Abstract
New, targeted imaging tracers enable improved diagnosis, staging, and planning of treatment of disease and represent an important step toward personalized medicine applications. The combination of radioisotopes for nuclear imaging with fluorophores for fluorescence imaging provides the possibility to noninvasively assess disease burden in a patient using positron emission tomography/single-photon emission computed tomography, followed by fluorescence imaging-assisted surgical intervention in close succession. Probes enabling imaging with both modalities pose a design, synthesis, and pharmacokinetics challenge. In this study, the authors strive to summarize recent efforts toward optimized, discrete, bimodal probes as well as a perspective on future directions of this burgeoning subfield of targeted imaging probe development.
Keywords: : fluorophores, molecular imaging, multimodality, radioisotopes
Introduction
The emergence of new, targeted nuclear imaging tracers enables improved diagnosis, staging, and planning of treatment of disease. Single-photon emission computed tomography (SPECT) tracers entered the clinical mainstay in the last century, with the cardiac perfusion agent, 99mTc sestamibi, gaining approval by the Food and Drug Administration in 1990.1 Nuclear imaging applications have further broadened after approval of the positron emission tomography (PET) tracer, 18F-fluorodesoxyglucose (18FDG), for the study of abnormal glucose metabolism in the context of cancerous growths, coronary artery disease, and neurological dysfunctions such as seizures in the year 2000.2–5 This has led to a continuously increasing SPECT and PET scanner base, which in turn has motivated the development of novel targeted imaging tracers incorporating various positron-emissive radioisotopes onto small molecules, synthetic peptides, and biologics. Furthermore, the incorporation of β− and α-emitting radionuclides can provide therapeutic analogs to β+/γ-based imaging tracers, specifically for personalized medicine applications in oncology, where targeted PET imaging is an indispensable treatment stratification tool.6 Often, evaluation of tumor burden is followed by surgical tumor debulking preceding chemotherapeutic intervention, where the surgeon relies on visual and tactile examination to identify tumor tissue. This impedes accurate delineation of margins and can result in nonresected malignancies leading to accelerated tumor metastasis and poor prognosis for the patient.7 This clinical challenge presents a formidable opportunity for probe development; specifically, the design of probes that provide a visual readout in the surgical suite. Optical probes are ideally suited for this purpose, providing high sensitivity and spatial resolution. The lack of depth penetration of excitation sources has thus far impeded widespread translation of targeted fluorescent tracers from murine models. However, if combined with a nuclear tracer, sites of tumor infiltration can be identified using noninvasive nuclear imaging techniques and guide the subsequent surgical intervention and fluorescence-guided surgery.8 Consequently, there is a need to combine nuclear and optical imaging tracers in one to improve patient outcomes. In an ideal clinical setting, one single bimodal compound could be administered to (1) assess total disease and operable tumor burden using nuclear imaging techniques, followed by (2) the intrasurgical identification of tumor margins using the optical modality to facilitate complete resection. The single component approach could be especially attractive to minimize burden on the patient and the healthcare provider; imaging immediately followed by image-guided surgery within a short time frame has the potential to drastically reduce inpatient time, which can accelerate improvement of health status and reduce the financial burden placed on healthcare. However, it is important to also note some of the disadvantages of the application of discrete bimodal systems (specifically when targeting vectors that are small molecules or peptides). Binding affinity and in vivo pharmacokinetics of targeted tracers incorporating large lipophilic chromophores are often drastically altered in comparison with the corresponding targeted, single-modality nuclear probe, thus control experiments that provide a quantitative comparison between bimodal and single-modality tracer are critically important. The detection limits for fluorescence versus nuclear tracer can differ by a minimum of two orders of magnitude, which limits the achievable specific activity for the reliable detection of both modalities. This indicates that dual modality tracer development is better suited for approaches incorporating radiometals onto molecules targeting receptors with enhanced accessibility and high expression levels over small molecules incorporating 11C or 18F. Furthermore, the lack of depth penetration and the nonquantitative nature of fluorescence emission remains one of the main impediments to fully implement this modality in a clinical setting.
This article places special emphasis on discrete, sequential molecular constructs; indeed, nanoparticle-mediated and single-center scaffold multimodal probes are also a widely explored and popular approach, but the authors defer to other excellent reviews on these topics.9,10 The single component assembly of two modalities in combination with a targeting vector often represents a formidable synthetic chemistry challenge. In this perspective, they aim to summarize approaches to targeted bimodal probes from recent literature and provide an outlook on future challenges and applications of single-component multimodality tracer systems.
Common Probe Design Strategies
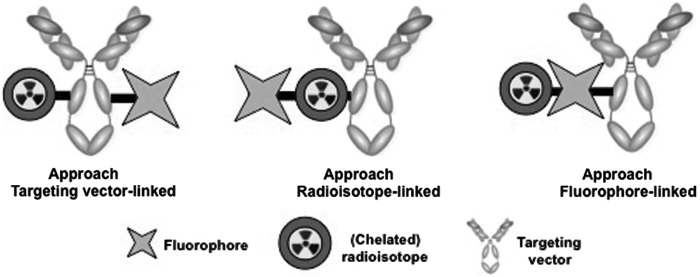
The incorporation of a nuclear and an optical beacon onto a targeting moiety can be achieved following a number of approaches. The primary challenge in designing an ideal bimodal tracer is to minimize the effect of the incorporation of both modalities on target affinity and off-target uptake. Specifically, the inclusion of fluorophores comprised of extended π systems can cause pronounced increase in lipophilicity and subsequent enhancement of hepatic probe clearance in vivo. Based on their survey of approaches employed thus far, the authors can identify three types of discrete, multimodal probe design: type A, where the targeting vector represents the link between fluorophore and radioisotope; type B, where the radioisotope (or more aptly, for radiometals, the chelator) links targeting vector and fluorophore; and type C, where the fluorophore joins targeting vector and radioisotope (Fig. 1). Type A has been explored most extensively, especially for large biomolecules with multiple available chemical conjugation sites in the context of nonspecific conjugation. Types B and C represent greater challenges in terms of chemical synthesis; the fluorophore and the site for incorporation of a radioisotope need to be joined before conjugation to the targeting vector. While more cumbersome to assemble, types B and C benefit from single-step conjugation to the targeting vector and direct control of targeting vector to radioisotope to fluorophore ratio. On the other hand, this approach can impede radiolabeling efficiency or alter fluorescence emission properties. All three approaches have been successfully utilized for multimodal, preclinical imaging. The ideal approach is determined by the properties of the conjugate, as the individual compound properties are not linearly cumulative. Below, they highlight work carried out using a variety of isotopes and fluorophores, grouped according to their linkage approach, and further broken down into categories based on targeting vector size and complexity; small molecules and peptides are typically more chemically inert to high-temperature reaction conditions involving nonphysiological pH or organic solvents, whereas biologics (antibodies, antibody fragments, and proteins) are more sensitive and constrain the scope of useable linker chemistry or molecular assembly considerably.
FIG. 1.
Schematic description of common multimodal probe design strategies based on linkage of individual components (radioisotope with prosthetic group/chelator, fluorophore, and targeting vector) discussed in this perspective.
Approach A: Targeting vector-linked bimodal probes
Targeting vector-linked bimodal probes have most extensively been explored in the context of monoclonal antibody (mAb) imaging. Primary linkage strategies include nonspecific incorporation of radioisotope and fluorophore by amide or thiourea bond formation with accessible lysines away from the variable region, as well as site-specific conjugation methods.11 Small molecules and peptides are also amenable to approach A, but will experience more pronounced alteration of in vitro and in vivo properties.
Small molecules and peptides
The incorporation of two imaging beacons on a comparatively small peptide can have significant consequences on binding affinity and clearance properties of the resulting construct. Josephson and coworkers addressed this issue by incorporation of a polyethyleneglycol (PEG)-cloud onto their bimodal RGDyK peptide (RGD) conjugate incorporating an indocyanine dye derivative, Cy5.5, and an 111In-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelate12 or 89Zr-desferrioxamine (DFO) chelate.13 The resulting conjugate exhibited enhanced quantum yields and improved pharmacokinetic properties, with the PEG-cloud exerting a shielding effect onto the lipophilic Cy5.5 fluorophore, preventing stacking interactions that would result in fluorescence quenching. PEG-clouded conjugates also exhibited longer blood residence time and improved tumor accumulation.
Caravan and coworkers reported the synthesis and in vitro characterization of a fibrin-targeting bimodal probe, where the fibrin-targeting peptide incorporates a fluorophore, fluorescein isothiocyanate (FITC), and a chelated radioisotope (64Cu(DOTA)) on each respective peptide terminus. This probe design was selected to address the accelerated metabolism of the peptide without C- and N-terminal “blocking groups.” Consequently, the bimodal probe shows excellent properties to visualize fibrin clots using fluorescence and PET without significant decrease of target affinity.14
Antibodies
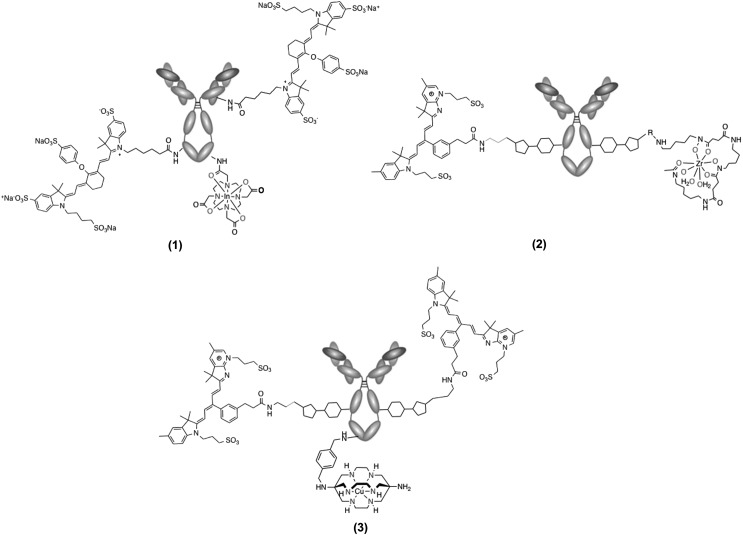
Application of approach A to antibodies represents both challenges and opportunities. The primary difficulty of the targeting vector-linked approach is close control of the number of imaging beacons incorporated, if a nonsite-specific conjugation approach is used. This is illustrated by the work of Rijpkema et al.,15 where the authors studied the effect of increasing the number of near-infrared (NIR) fluorophores on the in vivo behavior of an 111In-radiolabeled antibody (Fig. 2, 1); while a 1:1 ratio of fluorophore to DOTA provides ideal tumor uptake and high tumor-to-liver ratios, a 3:1 ratio results in significantly depressed tumor uptake while liver uptake increases drastically.
FIG. 2.
Antibody-linked multimodal probe approaches discussed in context of approach A.
Work by Zeglis et al.16 exemplifies applications of approach A using a more controlled incorporation of fluorophore and radioisotope (Fig. 2, 2). This method takes advantage of enzymatic modification of the heavy chain glycans of antibodies. In this work, huA33, an antibody targeting colorectal cancer cells, was modified to incorporate azide-bearing substrates, which then undergo strain-promoted click conjugation with dibenzocyclooctyne-desferrioxamine (DIBO-DFO) and DIBO-Dye680. By controlling the ratio of the two reacting DIBOs, the ratio of DFO and Dye680 conjugated on the mAb can be altered; however the degree of labeling of Dye680 cannot surpass 1.6 ± 0.1/mAb due to size or hydrophobicity constraints. Using a conjugation ratio of 2.0 ± 0.2 DFO/mAb and 1.0 ± 0.1 Dye680/mAb, the huA33 antibody was labeled with 89Zr, and the construct was then utilized to image SW1222 colorectal cancer xenografts in mice. PET and NIR fluorophore imaging were performed on both conjugates at 24, 48, 72, 96, and 120 h postinjection, showing comparable results with 89Zr-DFO-huA33-Dye680 immunoconjugates assembled using traditional, nonsite-specific methods.
This method for site-specific conjugation of DFO and fluorophore can be applied to other antibodies as well. Houghton et al.17 conjugated DIBO-DFO and DBCO-FL to hu5B1, human antibody that targets CA19.9 in pancreatic cancer. The immunoconjugates were injected into mice bearing both CA19.9-positive (BxPC3) and CA19.9-negative (MIAPaCa-2) xenografts on right or left flank, respectively. Subsequently, acquired PET images showed that the uptake in BxPC3 xenografts is eightfold higher than that in MIAPaCA-2. Fluorescence images show a tumor-to-background ratio of 25:1, whereas postsurgery fluorescence imaging revealed multiple micrometastases that were not visible with naked eye or PET imaging.
Similarly, 64Cu has also been utilized in context of a bimodal PET/NIR fluorophore immunoconjugate system, despite its relatively short half-life of 12.7 h. Adumeau et al.18 employed a pretargeting approach to overcome the difference between the physical half-life of 64Cu radioisotope and the extended pharmacokinetic half-life of targeting antibody. The huA33 antibody was first labeled with the NIR fluorophore Dye800 site specifically using the enzymatic heavy chain glycan modification; a trans-Cyclooctene (TCO) functional group was then attached separately through a lysine residue nonsite specifically to huA33 (Fig. 2, 3). The fluorophore immunoconjugate is injected to mice bearing SW1222 colorectal cancer xenografts and allowed to distribute, followed by delayed administration of tetrazine-SarAr-64Cu. The two components ligate in vivo through the tetrazine-TCO click reaction, with rapid elimination of residual unconjugated tetrazine-SarAr-64Cu through renal excretion. PET and fluorescence images obtained at 48 h following the administration of 64Cu indicated high target-specific accumulation in the tumor and low uptake in nontarget organs, exemplifying the key benefit gained from this pretargeting method being the significant reduction in radiation dose in conjunction with more rapid acquisition of high target-to-background ratios postisotope injection. The minor disadvantage of this approach is the need for the targeted cell surface receptor to exhibit no internalization or degradation after the initial binding event.
Approach B: Radioisotope-linked bimodal probes
Approach B incorporates the functionalization of a radioisotope-bearing prosthetic group, typically a chelator, with a targeting vector and a fluorophore. This approach has been explored extensively for peptides, due to the orthogonal protection group chemistry employed, which is typically less amenable to biomolecules. The primary challenge of this approach is to maintain optimized radiolabeling properties of the chelator even under the constraints of direct functionalization with two comparatively large molecules (fluorophore and targeting vector).
Small molecules and peptides
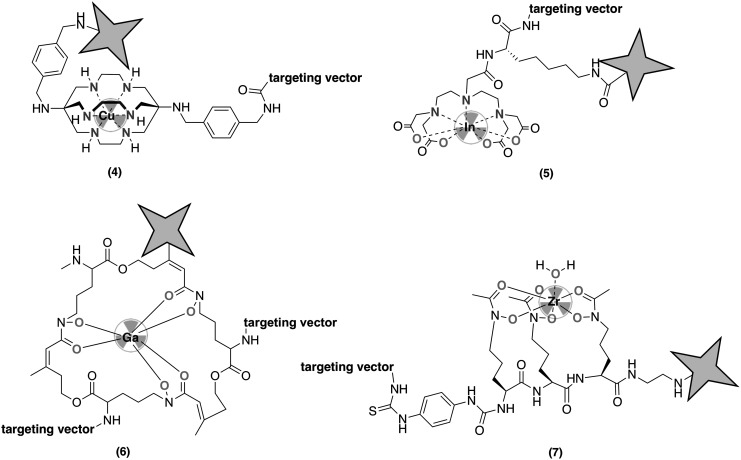
Work by Reiner et al.19 employed a 64Cu(SarAr) as a linker between a Cy5 fluorophore and exendin-4, a peptide targeting GLP-1 receptors expressed in pancreas and pancreatic islet cell tumors (Fig. 3, 4). The Cy5 fluorophore was coupled to an azide-functionalized sarcophagine (SarAr) chelator. The bioconjugation was carried out following prelabeling of the construct: The SarAr-azide was radiolabeled with 64Cu and conjugated to the exendin-4 peptide through site-specific Cu(I)-catalyzed click chemistry, which can result in the capture of oxidized Cu(II) within nonradiolabeled SarAr chelators. The resulting, click-conjugated bimodal tracer was administered to image insulinoma xenografts in mice. As exendin-4 is rapidly internalized into cells and clears from bloodstream within 5 h, PET images with satisfactory target-to-background ratio were obtained 4 to 5 h postinjection. Autoradiography and ex vivo fluorescence imaging performed with a pancreatic section showed good correlation between PET and optical probes.
FIG. 3.
Structures discussed in context of approach B, incorporating the radioisotope chelator as the linker between targeting vector and fluorophore.
Similarly, Ghosh et al.20 devised a novel derivative of DOTA that allows for incorporation of the radioisotope while also acting as a linker between octreotide and a triazole-appended IR dye. The corresponding bimodal conjugate did not experience a significant loss in binding affinity to SSTR2. In vitro experiments utilized the IR dye to visualize the intracellular localization of the construct in HCT116-SSTR2 cells; in vivo experiments in healthy mice with the 68Ga-radiolabeled probe show longer in vivo half-life of the bimodal construct when compared with 68Ga-DOTA-TOC. Targeted studies are forthcoming.
Kuil et al.21 conjugated Ac-TZ14011, a peptide that binds to chemokine receptor 4 (CXCR4), with a DTPA-Cy5.5 bimodal probe (Fig. 3, 5). The CXCR4 is a receptor that is present in various cells, but is 5.5 times overexpressed in breast cancer tissue. Because CXCR4 receptors are also found in healthy tissues, it is important that the bioconjugate can discriminate between tissues with basal level of CXCR4 and those with upregulation. When compared with the current clinical standard, 12G5-PE antibody conjugate, the Ac-TZ14011-DTPA-Cy5.5 performed comparatively, but the 111In-labeled bioconjugate showed less selectivity. This is likely due to the decreased negative charge of the 111In-conjugate, which increases hydrophobicity and in turn the nonspecific cellular uptake. In vivo PET and fluorescence images were obtained 24 h after injecting the bioconjugate to mice bearing either CXCR4+ or CXCR4− tumor. The tumor-to-muscle uptake ratio was 4.55 ± 0.68 for CXCR4+ and 1.20 ± 0.12 for CXCR4−, indicating that the Ac-tZ14011-DTPA-Cy5.5 bimodal probe can be used for determining sites of CXCR4+ overexpression.
Summer et al.22 employed asymmetric functionalization of the siderophore, fusarinine C, to append the NIR fluorophore sulfo-Cy7 and two RDG to target avb3 or MG (minigastrin) peptides to target cholecystokinin-2 (Fig. 3, 6). The corresponding construct maintained excellent radiolabeling properties with 68Ga, binding affinity to targets and in vivo imaging properties using both PET and fluorescence imaging of tumor xenografts in mice. The slow in vivo clearance of the constructs results in improved NIR imaging at later time points (>24 h), which is not ideal considering the short half-life of 68Ga.
Prostate-specific membrane antigen (PSMA)-targeting small molecules exhibit a faster clearance time. Baranski et al.23 focused in on the now clinically established PSMA-targeting PET tracer, PSMA-11 (Glu-urea-Lys-HBED-CC), and coupled it to each of IRDye800CW, Dylight800, FITC, and AlexaFluor488.23 The nanomolar binding affinity of PSMA-11 derivatives was only reduced twofold upon conjugation of fluorophore and radiolabeling, whereas cell-specific internalization and in vivo tumor uptake was improved upon fluorophore conjugation. The IRDye800CW- and DyLight800-conjugated bimodal probes show high tumor-to-blood and tumor-to-organ ratio 1 h after injection. Whereas the tumor uptake remains high over time, activity clears from other organs gradually, resulting in maximum tumor-to-background ratio after 6 h. The uptake in tumor was conspicuous in both in vivo PET and ex vivo fluorescence imaging.
Antibodies
Inspired by desferrichrome, a natural siderophore used by fungi and bacteria for abstracting Fe(III), their efforts have centered on the synthesis of linear desferrichrome derivatives Orn3-hx and Orn4-hx as Zr(IV) chelator molecules (Fig. 3, 7).24 Due to their peptidic nature, the linear desferrichromes can be conjugated to fluorophores and antibodies through amide bonding at either the C- or N-termini. To control the targeting vector to radioisotope to fluorophore ratio, Orn3-hx chelator is coupled to silicon rhodamine first, and the bimodal probe is conjugated to Trastuzumab through a lysine residue. The immunoconjugate was administered to naive C57Bl/6 mice to determine in vivo biodistribution and potential off-target accumulation. The bimodal immunoconjugate exhibits lower organ uptake and a significantly faster blood clearance compared with the gold standard DFO-mAb conjugate. Targeted imaging studies with the PET/fluorescence bimodal immunoconjugates using mouse xenograft tumor models are forthcoming.
Approach C: Fluorophore-linked bimodal probes
Approach C requires the functionalization of fluorophores to incorporate a biomolecule and a targeting vector. Besides the synthetic challenge of fluorophore modification, the primary concern is to not alter or even quench emissive properties of the linker fluorophore. Most approaches described herein have taken advantage of the chemical versatility of 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) derivatives.
Small molecules and peptides
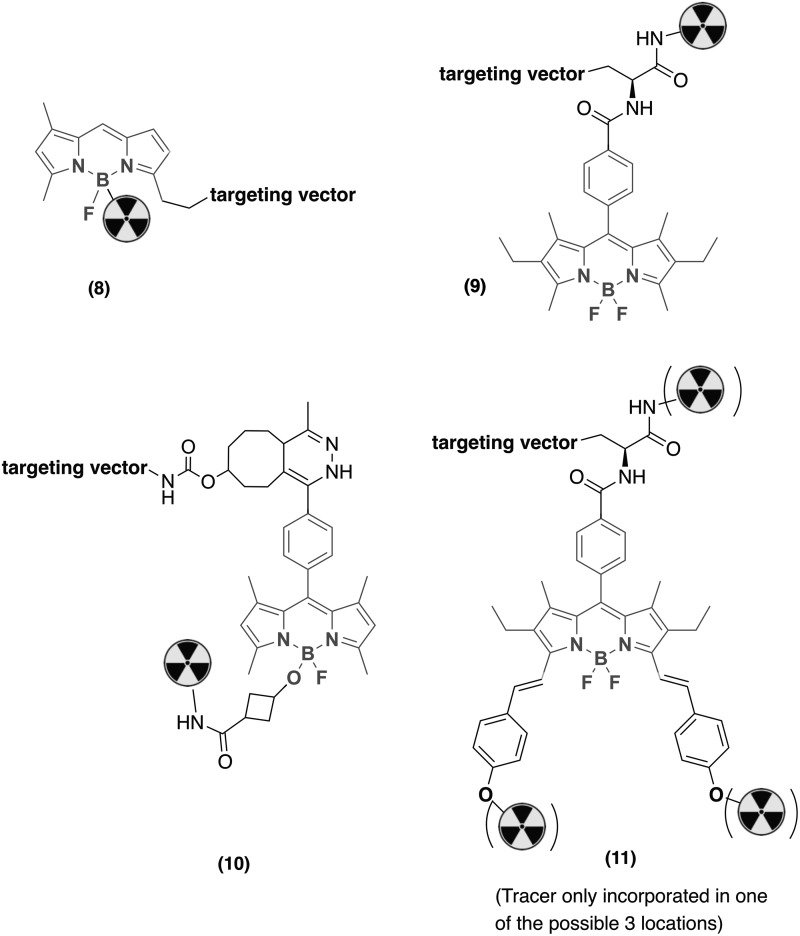
BODIPY dyes contain a BF2 core that allows for specific ligand exchange reactions on the B center with other hard Lewis basic donors. This has been exploited for the direct radiofluorination of BODIPY using an isotope exchange reaction. Reiner et al.19 and Carlucci et al.25 have developed PARPi-FL, a poly(ADP-ribose) polymerase 1 (PARP-1)-targeting bimodal PET/fluorescence probe. PARP-1 is a protein that is overexpressed upon DNA damage, and PARP-1 upregulation is observed in various cancers, including breast cancer, ovarian cancer, hepatocellular cancer, colorectal cancer, leukemia, and glioblastoma.26–29 The BODIPY fluorophore is covalently attached to the PARPi-FL-targeting scaffold and undergoes 18F/19F exchange to afford the bimodal [18F]PARPi-FL imaging agent (Fig. 4, 8).25 PET images obtained 90 min after injection of [18F]PARPi-FL in mice with U87 MG glioblastoma xenografts exhibited significant tumor uptake. The uptake in U87 xenografts in the brain was 0.78% ID/g, which was efficiently reduced to 0.1% ID/g by blocking of receptor binding by a large dose of cold inhibitor. The tumor-to-muscle and tumor-to-brain ratio are both high at 4 ± 0.6 and 12 ± 2.1, respectively, promising potential as an imaging tool for glioblastoma.
FIG. 4.
Structures discussed in context of approach C, incorporating the fluorophore as a linker between targeting vector and radioisotope.
Lhenry et al.30 have reported a monomolecular multimodal imaging probe, in which various DOTA derivatives were attached to aryl-BODIPYs (Fig. 4, 9). The BODIPY-chelator construct was coupled to octreotide, a peptide that targets neuroendocrine tumor cells. This bioconjugate was labeled with 111In and injected into mice with AR42J tumor. However, PET images obtained 24 h postinjection showed that uptake in tumor was comparatively low, likely due to lipophilicity of the conjugate resulting in higher uptake in liver and spleen.
Antibodies
Bimodal systems are useful not only for imaging tumors, but can also be applied in the study of the transport mechanism of lipopolysaccharides (LPS) in vivo.31 A DOTA-BODIPY probe conjugated to LPS retained proinflammatory property even after radiolabeling with 111In. The 111In-DOTA-BODIPY-LPS conjugate was injected into naive C57Bl/6 mice, and monitored with SPECT-CT and fluorescence imaging over a 24 h period. After 3.5 h, most of the activity was detected in the liver and spleen, and fluorescent imaging of liver sections showed numerous spots corresponding to 111In-DOTA-BODIPY-LPS, providing insight into the in vivo metabolism of LPS conjugates.
Meimetis et al.32 have designed a bimodal system in which BODIPY fluorophore was covalently linked to the Zr chelator DFO before conjugation to the antibody (Fig. 4, 10). The BODIPY is derivatized at the meso position of the aryl moiety with a tetrazine and simultaneously acid substituted through a fluorine abstraction. Coupling to DFO occurred at this carboxylic acid terminus to yield the bimodal unit, which was subsequently conjugated to trastuzumab-TCO at the tetrazine through inverse electron demand Diels–Alder addition. The fluorescence of BODIPY, initially quenched by the presence of the tetrazine, was unquenched upon conjugation to trastuzumab-TCO resulting in dearomatization of tetrazine. The fluorogenic probe thus acts both as a monitoring and quantification tool for the conjugation of Zr chelator to the antibody. The immunoconjugate was labeled with 89Zr and employed to image HER2+ and HER2− expressing breast cancer xenografts in a murine model. In vivo PET images, ex vivo PET, and fluorescence after 96 h revealed a strongly enhanced uptake of the probe in HER2+ tumors when compared with HER2− tumors.32
Maindron et al.33 also studied the antibody conjugates of the DOTA-BODIPY bimodal system (Fig. 4, 11). To address the enhanced lipophilicity when BODIPY is appended to biomolecules and to increase the radiolabeling yield, additional DOTA chelators were appended.33 Following radiolabeling with 111In, which allows for incorporation of the radioisotope into one of the three DOTA chelators present, the probe was utilized to image BT474 HER2+ xenografts in mice. In vivo fluorescence image obtained 24 and 48 h postinjection and ex vivo images of the organs show highest uptake in the tumor, followed by liver and kidneys.
The primary disadvantage of the approaches described above is the noticeable absence of more red-shifted fluorophores with better in vivo imaging properties. Xu et al.34 have successfully incorporated an indocyanine dye as a linking moiety. Evidently, one of the primary challenges with approach C is the limited chemical versatility of NIR dyes when compared with BODIPY derivatives and provides ample opportunities for creative synthetic approaches.
Conclusions and Outlook
Discrete bimodal probe systems have experienced a surge in interest over the past decade, which can be correlated with the availability of a wide array of imaging radionuclides in conjunction with the emergence of a myriad of small molecule, peptide, and antibody-targeting vectors for cell-surface receptor targeting. The ability to deliver an imaging tracer noninvasively to assess disease burden using the radionuclide, immediately followed by surgical intervention guided by the fluorophore is especially attractive from a logistical standpoint: In an ideal setting, a preliminary diagnosis can be made by less specific means of nuclear imaging (18FDG), followed by a more target-specific, personalized medicine approach using the bimodal, targeted tracer. Approaches A, B, and C to furnish bimodal probes each have their individual merits, thus the ideal approach has to be identified on a case-by-case basis. Specifically, the careful tuning of compound polarity and hydrophilicity is required to minimize off-target accumulation upon incorporation of lipophilic fluorophores. In addition to optimizing properties for ideal in vivo performance, other exciting challenges lie ahead to further enhance function and capability of multimodal probes. The incorporation of turn-on mechanisms or red shifting of emissive properties can act as additional, functional readouts on compound integrity and activity. This represents exciting opportunities to enhance the authors' understanding of the mechanism of action of targeted tracers and therapeutics and further improve the efficacy toward their ultimate goal to improving treatment outcomes for patients.
Acknowledgments
E.B. acknowledges funding sources, specifically the NIH for a pathway to independence award (Grant No. R00HL125728-03), a REACH award (Grant No. U01HL127522—18150031), and Stony Brook University for startup funds.
Disclosure Statement
There are no existing financial conflicts.
References
- 1.Schibli R, Schubiger PA. Current use and future potential of organometallic radiopharmaceuticals. Eur J Nucl Med Mol Imaging 2002;29:1529. [DOI] [PubMed] [Google Scholar]
- 2.Alavi A, Kung JW, Zhuang HM. Implications of PET based molecular imaging on the current and future practice of medicine. Semin Nucl Med 2004;34:56. [DOI] [PubMed] [Google Scholar]
- 3.Alavi A, Werner TJ. FDG-PET imaging to detect and characterize infectious disorders; an unavoidable path for the foreseeable future. Eur J Nucl Med Mol Imaging 2017;44:417. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 2009;50:88. [DOI] [PubMed] [Google Scholar]
- 5.Buck A, Schirrmeister H, Kühn T, et al. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 2002;29:1317. [DOI] [PubMed] [Google Scholar]
- 6.Schubiger PA, Grunberg J, Ametamey SM, et al. Radiopharmaceuticals: From molecular imaging to targeted radionuclide therapy. Chimia 2004;58:731 [Google Scholar]
- 7.Pandey SK, Gryshuk AL, Sajjad M, et al. Multimodality agents for tumor imaging (PET, fluorescence) and photodynamic therapy. A possible “see and treat” approach. J Med Chem 2005;48:6286. [DOI] [PubMed] [Google Scholar]
- 8.Rieffel J, Chitgupi U, Lovell JF. Recent advances in higher-order, multimodal, biomedical imaging agents. Small 2015;11:4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunschoten A, van Willigen DM, Buckle T, et al. Tailoring fluorescent dyes to optimize a hybrid RGD-tracer. Bioconjug Chem 2016;27:1253. [DOI] [PubMed] [Google Scholar]
- 10.Kuil J, Velders AH, van Leeuwen FWB. Multimodal tumor-targeting peptides functionalized with both a radio- and a fluorescent label. Bioconjug Chem 2010;21:1709. [DOI] [PubMed] [Google Scholar]
- 11.Zeglis BM, Lewis JS. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans 2011;40:6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Yuan H, Rice WL, et al. The PEG-fluorochrome shielding approach for targeted probe design. J Am Chem Soc 2012;134:19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilks MQ, Normandin MD, Yuan H, et al. Imaging PEG-like nanoprobes in tumor, transient ischemia, and inflammatory disease models. Bioconjug Chem 2015;26:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uppal R, Ciesienski KL, Chonde DB, et al. Discrete bimodal probes for thrombus imaging. J Am Chem Soc 2012;134:10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijpkema M, Bos DL, Cornelissen AS, et al. Optimization of dual-labeled antibodies for targeted intraoperative imaging of tumors. Mol Imaging 2015;14:348. [PubMed] [Google Scholar]
- 16.Zeglis BM, Davis CB, Abdel-Atti D, et al. Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconjug Chem 2014;25:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houghton JL, Zeglis BM, Abdel-Atti D, et al. Site-specifically labeled CA19. 9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc Natl Acad Sci U S A 2015;112:15850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adumeau P, Carnazza KE, Brand C, et al. A pretargeted approach for the multimodal PET/NIRF imaging of colorectal cancer. Theranostics 2016;6:2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiner T, Lacy J, Keliher EJ, et al. Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia 2012;14:IN1–IN3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh SC, Hernandez Vargas S, Rodriguez M, et al. Synthesis of a fluorescently labeled 68Ga-DOTA-TOC analog for somatostatin receptor targeting. ACS Med Chem Lett 2017;8:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuil J, Buckle T, Yuan H, et al. Synthesis and evaluation of a bimodal CXCR4 antagonistic peptide. Bioconjug Chem 2011;22:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summer D, Grossrubatscher L, Petrik M, et al. Developing targeted hybrid imaging probes by chelator scaffolding. Bioconjug Chem 2017;28:1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baranski A-C, Schäfer M, Bauder-Wüst U, et al. PSMA-11-derived dual-labeled PSMA inhibitors for preoperative PET imaging and precise fluorescence-guided surgery of prostate cancer. J Nucl Med 2018;59:639. [DOI] [PubMed] [Google Scholar]
- 24.Adams CJ, Wilson JJ, Boros E. Multifunctional desferrichrome analogues as versatile 89Zr(IV) chelators for immunoPET probe development. Mol Pharm 2017;14:2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlucci G, Carney B, Brand C, et al. Dual-modality optical/PET imaging of PARP1 in glioblastoma. Mol Imaging Biol 2015;17:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alanazi M, Pathan AAK, Arifeen Z, et al. Association between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi population. PLoS One 2014;8:e85541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bièche I, de Murcia G, Lidereau R. Poly(ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin Cancer Res 1996;2:1163. [PubMed] [Google Scholar]
- 28.Ossovskaya V, Koo IC, Kaldjian EP, et al. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer 2010;1:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojo F, García-Parra J, Zazo S, et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol 2012;23:1156. [DOI] [PubMed] [Google Scholar]
- 30.Lhenry D, Larrouy M, Bernhard C, et al. BODIPY: A highly versatile platform for the design of bimodal imaging probes. Chem Eur J 2015;21:13091. [DOI] [PubMed] [Google Scholar]
- 31.Duheron V, Moreau M, Collin B, et al. Dual labeling of lipopolysaccharides for SPECT-CT imaging and fluorescence microscopy. ACS Chem Biol 2013;9:656. [DOI] [PubMed] [Google Scholar]
- 32.Meimetis LG, Boros E, Carlson JC, et al. Bioorthogonal fluorophore linked DFO-technology enabling facile chelator quantification and multimodal imaging of antibodies. Bioconjug Chem 2015;27:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maindron N, Ipuy M, Bernhard C, et al. Near-infrared-emitting BODIPY-trisDOTA111In as a monomolecular multifunctional imaging probe: From synthesis to in vivo investigations. Chem Eur J 2016;22:12670. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Baidoo K, Gunn AJ, et al. Design, synthesis, and characterization of a dual modality positron emission tomography and fluorescence imaging agent for monoclonal antibody tumor-targeted imaging. J Med Chem 2007;50:4759. [DOI] [PMC free article] [PubMed] [Google Scholar]