Abstract
Background
The benefits of neurosurgery in Tourette Syndrome (TS) are still incompletely understood. Prefrontal cortical electrical stimulation offers a less invasive alternative to deep brain stimulation.
Objective
To perform a pilot assessment on safety and efficacy of prefrontal cortical bilateral electrical stimulation in TS using clinical and brain metabolic assessments.
Methods
Four adult TS patients underwent tic assessment using the Yale Global Tic Severity Scale and the Rush Video Rating Scale at baseline and 1, 3, 6, and 12‐months after implant; whereas FDG‐PET scans were acquired at baseline and after 6 and 12 months.
Results
Tic clinical scores were improved at 6 months after implant, meanwhile they showed a tendency to re‐emerge at the 12‐month follow‐up. There was a correlation between FDG‐PET and tics, mainly consisting in a reduction of baseline brain hypermetabolism, which paralleled tic score reduction.
Conclusion
Epidural stimulation in TS is safe and yields a modulation of tics, paralleled by FDG‐PET metabolic modulation.
Keywords: cerebral metabolism, prefrontal cortical stimulation, SPM analysis, tics, Tourette's syndrome
Introduction
Tourette disorder (TS) is an early‐onset neurological disorder characterized by motor and vocal tics that have persisted for more than one year since first tic onset.1 First‐line treatment is based on behavioral and medical therapy; deep brain stimulation (DBS) has been used for severe cases refractory to medical treatment2 as it can provide symptomatic improvement, but is not devoid of important adverse events.3 Moreover, there is uncertainty concerning the most appropriate DBS target, suggesting further treatment options are worth being explored. Among these, epidural cortical brain stimulation emerges as a possible alternative to DBS in hyperkinetic movement disorders.4, 5 Tics are the hallmark movement disorder observed in TS.6 Various clinical, neuroimaging, and neurophysiological observations indicate that abnormal functioning of the basal ganglia and related thalamocortical circuits constitutes the major pathophysiological basis of tics in TS.7 It is assumed that the basal ganglia play a major role in the “timing and sequencing” of motor and behavioral programs by selecting desired and by suppressing unwanted programs to be executed. In this way, the basal ganglia “assist” the prefrontal cortex by facilitating or suppressing behavioral or motor responses. Evidence that the motor cortex is hyperexcitable in TS patients comes from neurophysiological studies. Transcranial magnetic stimulation (TMS) studies demonstrated several changes of motor cortex excitability in TS, including reduced short‐interval intracortical inhibition, reduced short latency sensory afferent inhibition, and shortened cortical silent periods.8
Imaging studies in TS have revealed that, during tic execution, glucose metabolism is increased in premotor, prefrontal, and motor cortex, indicating neural hyperactivity in these brain areas.9, 10 A recent fMRI study in TS has shown that premotor and prefrontal areas have increased activation for hand motor tasks and that activation during motor imagery (a mental rehearsal of a motor task) also involved rostral prefrontal and temporoparietal regions in the right hemisphere.11 Positron emission tomography (PET) with 18F‐fluorodeoxyglucose (FDG‐PET) played an important role in revealing the involvement of brain circuits outside the basal ganglia.12 The main consistent finding in TS is a concurrent increase of glucose metabolism in the lateral and medial premotor cortices and cerebellum combined with a decrease in the basal ganglia and thalamus.13, 14
Building on these premises, we performed a proof‐of‐concept clinical trial on the safety and efficacy of bilateral epidural prefrontal cortex stimulation in TS patients.
Materials and Methods
We selected patients with a diagnosis of TS based on the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR) criteria:15 the patient was over the age of 18; the chronic tic disorder was adversely affecting quality of life; failure to reduce the severity of tics or intolerable side effects using adequate doses of oral medications administered for at least 3 months; and the absence of cognitive deficits or psychiatric disorders of Axis I. Patients with comorbid attention deficit hyperactivity disorder were excluded. Additional exclusion criteria were: significant structural brain lesions on imaging studies, history of severe head trauma preceding the onset of tics, use of dopamine receptor blockers before the recognition of tics, previously implanted electrical devices, electroconvulsion therapy in the past 24 months, suicide attempts in the past 12 months, significant sociopathic personality, and current or planned pregnancy. The study protocol was approved by the institutional review board and the patients signed an informed consent.
A multidisciplinary team, composed of a neurologist, a neurosurgeon, a neuropsychologist, and a psychiatrist examined all the patients. An independent neurologist and a psychiatrist reviewed screening data for each patient and certified their suitability for participation in the trial. The following scales were used: Yale Global Tic Severity Scale (YGTSS, which includes subscales for motor and phonic tics),16 Rush Video Rating Scale (RVRS),17 and the Hamilton Rating Scales for Depression and Anxiety (HAM‐D; HAM‐A).18, 19
An assessment of the acute effects stimulation with a frequency of 60 Hz and a pulse width of 60 μsec was initially performed in all patients one day after surgery. Based on previous reports,4, 20 stimulation settings were kept within the following ranges: amplitude 2.5 to 3.0 V; pulse width 60 to 90 μs; frequency 50 to 120 Hz. The internal pulse generators were set at constant voltage. At each visit, stimulation settings were reviewed and optimized to minimize side effects and maximize potential motor improvement (Supporting Table 1). The same stimulation settings were applied on both sides.
The epidural placement of the electrodes was performed under general anesthesia, as previously described.5 The cortical target was the prefrontal area, set between Brodmann areas 9 and 46. Cortical stimulation was delivered continuously for 24 hours, using individualized stimulation settings (Supporting Table 1).
FDG‐PET Study
FDG‐PET acquisitions were performed at baseline, 6‐months and 12‐months after implant, as previously described.21
Voxel‐based Single Subject Analysis
Single patient FDG‐PET scans were spatially normalized to a specific FDG‐PET template22 to allow voxel‐based comparisons based on a validated optimized statistical parametric mapping (SPM) procedure.21 This procedure statistically compares single‐subject FDG‐PET scans with a large database (N = 112) of healthy controls scans, delivering SPM‐t maps showing patterns of regional metabolic increases and decreases at the single‐subject level, which are thresholded at p < 0.05, corrected for multiple comparisons with Family‐Wise Error (FWE) correction.
Voxel Level Analysis
We adopted a second level flexible factorial design (repeated measures ANOVA) in SPM5, setting standardized clinical scales17, 23 as variables of interest. In the present design, three time levels (T0, T1, T2) were entered as factors, adding a covariate of interest‐containing clinical scores on the chosen scale and testing for positive and negative correlations. As for the within‐subject factor, given the dependency between the measurements over time, variance was coded as dependent and equal, thus accounting for non‐sphericity violations.24
The single‐subject SPM contrast images of hypermetabolism resulting from the first level analyses were used for the correlation analysis. In the present design, a positive correlation indicates that decreases in glucose metabolism are associated with decreases in tic severities, and vice versa. On the other hand, a negative correlation indicates that increases in glucose metabolism are associated with decreases in tic severities (e.g., interpreted as compensatory effect), and vice versa. A statistical threshold of p < 0.001 (uncorrected for multiple comparisons) was adopted, with a minimum cluster extent set at k:100 voxels. Additionally, the results were deemed significant only if surviving cluster‐level FWE‐corrected p < 0.05 threshold.
Region of Interest Analysis
Regions of Interest (ROIs) were obtained with the Wake Forest University PickAtlas (WFUPickAtlas) toolbox,25 adopting the Automated Anatomical Labeling atlas26 for SPM, while the ROI analysis was run with MarsBar Toolbox for SPM. Due to the limited number of patients, we decided for a confirmatory ROIs approach based on a priori hypothesis on the basis of the results in previous literature on TS, reporting alterations in specific neural networks.7, 13, 14, 27, 28 We focused on the following regions: (1) the sensorimotor network, including primary sensorimotor and the premotor cortex; (2) the associative cortices, namely the dorsolateral frontal cortex (inferior, middle, superior frontal gyri, and frontal operculum), the lateral temporal cortex (inferior, middle, and superior temporal gyri), the inferior and superior parietal cortex, and the rolandic operculum; (3) the limbic network, including insula, hippocampal structures, anterior cingulate cortex, and orbitofrontal cortex (inferior, middle, and superior frontal orbital definitions of the AAL atlas); and (4) the caudate nucleus, putamen, and the thalamus. At consistence with voxel‐based analysis, the same statistical threshold of p < 0.001 was adopted. Additionally, at the ROI‐level, a Bonferroni multiple comparisons correction was performed considering the total number of included ROIs.
Results
The population recruited consisted of four men aged 36.3 years (± 8.6 SD) at implant. Their demographic and clinical characteristics are summarized in Table 1. All had a severe tic disorder leading to functional impairment; in all, tics were considered the main source of disability and a main limitation to social integration and quality of life. Behavioral abnormalities were instead minor and not significantly disabling. Although none of them fulfilled DSM criteria for anxiety or depression, patients three and four scored positive at HAM‐A and patient four also at HAM‐D. Continuous epidural stimulation was well tolerated with no reported side effects. There were no surgical complications, device failures, or malfunctions.
Table 1.
Demographic and clinical features of the patients
| Patient number | Age at onset | Age at implant | Symptoms at onset | OCD | HAM‐A baseline |
HAM‐D baseline |
RVRS baseline | YGTSS Motor baseline | YGTSS Vocal baseline | Baseline medication | Medication after Cortical Stimulation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 14 | 43 | Motor tics: head, shoulder and arms Phonic Tic: Developed Later |
None | 15 | 3 | 12 | 16 | 9 | Pimozide, tetrabenazine, sulpiride, aripiprazole | None |
| P2 | 7 | 35 | Motor tics: limbs Phonic Tic: none |
None | 16 | 7 | 15 | 16 | 13 | Clonidine, olanzapine, quetiapine, risperidone | Quetiapine, fluvoxamina |
| P3 | 8 | 23 | Motor tics: head, shoulders, right limbs Phonic Tic: none |
None | 21 | 10 | 10 | 18 | 11 | Prazepam, pimozide, trihexyphenidyl, sulpiride, aripiprazole, haloperidol | Haloperidol |
| P4 | 8 | 37 | Motor tics: limbs Phonic Tic: none |
None | 22 | 15 | 14 | 25 | 12 | Haloperidol, pimozide, clonidine, paroxetine, sertraline, tetrabenazine | Quetiapine, Valproic acid |
Abbreviations: OCD (obsessive compulsive disorder according to DSMIV‐TR); HAM‐A, Hamilton scale for anxiety; HAM‐D, Hamilton scale for depression.
The individual RVRS scores are shown in Supporting Table 2. In summary, at month 6 RVRS improved in patient 1 (P1) by 33% compared to baseline; in patient 2 (P2) by 26%; in patient (P3) by 40%; and patient 4 (P4) by 7%. At 12 months, P1 and P4 stayed stable, while P2 and P3 relapsed compared to month 6. At 1 year, P1 was still 13% better than baseline, while P3 was 10% worse than baseline.
The YGTSS scores are shown in Supporting Table 2 with all components and totals. At month 6, the YGTSS total motor and total phonic tic score was reduced in P1by 52% compared to baseline, in P2 by 20%, in P3 by 62% and in P4 by 2.7%. At month 12, this score was still improved in P1 by 60% compared to baseline, it had deterioration in P2 by 7%, again improved by 24% in P3 and by 3% in P4. At month 6, the YGTSS Global score improved in P1 by 28% compared to baseline, in P2 by 44%, in P3 by 81% and in P4 by 12%; at month 12, there was a 77% improvement of P1 compared to baseline, a 3% deterioration of P2, a 28 % improvement and of P3, and a 13 % improvement of P4.
HAM‐A and HAM‐D values at baseline and at follow‐up visits are reported in Supporting Fig. 1.
At baseline, the FDG‐PET SPM analysis at single‐subject level did not reveal a significant reduction in regional brain glucose hypometabolism compared to healthy controls (pFWE < 0.05, Family‐Wise Error corrected for multiple comparisons). A significant (pFWE < 0.05) increase of brain metabolism was otherwise found in the primary sensorimotor cortex, premotor cortex, frontal and rolandic operculum, orbitofrontal cortex, lateral temporal cortex and parahippocampal structures, inferior parietal cortex, left primary auditory cortex, insula and posterior cingulate cortex, with some cerebellar involvement. These metabolic increases were more evident in P1 and P2 compared to P3 and P4, although anatomically co‐localized (Fig. 1B‐C).
Figure 1.
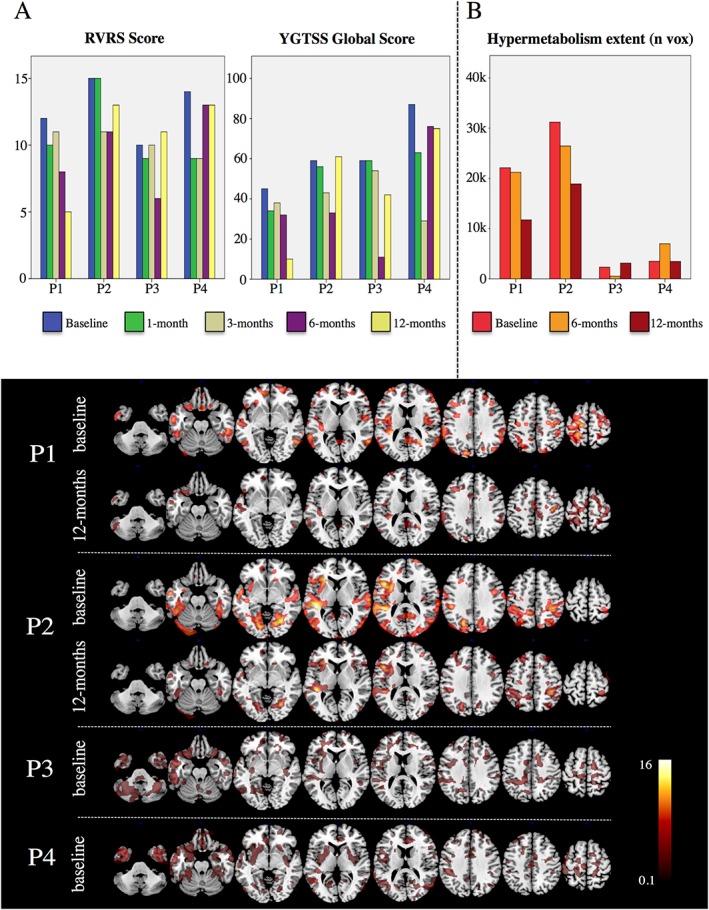
Longitudinal assessment of clinical and FDG‐PET brain metabolism in individual patients (listed from P1 to P4).
(A) Barplots show longitudinal variations of RVRS scores and YGTSS global scores at preset follow‐up times; (B) Barplot showing the longitudinal variations of hypermetabolic cluster extent (0‐40K number of voxels) at preset follow‐times, as measured at pFWE < 0.05 (minimum cluster extent 100 voxels); (C) Hypermetabolic patterns observed in patients at baseline and 12 months (only for patients 1 and 2, who showed the most significant changes at follow‐up. Patients 3‐4 showed limited regional metabolic increases and negligible FDG‐PET metabolism fluctuations a5 follow up (see Fig. 1B). SPM‐t maps (pFWE < 0.05) are overlaid on a standard anatomical T1 template with a multislice transaxial view.
The extent of the significant regional hypermetabolism was reduced 12 months after implant by 47% and 40%, respectively, in patients one and two. Instead, P3 and P4, who presented less severe hypermetabolism at baseline, had inconsistent changes after 12 months of cortical stimulation.
ROI‐based and voxel‐level correlation analyses showed consistent results. At the voxel‐level, significant association (p < 0.001 uncorrected, p < 0.05 FWE‐corrected at the cluster‐level) was found between clinical improvement and regional brain metabolism reduction, for both the RVRS and YGTSS scores (Fig. 2). Although these associations did not survive primary FWE‐correction for multiple comparisons, p < 0.001 was considered as a reasonably stringent threshold to balance Type I and Type II errors.
Figure 2.
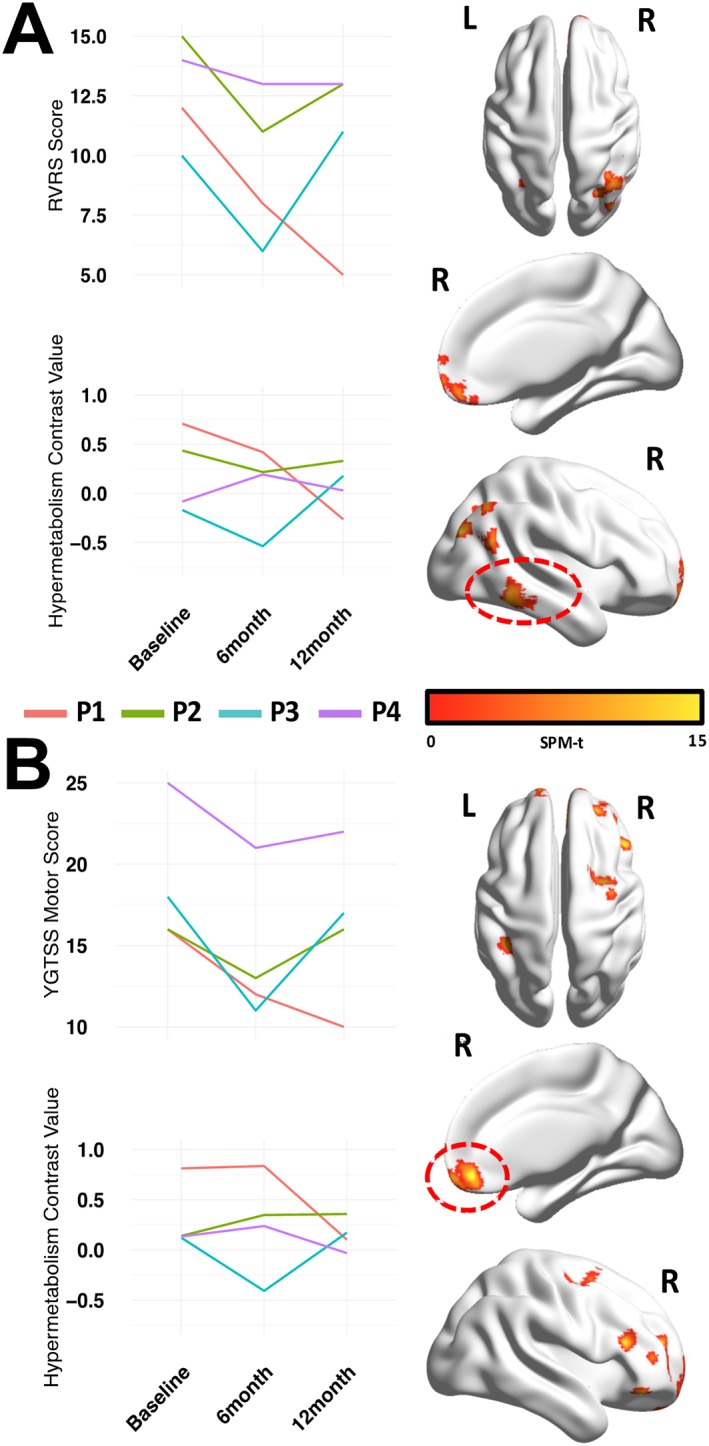
Clusters of significant association between changes in brain metabolism and changes in RVRS (panel A) and YGTSS Motor scores (panel B) at baseline, 6 and 12 months after implant.
Colored lines refer to individual patients (listed from P1 to P4). Figs on the right show thresholded (p < 0.001 uncorrected, k:100, p < 0.05 FWE cluster‐level) SPM‐t maps overimposed on an ICBM152 smoothed surface with BrainNet Software.34 Areas highlighted by the dashed red circle represent clusters with highest significance levels (lowest cluster‐corrected p‐value) in the model. Graphs on the left show comparisons between changes in brain glucose metabolism in the circled clusters and clinical scores measured at the different follow‐up time‐points.
ROI‐based analyses provided consistent results at the same statistical threshold, additionally surviving Bonferroni multiple comparisons correction. Table 2 and Supporting Table 3 provide further anatomical and statistical details.
Table 2.
Confirmatory ROI analysis. Significant correlations between decreases in regional HYPERmetabolism and clinical improvements are shown with respective p‐values and ROI xyz MNI stereotaxic coordinates. Significance for all the regions survived Bonferroni correction for multiple comparisons
| Correlations | Anatomical area | p‐value | x | Y | z |
|---|---|---|---|---|---|
| RVRS total score ‐ Positive correlation | Temporal_Inf_R | 0.000547 | 53 | −32 | −24 |
| YGTSS Motor – Positive correlation | Frontal_Mid_R | 0.000659 | 37 | 32 | 33 |
| Frontal_Sup_R | 0.000517 | 22 | 30 | 42 | |
| Frontal_Orb_R | 0.000479 | 33 | 41 | −14 |
A variability of tic scores was observed between patients after implant, consisting in improvements or relapses of symptomatology that were paralleled by decreases or increases of cortical metabolism in specific brain regions (Fig. 2). Such metabolic changes were significant in the following cortical regions with a marked prevalence in the right hemisphere: orbitofrontal, dorsolateral frontal, inferior/middle temporal and inferior parietal cortex.
Discussion
This study explored the safety and efficacy of bilateral epidural prefrontal cortex stimulation administered for one year to TS patients without psychiatric features. We aimed at influencing the generation of tics by stimulating frontal Brodmann areas 9 and 46. This minimally invasive treatment was well tolerated. P1 showed an overall marked reduction of tics, with an almost complete cessation of moderate to severe tics (Fig. 1A). However, in the remaining patients, an early beneficial response was followed by a progressive partial relapse after the first month. These results disappointed the hypothesis underlying the study design.
Considering that a YGTSS total tic severity score reduction of 35% is considered a reliable measure for determining treatment response,29 only P1 achieved a consistent tic reduction above such threshold. P3 had an improvement above 35% at month 6, which was not confirmed at month 12. The other patients were below such threshold.
Motor and phonic tics improved during the first 6 months of treatment and relapsed afterward in all but P1. By contrast, the main effect on FDG PET metabolism was at 12 months rather than at the time of maximum clinical effect. One year after implant, neuroimaging changes were still appreciable. FDG‐PET data at baseline showed metabolic increases in sensorimotor, premotor, and primary auditory cortex, with some cerebellar involvement, as expected.7, 14, 28
All the stimulation settings tested were safe. No immediate changes of tic severity were observed when stimulation settings were changed. Of note, comparable changes in stimulation settings were adopted for all the patients, in particular for the first three patients (Supporting Table 1), in terms of gradual increases of both frequency and amplitude of the stimulation along the follow‐up. Despite this, the patients presented individual variable trajectories in terms of modulation of metabolism, thus making it unlikely that stimulation changes could have played a primary role in the PET results.
We found a correlation between changes in FDG‐PET brain metabolism and variations in RVRS and YGTSS motor scores, mainly consisting in a reduction of baseline regional brain hyper‐metabolism that paralleled tic score reduction. This suggests that prefrontal cortical stimulation modulated brain metabolism in correlation with clinical improvement, an action likely exerted by influence on the cortico‐striato‐pallido‐thalamic network that is abnormal in TS.30 Changes in motor scores were directly associated with metabolic changes in premotor regions, which are known to be crucial for motor imagery31 and are hyperactive during tics.10 To sum up, when clinical amelioration was observed, it was associated with a modulation of brain glucose metabolism. This finding also supports a pathogenic role of abnormal premotor and motor cortex activations in the pathophysiology of tics, possibly dysregulating prefrontal suppression of involuntary movements.32
In our study, we selected patients who had no significant psychiatric comorbidity such as OCD and depression which indeed seem to benefit significantly from DBS. Given to the wide‐ranging of the clinical course of TS, the lower percentage of clinical improvement might be due to the restriction on the type of patients enrolled in this study. At baseline, two patients had a HAM‐A total score of 21 and 22, respectively and one had a HAM‐D total score of 15. These patients did not fulfil DSM‐IV‐TR criteria for Depressive Disorder or Anxiety Disorder. In all, however, anxiety and depressive scores improved after surgery (Supporting Fig. 1).
Prefrontal cortical stimulation may possibly be useful in a subset of TS patients with a predominantly motor phenotype. This proof‐of‐concept study in a highly selected TS population provides heterogeneous outcomes. Two main issues need to be addressed before planning further controlled trials based on cortical stimulation. First, long‐term efficacy is still untested: this one‐year observation showed a partial relapse that warrants longer follow‐up observations. Second, heterogeneity of tic improvement following prefrontal cortical stimulation suggests that different brain network abnormalities may produce overlapping phenotypes. This may indicate that better‐refined selection of patients, based on some yet undiscovered endophenotype, may be needed. A similar explanation may also apply to inconsistent outcomes observed after DBS implants in TS.33
Whether prefrontal cortical stimulation is specifically indicated in tics or applies instead to the broader category of hyperkinetic disorders is a matter for investigation. In patients with “mobile” cervical dystonia motor and FDG‐PET improvement were observed over one year after implant.5 In patients with fixed cervical dystonia, including a previously published patient who had transient benefit after cortical stimulation,20 no appreciable improvement occurs. Tics have different pathophysiology from dystonia, although they may have similarities in the common final pathway to the motor cortex.
Author Roles
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
D.P.: 3A, 3B
S.L.: 1B, 1C, 3A, 3B
L.I.: 2B
P.A.: 2B
O.G.: 3B
A.F.: 1C, 3B
A.A.: 1A, 1B, 2C, 3B
Disclosures
Ethical Compliance Statement: The ethics committee of the Istituto Neurologico Carlo Besta approved the study. Patient consent was obtained in written form and documented in the hospital record. The authors confirm that have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This research has received funding from the Italian Ministero della Salute (ALISEI IVASCOMAR Project 2013‐2017).
Financial Disclosures for the previous 12 months: Orsola Gambini reports speaker's honoraria from Chiesi. Alberto Albanese has active grant support from Italian government (Ministero della Salute) and private philanthropic organizations (Cariplo Foundation, Jacques and Gloria Gossweiler Foundation, Beneficentia Stiftung). He has received speaker's honoraria from Boston Scientific, Chiesi, Medtronic Inc, Merz Pharma, Ipsen Pharma, Zambon, Teva Pharmaceuticals.
Supporting information
Supporting information may be found in the online version of this article.
Supporting File 1. This file contains Supporting Tables 1‐3 and Supporting Fig. 1.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Association AP. Diagnostic and statistical manual of mental disorders (DSM‐5): Washington DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 2. Schrock LE, Mink JW, Woods DW, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. 2015;30:448–471. [DOI] [PubMed] [Google Scholar]
- 3. Martinez‐Ramirez D, Jimenez‐Shahed J, Leckman JF, et al. Efficacy and safety of deep brain stimulation in Tourette syndrome: the International Tourette Syndrome Deep Brain Stimulation public database and registry. JAMA Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurology. 2007;6:279–286. [DOI] [PubMed] [Google Scholar]
- 5. Lalli S, Piacentini S, Franzini A, et al. Epidural premotor cortical stimulation in primary focal dystonia: clinical and 18F‐fluoro deoxyglucose positron emission tomography open study. Mov Disord. 2012;27:533–538. [DOI] [PubMed] [Google Scholar]
- 6. Singer HS. Tourette syndrome and other tic disorders. Handb Clin Neurol. 2011;100:641–657. [DOI] [PubMed] [Google Scholar]
- 7. McNaught KS, Mink JW. Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol. 2011;7:667–676. [DOI] [PubMed] [Google Scholar]
- 8. Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. JNeurol Neurosurg Psychiatry. 2009;80:29–34. [DOI] [PubMed] [Google Scholar]
- 9. Stern E, Silbersweig DA, Chee KY, et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–748. [DOI] [PubMed] [Google Scholar]
- 10. Peterson BS. Neuroimaging studies of Tourette syndrome: a decade of progress. Adv Neurol. 2001;85:179–196. [PubMed] [Google Scholar]
- 11. Zapparoli L, Porta M, Gandola M, et al. A functional magnetic resonance imaging investigation of motor control in Gilles de la Tourette syndrome during imagined and executed movements. Eur J Neurosci. 2016;43:494–508. [DOI] [PubMed] [Google Scholar]
- 12. Alongi P, Iaccarino L, Perani D. PET neuroimaging: insights on dystonia and Tourette syndrome and potential applications. Front Neurol. 2014;5:183‐181–183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eidelberg D, Moeller JR, Antonini A, et al. The metabolic anatomy of Tourette's syndrome. Neurology. 1997;48:927–934. [DOI] [PubMed] [Google Scholar]
- 14. Pourfar M, Feigin A, Tang CC, et al. Abnormal metabolic brain networks in Tourette syndrome. Neurology. 2011;76:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Association AP. DSM‐IV‐TR Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association, 2000. [Google Scholar]
- 16. Storch EA, Murphy TK, Geffken GR, et al. Reliability and validity of the Yale Global Tic Severity Scale. Psychol Assess. 2005;17:486–491. [DOI] [PubMed] [Google Scholar]
- 17. Goetz CG, Tanner CM, Wilson RS, Shannon KM. A rating scale for Gilles de la Tourette's syndrome: description, reliability, and validity data. Neurology. 1987;37:1542–1544. [DOI] [PubMed] [Google Scholar]
- 18. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. [DOI] [PubMed] [Google Scholar]
- 19. Hamilton M. A rating scale for depression. JNeurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romito LM, Franzini A, Perani D, et al. Fixed dystonia unresponsive to pallidal stimulation improved by motor cortex stimulation. Neurology. 2007;68(11):875–876. [DOI] [PubMed] [Google Scholar]
- 21. Perani D, Della Rosa PA,Cerami C, et al. Validation of an optimized SPM procedure for FDG‐PET in dementia diagnosis in a clinical setting. Neuroimage Clin. 2014;6:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Della Rosa PA,Cerami C, Gallivanone F, et al. A standardized 18F‐FDG‐PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–593. [DOI] [PubMed] [Google Scholar]
- 23. Storch EA, Murphy TK, Bagner DM, et al. Reliability and validity of the Child Behavior Checklist Obsessive‐Compulsive Scale. JAnxiety Disord. 2006;20(4):473–485. [DOI] [PubMed] [Google Scholar]
- 24. Gläscher J, Gitelman D. Contrast weights in flexible factorial design with multiple groups of subjects. http://www.sbirc.ed.ac.uk/cyril/download/Contrast_Weighting_Glascher_Gitelman_2008.pdf 2008, accessed on July 12, 2018.
- 25. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 26. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 27. Worbe Y, Gerardin E, Hartmann A, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010;133:3649–3660. [DOI] [PubMed] [Google Scholar]
- 28. Worbe Y, Malherbe C, Hartmann A, et al. Functional immaturity of cortico‐basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. [DOI] [PubMed] [Google Scholar]
- 29. Storch EA,De Nadai AS,Lewin AB, et al. Defining treatment response in pediatric tic disorders: a signal detection analysis of the Yale Global Tic Severity Scale. JChild Adolesc Psychopharmacol. 2011;21(6):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Worbe Y, Marrakchi‐Kacem L, Lecomte S, et al. Altered structural connectivity of cortico‐striato‐pallido‐thalamic networks in Gilles de la Tourette syndrome. Brain. 2015;138:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Rienzo F,Collet C, Hoyek N, Guillot A. Impact of neurologic deficits on motor imagery: a systematic review of clinical evaluations. Neuropsychol Rev. 2014;24:116–147. [DOI] [PubMed] [Google Scholar]
- 32. Ganos C, Roessner V, Munchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev. 2013;37:1050–1062. [DOI] [PubMed] [Google Scholar]
- 33. Baldermann JC, Schuller T, Huys D, et al. Deep brain stimulation for Tourette‐syndrome: a systematic review and meta‐analysis. Brain Stimul. 2016;9:296–304. [DOI] [PubMed] [Google Scholar]
- 34. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910.68911–e68910.68915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information may be found in the online version of this article.
Supporting File 1. This file contains Supporting Tables 1‐3 and Supporting Fig. 1.


