Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) is a common cause of morbidity and associated with a significant burden of comorbidities. Although anemia is associated with adverse outcomes in COPD, its contribution to outcomes in individuals with other comorbid chronic diseases is not well understood.
Objectives: This study examines the association of anemia with outcomes in a large, well-characterized COPD cohort, and attempts to understand the contribution of anemia to outcomes and phenotypes in individuals with other comorbidities.
Methods: Participants with COPD from SPIROMICS (the Subpopulations and Intermediate Outcome Measures in COPD Study) were analyzed in adjusted models to determine the associations of normocytic anemia with clinical outcomes, computed tomographic measures, and biomarkers. Analysis was additionally performed to understand the independence and possible interactions related to cardiac and metabolic comorbidities.
Results: A total of 1,789 individuals with COPD from SPIROMICS had data on hemoglobin, and of these 7.5% (n = 135) were found to have normocytic anemia. Anemic participants were older with worse airflow obstruction, a higher proportion of them were African Americans, and they had a higher burden of cardiac and metabolic comorbidities. Anemia was strongly associated with 6-minute walk distance (β, −61.43; 95% confidence interval [CI], −85.11 to −37.75), modified Medical Research Council dyspnea questionnaire (β, 0.27; 95% CI, 0.11–0.44), and St. George’s Respiratory Questionnaire (β, 3.90; 95% CI, 1.09–6.71), and these adjusted associations were stronger among those with two or more cardiac and metabolic comorbidities. Anemia was associated with higher levels of serum C-reactive protein, soluble receptor for advanced glycosylation end-products, and epithelial cadherin-1, findings that persisted when in those with a high burden of comorbidities.
Conclusions: Anemia is associated with worse exercise capacity, greater dyspnea, and greater disease severity among adults with COPD, particularly among those with comorbid chronic cardiac and metabolic diseases. The biomarkers found in anemic individuals suggest inflammation, lung tissue injury, and oxidative stress as possible pathways for the adverse correlations of anemia with outcomes in COPD; however, substantial further study is required to better understand these potential mechanisms.
Clinical trial registered with www.clinicaltrials.gov (NCT01969344).
Keywords: chronic obstructive pulmonary disease, anemia, comorbidities, systemic inflammation
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States (1) and is associated with a significant burden of comorbidities (2). The estimated prevalence of anemia has varied between studies but has ranged between 7.5 and 33% in COPD (3). Ferrari and colleagues found that anemic patients with COPD report a worse quality of life, lower exercise capacity, and increased dyspnea compared with nonanemic patients with COPD (4). Others have also described a higher risk for mortality (5, 6) and health care utilization (7) in individuals with COPD also having anemia. In addition, there has been an increasing focus on describing a phenotype of individuals with COPD having a high burden of comorbid chronic disease (8–10) and systemic inflammation. The role of anemia in contributing to phenotypic features and adverse outcomes in this subgroup of individuals with a high burden of comorbid cardiac and metabolic disease has not clearly been elucidated.
SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) is an ideal population in which to study the association of anemia with COPD morbidity, due to the extensive phenotyping data which were collected. The purpose of this study is to evaluate the association of anemia and phenotypic characteristics including clinical and biologic markers among a contemporary cohort of individuals with COPD. We hypothesized that those individuals with COPD having comorbid anemia would have more systemic inflammation, dyspnea, and exercise limitation compared with those with normal hemoglobin concentrations.
Methods
SPIROMICS (11) is a multicenter prospective study of current and former smokers (≥20 pack-years) with and without obstructive lung disease and of nonsmokers without obstructive lung disease (age, 40–80 yr) enrolled between November 2010 and July 2015 and monitored quarterly for up to 3 years. The original goals of the study were to identify subgroups of individuals with COPD to develop future targeted therapeutic strategies and treatments. This study includes all individuals with COPD including current and former smokers with FEV1/FVC less than 0.70 (FEV1 ≥ 50% predicted, n = 1,193; and FEV1 < 50% predicted, n = 596).
Anemia Characterization
Data on hemoglobin concentration, reported as grams per deciliter (g/dl), from complete blood counts drawn at the baseline visit, were analyzed. Normocytic anemia was defined as a hemoglobin concentration below 12 g/dl if female and below 13 g/dl if male while also having mean corpuscular volume in the normal range (80–100 fl) (12). Anemic individuals with abnormal mean corpuscular volume were excluded from this analysis given the low prevalence of microcytic and macrocytic anemia (n = 20 and four individuals, respectively).
Computed Tomography Measures
Participants underwent whole-lung multidetector helical computed tomography (CT) at full inspiration and expiration at the baseline study visit. Measurements of interest included percent emphysema (percent voxels less than −950 Hounsfield units in the inspiratory phase), percent gas-trapping (percent voxels less than −856 Hounsfield units in the expiratory phase), upper-to-lower lobe ratio of emphysema, and Pi10 (a measure of bronchial wall thickening which correlates to the square root of the wall area of a hypothetical airway with a 10 mm internal perimeter) (13).
Biomarker Measurements
Blood was collected and 12 multiplex platforms and one simplex platform (Myriad-RBM) were utilized on baseline samples to analyze 105 biomarkers, which were preselected on the basis of possible relevance to COPD (14). Of the 105 biomarkers, a subgroup (n = 13) of preselected biomarkers thought to be associated with systemic inflammation and COPD subphenotyping in previous analyses was analyzed, including von Willebrand factor (VWF), vascular endothelial growth factor (VEGF)-α, tumor necrosis factor (TNF)-α, IL-15, interferon (IFN)-γ, immunoglobulin A (IgA), C-reactive protein (CRP), apolipoprotein A-IV (ApoA4), soluble receptor for advanced glycosylation endproducts (sRAGE, encoded by the AGER gene), vascular cell adhesion molecule 1 (VCAM1), superoxide dismutase 1 (SOD1), sex hormone–binding globulin (SHBG), and epithelial cadherin 1 (E-cadherin, CDH1).
Outcomes
Outcomes of interest were respiratory-specific quality of life (St. George’s Respiratory Questionnaire [SGRQ]) (15), exercise capacity (6-min walk distance [6MWD], in meters) (16), dyspnea (Modified Medical Research Council [MMRC] questionnaire) (17), and COPD health status (COPD Assessment Test [CAT]) (18). Exacerbations in the previous year were based on participant report of new prescription of antibiotics and/or steroids, unscheduled doctor visits, emergency room visits, and hospitalizations for COPD exacerbations and frequency of these instances over the past year. Severe exacerbations were defined as events requiring emergency room visit or hospitalization. In addition to baseline exacerbation data, available data regarding prospective exacerbations from study enrollment to most recent follow-up contact were analyzed where available.
Statistical Methods
The cross-sectional associations between anemic status and outcomes were analyzed using multivariable linear or logistic regression models (in which mean differences and odds ratios were estimated, respectively). Longitudinal analyses of exacerbation risk were analyzed as count data using adjusted zero-inflated negative binomial regression with an offset for time to account for individuals’ varying follow-up time to estimate incidence rate ratios. To examine whether there was a dose–response relationship between hemoglobin and each outcome, hemoglobin was also examined as a continuous variable in all models. Individuals with polycythemia were removed from this analysis because this represents a separate clinical phenotype. All models were adjusted for age, sex, race (African American vs. other), education (high school education or less vs. more than high school), FEV1 % predicted, current smoking, and number of comorbidities (19). Figures of adjusted associations of hemoglobin concentration (continuous) with selected outcomes for those with hemoglobin below 15 for females and 17 for males (given the hypothesis that those with polycythemia represent a distinct phenotype), modeled continuously, were constructed using fractional polynomials to test for linearity. The point estimates and 95% confidence interval represent the predicted level of the outcome across hemoglobin, which has been power transformed based on a fractional polynomial model (m = 2) with power selected from −2, −1, −0.0.5, 0, 0.5, 1, 2, and 3. For each figure, the best fitting fractional polynomial model was used, with hemoglobin transformed to its appropriate power terms. The predicted levels represent the average predicted levels with all other covariates held at their observed values. The plots were drawn with the Stata package “margincontplot” (StataCorp), which handles fractional polynomial transformation for margins plotting (20).
Given the high prevalence of cardiac and metabolic comorbidities in the group with anemia, sensitivity analysis was performed, repeating models with adjustment for “cardiometabolic phenotype,” defined as having two or more cardiac or metabolic comorbidities including self-report of hypertension, coronary heart disease (including self-report of doctor-diagnosed coronary artery disease, angina, or heart attack), diabetes, congestive heart failure (not specific to diastolic or systolic), stroke, and obesity (defined as body mass index greater than or equal to 30 kg/m2). To evaluate whether there was a heightened susceptibility to adverse outcomes due to anemia among those with the cardiometabolic phenotype, interaction terms between anemia and the presence of this phenotype were included in fully adjusted models, with a threshold for significance for reporting of 0.10 (21). The association of anemia with biomarker concentrations was analyzed in a similar manner using unadjusted and adjusted models as previously described (which also included adjustment for the batch number of biomarker run), and interaction terms for anemia status and the cardiometabolic phenotypes. Sensitivity analyses were performed including those with micro- and macrocytic anemia in the group with anemia for main analysis.
All analyses were conducted with Stata 12 (22), and the thresholds for statistical significance for main effects and interactions were 0.05 and 0.10, respectively (21, 23, 24). Given that multiple biomarkers were tested (13 in total), Bonferroni correction was utilized for biomarker models, with a threshold for statistical significance of 0.00384. SPIROMICS was approved by institutional review boards at each center, and all participants provided written informed consent.
Results
Cohort Characteristics
Data from 1,789 participants with COPD and having data on hemoglobin concentration were analyzed (Table 1). The prevalence of normocytic anemia was 7.5% (n = 135). Those with anemia had a slightly higher and more variable red cell distribution width (RDW) compared with those without anemia (mean RDW, 14.5 [SD, 2.2] in individuals with anemia compared with mean RDW, 13.8 [SD, 1.2] in those with normal hemoglobin). Compared with those without anemia, those with anemia were older (mean age, 68.2 [SD, 8.1] vs. 64.9 [SD, 7.1]), had lower lung function (mean FEV1 % predicted, 56.1 [SD, 24.7] vs. 61.6 [SD, 22.8]), had lower education attainment (49% with more than high school education vs. 62%), were more likely to be African American (30 vs. 14%), were more likely to have quit smoking (22% current smokers vs. 35%), and were more likely to require oxygen supplementation (37 vs. 19%). Those with anemia had a higher overall comorbidity burden (count of comorbidities: 3.6 [SD, 1.7] vs. 2.4 [SD, 1.6]) and in particular a higher prevalence of cardiac and metabolic comorbidities, specifically a higher prevalence of diabetes (21%), CHD (25%), CHF (8%), and stroke (10%) compared with those without anemia. Mean follow-up time for exacerbation data was 2.1 years (SD, 0.95).
Table 1.
Cohort characteristics
| Total N = 1,789 |
||
|---|---|---|
| |
Normocytic Anemia |
Not Anemic |
| (N = 135; 7.5%) | (N = 1,654; 92.5%) | |
| Hemoglobin | 11.6 (0.95) | 14.6 (1.33) |
| Red cell distribution width (RDW) | 13.8 (1.2) | 14.5 (2.2) |
| Demographics and smoking | ||
| Age, yr | 68.2 (8.1) | 64.9 (7.1) |
| Female, n (%) | 52 (39%) | 708 (43%) |
| More than high school education, n (%) | 66 (49%) | 1,026 (62%) |
| African American, n (%) | 40 (30%) | 229 (14%) |
| Pack-years | 52.7 (25.2) | 52.7 (27.7) |
| Current smoker, n (%) | 30 (22%) | 575 (35%) |
| Spirometry, oxygenation, exercise, disease status | ||
| FEV1 % predicted | 56.1 (24.7) | 61.6 (22.8) |
| 6MWD, m | 319.1 (134.9) | 398.2 (126.6) |
| Oxygen use, n (%) | 48 (37%) | 309 (19%) |
| Resting oxygen saturation | 95.2 (2.6) | 94.7 (4.2) |
| COPD Assessment Test | 17.06 (8.02) | 15.25 (7.96) |
| St. George’s Respiratory Questionnaire, total score | 42.93 (18.85) | 37.46 (19.81) |
| MMRC dyspnea score | 1.61 (1.21) | 1.23 (1.01) |
| CT measures | ||
| % emphysema | 12.1 (11.8) | 11.1 (11.3) |
| Upper-to-lower lobe ratio of emphysema | 2.36 (4.22) | 2.30 (4.62) |
| Normalized Pi10 | 0.25 (1.12) | −0.0046 (1.01) |
| Comorbidities | ||
| Stroke, n (%) | 13 (10%) | 63 (4%) |
| Diabetes, n (%) | 28 (21%) | 211 (13%) |
| Reported OSA, n (%) | 22 (16%) | 307 (19%) |
| Hypertension, n (%) | 77 (57%) | 819 (50%) |
| CHD, n (%) | 34 (25%) | 299 (18%) |
| CHF, n (%) | 11 (8%) | 42 (3%) |
| Obesity, n (%) | 40 (30%) | 515 (31%) |
| Depression, n (%) | 38 (28%) | 447 (27%) |
| Anxiety, n (%) | 32 (24%) | 436 (26%) |
| GERD, n (%) | 44 (33%) | 514 (31%) |
| Allergies, n (%) | 29 (21%) | 487 (29%) |
| Asthma, n (%) | 27 (20%) | 267 (17%) |
| Comorbidity count | 3.6 (1.7) | 2.4 (1.6) |
Definition of abbreviations: 6MWD = 6-minute walk distance; CHD = coronary heart disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CT = computed tomography; FEV1 = forced expiratory volume in 1 second; GERD = gastroesophageal reflux disease; MMRC = Modified Medical Research Council; OSA = obstructive sleep apnea; Pi10 = square root of the wall area of a hypothetical airway with a 10 mm internal perimeter.
Associations of Anemia with Clinical Outcomes
Normocytic anemia was associated with several clinical outcomes in those with COPD (Table 2). After adjustment for covariates including comorbidity burden, those with anemia had lower 6MWD (mean difference, −61.4; 95% confidence interval [CI], −85.1 to −37.8), higher SGRQ (mean difference, 3.9; 95% CI, 1.1–6.7), higher total CAT score (mean difference, 1.3; 95% CI, 0.04–2.5), and higher MMRC score (mean difference, 0.27; 95% CI, 0.1–0.4) compared with those without anemia. Anemia was not associated with an increased risk of exacerbations over follow-up. Anemia was not significantly associated with any CT phenotypes (data not shown). After accounting for multiple comparisons of outcomes, statistically significant associations between anemia and 6MWD, FEV1 % predicted, and MMRC were observed.
Table 2.
Associations of normocytic anemia with outcomes
| No. Included in Model | Coefficient | 95% CI | P Value | |
|---|---|---|---|---|
| 6MWD | 1,631 | −61.4 | −85.1 to −37.8 | <0.01 |
| FEV1 % predicted | 1,719 | −6.7 | −10.8 to −2.7 | 0.01 |
| SGRQ | 1,618 | 3.9 | 1.1 to 6.7 | 0.01 |
| Total CAT | 1,647 | 1.3 | 0.04 to 2.5 | 0.04 |
| No. of exacerbations in past year | 1,719 | 0.1 | −0.04 to 0.3 | 0.14 |
| No. of severe exacerbations in past year | 1,719 | 0.1 | 0.01 to 0.3 | 0.03 |
| MMRC | 1,710 | 0.3 | 0.1 to 0.4 | 0.01 |
| Prewalk oxygen saturation | 1,641 | 0.9 | 0.1 to 1.7 | 0.02 |
| Postwalk oxygen saturation | 1,627 | 1.4 | 0.4 to 2.4 | 0.01 |
| Exacerbations over follow-up, IRR | 1,625 | 1.1 | 0.8 to 1.4 | 0.59 |
| Severe exacerbations over follow-up, IRR | 1,625 | 1.2 | 0.8 to 1.8 | 0.49 |
Definition of abbreviations: 6MWD = 6-minute walk distance; CAT = COPD Assessment Test; CI = confidence interval; FEV1 = forced expiratory volume in 1 second; IRR = incidence rate ratio; MMRC = Modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
All regression models adjusted for age, sex, race, FEV1 % predicted, current smoking status, education level, and comorbidity count. Odds ratios were estimated by adjusted logistic regression. Incident rate ratios were estimated by adjusted zero-inflated negative binomial regression.
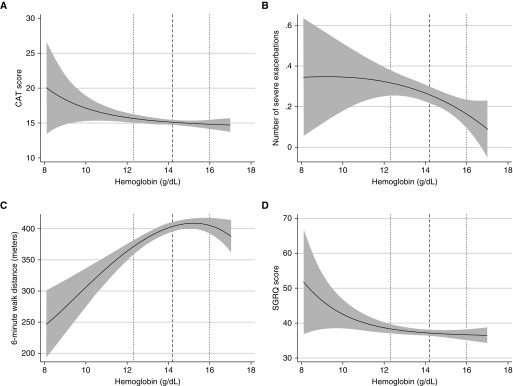
Sensitivity analysis performed including those with micro- and macrocytic anemia and adjusting for cardiometabolic comorbidities did not substantially change the analysis results (data not shown). Findings were similar when hemoglobin was treated as a continuous variable. For each 1-g/dl increment in hemoglobin, there were statistically significant increments in 6MWD (mean difference, 11.4; 95% CI, 6.1–16.7) and FEV1 % predicted (mean difference, 1.7; 95% CI, 0.4–0.7) and significant decreases in SGRQ (mean difference, −0.7; 95% CI, −1.3 to −0.04), CAT (mean difference, −0.3; 95% CI, −0.6 to −0.006), number of severe exacerbations in the past year (mean difference in number of exacerbations, −0.04; 95% CI, −0.07 to −0.0096), MMRC (β, −0.07; 95% CI, −0.1 to −0.03), prewalk oxygen saturation (mean difference, −0.3; 95% CI, −0.5 to −0.1), and postwalk oxygen saturation (mean difference, −0.4; 95% CI, −0.6 to −0.2) (see Table E1 in the online supplement). Using fractional polynomials and restricted cubic splines and when excluding those with high hemoglobin (>15 g/dl for females, >17 for males), the linearity of associations is depicted in Figure 1, which displays the adjusted linear associations of hemoglobin with 1) total CAT, 2) number of severe exacerbations in the past year, 3) 6MWD, and 4) SGRQ. With these methods, the associations appeared to be linear in nature, with the exception of 6MWD, which appeared to have some nonlinearity particularly in the higher range of hemoglobin, reflecting that the effect of increasing hemoglobin attenuates at the higher range.
Figure 1.
Adjusted associations of continuous hemoglobin with selected outcomes of interest. (A) Total CAT score; (B) number of severe exacerbations in the past year; (C) 6-minute walk distance; (D) SGRQ. Dotted lines indicate 10th and 90th percentiles for hemoglobin distribution, and the dashed line indicates the median for hemoglobin distribution. CAT = COPD Assessment Test; SGRQ = St. George’s Respiratory Questionnaire.
Effect Modification by Cardiometabolic Phenotype
Significant interactions with the cardiometabolic phenotype were identified in adjusted models for several outcomes, with anemia more strongly associated with outcomes among individuals with the cardiometabolic phenotype compared with those without (Table 3). Specifically, the adjusted mean 6MWD difference was 85.9 m (95% CI, −122.5 to −49.5) among those with the cardiometabolic phenotype compared with 37.7 m (β, −37.7; 95% CI, −68.0 to −7.3) among those without the cardiometabolic phenotype (P value for interaction = 0.02). Although anemia was not associated with increased exacerbation risk in the overall cohort, anemia was associated with increased number of any exacerbations (mean difference in number of any exacerbations, 0.3; 95% CI, 0.004–0.6) and severe exacerbations (mean difference in number of severe exacerbations, 0.2; 95% CI, 0.04–0.4) among those with the cardiometabolic phenotype, with weaker associations among those without the phenotype (for all interactions, P value for interaction < 0.10).
Table 3.
Associations of normocytic anemia with outcomes, based on subgroups
| Group with Two or More Cardiometabolic Comorbidities (n = 644) |
All Others (n = 1,145) |
||||||
|---|---|---|---|---|---|---|---|
| Difference in Outcome Based on Anemia Status | 95% CI | P Value | Difference in Outcome Based on Anemia Status | 95% CI | P Value | P-int | |
| 6MWD | −86.0 | −122.5 to −49.5 | <0.01 | −37.7 | −68.0 to −7.3 | 0.02 | 0.02 |
| Exacerbations in past year (continuous, β) | 0.3 | 0.004 to 0.6 | 0.047 | −0.03 | −0.3 to 0.2 | 0.83 | 0.04 |
| Severe exacerbations in past year (continuous, β) | 0.2 | 0.04 to 0.4 | 0.02 | 0.04 | −0.1 to 0.2 | 0.65 | 0.06 |
Definition of abbreviations: 6MWD = 6-minute walk distance; CI = confidence interval; P-int = P value for interaction.
All models adjusted for age, sex, race, FEV1 % predicted, current smoking status, and educational level.
Associations with Serum Biomarker Levels
Biomarker data were available for a subgroup of participants (n = 1,042, 74 with normocytic anemia). After correction for multiple comparisons and adjustment for covariates, anemia was associated with higher CRP (adjusted mean difference, 7.0; 95% CI, 4.4–9.6), sRAGE (adjusted mean difference, 1.4; 95% CI, 0.8–1.9), and CDH1 (adjusted mean difference, 1,213.2; 95% CI, 832.9–1,593.6) (Table E2). There was evidence of effect modification by the cardiometabolic phenotype with stronger associations of anemia with sRAGE, CDH1, IL-15, and ApoA4 among those with the cardiometabolic phenotype compared with those without (P for interaction < 0.1) (Table E3).
Discussion
In this analysis of the large cohort of adults with COPD in SPIROMICS, normocytic anemia was a prevalent condition and associated with adverse outcomes, including low exercise capacity, health status measured by SGRQ score, and increased dyspnea. In the COPD population, those with normocytic anemia had a significantly higher burden of cardiac and metabolic comorbidities, and not only was the association of anemia with poor outcomes independent of these comorbidities, but unexpectedly, anemia appeared to have stronger associations among those with cardiac and metabolic comorbidities compared with those without. Finally, differing levels of select biomarkers found in those with normocytic anemia suggest that anemia may be associated with a specific subphenotype of COPD with a higher level of inflammation and epithelial injury, associated with poor clinical outcomes.
Among those with anemia, there was a significantly higher burden of comorbidities, suggesting that anemia is likely part of a larger phenotype of increasing systemic illness and comorbidity burden. Interestingly, the group with anemia had a higher proportion of African Americans and lower educational attainment, further suggesting that anemia is likely part of a larger systemic and environmental picture that cannot be explained by COPD alone. Regardless, the finding of worse outcomes among anemic individuals with COPD is consistent with findings of previous smaller studies. Anemia has been associated with mortality in a wide range of severity of COPD including stable outpatients (5), inpatients with acute exacerbations (25), and those with respiratory failure on mechanical ventilation (26). One previous study showed that anemia of chronic disease was associated with dyspnea and poor circulatory efficiency in COPD, shown by significantly lower peak oxygen uptake and peak work rate on cardiopulmonary exercise testing (27), suggesting a mechanistic link between low hemoglobin and oxygen carrying capacity with reduced exercise capacity. Demonstrating the association of anemia with impairment in exercise capacity and heightened symptoms such as dyspnea in a large, well-characterized cohort such as SPIROMICS is an important step in establishing the impact of anemia on disease burden in COPD.
A key finding was of heightened dyspnea among individuals with COPD having comorbid anemia. It is possible that those with respiratory limitation such as individuals with COPD are more likely to be burdened by comorbid anemia, particularly regarding symptoms commonly thought to impact individuals with respiratory disease, such as exercise limitation and dyspnea. A previous study demonstrated that individuals with COPD having anemia had lower peak Vo2 during maximal cycle ergometer testing, suggesting that anemia physiologically impacts individuals with COPD to drive symptoms such as dyspnea and exercise limitation (4). The study by Ferrari and colleagues is consistent with the current study in demonstrating a significant decrement in exercise capacity despite the degree of anemia being relatively mild. Other factors may also contribute, such as increased respiratory rate and depth of inspiration, which in normal individuals could lead to the sensation of exertional dyspnea (28) and in those with COPD heighten existing exertional dyspnea.
Individuals with COPD and comorbid anemia also had a significantly higher burden of comorbid conditions. Another study also showed a high prevalence of cardiac and metabolic comorbidities in individuals with COPD and anemia (9). It is important to note that the adverse effects of anemia were independent of the impact of overall comorbidity burden including comorbid cardiac and metabolic diseases specifically, which are known to cause significant morbidity in patients with COPD (29, 30). Taking these findings further, there was a synergistic effect of the presence of both comorbid cardiac/metabolic conditions and anemia, with anemia having a worse impact among those with a high burden of comorbid cardiac and metabolic conditions. In the subjects with these comorbidities, anemia had a stronger adverse association with exercise capacity, respiratory health status, and exacerbation risk. This may be explained by worsened oxygen delivery to tissues due to both decreased cardiac output and oxygen carrying capacity. In addition, anemia may lead to further adverse impacts on cardiac or vascular (micro or macro) function, contributing to worse morbidity in patients with COPD who have concomitant cardiac disease. Interestingly, anemic patients with COPD had a lower prevalence of current smoking than those without anemia. Although the etiology for this finding is not clear, it is possible that the higher burden of comorbid chronic disease, symptom burden, and overall morbidity are associated with increased prevalence of smoking cessation.
This study’s findings of a higher level of certain biomarkers in the group with anemia suggest possible mechanisms for the adverse impacts of anemia on COPD outcomes as well as further identify a subphenotype of individuals with COPD suffering from anemia, comorbidity burden, and systemic inflammation. Previous studies have described possible systemic inflammatory phenotypes of COPD in those with a high prevalence of comorbid chronic conditions (8–10). Garcia-Aymerich and colleagues showed in a subgroup having a high burden of comorbid chronic diseases and systemic inflammation, but less substantially impaired airflow obstruction, that exercise capacity was similarly impaired in comparison with a group having less comorbidity but more substantial airflow obstruction (8). However, anemia was not specifically explored regarding its contribution to outcomes and phenotyping in these studies; and our results suggest that anemia may have an additive effect to other comorbid conditions.
Individuals with anemia had higher levels of C-reactive protein, previously shown to be associated with systemic inflammation in COPD (8, 9). In addition, two biomarkers were differentially expressed not only between those with and without anemia but also between those with and without the cardiometabolic phenotype: sRAGE and CDH1. The soluble receptor for advanced glycation end-products (sRAGE) is a marker of systemic inflammation that has been traditionally associated with metabolic diseases such as diabetes (31, 32), has also more recently been studied in populations with COPD where sRAGE has been inversely associated with emphysema severity (31). This analysis finds that not only are anemia and cardiometabolic comorbidities independently associated with sRAGE but that the existence of both together is associated with an even greater elevation of sRAGE levels. These findings help to establish anemia as an independent and important correlate of systemic inflammation, which in turn appears to be part of a larger systemic inflammatory subphenotype of COPD having a high burden of comorbid chronic disease (8–10). This analysis further demonstrates that anemia is one of several possible independent factors that contribute to the adverse outcomes noted in this subgroup.
The consistent finding of higher serum E-cadherin levels in those with anemia as well as the positive interaction between anemia and cardiometabolic phenotype for E-cadherin levels is more difficult to understand given the limited information currently available about the role of serum E-cadherin levels in COPD. E-cadherin is a component of epithelial cells adherens junctions, which allow for organization of membrane proteins (32). The role of E-cadherin in COPD is not clear, but decreased expression of E-cadherin on the epithelium is associated with a higher risk for development of COPD in the setting of cigarette smoke exposure (33–36). Analysis of the SPIROMICS cohort showed that higher serum E-cadherin levels were associated with lower percent emphysema (37). It is possible that increased lung inflammation and epithelial injury leads to lower E-cadherin levels specifically in the epithelium, but higher released or soluble levels of E-cadherin. The findings of higher levels of serum E-cadherin in COPD participants with anemia are intriguing, particularly taken with concurrent findings of a trend for less emphysema in this group. Regardless of the mechanism, the strength and significance of this association are compelling and worthy of further study.
This study is subject to some limitations. There are several factors that likely confound the association of anemia with outcomes in the population with COPD, and as with any study not all factors are measured. Accordingly, we have made adjustments for many potential confounding factors, and have paid special attention to confounding from comorbid chronic disease in our adjustments, but as with any epidemiologic study, residual confounding is possible. Ultimately, randomized trials targeted at both identifying and treating anemia would be required to provide further insight into the importance of anemia and whether improving hemoglobin would impact these outcomes. Until such trials are conducted it is difficult to know whether screening for or treating anemia would be of value to patients with COPD and should be incorporated in clinical practice guidelines. In addition, there is a high prevalence of normocytic anemia in patients with chronic kidney disease (CKD), but there are few biomarker data on CKD such as serum creatinine or glomerular filtration rate, and also no data on carbon monoxide and carboxyhemoglobin. In addition, there were no data on erythropoietin levels or iron indices, limiting our ability to determine the underlying cause for anemia when present. Regardless, the strength of the associations noted between anemia and adverse outcomes, as well as the independence of these associations from other comorbid chronic disease, is ultimately reassuring that these findings are not merely due to uncontrolled confounding related to unmeasured chronic diseases such as CKD. Several data points were also collected using self-report, including report of exacerbation events as well as the presence of comorbid chronic disease and accordingly are subject to some limitation. Another important limitation is the overall low prevalence of anemia. However, despite this limitation, strong associations with outcomes were found between anemia and clinical outcomes. The results described largely represent a cross-sectional analysis in which exposures and outcomes were ascertained at the same point in time; we are therefore limited in inferring causality or directionality of the observed associations due to the unknown temporal ordering of exposure and outcome. Finally, a small proportion of people having anemia had microcytic or macrocytic anemia, and because of this small proportion as well as the hypothesis that the etiology driving these different types of anemia would be different than those causing normocytic anemia, these individuals were not included in the main analysis. However, sensitivity analyses were performed including these individuals, and the results were minimally changed.
In conclusion, in this large, multicenter study, normocytic anemia is shown to be strongly associated with adverse functional measures and outcomes in COPD, and this association appears to be independent of comorbid chronic disease, which is prevalent in the group with anemia. Also, the biomarker analysis suggests a few possible mechanisms, such as increased systemic inflammation and airway epithelial injury, which may contribute to the adverse impacts of anemia on COPD outcomes. Ultimately, anemia could be one of several independent contributors to adverse outcomes in the subgroup of individuals with COPD having high levels of systemic inflammation and comorbidity burden. This analysis is an important step in establishing the scope of the problem of anemia in COPD so that future studies can understand which populations are at highest risk and could best be targeted for screening and intervention.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, Ph.D.; Wayne H. Anderson, Ph.D.; R. Graham Barr, M.D., Dr.P.H.; Eugene R. Bleecker, M.D.; Richard C. Boucher, M.D.; Russell P. Bowler, M.D., Ph.D.; Elizabeth E. Carretta, M.P.H.; Stephanie A. Christenson, M.D.; Alejandro P. Comellas, M.D.; Christopher B. Cooper, M.D., Ph.D.; David J. Couper, Ph.D.; Gerard J. Criner, M.D.; Ronald G. Crystal, M.D.; Jeffrey L. Curtis, M.D.; Claire M. Doerschuk, M.D.; Mark T. Dransfield, M.D.; Christine M. Freeman, Ph.D.; MeiLan K. Han, M.D., M.S.; Nadia N. Hansel, M.D., M.P.H.; Annette T. Hastie, Ph.D.; Eric A. Hoffman, Ph.D.; Robert J. Kaner, M.D.; Richard E. Kanner, M.D.; Eric C. Kleerup, M.D.; Jerry A. Krishnan, M.D., Ph.D.; Lisa M. LaVange, Ph.D.; Stephen C. Lazarus, M.D.; Fernando J. Martinez, M.D., M.S.; Deborah A. Meyers, Ph.D.; John D. Newell, Jr, M.D.; Elizabeth C. Oelsner, M.D., M.P.H.; Wanda K. O’Neal, Ph.D.; Robert Paine III, M.D.; Nirupama Putcha, M.D., M.H.S.; Stephen I. Rennard, M.D.; Donald P. Tashkin, M.D.; Mary Beth Scholand, M.D.; J. Michael Wells, M.D.; Robert A. Wise, M.D.; and Prescott G. Woodruff, M.D., M.P.H. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, Ph.D., and Thomas Croxton, Ph.D., M.D. SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici SpA; Forest Research Institute, Inc.; GSK; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; and Sanofi.
Footnotes
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute K23HL123594 (N.P.). SPIROMICS is funded by contract from the National Heart, Lung, and Blood Institute (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, and HHSN268200900020C).
Author Contributions: N.P., G.G.P., and N.N.H. were involved with conception, design, analysis and interpretation of data, drafting and revisions, and final approval, and agree to be accountable for all aspects of the work. A.A.L., A.F., K.J.P., V.K.S., J.W., J.M.W., W.W.L., C.M., and L.M.P. were involved with design and interpretation of data and revisions of the work, and provided final approval. C.M.D., R.E.K., M.K.H., F.J.M., R.A.W., W.K.O’N., and R.G.B. were involved with design, analysis and interpretation of data, collection of data, and revisions to the work, and provided final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the SPIROMICS Investigators, Neil E. Alexis, Wayne H. Anderson, R. Graham Barr, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Elizabeth E. Carretta, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Christine M. Freeman, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Robert J. Kaner, Richard E Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, John D. Newell, Jr, Elizabeth C. Oelsner, Wanda K. O’Neal, Robert Paine, III, Nirupama Putcha, Stephen I. Rennard, Donald P. Tashkin, Mary Beth Scholand, J. Michael Wells, Robert A. Wise, Prescott G. Woodruff, Lisa Postow, Thomas Croxton, and Nycomed GmbH
References
- 1.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59:1–51. [PubMed] [Google Scholar]
- 2.Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar M, Rajta PN, Khatana J. Anemia in chronic obstructive pulmonary disease: prevalence, pathogenesis, and potential impact. Lung India. 2015;32:142–151. doi: 10.4103/0970-2113.152626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari M, Manea L, Anton K, Bruzzone P, Meneghello M, Zamboni F, et al. Anemia and hemoglobin serum levels are associated with exercise capacity and quality of life in chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:58. doi: 10.1186/s12890-015-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutou AK, Karrar S, Hopkinson NS, Polkey MI. Anemia and survival in chronic obstructive pulmonary disease: a dichotomous rather than a continuous predictor. Respiration. 2013;85:126–131. doi: 10.1159/000338792. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Rivera C, Portillo K, Muñoz-Ferrer A, Martínez-Ortiz ML, Molins E, Serra P, et al. Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. COPD. 2012;9:243–250. doi: 10.3109/15412555.2011.647131. [DOI] [PubMed] [Google Scholar]
- 7.Barba R, de Casasola GG, Marco J, Emilio Losa J, Plaza S, Canora J, et al. Anemia in chronic obstructive pulmonary disease: a readmission prognosis factor. Curr Med Res Opin. 2012;28:617–622. doi: 10.1185/03007995.2012.675318. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Aymerich J, Gómez FP, Benet M, Farrero E, Basagaña X, Gayete À, et al. PAC-COPD Study Group. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66:430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 9.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 10.Rennard SI, Locantore N, Delafont B, Tal-Singer R, Silverman EK, Vestbo J, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12:303–312. doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 11.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 13.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neal WK, Anderson W, Basta PV, Carretta EE, Doerschuk CM, Barr RG, et al. SPIROMICS Investigators. Comparison of serum, EDTA plasma and P100 plasma for Luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9. doi: 10.1186/1479-5876-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 16.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 17.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 19.Putcha N, Puhan MA, Drummond MB, Han MK, Regan E, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royston P. marginscontplot: plotting the marginal effects of continuous predictors. Stata J. 2013;13:510–527. [Google Scholar]
- 21.McCormack MC, Belli AJ, Kaji DA, Matsui EC, Brigham EP, Peng RD, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J. 2015;45:1248–1257. doi: 10.1183/09031936.00081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp Stata Statistical Software, release 12College Station, TX: StataCorp2011 [Google Scholar]
- 23.Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. 2013;188:1210–1215. doi: 10.1164/rccm.201305-0889OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvin S. Statistical analysis of epidemiologic data. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Haja Mydin H, Murphy S, Clague H, Sridharan K, Taylor IK. Anemia and performance status as prognostic markers in acute hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:151–157. doi: 10.2147/COPD.S39403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen L, Christensen S, Lenler-Petersen P, Johnsen SP. Anemia and 90-day mortality in COPD patients requiring invasive mechanical ventilation. Clin Epidemiol. 2010;3:1–5. doi: 10.2147/CLEP.S12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutou AK, Stanopoulos I, Pitsiou GG, Kontakiotis T, Kyriazis G, Sichletidis L, et al. Anemia of chronic disease in chronic obstructive pulmonary disease: a case–control study of cardiopulmonary exercise responses. Respiration. 2011;82:237–245. doi: 10.1159/000326899. [DOI] [PubMed] [Google Scholar]
- 28.Blumgart HL, Altschule MD. Clinical significance of cardiac and respiratory adjustments in chronic anemia. Blood. 1948;3:329–348. [PubMed] [Google Scholar]
- 29.Patel ARC, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141:851–857. doi: 10.1378/chest.11-0853. [DOI] [PubMed] [Google Scholar]
- 30.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 31.Cheng DT, Kim DK, Cockayne DA, Belousov A, Bitter H, Cho MH, et al. TESRA and ECLIPSE Investigators. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 32.Ragkousi K, Gibson MC. Cell division and the maintenance of epithelial order. J Cell Biol. 2014;207:181–188. doi: 10.1083/jcb.201408044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohal SS, Walters EH. Role of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD) Respir Res. 2013;14:120. doi: 10.1186/1465-9921-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohal SS, Walters EH. Epithelial mesenchymal transition (EMT) in small airways of COPD patients. Thorax. 2013;68:783–784. doi: 10.1136/thoraxjnl-2013-203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldenburger A, Poppinga WJ, Kos F, de Bruin HG, Rijks WF, Heijink IH, et al. A-kinase anchoring proteins contribute to loss of E-cadherin and bronchial epithelial barrier by cigarette smoke. Am J Physiol Cell Physiol. 2014;306:C585–C597. doi: 10.1152/ajpcell.00183.2013. [DOI] [PubMed] [Google Scholar]
- 36.Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J Biol Chem. 2012;287:42288–42298. doi: 10.1074/jbc.M112.387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishida K, Brune KA, Putcha N, Mandke P, O’Neal WK, Shade D, et al. Cigarette smoke disrupts monolayer integrity by altering epithelial cell–cell adhesion and cortical tension. Am J Physiol Lung Cell Mol Physiol. 2017;313:L581–L591. doi: 10.1152/ajplung.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.