Abstract
Children with reading difficulties (RDs) often receive related accommodations in schools, such as additional time for examinations and reading aloud written material. Existing data suggest that these readers share challenges in executive functions (EFs). Our study was designed to determine whether children with RDs have specific challenges in EFs and define neurobiological signatures for such difficulties using magnetic resonance imaging (MRI) data. Reading and EFs abilities were assessed in 8–12-year-old children with RDs and age-matched typical readers. Functional MRI data were acquired during a Stroop task, and functional connectivity of the EFs defined network was calculated in both groups and related to reading ability. Children with RDs showed lower reading and EFs abilities and demonstrated greater functional connectivity between the EFs network and visual, language, and cognitive control regions during the Stroop task, compared to typical readers. Our results suggest that children with RDs utilize neural circuits supporting EFs more so than do typical readers to perform a cognitive task. These results also provide a neurobiological explanation for the challenges in EFs shared by children with RDs and explain challenges this group shares outside of the reading domain.
Keywords: : children, executive functions, fMRI, language, visual processing
Introduction
Intact reading is fundamental for academic success. For this reason, providing appropriate accommodations and interventions for children with reading difficulties (RDs) is crucial. RDs are defined as ongoing challenges in reading accurately and fluently despite exposure to the written language and provision of appropriate intervention (IDA, 2011).
Many theories have been raised regarding the primary cause for RDs, including phonological deficit (Snowling et al., 1997), asynchrony (Breznitz, 2006), orthographic (Brunswick et al., 1999), and morphological (Nagy et al., 2006) theories. Although the main deficit in individuals with RDs is within the reading domain, studies conducted during the past decade have identified deficits in executive functions (EFs) in this group of readers, both behaviorally (Altemeier et al., 2008; Brosnan et al., 2002; Gooch et al., 2011) and with evidence of alterations in neural circuits involved in executive dysfunctions (Horowitz-Kraus, 2014, 2015; Horowitz-Kraus et al., 2015c, 2016).
Relationship between reading ability and EFs
The ability to read fluently involves intact subcomponents of reading, such as phonology, orthography, and semantics, but it also requires higher order cognitive ability, usually referred to as the umbrella term “executive functions” (EFs) (Breznitz, 2006). EFs are cognitive processes used to optimize performance through planning, organizing, and learning that include working memory, speed of processing, switching, and visual attention (Anderson, 2002). In the past decade, accumulated literature suggests that children with RDs demonstrate challenges in EFs in addition to their reading problems (Horowitz-Kraus, 2014, 2015; Horowitz-Kraus and Breznitz, 2008, Horowitz-Kraus and Holland, 2015, Horowitz-Kraus et al., 2015a, 2015c). However, there is still a gap in knowledge as to how general this deficit in EF is or if it is restricted to tasks involving reading, which obviously has clinical and educational implications.
Defining whether there is a specific underlying neurobiological deficit in EF has the potential to affect diagnosis of RD early in life (even before reading is acquired) and treatment in early childhood. Therefore, the aim of this study was to determine neurobiological markers for a specific EF deficit in children with RDs.
The neurobiological basis of the Stroop effect and EFs
An examination of EFs in children with RDs is challenging since finding one task that engages all EFs is itself challenging. In the past decade, the Stroop task was suggested as a paradigm that engages multiple EF domains, such as inhibition and switching (Stroop, 1935). For this task, participants are first presented with words representing colors (red, blue, green, etc.) that are each colored in the same ink as the presented word and then are asked to name the words. Next, words representing colors are presented in different-colored fonts, and participants are asked to identify the font color and ignore the word, which is referred to as the interference effect (Stroop, 1935). By inhibiting their automatic response and then determining the color of the font, subjects rely on their working memory and speed of processing (Stuss and Knight, 2002). This automatic timely response inhibition was coined “the Stroop phenomenon” and has been observed in both the colors and numerical Stroop tasks (Liua et al., 2014).
Neuroimaging studies have shown increased brain activation in several brain regions related to EF, such as the left lateral prefrontal cortex, left anterior cingulate, and left parietal and parieto-occipital cortices, in typical readers performing this task (Adleman et al., 2002). Previous studies identified different populations with an EF deficit who demonstrated an altered performance in this task [e.g., children with attention-deficit/hyperactive disorder (Homack and Riccio, 2004) and individuals with post-traumatic syndrome disorder (Moradi et al., 2000)]. Children with RDs also demonstrated decreased performance during this task compared with typical readers (Faccioli et al., 2008; Horowitz-Kraus, 2014, 2015; Protopapasa et al., 2007), even in the nonlinguistic version of the task (Liua et al., 2014). However, the involvement of neural circuits related to RDs during the Stroop task remains elusive, but are crucial for an objective early detection of such deficit.
The neurobiological basis of RDs and EFs
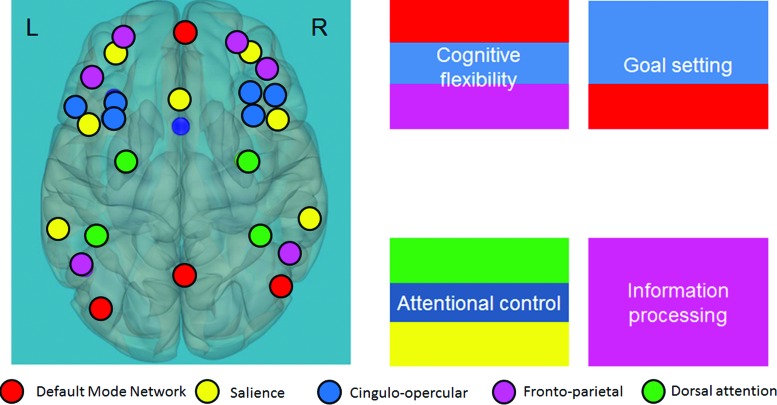
Several neuroimaging studies focusing on individuals with RDs suggest a controversy of inconsistent patterns of activation and connectivity of frontal regions and cognitive control networks, respectively, during verbal and nonverbal tasks. Some studies have reported an overactivation in anterior regions, usually related to EFs, during a written nonword rhyming task, such as in the inferior frontal gyrus, part of the cingulo-opercular cognitive control network (Shaywitz et al., 1998). Similar results were seen in a reading-aloud task (Brunswick et al., 1999). Others have reported increased functional connectivity between seeds in the occipital and frontal (inferior frontal gyrus) lobes in children with dyslexia during naming and reading tasks (Morken et al., 2017), as well as during functional magnetic resonance imaging (fMRI) sentence-reading comprehension, which poses a heavier load on cognitive processes (Yagle et al., 2017). And yet other studies suggest a decreased functional connectivity in cognitive networks involving these frontal regions during both reading and rest [cingulo-opercular (Horowitz-Kraus et al., 2015c) and frontoparietal networks (Horowitz-Kraus et al., 2015a)]. These networks are related to error monitoring and response evaluation (cingulo-opercular), as well as speeded response and working memory (frontoparietal) (Dosenbach et al., 2008). Other networks related to the involvement of cognitive control abilities during reading are the dorsal attention (Vogel et al., 2012) and salience (Li et al., 2012) networks, as well as the default-mode network (DMN) (Koyama et al., 2010), corresponding separately and in combination with cognitive flexibility, goal setting, information processing, and attentional control (Fig. 1) (Anderson, 2002). Owing to the existing controversy and the limited number of published studies examining specifically the functional connectivity patterns during an EF task rather than during a reading paradigm, and since only subnetworks related to EF have been investigated while those associated with EF required for the Stroop task were not, it remains unclear whether there is a neurobiological difference in the functional connections within these networks in children with RDs and the relationship with reading ability. This was the focus of this study.
FIG. 1.
The EFs network. The EFs network is composed of the default-mode network (in red); the cingulo-opercular (in blue), frontoparietal (in pink), salience (in yellow), and dorsal attention (in green) networks; and the corresponding abilities (cognitive flexibility, goal setting, attentional control, and information processing). Adapted from the proposed developmental model for EFs (Anderson and Reidy, 2012). EFs, executive functions. Color images available online at www.liebertpub.com/brain
The study was designed to identify the neural circuits underlying the differences in EFs in children with RDs compared with typical readers during a Stroop task. To determine the differences in functional connections within the EF network and between the EF network and other regions related to the reading (i.e., cognitive, language, and visual processing regions) in children with RDs compared with typical readers, we defined the “EF network” as being composed of functional networks related to a variety of EFs and that were previously related to reading (i.e., frontoparietal, cingulo-opercular, dorsal attention, and salience networks and the DMN), and view these as a set of a priori functional networks involved in higher level cognitive abilities. Grouping all of the networks into one EF network enabled us to focus only on the role of the EF network as a whole and its engagement with other regions in the brain during an EF task, without referring to each network separately. Unlike previous work looking at the correspondence between cognitive control networks (Horowitz-Kraus et al., 2015c), the main aim of this study was to generate one network that includes all subnetworks of the executive system within the whole brain to understand the overall reliance on this network in children with RDs versus typical readers during an EF task. Since we were interested in within versus outside network connectivity for a set of a priori networks of interest, we explored outside network connectivity and also global efficiency exploring within-network differences. Global efficiency is a graph theory-driven measure that provides information on the average inverse shortest path length and can be computed on disconnected networks, assuming that the paths between disconnected nodes will have infinite length and, therefore, zero efficiency (Rubinov and Sporns, 2010).
We hypothesized that challenges in EFs in children with RDs would be accompanied by greater functional connections between the EF network and regions related to reading, as was previously suggested in other populations (Deslauriers et al., 2017). This hypothesis is based on the assumption that greater effort in performing the task will be accompanied by increased engagement and reliance on the EF network and increased synchronization within the reading and language regions. Owing to the inverse relationship between graph theory measures for network connectivity (Wong et al., 2007), we anticipated that greater global efficiency scores would be related to increased reading and EF scores. EFs develop before reading is acquired and support reading later in life. It is therefore important to define their involvement in reading in children with RDs. This will support detection of these challenges earlier in life to provide intervention before the evidence of reading failures.
Materials and Methods
Participants
Children between the ages of 8 and 12 years participated in this study: children with RDs (n = 28, mean age 9.86 years, SD = 1.46, 13 males, 28 right handed) and typical readers (n = 17, mean age 9.77 years, SD = 1.4, 9 males, 17 right handed) who were native English speakers participated in this study. Children attended the second to sixth grades without any significant difference in grade level between the two groups (children with RDs: mean grade level = 3.79, SD = 0.833; typical readers: mean grade level = 3.82, SD =1.058, t(45) = −0.123, p = 0.903). Written informed consents and assents were received from the parents and the participants, respectively. The appropriate Institutional Review Board approved the study. Participants had no history of psychiatric or neurological impairment, including attention difficulties.
Behavioral and neurocognitive measures
The nonverbal and verbal IQ of the participants was determined through administration of the Test of Nonverbal Intelligence (TONI-3) (Brown et al., 1997) and a vocabulary task (Peabody Picture Vocabulary Test [PPVT-4]) (Dunn and Dunn, 2007), respectively, to verify normal (>85) nonverbal and verbal IQ. Current RD literature suggests that children with RDs are defined as such if they demonstrate a score of −1 standard score and lower in at least two reading measures (after; Kovelman et al., 2012). These reading measures include several subdomains of reading, such as automatic and nonautomatic orthographical, decoding, reading comprehension, and fluency or phonemic awareness abilities. Therefore, in this study, children performed the following behavioral reading measures: (1) word and (2) nonword reading, as well as (3) reading fluency from the Test of Word Reading Efficiency second edition (TOWRE II) (Torgesen et al., 1999), (4) reading comprehension, and use of the (5) word-attack subtest, and (6) letter word subtest from the WJ-III (Woodcock and Johnson, 1989).
Neurocognitive testing
To evaluate differences between children with RDs and typical readers in EF abilities, the following EF tests were administered: (1) working memory (digit span; Wechsler, 1999), (2) speed of processing (symbol search/coding subtests; Wechsler, 1999), (3) naming ability (naming and letter naming from the Comprehensive Test of Phonological Processing [CTOPP]; Wagner et al., 1999), (4) switching and inhibition (Stroop, from Delis–Kaplan Executive Function System [D-KEFS]; Dellis et al., 2001), and (5) visual attention (SkySearch; Manly et al., 1999). Typical readers showed intact scores in all reading tests.
To determine the differences in reading and EF measures between the two groups of readers, independent t-test analysis was performed. To define the relationship between reading and EFs, a Pearson correlation was performed for both reading groups. Data were corrected for multiple comparisons using a Bonferroni correction.
Neuroimaging measures
Stroop task
In this task, participants were shown a series of color words in the center of the screen. Each word had a colored font, and participants were asked to identify the color of the font. Participants were given a 2-min practice session before beginning the scan. During the scan, they were asked to focus on the task and avoid sleeping and/or closing their eyes. The stimulation included three conditions: interference, control, and rest.
In the interference condition, the participants were presented with words colored in a different color than the actual word (i.e., the word “blue” was printed in a red ink). The participants were instructed to “push the button on the response box that matches the color of the ink and not the word.” The buttons on the response box corresponded by color and order to three colored circles on the screen (red, blue, and green) so that the participants would not be tempted to gaze toward their hands. Each stimulus was presented for 2 s, and the response screen (the three circles) was also presented on the screen for 2 s.
In the control condition, the participants were presented with a string of four “X” characters (“XXXX”) printed in different colors and asked to “push the button that matches the color of the string of X's”.
In the rest condition, the participants were presented with a cross on the screen and instructed to look at the cross.
There were five blocks of words for the interference condition (15 stimuli per block), five blocks of X's for the control condition (15 stimuli per block), and two blocks of rest (i.e., a cross), which alternated randomly. The duration for each block was 60 s. The task lasted for a total time of 12 min and 22 s.
MRI acquisition and data preparation
Participants were given time to acclimate to the MRI scanner and practice lying still. Head motion was controlled using elastic straps attached to the head-coil apparatus; along the child's forehead, a headband further reduced motion. Once comfortable inside the machine, the child began watching a movie through the MRI-compatible audiovisual system. At this point, image acquisition commenced. Headphones equipped with a built-in microphone were used to communicate with the child, to provide positive feedback throughout the scan. If the child did not wish to continue at any point, the process was stopped. All children were awake throughout the scan. All participants were scanned using a 3T Philips Achieva MRI scanner accompanied by the Avotec SS3150/SS7100 audio/visual system. A gradient echo planar sequence was used for T2-weighted blood oxygen level-dependent (BOLD) fMRI scans with the following parameters: TR/TE = 2000/38 ms, BW = 125 kHz, FOV = 25.6 × 25.6 cm, matrix 64 × 64, and slice thickness of 5 mm. Three hundred sixty image volumes were acquired during the fMRI experiment consisting of 60 s per condition for a total time of 300 s (150 volumes) for each stimulus (i.e., “word/color” interference and “XXXX” control). A T1-weighted inversion recovery gradient echo anatomical whole brain was acquired for each participant for an anatomical coregistration and used in spatial normalization of the functional data.
MRI data analysis
Data preprocessing and first-level analyses were performed using SPM12 implemented in the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). Images were slice-time corrected and realigned. The data were normalized using a 3D anatomical whole-brain scan to match the Montreal Neurological Institute standard template, resampled (3-mm3 voxels), and smoothed with 8 mm full width at half maximum (FWHM). Voxels activated by the Stroop task were identified using a general linear model. Six motion parameters were determined through 3D affine transformation. Excessive motion led to removal from the postprocessing pipeline. All data met the criterion of median voxel displacement in the center of the brain (0.2 mm). Voxel-wise temporal denoising of the BOLD signal was applied through regression of zero- and first-order derivatives of the six motion parameters, regression of the five principle components of the white matter, and cerebrospinal fluid BOLD signal using a component-based noise correction approach (Behzadi et al., 2007). Images regarded as movement outliers were regressed out. Outliers detection was performed using the ART toolbox (http://nitrc.org/projects/artifact_detect/) and defined as volumes with frame-wise displacement >0.5 mm or signal intensity changes >2 standard deviations. Functional connectivity during the interference condition was examined in CONN in the second-level analysis.
Functional connectivity analysis
Based on the relationship between the cingulo-opercular, frontoparietal, salience, dorsal attention networks, the DMN, and reading ability, these networks were included in the EF network (i.e., we chose only the edges of these networks to create the utilized “EF network”). This means that each network received the same “weight” within the large EF network (for additional information, see Rubinova and Spornsd, 2010). The networks, except for the cingulo-opercular, were defined by the networks atlas implemented in CONN (www.nitrc.org/projects/conn/) (Whitfield-Gabrieli and Nieto-Castanon, 2012). The cingulo-opercular network was defined after Dosenbach's model (Dosenbach et al., 2008), see Figure 1, and created using WFU PickAtlas (http://fmri.wfubmc.edu/software/pickatlas). Functional connectivity between the newly formed EF network and the whole brain was defined for the interference condition, noted in both Brodmann areas (BAs) or the corresponding anatomical regions, and performed in a seed-based functional connectivity analysis.
Global efficiency calculation was performed for the entire EF network. Global efficiency was calculated in CONN using the formula (from Latora and Marchiori, 2001):
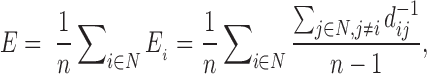 |
where Ei is the efficiency of node i, n is the number of network nodes, N is the set of all network nodes, and dij−1 is the inverse shortest path length between nodes i and j.
The shortest path length was calculated using a binarized connectivity matrix, as defined in CONN, where a value of 1 means a direct connection between two nodes, while a value of 0 would mean that a direct connection between the two nodes is absent. Adjacency matrix threshold of 0.15, two-sided was set in CONN. To measure the difference in global efficiency between the groups, two-sample t-test was performed and significance level was set at p < 0.05, false discovery rate (FDR) corrected for multiple comparisons.
Correlation of global efficiency with behavioral scores
To determine the associations between global efficiency of the EF network and reading and EF measures, a Pearson correlation between these measures was performed for both reading groups. Data were corrected for multiple comparisons using a Bonferroni correction.
Results
Behavioral results
Children with RDs demonstrated significantly lower reading ability, including orthographic ability (word reading), decoding (nonword reading), phonemic awareness, and comprehension, than typical readers. Children with RDs also demonstrated lower scores in all EF domains (switching, inhibition, working memory, speed of processing, attention, and naming). See Table 1 for these results.
Table 1.
Baseline Behavioral Reading and Executive Function Scores for Children with Reading Difficulties and Typical Readers
| Cognitive ability | Children with RDs | Typical readers | t-Test |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| General ability | |||
| Subability | |||
| Language ability (PPVT, standard score) | 98.13 (10.54) | 112.82 (10.50) | −5.365*** |
| Nonlinguistic ability (TONI, scaled score) | 99.57 (6.72) | 103.13 (7.24) | −1.640 ns |
| Reading | |||
| Word-level reading | |||
| Word reading (TOWRE, scaled score) | 76.64 (12.37) | 101.59 (9.68) | −7.018*** |
| Nonword reading (TOWRE, scaled score) | 74.84 (10.71) | 103.24 (8.48) | −9.270*** |
| Word reading, untimed (WJ, letter word, standard score) | 91.50 (9.70) | 104.12 (14.94) | −5.078*** |
| Contextual reading | |||
| Reading comprehension (WJ, standard score) | 85.13 (8.95) | 105.30 (7.20) | −9.787*** |
| Phonological processing | |||
| Phonological processing (CTOPP, Ellison, scaled score) | 7.00 (2.76) | 11.98 (2.06) | −6.439*** |
| Executive functions | |||
| Switching inhibition | |||
| D-KEFS color naming (standard score) | 7.68 (3.31) | 10.47 (3.37) | −2.273** |
| D-KEFS word reading (standard score) | 7.04 (2.98) | 10.82 (2.21) | −4.520*** |
| D-KEFS color/word switching (standard score) | 6.21 (3.41) | 10.18 (3.02) | −3.935*** |
| Working memory | |||
| Digit span forward (WISC, maximal number of digits reached) | 5.11 (0.83) | 5.41 (1.12) | −1.043 ns |
| Digit span backwards (WISC, maximal number of digits reached) | 3.07 (0.90) | 3.18 (1.01) | −0.302 ns |
| Speed of processing | |||
| Symbol search (WISC, maximal number of digits reached) | 8.82 (2.56) | 10.59 (2.42) | 0.453* |
| Coding (WISC, standard score) | 7.18 (2.22) | 9.00 (3.18) | 0.149* |
| Attention | |||
| Sky Search Attention (TEA-Ch, accuracy, scaled score) | 6.54 (2.43) | 8.65 (2.26) | −2.852** |
| Naming | |||
| Number Naming (CTOPP, percentile) | 18.00 (16.81) | 49.06 (24.84) | −4.995*** |
| Letter naming (CTOPP, scaled score) | 6.70 (2.02) | 9.70 (2.84) | −4.018* |
p < 0.05; **p < 0.01; ***p < 0.001.
CTOPP, Comprehensive Test of Phonological Processing; D-KEFS, Delis–Kaplan Executive Function System; PPVT, Peabody Picture Vocabulary Test; RDs, reading difficulties; SD, standard deviation; TEA-Ch, Test of Everyday Attention for Children; TONI, Test of Nonverbal Intelligence; TOWRE, Test of Word Reading Efficiency; WISC, Wechsler Intelligence Scale for Children; WJ, Woodcock–Johnson Test.
Correlation between reading and EF measures
A Pearson correlation across both reading groups revealed significant positive correlations between measures related to orthographic ability (timed: TOWRE, nontimed: letter word) and EF measures for (1) memory (digit span) [r(45) = 0.343, p < 0.05; r(45) = 0.299, p < 0.05, respectively], (2) speed of processing (coding) [r(45) = 0.351, p < 0.05; r(45) = 0.341, p < 0.05, respectively], and (3) visual attention (Sky-search) [r(45) = 0.401, p < 0.01; r(45) = 0.39, p < 0.05, respectively]. Results suggest that increased reading ability is related to greater working-memory, speed-of-processing, and visual-attention scores.
Imaging data
Functional connectivity between the EF network and regions related to reading for typical readers: results suggest positive functional connectivity between the EF network and regions related to cognitive control (e.g., BAs 8, 9, 10, 24, 32), language (e.g., BAs 7, 21, 22, 41, 42), and visual processing (e.g., BAs 17, 18, 19); p < 0.05, FDR corrected. For the BA-related anatomical regions, see Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/brain) and Figure 2.
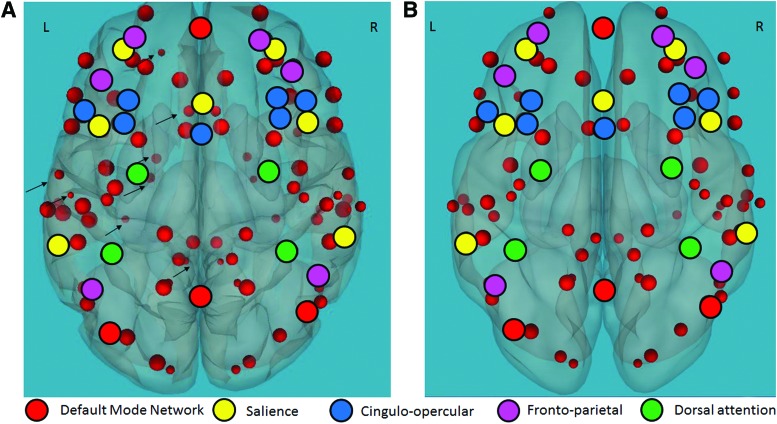
FIG. 2.
Functional connectivity of the EFs network and reading-related regions in typical readers and children with RDs. Positive correlation between functional connectivity of the EFs network (black circles) and reading-related language, visual, and cognitive control regions (red circles) during the Stroop task in (A) typical readers and (B) children with RDs. Orientation; L, left, R, right. p < 0.05, false discovery rate corrected for multiple comparisons. The red circles represent regions that are positively active when the EF network is active and illustrate the additional functional connections between the EF network and Brodmann areas in the left hemisphere in typical readers versus children with RDs. RDs, reading difficulties. Color images available online at www.liebertpub.com/brain
Functional connectivity between the EF network and regions related to reading for children with RDs: results suggest positive functional connectivity between the EF network and regions related to cognitive control (e.g., BAs 8, 9, 10, 24, 32), language (e.g., BAs 7, 21, 22, 41, 42), and visual processing (e.g., BAs 17, 18, 19); p < 0.05, FDR corrected. For the BA-related anatomical regions, see Supplementary Table S1 and Figure 2.
Differences in functional connectivity between children with RDs and typical readers: children with RDs demonstrated greater functional connectivity between the EF network and regions related to reading, p < 0.05 FDR corrected, including the left BAs 7, 39 and the right BA 21 in the language network, left BA 19 and right BAs 18, 19 in the visual network, and left BAs 31, 2, 8 and right BAs 9, 35, 43, 30, 31 in the cognitive control network. For the BA-related anatomical regions, see Supplementary Table S1 and Figure 3.
FIG. 3.
Difference in functional connectivity of the EFs network and reading-related regions in typical readers compared with children with RDs. Negative correlation between functional connectivity of the EFs network (black circles) and reading-related language and cognitive control regions (blue circles) during the Stroop task in children with RDs compared with typical readers. Orientation; L, left, R, right. p < 0.05, false discovery rate corrected for multiple comparisons. The blue circles represent regions that are more functionally connected with the EF network in children with RDs versus typical readers. Color images available online at www.liebertpub.com/brain
Graph theory measures for the EFs network as related to reading and EFs
Functional connectivity within the EF network was measured by the global efficiency measures and calculated for each participant separately, which was then fed into a two-way independent t-test analysis. Global efficiency values did not differ between the groups (mean global efficiency for typical readers: X = 0.667, SD = 0.018; children with RDs: X = 0.66, SD = 0.17, t(45) = −0.75, ns).
A Pearson correlation between global efficiency scores and reading measures among the two groups revealed a positive correlation between timed [TOWRE: r(45) = 0.387, p < 0.01] and nontimed[(letter word, WJ: r(45) = 0.404, p < 0.01] reading measures. Higher global efficiency scores were associated with greater reading ability.
Positive correlation between global efficiency measures and EF measures was also observed [working memory: digit span backwards r = (45) = 0.269, p < 0.05; speed of processing: coding r(45) = 0.355, p < 0.05; letter naming r(45) = 0.339, p < 0.05] and overall switching/inhibition ability [r(45) = 0.306, p < 0.05]. Greater global efficiency measures were related to overall better working memory, speed of processing, and EF measures among the entire study cohort.
Discussion
The aim of this study was to determine the different engagement of the EF network in children with RDs compared with typical readers, as related to their reading and EF abilities. We hypothesized that challenges in EFs in children with RDs would be accompanied by greater functional connections between the EF network and regions related to reading, and greater global efficiency scores would be related to increased reading and EF scores. Results did show that children with RDs performed significantly lower in all reading and EF measures tested in this study. The lower Stroop scores were accompanied by increased functional connectivity of the EF network and regions related to reading in children with RDs, as compared with typical readers. This may indicate a compensatory mechanism for individuals with RDs. Moreover, greater global efficiency of the EF network was related to higher reading and EF abilities in both groups, which highlights the role of the EF network in these academic abilities, as has been previously suggested (Horowitz-Kraus, 2016).
Increased functional connectivity between the EF network and regions related to reading: a possible compensatory mechanism?
Reading is a human invention that demanded an adaptation and a “recycling” of existing neural networks, as previously suggested by Dehaene and colleagues (2010). When examining the “highways,” our brains had to create to read, research shows the involvement of neural circuits related to EFs (Horowitz-Kraus and Holland, 2015; Vogel et al., 2012), language (Horowitz-Kraus et al., 2013, 2015b), and visual processing (Vogel et al., 2013, 2012, 2014). As reading develops, children rely on their language skills, visual and auditory attention, inhibition, memory, and processing speed to comprehend stories read by their parents (see Horowitz-Kraus and Hutton, 2015; Horowitz-Kraus et al., 2017; for review]. This activity of active stories listening was reported to facilitate future reading ability (Bus et al., 1995). These cognitive abilities have to synchronize in time to create the orchestra of reading, and in the case of a failure in synchronization, a reading failure may occur (see Horowitz-Kraus and Holland, 2015).
In addition to several theories to explain RDs, it seems that the overall slowness that characterizes readers with RDs may be related to a much more basic nonlinguistic challenge in EFs (Pennington, 2006; Welsh et al., 1991; Willcutt et al., 2001; and as also indicated by this study). In our study, this challenge in EFs was accompanied by an increased functional connectivity between the EF network and regions related to reading during the Stroop task in children with RDs that may serve as a compensatory mechanism for these readers, as has been observed in other pathologies and with an aging population (Deslauriers et al., 2017). Therefore, increased functional connectivity is often used as a compensation pathway to maintain the level of performance, which seems to still be impaired in children with RDs. However, since we included several EF networks in the current analyses, a future study should examine the relative contribution of each network included in the global EF network to the Stroop task. This contribution should then also be compared with the functional connectivity of each separate network during an fMRI reading task. Interestingly, greater involvement of regions in the left hemisphere was observed in typical readers during the Stroop task, which was not observed in children with RDs (Fig. 2) as was previously observed in the literature for reading tasks (Pugh et al., 2000). It may be that this differential involvement in the left hemisphere in children with RDs was not specific for reading, but generalized also to EF tasks. However, since the current version of the Stroop task did involve an exposure to words (even though the participants were requested to ignore them), an additional study using a word-free EF task specific to lateralization should focus on clarifying this point.
EFs as the infrastructure for reading ability
Previous studies suggest that reading and reading comprehension rely on intact language and EFs (Horowitz-Kraus, 2016). Others showed that an EF training (Horowitz-Kraus and Breznitz, 2009), video games (Franceschini et al., 2013), or EF-based reading training (Horowitz-Kraus, 2015; Horowitz-Kraus and Holland, 2015; Horowitz-Kraus et al., 2014a) improved reading ability in both individuals with RDs and typical readers. These intervention studies showing the positive effect of EF-based trainings on reading in typical readers as well emphasize that this effect is not attributed to the proposed joint impairment in EF and reading in individuals with RDs. It suggests a possible stronger linkage between these two abilities, even in the typically developing child, and future studies examining the relationship between an EF training before reading are acquired and future reading abilities may reveal whether there exists causality between training EFs and reading abilities.
The findings of this study provide the neurobiological evidence for the relationship between EFs and reading and may provide an explanation for previous intervention findings. The global efficiency of the EF network (i.e., the inverse average shortest path length of all pairs of nodes in the EF network; Watts and Strogatz, 1998) was found to be related to higher reading and EF scores. Greater global efficiency reflects the efficiency of the network and stems from improved learning abilities (Yang et al., 2015). These results support previous findings, suggesting that improved reading after an EF-based reading intervention was related to increased global efficiency in the cingulo-opercular network in 8–12-year-old children with RDs (Horowitz-Kraus et al., 2015c). However, the authors suggest that not only the cingulo-opercular network is related to higher reading scores, but also regions in the frontoparietal, salience, and visual-attention networks and the DMN are also critical for better reading and EF abilities. The relative contribution of each of these networks to the reading process warrants further research.
Another intriguing finding is that only orthographic abilities (i.e., timed and nontimed word-reading tests) were associated with the EF network global efficiency measures, but not comprehension. Recent findings regarding the critical relationship between planning abilities and reading comprehension (Georgiou and Das, 2016) and the role of the right hemisphere (as opposed to the left hemisphere for word reading) in reading comprehension both in adults and children (Horowitz-Kraus et al., accepted; Horowitz-Kraus et al., 2014b) indicate the challenge that reading comprehension has over word reading (see also the simple-view theory, Gough and Tunmer, 1986). It may be that selective networks that compose the EF network do not include specific network/regions related to this executive ability. A future study looking specifically into this point is warranted.
As previously suggested, speed-of-processing measures, working memory, and switching/inhibition were positively associated with higher global efficiency measures of the EF network. These findings reinforce previous findings highlighting the importance of fast and efficient words processing, which then decreases the working-memory bottleneck that often occurs in reading impairment (Breznitz and Share, 1992). Visual attention, which was previously reported to be related to reading ability (Facoetti and Molteni, 2001) and improvement after an EF-based reading intervention (Horowitz-Kraus et al., 2015a), was absent in this study. We suggest that the involvement of visual attention should include visual regions, and since these were absent in the EF network, global efficiency of this network did not show positive correlation with visual attention.
Study limitations
The results of this study should be considered with the following limitations. First, although the Stroop task is indeed an EF task, the condition we examined did involve reading. Despite the fact that the participants were explicitly required to ignore the word and respond to the color, reading is involved while performing the task (see Adleman et al., 2002). Therefore, to better demonstrate the neural engagement of EF networks during an EF task, a nonlinguistic Stroop task should be used in an MRI environment. Second, this study examined the functional connectivity and global efficiency of a network composed of several networks. Therefore, a future study should examine the relative contribution of each network to the overall network functional connectivity and task performance in children with RDs. Third, as noted, the definition of EFs is under debate. Although we chose to focus on the Andersen model for the development of EFs (Anderson, 2002) and the Dosenbach model for the corresponding neural circuits to EFs (Dosenbach et al., 2008), one must keep in mind that there are additional neuropsychological theories claiming that working memory and EFs are distinct constructs (Lezak et al., 2012) and that both are unrelated to processing speed (Salthouse, 1996). A future longitudinal neurobiological study examining the functional connections of each of the suggested networks during tasks specifically examining each of the mentioned EFs should be conducted in an attempt to separate the subcomponents of EFs. Another possible limitation is the definition of the EF network based on other networks defined using the CONN network atlas. A future study may be able to define this network using a functional approach (e.g., Cradock et al., 2004, functionally defined parcells that can be the basis for the EF network). Lastly, a controversy still exists between studies suggesting the decreased activation of the DMN and those suggesting an increased activation of this network during cognitive tasks (see Spreng et al., 2010, for further information). Sperg and colleagues suggested the coupled activation of the DMN with the frontoparietal network during a planning task, supporting the inclusion of this network as part of the EF network in this study. However, a future analysis focusing on the role of the DMN might demonstrate the exact involvement of this network in the EF network.
Conclusions
The results of this study suggest that children with RDs more heavily utilize the EF network to perform an EF task than do typical readers and that this may be an attempt to compensate for challenges performing the Stroop task. This connection between reading and EF suggests two important points: (1) A specific EF training even before reading is acquired may “set the stage” for future reading acquisition (e.g., see Rueda et al., 2005, 2012) and (2) functional connectivity of the EF network with the rest of the brain may serve as an early marker for RD. Both points should be examined in depth. Also, the relative contribution of each of the networks separately to the performance an EF task should be determined. The results of this study have several implications related to the educational and clinical fields: (1) The challenges children with RDs share in EFs should be taken into consideration in the classroom: teachers should try to assist children with RDs with avoiding visual and auditory distractions that may harm the child's attention. Since speed of processing is also slow, teachers may choose sharing the questions they are about to ask the student with RD before the formal lesson to allow an adequate processing time. (2) The results support the inclusion of neuropsychological tests related to EFs as part of the reading assessment battery to evaluate the strengths and weaknesses of students with RDs, which may influence their reading achievements as well. (3) Accommodations for children with RDs may not need to be specific only for topics involving reading, but may have to be extended to other topics learned in school that rely on EFs (e.g., mathematics).
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. 2002. A developmental fMRI study of the Stroop color-word task. Neuroimage 16:61–75 [DOI] [PubMed] [Google Scholar]
- Altemeier LE, Abbott RD, Berninger VW. 2008. Executive functions for reading and writing in typical literacy development and dyslexia. J Clin Exp Neuropsychol 30:588–606 [DOI] [PubMed] [Google Scholar]
- Anderson P. 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8:71–82 [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Reidy N. 2012. Assessing executive function in preschoolers. Neuropsychol Rev 22:345–360 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznitz Z. 2006. Fluency in Reading: Synchronization of Processes. Mahwah, New Jersey: Lawrence Erlbaum Associates [Google Scholar]
- Breznitz Z, Share DL. 1992. Effects of accelerated reading rate on memory for text. J Educ Psychol 84:193–199 [Google Scholar]
- Brosnan M, Demetre J, Hamill S, Robson K, Shepherd H, Cody G. 2002. Executive functioning in adults and children with developmental dyslexia. Neuropsychologia 40:2144–2155 [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou R, Johnsen S. 1997. Test of Nonverbal Intelligence, 3rd ed. Austin: Pro-Ed [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. 1999. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain 122 (Pt 10):1901–1917 [DOI] [PubMed] [Google Scholar]
- Bus AG, van IJzendoorn MH, Pellegrini AD. 1995. Joint book reading makes for success in learning to read: a meta-analysis on intergenerational transmission of literacy. Rev Educ Res 65:1–21 [Google Scholar]
- Cradock AL, Wiecha JL, Peterson KE, Sobol AM, Colditz GA, Gortmaker SL. 2004. Youth recall and TriTrac accelerometer estimates of physical activity levels. Med Sci Sports Exerc 36:525–532 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes G, Jobert A, et al. 2010. How learning to read changes the cortical networks for vision and language. Science 330:1359–1364 [DOI] [PubMed] [Google Scholar]
- Dellis DC, Kaplan E, Kramer JH. 2001. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation [Google Scholar]
- Deslauriers J, Ansado J, Marrelec G, Provost J, Joanette Y. 2017. Increase of posterior connectivity in aging within the Ventral Attention Network: a functional connectivity analysis using independent component analysis. Brain Res 1657:288–296 [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci 12:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. 2007. Peabody Picture Vocabulary Test. Bloomington, MN: NCS Pearson, Inc [Google Scholar]
- Faccioli C, Peru A, Rubini E, Tassinari G. 2008. Poor readers but compelled to read: stroop effects in developmental dyslexia. Child Neuropsychol 14:277–283 [DOI] [PubMed] [Google Scholar]
- Facoetti A, Molteni M. 2001. The gradient of visual attention in developmental dyslexia. Neuropsychologia 39:352–357 [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Viola S, Molteni M, Facoetti A. 2013. Action video games make dyslexic children read better. Curr Biol 23:462–466 [DOI] [PubMed] [Google Scholar]
- Georgiou GK, Das JP. 2016. What component of executive functions contributes to normal and impaired reading comprehension in young adults? Res Dev Disabil 49–50:118–128 [DOI] [PubMed] [Google Scholar]
- Gooch D, Snowling M, Hulme C. 2011. Time perception, phonological skills and executive function in children with dyslexia and/or ADHD symptoms. J Child Psychol Psychiatry 52:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PB, Tunmer WE. 1986. Decoding, reading, and reading disability. Remedial Spec Educ 7:6–10 [Google Scholar]
- Homack S, Riccio CA. 2004. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Arch Clin Neuropsychol 19:725–743 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T. 2014. Pinpointing the deficit in executive functions in adolescents with dyslexia performing the Wisconsin card sorting test: an ERP study. J Learn Disabil 47:208–223 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T. 2015. Improvement in non-linguistic executive functions following reading acceleration training in children with reading difficulties: An ERP study. Trends Neurosci Educ
- Horowitz-Kraus T. 2016. The role of executive functions in the reading process. In: Bar-Kochva I, Khateb A. (eds.) Reading Fluency: Current Insights from Neuro-Cognitive Research and Intervention Studies. Netherlands: Springer [Google Scholar]
- Horowitz-Kraus T, Breznitz Z. 2008. An error-detection mechanism in reading among dyslexic and regular readers—an ERP study. Clin Neurophysiol 119:2238–2246 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Breznitz Z. 2009. Can the error detection mechanism benefit from training the working memory? A comparison between dyslexics and controls—an ERP study. PLoS One 4:e7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, DiFrancesco M, Kay B, Wang Y, Holland SK. 2015a. Increased functional connectivity of specific brain networks after reading training in dyslexic children. Clin Neuroimage 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Eaton K, Farah R, Hajinazarian A, Vannest J, Holland SK. 2015b. Predicting better performance on a college preparedness test from narrative comprehension at the age of 6 years: an fMRI study. Brain Res 1629:54–62 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Grainger M, DiFrancesco M, Holland SK. 2015. Right is not always wrong: DTI and fMRI evidence for the reliance of reading comprehension on language comprehension networks in the right hemisphere. Brain Imaging Behav 9:19–31 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Holland SK. 2015. Greater functional connectivity between reading and error-detection regions following training with the reading acceleration program in children with reading difficulties. Ann Dyslexia 65:1–23 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Hutton JS. 2015. From emergent literacy to reading: how learning to read changes a child's brain. Acta Paediatr 104:648–656 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Kiefer A, DiCesare C, Dorrmann D. 2016. Eye-movements and decreased connectivity in cognitive-control regions during rest in children. In: Organization of Human Brain Mapping. Geneva, Switzerland [Google Scholar]
- Horowitz-Kraus T, Schmitz R, Hutton JS, Schumacher J. 2017. How to create a successful reader? Milestones in reading development from birth to adolescence. Acta Paediatr 106:534–544 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Toro-Serey C, DiFrancesco M. 2015c. Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PLoSOne 10:e0133762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Vannest JJ, Holland SK. 2013. Overlapping neural circuitry for narrative comprehension and proficient reading in children and adolescents. Neuropsychologia 51:2651–2662 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Vannest JJ, Kadis D, Cicchino N, Wang YY, Holland SK. 2014a. Reading acceleration training changes brain circuitry in children with reading difficulties. Brain Behav 4:886–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Wang Y, Plante E, Holland SK. 2014b. Involvement of the right hemisphere in reading comprehension: a DTI study. Brain Res 1582:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDA. 2011. Definition of Dyslexia-Based in the Initial Definition of the Research Committee of the Orton Dyslexia Society, Former Name of the IDA, Done in 1994. International Dyslexia Association [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, et al. 2012. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb Cortex 22:754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. 2010. Reading networks at rest. Cereb Cortex 20:2549–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. 2001. Efficient behavior of small-world networks. Phys Rev Lett 87:198701. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Bigler E, Tranel D. 2012. Neuropsychological Assessment, 5th ed. New York: Oxford University Press [Google Scholar]
- Li R, Qin W, Zhang Y, Jiang T, Yu C. 2012. The neuronal correlates of digits backward are revealed by voxel-based morphometry and resting-state functional connectivity analyses. PLoS One 7:e31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liua C, Yao B, Wang B, Zhouac R. 2014. N450 as a candidate neural marker for interference control deficits in children with learning disabilities. Int J Psychophysiol 93:70–77 [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Anderson V, Nimmo-Smith I. 1999. TEA-Ch: The Test of Everyday Attention for Children Manual. Bury St. Edmunds, United Kingdom: Thames Valley Test Company Limited [Google Scholar]
- Moradi AR, Taghavi R, Neshat-Doost HT, Yule W, Dalgleish T. 2000. Memory bias for emotional information in children and adolescents with posttraumatic stress disorder: a preliminary study. J Anxiety Disord 14:521–534 [DOI] [PubMed] [Google Scholar]
- Morken F, Helland T, Hugdahl K, Specht K. 2017. Reading in dyslexia across literacy development: a longitudinal study of effective connectivity. Neuroimage 144:92–100 [DOI] [PubMed] [Google Scholar]
- Nagy W, Berninger VW, Abbott RD. 2006. Contributions of morphology beyond phonology to literacy outcomes of upper elementary and middle-school students. J Educ Psychol 98:134–147 [Google Scholar]
- Pennington BF. 2006. From single to multiple deWcit models of developmental disorders. Cognition 101:385–413 [DOI] [PubMed] [Google Scholar]
- Protopapasa A, Archonti A, Skaloumbakas C. 2007. Reading ability is negatively related to Stroop interference. Cogn Psychol 54:251–282 [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. 2000. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6 207–213 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069 [DOI] [PubMed] [Google Scholar]
- Rueda MR, Checa P, Cómbita LM. 2012. Enhanced efficiency of the executive attention network after training in preschool children: immediate changes and effects after two months. Dev Cogn Neurosci 2S:S192–S204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. 2005. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci 102:14931–14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. 1996. The processing-speed theory of adult age differences in cognition. Psychol Rev 103:403–428 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. 1998. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A 95:2636–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M, Nation K, Moxham P, Gallagher A, Frith U. (1997). Phonological processing skills of dyslexic students in higher education: a preliminary report. J Res Read 20:31–41 [Google Scholar]
- Spreng SN, Stevens NS, Chamberlain JP, Gilmore AP, Schacter DL. 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage Clin 53:303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. 1935. Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662 [Google Scholar]
- Stuss DT, Knight RT. (eds.). 2002. Principles of Frontal Lobe Function. (New York, NY: Oxford University Press; ) [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. 1999. Test of Word Reading Efficiency (TOWRE). Austin, TX: Pro-Ed [Google Scholar]
- Vogel AC, Church JA, Power JD, Miezin FM, Petersen SE, Schlaggar BL. 2013. Functional network architecture of reading-related regions across development. Brain Lang 125:231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. 2012. The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex 22:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL. 2014. The VWFA: its not just for words anymore. Front Hum Neurosci 8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. 1999. Comprehensive Test of Phonological Processing (CTOPP). Austin, TX: Pro-Ed [Google Scholar]
- Watts DJ, Strogatz SH. 1998. Collective dynamics of ‘small-world’ networks. Nature 393:440–442 [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Intelligence Scale for Children—Third Edition (WISC-III). New York: The Psychological Corporation [Google Scholar]
- Welsh M, Pennington B, Groisser D. 1991. A normative developmental-study of executive function-a window on prefrontal function in children. Dev Neuropsychol 7:131–149 [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, Olson RK. 2001. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. J Abnorm Psychol 110:157–172 [DOI] [PubMed] [Google Scholar]
- Wong P, Perrachione TK, Parrish TB. 2007. Neural characteristics of successful and less successful speech and word learning in adults. Hum Brain Mapp 28:995e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. 1989. Woodcock-Johnson Psycho-Educational Battery-Revised (WJ-R). Allen, TX: Developmental Learning Materials [Google Scholar]
- Yagle K, Richards T, Askren K, Mestre Z, Beers S, Abbott R, et al. 2017. Relationships between eye movements during sentence reading comprehension, word spelling and reading, and DTI and fmri connectivity in students with and without dysgraphia or dyslexia. J Syst Integr Neurosci 3:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Gates KM, Molenaar P, Li P. 2015. Neural changes underlying successful second language word learning: an fMRI study. J Neurolinguistics 33:29e49 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.