Acute exacerbations of COPD (AECOPD) are a major contributor to the morbidity and mortality of the disease (3, 4). Reduced lung function and a history of AECOPD are established risk factors for future development of AECOPD (5, 6). Persons living in rural areas of the United States face unique and important healthcare challenges. They experience higher COPD mortality rates than their urban counterparts across all regions of the United States (7), and a comparatively higher burden of chronic diseases (8–10). The potential mechanisms for poorer health outcomes in rural populations include reduced access to care, differential behavioral risk factors, and unique environmental exposures. With emergency department expenditures for COPD increasing, limited access to care among rural patients with COPD, and more AECOPD-related morbidity and mortality in the rural areas of the United States over the preceding three decades, determining the role rurality plays in AECOPD is important (11–15). The impact of rural residence on AECOPD has yet to be established in a prospective, nationwide cohort.
SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) is a multicenter, observational cohort study of current and former smokers and nonsmoking control subjects with extensive physiologic, clinical, and biochemical metrics (16). SPIROMICS affords the opportunity to independently determine associations between demographic factors and COPD outcomes in a rigorous manner, including access to geocoding for patient residence and a robust assessment of environmental and work exposures. For this analysis, using home addresses obtained at the time of enrollment, urban-rural status was defined through geocoding to the block level in individuals with COPD.
We hypothesized that rural residence would be independently associated with an increased risk of total and severe COPD exacerbations. Rurality can impact COPD outcomes through several mechanisms, including environmental and occupational exposures, socioeconomic status, geographic location, smoking patterns, and access to care. This study explores how these factors impact the independent association of rural residence on 1-year COPD exacerbation outcomes.
Methods
Study Cohort
SPIROMICS is a multicenter cohort study that includes current and former smokers (>20 pack years) and nonsmoking control subjects between 40 and 80 years of age, with and without COPD (defined as postbronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] ratio < 0.70), who were recruited from 12 clinical centers (16). The analytical cohort for this study is limited to SPIROMICS participants with spirometry-defined COPD, complete 1-year exacerbation data from the baseline visit, and rural-urban residence status determined via geocoding as described below (N = 1,684). Institutional review boards at each center approved SPIROMICS, and all participants provided written informed consent.
Data Collection
Demographic data, smoking status and history, inhaler mediation use, and AECOPD occurrence in the year before the baseline visit were obtained via self-report at enrollment. Medical comorbidities were defined via self-report of a physician’s diagnosis. Respiratory symptom assessments were performed using the modified Medical Research Council (mMRC) dyspnea scale (17); body mass index, airflow obstruction, dyspnea, and exercise (BODE) index (18); and COPD assessment tool (CAT) (19). Chronic bronchitis was defined using subdomains of the St. George’s Respiratory Questionnaire (20). All analyses incorporated postbronchodilator spirometry data from the baseline visit. Analyses of lung computed tomography (CT) scans were performed per protocols described previously (21), including the extent of emphysema (defined as voxels below −950 Hounsfield units) and functional small-airways disease (22, 23) evaluated on a whole-lung basis. Occupation and occupational exposures were assessed by asking the following questions: 1) have you ever worked in a particular environment, 2) have you ever worked in a specific occupation, and 3) in your job, do/did you come into regular contact with any of the following specific examples of vapors, gas, dust, or fumes? A composite variable to capture any agricultural exposure was generated and defined as present with an affirmative response to any of the following questions: 1) have you ever had a profession in agriculture, 2) have you ever worked as a pig farmer, and 3) have you ever been exposed to wheat, flower, grain, animal feed, or cotton dust? (24, 25).
The binary urban/rural classification was determined from the 2010 U.S. Census. The U.S. Census generally categorizes census blocks as “urban” in areas containing 500 people per square mile and at least 2,500 people total (26), or “rural” otherwise. Primary residency at the initial visit was geocoded using the ArcGIS 10.3 system (Esri), which is based primarily on parcel data. Addresses that could not be automatically geocoded (6% of the cohort) were assigned based on a Google or Bing Maps application search. Census data were obtained from the U.S. Census via the University of Washington library. The demographic variable for rurality was compiled at the block level using geocoded addresses.
Longitudinal data on AECOPD were collected by quarterly telephone calls and an annual clinic visit. Two AECOPD outcomes that occurred within the first year of study follow-up were assessed as defined previously (6, 27): 1) exacerbations requiring treatment with medications, including oral steroids or antibiotics (total exacerbations), and 2) exacerbations that led to an emergency department visit or hospital admission (severe exacerbations).
Statistical Methods
Chi-square tests or t tests (for normally distributed data) or the Kruskal-Wallis test (for skewed data) were used to identify differences in demographic and clinical factors between rural and urban participants. To determine the independent association between rural residence (exposure) and COPD exacerbations (outcomes), logistic regression and negative-binomial regression modeling approaches were employed separately. For logistic regression, exacerbations were dichotomized as either no exacerbation or any exacerbations over the first year after enrollment, with odds ratios (ORs) representing the odds of any exacerbation related to exposure variables. For negative-binomial regression models, exacerbations were modeled continuously as the number of exacerbations, with incidence rate ratios (IRRs) representing the incidence rate of an additional exacerbation related to exposure variables. For model construction, univariate models were first constructed. Covariates defined as clinically relevant from a literature review or statistically associated with outcomes of interest (threshold P < 0.20) were included in multivariate models. Sensitivity analyses were performed to adjust for study site, removal of previous exacerbation history from models, and addition of the agricultural occupation variable. A P value < 0.05 was used to determine statistical significance. All analyses were completed with SAS 9.4.
Results
Participant Characteristics
The total SPIROMICS cohort (N = 2,974) included 1,831 individuals with spirometry-confirmed COPD (FEV1/FVC < 0.7). The analytical cohort included here comprised 1,684 participants with geocoding and exacerbation data, representing 92% of the COPD participants in SPIROMICS (see Figure E1 in the online supplement). There were no substantial differences in clinical or demographic characteristics between the analytical cohort and the larger cohort of COPD participants in SPIROMICS (Tables E1 and E2). Of the 1,684 SPIROMICS participants included in the analysis, 204 (12%) resided in rural areas and 1,480 (88%) resided in urban areas. More than 20% of the enrolled patients at four SPIROMICS centers (University of Alabama-Birmingham, Wake Forest University, University of Michigan at Ann Arbor, and University of Iowa at Iowa City) qualified as rural, constituting 81% of the entire rural cohort. Rural participants were more likely to be white and less likely to have an annual income below $35,000 (Table 1). There was no difference in sex, age, pack-years of smoking history, body mass index (BMI), current smoking status, or education between rural and urban residents. Nonpulmonary medical comorbidities were similar at baseline (Table E3). Rural participants were more likely to report ever being employed in synthetic-fiber manufacturing, pig farming, carpentry, agriculture, and beauty care (Table E4). Rural participants reported a higher prevalence of regular contact with wheat flour or grain dust, metal dust or fumes, cotton dust, and animal feed (Table E5).
Table 1.
Cohort demographic and clinical characteristics
| n | Urban 1,480 | Rural 204 | P Value |
|---|---|---|---|
| Age, yr | 65.3 (8.1) | 65.4 (7.2) | 0.89 |
| Race | <0.001 | ||
| White | 1175 (79) | 200 (98) | |
| Black | 244 (16) | 1 (0) | |
| Asian | 22 (1) | 0 (0) | |
| American Indian | 7 (0) | 0 (0) | |
| Mixed | 23 (2) | 3 (1) | |
| Missing | 9 (1) | 0 (0) | |
| Female | 622 (42) | 96 (47) | 0.17 |
| Body mass index, kg/m2 | 27.3 (5.3) | 27.5 (4.9) | 0.73 |
| Pack-years smoked | 52.5 (26.6) | 54.0 (23.8) | 0.16 |
| Current smoker | 490 (34) | 66 (33) | 0.78 |
| Education | 0.12 | ||
| Eighth grade or below | 25 (1) | 6 (3) | |
| Trade/business/some high school | 154 (10) | 24 (12) | |
| High school graduate | 374 (25) | 56 (27) | |
| Trade/business after high school | 73 (5) | 16 (8) | |
| Some college | 444 (30) | 64 (31) | |
| Bachelor’s degree | 190 (13) | 21 (10) | |
| Post-Bachelor’s education | 215 (15) | 17 (8) | |
| Declined to answer | 2 (0) | 0 (0) | |
| Total yearly income | 0.005 | ||
| <$15,000 | 255 (17) | 35 (17) | |
| $15,000–$34,999 | 310 (21) | 28 (14) | |
| $35,000–$49,999 | 179 (12) | 38 (19) | |
| $50,000–$74,999 | 212 (14) | 36 (18) | |
| $75,000+ | 260 (18) | 24 (12) | |
| Declined to answer | 250 (17) | 42 (21) |
All values are mean (SD) or n (%) unless otherwise indicated.
Association between Rural Residence and COPD-related Outcomes
At the baseline visit, there was no difference in measures of postbronchodilator FEV1 between rural and urban participants (Table 2). Postbronchodilator FVC% predicted was lower in rural participants (85.5 vs. 89.2% predicted; P = 0.01). There was no difference in the BODE index, CAT scores, mMRC, or total count of AECOPD requiring medications or severe exacerbations in the 12 months before the baseline visit between the groups. Self-report of a physician’s diagnosis of asthma was less prevalent among rural participants than among urban ones (16% vs. 24%; P = 0.005), whereas self-reported diagnosis of emphysema, COPD, or chronic bronchitis did not differ between the two groups. Report of any inhaler use, short-acting bronchodilator use, or long-acting inhaler use did not differ between residence groups (Table E6). There was no difference in CT measures of emphysema or functional small-airways disease between rural and urban participants. Over the first year of follow-up, there was no difference in the rate of decline of FEV1 between rural and urban participants (−51 ± 213 ml vs. −42 ± 213 ml; P = 0.63).
Table 2.
Baseline pulmonary function and chronic obstructive pulmonary disease symptom characteristics
| Urban | Rural | P Value | |
|---|---|---|---|
| FEV1/FVC, postbronchodilator | 67.4 (17.5) | 68.5 (16.6) | 0.40 |
| FEV1, postbronchodilator | |||
| Absolute, L | 1.75 (0.80) | 1.70 (0.68) | 0.30 |
| % predicted | 61.4 (23.4) | 59.6 (20.6) | 0.25 |
| FVC, postbronchodilator | |||
| Absolute, L | 3.37 (1.08) | 3.26 (0.93) | 0.15 |
| % predicted | 89.2 (20.5) | 85.5 (17.3) | 0.005 |
| GOLD category* | 0.34 | ||
| A | 353 (24) | 59 (29) | |
| B | 545 (37) | 70 (34) | |
| C | 87 (6) | 8 (4) | |
| D | 486 (33) | 67 (33) | |
| CAT score, median (IQR) | 15 (12) | 15 (11) | 0.84 |
| CAT score ≥10 | 1,035 (73) | 137 (71) | 0.67 |
| Chronic bronchitis by SGRQ | 650 (46) | 101 (53) | 0.09 |
| mMRC, median (IQR) | 1 (1) | 1 (2) | 0.28 |
| mMRC ≥ 2 | 465 (32) | 63 (31) | 0.83 |
| BODE index | 2.08 (2.1) | 1.95 (1.7) | 0.23 |
| Total COPD exacerbations in prior 12 mo | 0.59 | ||
| 0 | 1,043 (72) | 139 (70) | |
| 1 | 213 (15) | 35 (18) | |
| 2+ | 184 (13) | 25 (13) | |
| Severe COPD exacerbations in prior 12 mo | 0.73 | ||
| 0 | 1,233 (84) | 168 (83) | |
| 1 | 158 (11) | 23 (11) | |
| 2+ | 69 (5) | 12 (6) | |
| Patient-reported physician diagnosis of chronic bronchitis | 374 (27) | 53 (27) | 0.96 |
| Patient-reported physician diagnosis of emphysema | 697 (50) | 97 (49) | 0.70 |
| Patient-reported physician diagnosis of COPD | 1,086 (76) | 144 (72) | 0.14 |
| Patient-reported physician diagnosis of chronic bronchitis, emphysema, or COPD | 1,193 (84) | 158 (79) | 0.091 |
| Diagnosis of asthma | 348 (25) | 30 (16) | 0.005 |
| Diagnosis of asthma as a child | 144 (10) | 14 (7) | 0.19 |
| Diagnosis of asthma as an adult | 196 (14) | 15 (8) | 0.018 |
| Asthma status | 0.014 | ||
| Current asthma | 251 (19) | 19 (10) | |
| Past asthma | 58 (4) | 6 (3) | |
| Never asthma | 1,043 (77) | 160 (86) |
Definition of abbreviations: BODE = body mass index, airflow obstruction, dyspnea, exercise; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GOLD = Global Initiative for Obstructive Lung Disease; IQR = interquartile range; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
All values are mean (SD) or n (%) unless otherwise indicated.
GOLD categorization using COPD Assessment Test criteria.
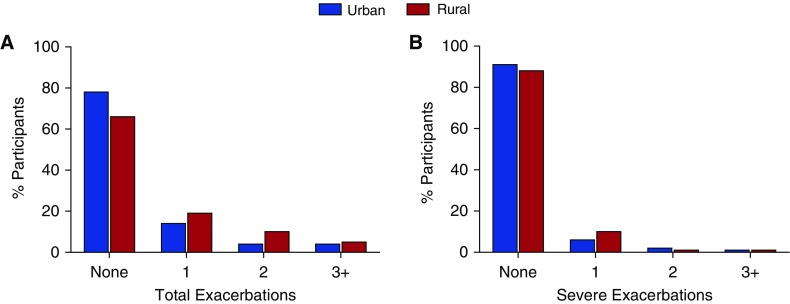
As shown in Figure 1, in the 1 year after follow-up from cohort entry, 78% of urban patients had no exacerbations, whereas 14% had one, 4% had two, and 5% had three or more AECOPD. Among the rural cohort, 66% had no exacerbations, whereas 19% had one, 10% had two, and 5% had three or more AECOPD (overall chi-square P = 0.002). For severe exacerbations, 91% of the urban participants had no severe exacerbations, 6% had one, 2% had two, and 1% had three or more severe AECOPD. In the rural cohort, 88% had no severe exacerbations, whereas 10% had one, 1% had two, and 1% had three or more severe exacerbations (overall chi-square P = 0.37). There was no difference in severe exacerbations between rural and urban participants who experienced an exacerbation during follow-up (34% vs. 36%; P = 0.71).
Figure 1.
Percentage of urban (blue bars) and rural (red bars) SPIROMICS participants experiencing total (A) or severe (B) COPD exacerbations within the first year of study follow-up. Overall chi-square P = 0.002 (total) and P = 0.37 (severe) exacerbations. COPD = chronic obstructive pulmonary disease.
Association between Rural Residence and Total Exacerbations
In univariate logistic regression, several factors were associated with increased odds of total exacerbations (Table E7). These included never having graduated from high school, a reported history of asthma, lower baseline FEV1, and a history of two or more exacerbations in the year before enrollment. Older age and male sex were associated with reduced odds of total AECOPD. In a univariate analysis, rural residence was associated with an 81% increase in the odds of total exacerbations (OR, 1.81 [95% CI, 1.32–2.47]; P < 0.001). A multivariate logistic regression model was generated that included age, race, sex, current smoking status, education, income, asthma history, baseline FEV1, number of exacerbations in the year preceding enrollment, and rural status (Table 3). In this model, white race, reported history of asthma, lower baseline FEV1, younger age, and two or more exacerbations in the year preceding enrollment were associated with significantly increased odds of total exacerbations. Rural residence was independently associated with a 70% increase in odds of total AECOPD (OR, 1.70 [95% CI, 1.34–2.56]; P = 0.012).
Table 3.
Multivariate regression models* for chronic obstructive pulmonary disease total exacerbations in the first 365 days of follow-up
| Logistic Regression |
Negative Binomial
Regression |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | IRR (95% CI) | P Value | |
| Age (per 10 yr) | 0.69 (0.57–0.84) | <0.001 | 0.74 (0.62–0.88) | 0.001 |
| White race (vs. others) | 1.79 (1.19–2.68) | 0.005 | 1.74 (1.23–2.46) | 0.002 |
| Male | 1.19 (0.88–1.63) | 0.27 | 1.06 (0.81–1.39) | 0.68 |
| Current smoking | 0.77 (0.55–1.08) | 0.13 | 0.95 (0.71–1.26) | 0.71 |
| Not graduating high school | 1.32 (0.86–2.01) | 0.20 | 1.32 (0.92–1.89) | 0.13 |
| Income < $50,000 | 1.03 (0.75–1.42) | 0.84 | 1.05 (0.8–1.39) | 0.71 |
| History of asthma | 1.53 (1.11–2.12) | 0.011 | 1.49 (1.12–1.98) | 0.006 |
| Baseline FEV1 (per 100 ml) | 0.93 (0.91–0.95) | <0.001 | 0.93 (0.91–0.95) | <0.001 |
| More than two exacerbations at baseline | 3.03 (2.11–4.36) | <0.001 | 2.71 (2–3.68) | <0.001 |
| Rural residence | 1.70 (1.13–2.56) | 0.012 | 1.46 (1.02–2.10) | 0.039 |
Definition of abbreviations: CI = confidence interval; FEV1 = forced expiratory volume in 1 second; IRR = incidence rate ratio; OR = odds ratio.
Adjusted for all covariates in the table.
Using negative binomial regression modeling, factors associated with an increased incidence rate of total AECOPD in the univariate analysis included not graduating from high school, reduced FEV1, two or more exacerbations in the year before enrollment, yearly income less than $50,000, and history of asthma (Table E7). Older age and male sex were associated with a lower incidence of total exacerbations. In a univariate analysis, rural residence was associated with a 46% increase in the incidence rate of total exacerbations (IRR, 1.46 [95% CI, 1.06–2.00]; P = 0.02). In a multivariate analysis (Table 3) incorporating the same covariates as in the logistic regression models, an increased incidence rate of total exacerbations was seen with white race, reported history of asthma, lower baseline FEV1, younger age, and two or more exacerbations in the year before enrollment. Rural residence was independently associated with a 46% higher incidence rate of total exacerbations (IRR, 1.46 [95% CI, 1.02–2.10]; P = 0.039).
Association between Rural Residence and Severe Exacerbations
In univariate logistic regression, several factors were associated with severe AECOPD (Table E8). These factors included not graduating from high school, yearly income less than $50,000, lower baseline FEV1, reported history of asthma, and having two or more exacerbations in the year before enrollment. Factors associated with lower odds of severe exacerbation included older age and white race. Rural residence was not associated with differential odds of severe exacerbation (OR, 1.38 [95% CI, 0.88–2.17]; P = 0.16). In multivariate logistic regression (Table 4), only a low baseline FEV1 and not graduating from high school were associated with an increased odds of severe exacerbation. Rural residence was not associated with odds of severe exacerbation at 1-year follow-up (OR, 1.15 [95% CI, 0.63–2.11]; P = 0.66) in a multivariate analysis.
Table 4.
Multivariate regression models* for severe chronic obstructive pulmonary disease exacerbations in the first 365 days of follow-up
| Logistic Regression |
Negative Binomial
Regression |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | IRR (95% CI) | P Value | |
| Age (per 10 yr) | 0.78 (0.60–1.02) | 0.07 | 0.79 (0.60–1.03) | 0.085 |
| White race (vs. others) | 0.85 (0.52–1.39) | 0.52 | 0.98 (0.59–1.63) | 0.93 |
| Male | 1.32 (0.86–2.02) | 0.21 | 1.37 (0.88–2.12) | 0.16 |
| Current smoking | 0.97 (0.61–1.54) | 0.89 | 1.01 (0.64–1.61) | 0.95 |
| Not graduating high school | 1.83 (1.09–3.07) | 0.022 | 1.36 (0.78–2.38) | 0.28 |
| Income < $50,000 | 1.32 (0.82–2.13) | 0.25 | 1.58 (0.98–2.55) | 0.06 |
| History of asthma | 1.24 (0.79–1.94) | 0.36 | 1.49 (0.94–2.36) | 0.09 |
| Baseline FEV1 (per 100 ml) | 0.91 (0.88–0.95) | <0.001 | 0.90 (0.87–0.94) | <0.001 |
| More than two exacerbations at baseline | 1.54 (0.95–2.51) | 0.083 | 1.55 (0.93–2.58) | 0.091 |
| Rural residence | 1.15 (0.63–2.11) | 0.66 | 1.14 (0.60–2.17) | 0.68 |
Definition of abbreviations: CI = confidence interval; FEV1 = forced expiratory volume in 1 second; IRR = incidence rate ratio; OR = odds ratio.
Adjusted for all covariates in table.
Using negative binomial regression modeling, factors associated with an increased incidence rate of severe exacerbations in a univariate analysis (Table E8) included not graduating from high school, annual income of less than $50,000, reported history of asthma, lower baseline FEV1, and two or more exacerbations in the year before enrollment. Factors associated with a reduced incidence rate of severe exacerbations in univariate analysis included age and white race. Rural residence was not associated with an increased incidence rate of severe exacerbations in the univariate analysis (IRR, 1.09 [95% CI, 0.65–1.83]; P = 0.75). In multivariate negative binomial regression (Table 4), lower baseline FEV1 was independently associated with a higher incidence of severe exacerbations. Rural residence was not independently associated with an increased incidence rate of severe exacerbations (IRR, 1.14 [95% CI, 0.60–2.17]; P = 0.68).
Impact of Agricultural Exposures on COPD Exacerbations and Rural Associations
Because substantial differences in occupation and occupational exposures were associated with rurality, a composite agricultural exposure variable was generated (see Methods). The prevalence of agriculture exposure was higher in rural compared with urban participants (25% vs. 12%; P < 0.001). In a univariate analysis, agricultural exposure was associated with increased odds (OR, 1.60 [1.19–2.15]; P = 0.002) and incidence (IRR, 1.75 [1.30–2.36]; P < 0.001) of total exacerbations. Agriculture exposure was also associated with increased odds (OR, 1.82 [1.16–2.85]; P = 0.009) and incidence (IRR, 1.94 [1.30–2.89]; P = 0.001) of severe exacerbations. Inclusion of agricultural exposure in multivariate models demonstrated that agriculture exposure was independently associated with increased odds (OR, 2.18 [95% CI, 1.51–3.16]; P < 0.001) and incidence (IRR, 1.53 [95% CI, 1.11–2.11]; P = 0.01) of total exacerbations (Table E9). Agricultural exposure was also independently associated with increased odds (OR, 2.23 [95% CI, 1.36–3.56]; P = 0.002) and incidence (IRR, 2.00 [95% CI, 1.20–3.33]; P = 0.01) of severe exacerbations. Addition of agricultural exposure to the multivariate analysis attenuated the association between rural residence and increased odds of total exacerbations (OR, 1.52 [95% CI, 1.00–2.32]; P = 0.05) and the incidence rate of total exacerbations (IRR, 1.39 [95% CI, 0.97–1.99]; P = 0.07).
Sensitivity Analysis
The association between rural residence and total exacerbations was attenuated with inclusion of study site (OR, 1.30 [95% CI, 0.84–2.01]; P = 0.24; and IRR, 1.21 [95% CI, 0.84–1.76]; P = 0.31). Restricting analysis to the four sites with the highest rural prevalence attenuated the association between rural residence and total exacerbations (OR, 1.33 [95% CI, 0.81–2.17]; P = 0.26; and IRR, 1.35 [95% CI, 0.92–1.97]; P = 0.12). Inclusion of a metal dust/fume exposure variable did not alter the rural associations, and in these models, metal fumes were not significantly correlated with AECOPD. Inclusion of prior exacerbation history could overadjust for future exacerbations. In multivariate models excluding prior exacerbation history, rural residence remained independently associated with increased odds (OR, 1.68 [95% CI, 1.18–2.39]; P = 0.004) and incidence (IRR, 1.42 [95% CI, 1.03–1.95]; P = 0.031) of total exacerbations. Rurality remained unassociated with severe exacerbations in all sensitivity models. Smoking history measured by pack-years was not independently associated with total exacerbations (P = 0.33 for logistic regression and P = 0.4 for negative binomial model) or severe exacerbations (P = 0.90 for logistic regression and P = 0.85 for negative binomial model). Given the difference in income categories at enrollment, multivariate models incorporating income with five categories were generated. These models did not impact the associations between rural status and total exacerbations, nor was income statistically significant in these models. There was no impact of alternative income covariate modeling on the lack of a rural association with increased odds of severe exacerbations. In negative binomial modeling, only the highest income category compared with the lowest was associated with a reduced incidence of severe exacerbations (IRR, 0.33 [95% CI, 0.14–0.75]; P = 0.008).
Discussion
In this analysis of individuals with spirometry-confirmed COPD participating in the observational SPIROMICS cohort, we observed that rural residence was independently associated with increased odds and incidence of AECOPD requiring treatment, but not severe exacerbations requiring an emergency room visit or hospitalization. Rural residence independently conferred a 70% increase in the odds and a 46% increase in the incidence of total AECOPD. The association between rural residence and exacerbation risk persisted after we accounted for potential confounders, including smoking patterns, lung function, prior exacerbation history, and income. Rural residence was associated with agriculture-related exposures; however, the association between rural residence and exacerbation risk was not fully explained after we accounted for agriculture-related exposures.
Investigations of rural healthcare outcomes in the United States have described disparate outcomes in chronic diseases such as heart disease, stroke, and cancer (28–30). From 1980 to 2014, the rates of mortality from chronic respiratory disease have increased, as illustrated by COPD-related deaths in the rural locales of central Appalachia (15). Rural patients with COPD across the United States have more frequent hospitalizations, more severe dyspnea, worse quality of life, and access to fewer pulmonary specialists (7, 12, 13, 31–34). Rural patients in the Veterans Affairs system have been shown to have increased mortality related to acute exacerbations (14). Furthermore, patients in rural and urban hospitals experience differential outcomes (35). The adverse pulmonary outcomes seen in rural patients with COPD are due to multiple factors, including a lack of adequate diagnoses and treatments, differential access to acute and chronic care, and limited availability of pulmonary specialists (13). This study extends previous investigations by demonstrating that rural status is independently associated with more frequent COPD exacerbations requiring treatment. This finding persisted after we accounted for medication use patterns, comorbidities, and markers of socioeconomic status that are often implicated in health outcome disparities in rural populations. Although income did differ between rural and urban participants, we observed no consistent association between income and AECOPD or the impact of income on rural associations with AECOPD. Poor access to healthcare is often implicated as a cause of adverse health outcomes in chronic disease, as demonstrated by the increased rate of hospital readmissions for patients with heart failure who do not have ready access to healthcare due to rurality (36). However, the participants enrolled in SPIROMICS may have been less subject to limited access than the general rural population given their involvement in a study at an academic center. Thus, the estimates of association between rurality and COPD outcomes in SPIROMICS may underestimate the strength of associations in the general rural population.
Environmental exposures may explain the association between rural residence and increased AECOPD risk. Globally, exposure to biomass fuel is associated with respiratory symptoms and poor pulmonary function (37). Individuals in professions with a high risk of exposure based on job exposure matrices have been shown to have more episodes of AECOPD requiring emergency department care and hospital admission (38, 39). Agricultural work produces dusts and vapors that can cause and/or exacerbate underlying lung disease (40, 41). Agricultural workers may be exposed to ambient gasses and particles such as ammonia, pesticides, bacterial products (including peptidoglycan and endotoxin), and fungal spores, which collectively play a role in the development of COPD and respiratory symptoms (39, 40, 42). Specific farm-animal exposures may impair respiratory health. Swine farm practices over the last few decades have been associated with worsening respiratory symptoms (43, 44). Exposure to poultry and livestock has been shown to increase the risk of COPD (45) and worsen respiratory symptoms (46, 47). Higher exposure to indoor endotoxin is also associated with reduced lung function in the U.S. farming population (48). Rural populations may be exposed to an increased load of ambient endotoxin and small particulate matter that may increase respiratory symptoms in patients with COPD (49, 50). The exposure profiles of the rural participants in SPIROMICS were substantially different from those of their urban counterparts, and agricultural exposures were statistically associated with AECOPD, suggesting that these exposures may underlie some of the increased exacerbation risk. FVC was also reduced in rural participants. This reduction, which was not explained by differences in BMI, may represent early interstitial changes associated with chronic respiratory exposures.
The findings presented here confirm the results of prior studies of AECOPD risk factors, including reduced FEV1 and previous exacerbation history (6, 51–56). Although white race was also associated with an increased exacerbation risk, the underrepresentation of other racial groups limits interpretation of these findings. Older age was associated with a reduced risk of total and severe AECOPD in this analysis. There are several potential explanations for this association: 1) in this cohort, older individuals had fewer exacerbations in the year before enrollment and less severe airflow obstruction, and were less likely to be current smokers; 2) younger participants may have better functional status, allowing them to work in the agricultural sector and thereby increasing their exposure profile; 3) the older population in this study may represent a “survivor” population with overall better health; and 4) the availability of Medicare insurance to the elderly may afford these patients better access to care and reduce the risk of exacerbations (57).
Although rural participants experienced more overall exacerbations than urban participants, severe exacerbations did not differ between these groups. There are several potential explanations for this observation. First, the factors associated with increased exacerbation rates in rural participants (i.e., agricultural exposures) may lead to milder exacerbation severity. Second, differential access to care among rural participants could lead to fewer hospital encounters, which are required to meet the criteria for severe exacerbation. Finally, this observation may be explained by the low overall prevalence of severe exacerbations in SPIROMICS, leading to insufficient power to detect this difference.
This study has several limitations. Although the reported data support the conclusion that agricultural exposures substantially contribute to pulmonary outcomes in rural participants, we are not able to fully determine the potential impact of access to healthcare on rural participants. We do not have robust data regarding health insurance at the time of recruitment or at younger ages. Because recruitment occurred primarily in urban academic medical centers, rural participants may have been required to travel to these centers for participation. We do not have data on distance from residence to recruitment center. However, participant enrollment suggests access to the recruiting medical centers. This mobility may not be reflective of a broader rural population. Alternatively, rural participants may have resided closer to urban centers, resulting in characteristics that more closely resemble urban individuals. These selection factors may result in a biased cohort of rural individuals who may not be representative of the larger U.S. rural population. Although this is supported by demographic similarities between the cohorts, agricultural and occupational profiles did differ between rural and urban participants. The impact of rural participants being more urbanized would attenuate any observed associations. In a more representative rural population, observed differences in pulmonary outcomes may be greater. Although there was no observed association between self-reported income and pulmonary outcomes, this metric may not accurately reflect expendable income, as living wages differ across multiple U.S. geographic regions. The relatively low number of rural participants and low exacerbation rates in this cohort may limit the power to detect a true association between rural residence and severe exacerbations. Self-reported data elements (exacerbation data, comorbidities, and medication use) are susceptible to recall bias. Occupational exposures were assessed as ever-exposure, but did not include the duration of exposure or current exposure status and may not fully reflect cumulative occupational exposures. It is possible that the geographical location in the country may impact some of our observed associations. This is supported by the attenuation of our observations when we adjusted for site. However, the site variable likely encompasses many confounders relevant to the rural-COPD relationship (geographic location, exposures, and access to care), making this attenuation somewhat nonspecific. As geocoding was performed only on baseline residence, this analysis does not account for geographic migration during follow-up.
In conclusion, we have observed that rural residence is associated with an increased risk of COPD exacerbations requiring treatment. Agriculture-related exposures were more prevalent in rural populations and strongly associated with an increased risk of COPD exacerbations, but did not fully explain the association between rural residence and COPD exacerbations. Through these findings, this study advances our understanding of the impact of rural residence on COPD exacerbations and identifies areas of further investigation into the prevention of pulmonary symptoms in rural patients with COPD. These findings highlight the need for robust epidemiological cohorts to investigate within the rural COPD population factors such as access to healthcare, the impact of regional air pollution and particulate matter, the influence of longitudinal agricultural exposures, and potential biomarkers and radiographic parameters specific to rural populations.
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at https://www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, Ph.D.; Wayne H. Anderson, Ph.D.; R. Graham Barr, M.D., Dr.P.H.; Eugene R. Bleecker, M.D.; Richard C. Boucher, M.D.; Russell P. Bowler, M.D., Ph.D.; Elizabeth E. Carretta, M.P.H.; Stephanie A. Christenson, M.D.; Alejandro P. Comellas, M.D.; Christopher B. Cooper, M.D., Ph.D.; David J. Couper, Ph.D.; Gerard J. Criner, M.D.; Ronald G. Crystal, M.D.; Jeffrey L. Curtis, M.D.; Claire M. Doerschuk, M.D.; Mark T. Dransfield, M.D.; Christine M. Freeman, Ph.D.; MeiLan K. Han, M.D., M.S.; Nadia N. Hansel, M.D., M.P.H.; Annette T. Hastie, Ph.D.; Eric A. Hoffman, Ph.D.; Robert J. Kaner, M.D.; Richard E. Kanner, M.D.; Eric C. Kleerup, M.D.; Jerry A. Krishnan, M.D., Ph.D.; Lisa M. LaVange, Ph.D.; Stephen C. Lazarus, M.D.; Fernando J. Martinez, M.D., M.S.; Deborah A. Meyers, Ph.D.; Wendy C. Moore, M.D.; John D. Newell Jr., M.D.; Laura Paulin, M.D., M.H.S.; Stephen Peters, M.D., Ph.D.; Elizabeth C. Oelsner, M.D., M.P.H.; Wanda K. O’Neal, Ph.D.; Victor E. Ortega, M.D., Ph.D.; Robert Paine III, M.D.; Nirupama Putcha, M.D., M.H.S.; Stephen I. Rennard, M.D.; Donald P. Tashkin, M.D.; Mary Beth Scholand, M.D.; J. Michael Wells, M.D.; Robert A. Wise, M.D.; and Prescott G. Woodruff, M.D., M.P.H. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, Ph.D., and Thomas Croxton, Ph.D., M.D.
Footnotes
Supported by National Institutes of Health (NIH) grants R01HL125432-01A1 (M.B.D.) and T32HL007106-41 (R.M.B.). SPIROMICS was supported by contracts from the National Heart, Lung, and Blood Institute, NIH (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C) and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc..; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; and Sunovion.
A complete list of SPIROMICS Investigators may be found before the beginning of the References.
Author Contributions: M.B.D. had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis. W.K.O.’N., P.G.W., J.A.K., R.G.B., M.K.H., F.J.M., M.T.D., R.E.K., R.P., E.R.B., and N.N.H. contributed to the conception and design of the study. A.J.G., W.K.O.’N., P.G.W., J.A.K., R.G.B., M.K.H., F.J.M., A.P.C., J.D.K., M.T.D., R.E.K., R.P., E.R.B., and N.N.H. contributed to the acquisition of the data. R.M.B., A.J.G., A.S.C., W.A., W.K.O.’N., A.A.L., J.D.K., L.M.P., N.N.H., and M.B.D. contributed to the drafting of the manuscript. R.M.B., A.J.G., W.A., W.K.O.’N., P.G.W., J.A.K., R.G.B., M.K.H., F.J.M., A.P.C., A.A.L., J.D.K., M.T.D., J.M.W., R.E.K., R.P., L.M.P., N.N.H., and M.B.D. contributed to revisions of the manuscript for critically important intellectual content. All of the authors approved this version of the manuscript to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the SPIROMICS Investigators, Neil E. Alexis, Wayne H. Anderson, R. Graham Barr, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Elizabeth E. Carretta, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Christine M. Freeman, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Laura Paulin, Stephen Peters, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Robert Paine, Nirupama Putcha, Stephen I. Rennard, Donald P. Tashkin, Mary Beth Scholand, J. Michael Wells, Robert A. Wise, Prescott G. Woodruff, The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were, Lisa Postow, and Thomas Croxton
References
- 1. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Chronic Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2015 [accessed 2017 Oct 31] Available from: www.goldcopd.org.
- 3. Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis. 2014;5:212–227. doi: 10.1177/2040622314532862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 6. Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. SPIROMICS Investigators. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia MC, Faul M, Massetti G, Thomas CC, Hong Y, Bauer UE, et al. Reducing potentially excess deaths from the five leading causes of death in the rural United States. MMWR Surveill Summ. 2017;66:1–7. doi: 10.15585/mmwr.ss6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Logan H, Guo Y, Dodd VJ, Muller K, Riley J., III The burden of chronic diseases in a rural north Florida sample. BMC Public Health. 2013;13:906. doi: 10.1186/1471-2458-13-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goeres LM, Gille A, Furuno JP, Erten-Lyons D, Hartung DM, Calvert JF, et al. Rural-urban differences in chronic disease and drug utilization in older Oregonians. J Rural Health. 2016;32:269–279. doi: 10.1111/jrh.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan EH, Graham ML, Folta SC, Seguin RA. A qualitative study of factors related to cardiometabolic risk in rural men. BMC Public Health. 2016;16:305. doi: 10.1186/s12889-016-2977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh JA, Yu S. Utilization due to chronic obstructive pulmonary disease and its predictors: a study using the U.S. National Emergency Department Sample (NEDS) Respir Res. 2016;17:1. doi: 10.1186/s12931-015-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29:s62–s69. doi: 10.1111/jrh.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Croft JB, Lu H, Zhang X, Holt JB. Geographic accessibility of pulmonologists for adults with COPD: United States, 2013. Chest. 2016;150:544–553. doi: 10.1016/j.chest.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Ann Intern Med. 2011;155:80–86. doi: 10.7326/0003-4819-155-2-201107190-00003. [DOI] [PubMed] [Google Scholar]
- 15. Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Kutz MJ, Huynh C, et al. US county-level trends in mortality rates for major causes of death, 1980-2014. JAMA. 2016;316:2385–2401. doi: 10.1001/jama.2016.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 19. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 20. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 21. Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez CH, Diaz AA, Meldrum C, Curtis JL, Cooper CB, Pirozzi C, et al. SPIROMICS Investigators. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017;195:464–472. doi: 10.1164/rccm.201604-0871OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iversen M, Pedersen B. Relation between respiratory symptoms, type of farming, and lung function disorders in farmers. Thorax. 1990;45:919–923. doi: 10.1136/thx.45.12.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langley RL. Consequences of respiratory exposures in the farm environment. N C Med J. 2011;72:477–480. [PubMed] [Google Scholar]
- 26. Department of Commerce. Urban area criteria for the 2010 census. Fed Regist. 2011;76:53029–53043. [Google Scholar]
- 27. Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, et al. COPDGene and SPIROMICS Investigators ‡. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng D, You W, Mills B, Alwang J, Royster M, Anson-Dwamena R. A closer look at the rural-urban health disparities: insights from four major diseases in the Commonwealth of Virginia. Soc Sci Med. 2015;140:62–68. doi: 10.1016/j.socscimed.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 29. Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality. Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc. 2017;14:403–411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 30. Lauckner HM, Hutchinson SL. Peer support for people with chronic conditions in rural areas: a scoping review. Rural Remote Health. 2016;16:3601. [PubMed] [Google Scholar]
- 31. Jackson BE, Suzuki S, Lo K, Su F, Singh KP, Coultas D, et al. Geographic disparity in COPD hospitalization rates among the Texas population. Respir Med. 2011;105:734–739. doi: 10.1016/j.rmed.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim M, Ren J, Tillis W, Asche CV, Kim IK, Kirkness CS. Explaining the link between access-to-care factors and health care resource utilization among individuals with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:357–367. doi: 10.2147/COPD.S95717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Faganello MM, Tanni SE, Sanchez FF, Pelegrino NR, Lucheta PA, Godoy I. BODE index and GOLD staging as predictors of 1-year exacerbation risk in chronic obstructive pulmonary disease. Am J Med Sci. 2010;339:10–14. doi: 10.1097/MAJ.0b013e3181bb8111. [DOI] [PubMed] [Google Scholar]
- 34. Joo MJ, Lee TA, Weiss KB. Geographic variation in chronic obstructive pulmonary disease exacerbation rates. J Gen Intern Med. 2007;22:1560–1565. doi: 10.1007/s11606-007-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahsan S, Dooley C, Gholitabar N, Gholitabar F, Matthew J. A nationwide analysis of outcomes of COPD exacerbations between rural and urban hospitals. Chest. 2017;152:A773. [Google Scholar]
- 36. Muus KJ, Knudson A, Klug MG, Gokun J, Sarrazin M, Kaboli P. Effect of post-discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10:1447. [PubMed] [Google Scholar]
- 37. Balcan B, Akan S, Ugurlu AO, Handemir BO, Ceyhan BB, Ozkaya S. Effects of biomass smoke on pulmonary functions: a case control study. Int J Chron Obstruct Pulmon Dis. 2016;11:1615–1622. doi: 10.2147/COPD.S109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD. Short term effects of particle exposure on hospital admissions in the mid-Atlantic states: a population estimate. PLoS One. 2014;9:e88578. doi: 10.1371/journal.pone.0088578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paulin LM, Diette GB, Blanc PD, Putcha N, Eisner MD, Kanner RE, et al. SPIROMICS Research Group. Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557–565. doi: 10.1164/rccm.201408-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med. 2016;22:144–149. doi: 10.1097/MCP.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bailey KL, Meza JL, Smith LM, Von Essen SG, Romberger DJ. Agricultural exposures in patients with COPD in health systems serving rural areas. J Agromed. 2007;12:71–76. doi: 10.1300/J096v12n02_10. [DOI] [PubMed] [Google Scholar]
- 42. Alif SM, Dharmage SC, Benke G, Dennekamp M, Burgess JA, Perret JL, et al. Occupational exposure to pesticides are associated with fixed airflow obstruction in middle-age. Thorax. 2017;72:990–997. doi: 10.1136/thoraxjnl-2016-209665. [DOI] [PubMed] [Google Scholar]
- 43. Donham KJ, Wing S, Osterberg D, Flora JL, Hodne C, Thu KM, et al. Community health and socioeconomic issues surrounding concentrated animal feeding operations. Environ Health Perspect. 2007;115:317–320. doi: 10.1289/ehp.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pavilonis BT, Sanderson WT, Merchant JA. Relative exposure to swine animal feeding operations and childhood asthma prevalence in an agricultural cohort. Environ Res. 2013;122:74–80. doi: 10.1016/j.envres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guillien A, Puyraveau M, Soumagne T, Guillot S, Rannou F, Marquette D, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2016;47:95–103. doi: 10.1183/13993003.00153-2015. [DOI] [PubMed] [Google Scholar]
- 46. Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Animal production and wheeze in the Agricultural Health Study: interactions with atopy, asthma, and smoking. Occup Environ Med. 2003;60:e3. doi: 10.1136/oem.60.8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoppin JA, Umbach DM, Long S, Rinsky JL, Henneberger PK, Salo PM, et al. Respiratory disease in United States farmers. Occup Environ Med. 2014;71:484–491. doi: 10.1136/oemed-2013-101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carnes MU, Hoppin JA, Metwali N, Wyss AB, Hankinson JL, O’Connell EL, et al. House dust endotoxin levels are associated with adult asthma in a U.S. farming population. Ann Am Thorac Soc. 2017;14:324–331. doi: 10.1513/AnnalsATS.201611-861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pavilonis BT, Anthony TR, O’Shaughnessy PT, Humann MJ, Merchant JA, Moore G, et al. Indoor and outdoor particulate matter and endotoxin concentrations in an intensely agricultural county. J Expo Sci Environ Epidemiol. 2013;23:299–305. doi: 10.1038/jes.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB. Respiratory Effectiveness Group. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439–2450. doi: 10.2147/COPD.S94259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Margüello MS, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, et al. Independent effect of prior exacerbation frequency and disease severity on the risk of future exacerbations of COPD: a retrospective cohort study. NPJ Prim Care Respir Med. 2016;26:16046. doi: 10.1038/npjpcrm.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomioka R, Kawayama T, Suetomo M, Kinoshita T, Tokunaga Y, Imaoka H, et al. “Frequent exacerbator” is a phenotype of poor prognosis in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:207–216. doi: 10.2147/COPD.S98205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Make BJ, Eriksson G, Calverley PM, Jenkins CR, Postma DS, Peterson S, et al. A score to predict short-term risk of COPD exacerbations (SCOPEX) Int J Chron Obstruct Pulmon Dis. 2015;10:201–209. doi: 10.2147/COPD.S69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi: 10.2147/COPD.S122382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zider AD, Wang X, Buhr RG, Sirichana W, Barjaktarevic IZ, Cooper CB. Reduced COPD exacerbation risk correlates with improved FEV1: a meta-regression analysis. Chest. 2017;152:494–501. doi: 10.1016/j.chest.2017.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simmering JEPL, Polgreen LA, Comellas AP, Cavanaugh JE, Polgreen PM. Identifying patients with COPD at high risk of readmission. Chronic Obstr Pulm Dis (Miami) 2016;3:729–738. doi: 10.15326/jcopdf.3.4.2016.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]