Abstract
Significance: Humans are exposed daily to polyphenols in milligram-to-gram amounts through dietary consumption of fruits and vegetables. Polyphenols are also available as components of dietary supplements for improving general health. Although polyphenols are often advertised as antioxidants to explain health benefits, experimental evidence shows that their beneficial cancer preventing and controlling properties are more likely due to stimulation of pro-oxidant and proapoptotic pathways.
Recent Advances: The understanding of the biological differences between cancer and normal cell, and especially the role that mitochondria play in carcinogenesis, has greatly advanced in recent years. These advances have resulted in a wealth of new information on polyphenol bioactivity in cell culture and animal models of cancer. Polyphenols appear to target oxidative phosphorylation and regulation of the mitochondrial membrane potential (MMP), glycolysis, pro-oxidant pathways, and antioxidant (adaptive) stress responses with greater selectivity in tumorigenic cells.
Critical Issues: The ability of polyphenols to dissipate the MMP (Δψm) by a protonophore mechanism has been known for more than 50 years. However, researchers focus primarily on the downstream molecular effects of Δψm dissipation and mitochondrial uncoupling. We argue that the physicochemical properties of polyphenols are responsible for their anticancer properties by virtue of their protonophoric and pro-oxidant properties rather than their specific effects on downstream molecular targets.
Future Directions: Polyphenol-induced dissipation of Δψm is a physicochemical process that cancer cells cannot develop resistance against by gene mutation. Therefore, polyphenols should receive more attention as agents for cotherapy with cancer drugs to gain synergistic activity. Antioxid. Redox Signal.
Keywords: : glycolysis, mitochondria, oxidative stress, polyphenols, protonophore, ROS signaling
Introduction
Epidemiological studies have revealed an inverse relationship between cancer risk and diets consisting of grains, fruits, and vegetables (12, 39, 218). It is more difficult to establish such a relationship with dietary constituents because food products contain thousands of phytochemicals with diverse actions on normal and abnormal physiology. Many published studies have promoted the popular view that dietary phytochemicals, especially polyphenols, act as antioxidants to repress oxidative stress. This postulate is oversimplified and needs refinement.
Oxidative stress, or the imbalance between the production and removal of reactive oxygen species (ROS), can result from chronic inflammation and dysfunctional metabolism. NADPH oxidase, xanthine oxidase, and the electron transport chain (ETC) are prominent sources of superoxide, which is subsequently transformed by superoxide dismutase (SOD) into hydrogen peroxide (H2O2) and oxygen (52, 63). ROS play a crucial role in innate immunity to fight infections, but can also cause harm to the organism's own biomolecules. To protect cells from damage from excess ROS, the body relies on the antioxidant vitamins C and E to scavenge ROS or to interfere with the propagation of radical chain reactions, which are most likely to initiate in lipid bilayers (195).
Another important cellular antioxidant is glutathione (GSH) (63). Vitamin C and GSH are present in the cytosol at millimolar concentrations while dietary polyphenols or their metabolites might reach cellular concentrations in the low micromolar range. Therefore, it is unlikely that dietary polyphenols contribute significantly to any antioxidant activity in vivo in the sense that they scavenge ROS (62). This is not to say that dietary polyphenols exert no beneficial health effects in vivo. On the contrary, many studies have demonstrated that dietary polyphenols and other phytochemicals exert bioactivity through stimulating or interfering with cell signaling (86, 112). They affect cell signaling by interaction with nuclear receptors and other molecular targets, processes that require far smaller concentrations than the cellular concentrations of the endogenous antioxidants.
Mechanistic studies on the bioactivity of dietary polyphenols suggest that they can act as pro-oxidants in vivo (62). Under normal, physiological conditions, a pro-oxidant effect will deplete cells of GSH, which triggers an adaptive stress response leading to GSH synthesis to protect cells from pre-existing ROS (140). In this scenario, polyphenols exert an indirect antioxidant effect (137, 186), sometimes referred to as “para-hormesis” (45, 46). Under pathophysiological conditions, such as existing in tumor cells where steady-state levels of ROS are abnormally high, dietary polyphenols may actually enhance oxidative stress and drive tumor cells into apoptosis (50).
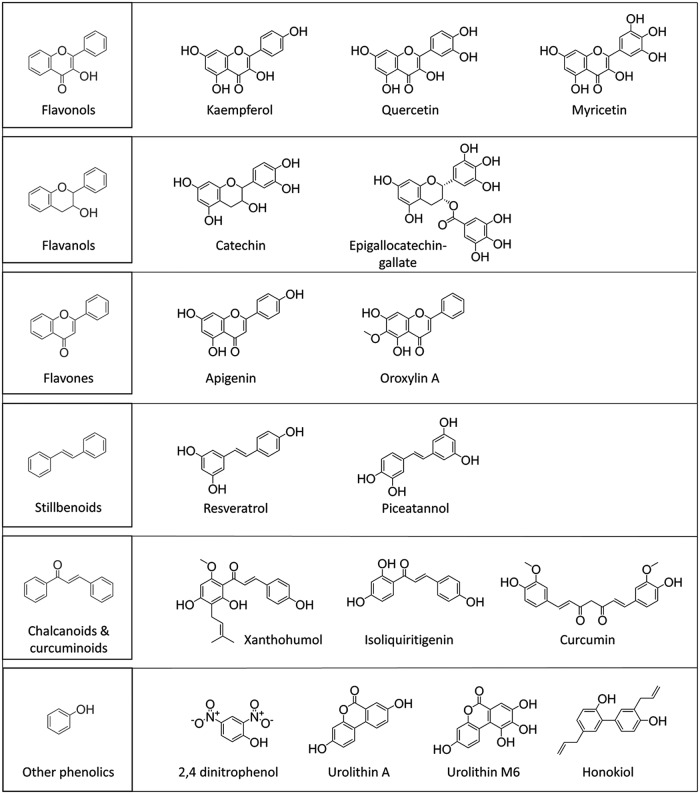
Regardless of the outcome of polyphenol bioactivity, the chemical moiety responsible for any effect is invariably the hydroxyl group found in all polyphenol classes (Fig. 1). Oxidation of a phenol involves the loss of a proton and an electron to generate a phenoxy radical that then reacts with ascorbate or GSH, or participates in radical propagation reactions (44). It can dissociate into a proton and a phenolate anion, which can dissipate the mitochondrial membrane potential (MMP) and cause uncoupling of the ETC from adenosine triphosphate (ATP) production (185).
FIG. 1.
Chemical structures of polyphenols discussed in this review.
When polyphenol molecules contain a catechol functionality, they are subject to oxidative conversion into ortho-quinones that can interfere with cellular processes through covalent interactions with various biomolecules (15, 77). Several comprehensive reviews provide examples of how ortho-quinones react with sulfhydryl groups in the Kelch-like ECH-associated protein 1 (Keap1) protein to induce the Keap1/antioxidant–nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling pathway (5, 34, 130, 181). Other reviews (89, 104, 114, 141) describe how polyphenols interfere with the proinflammatory NFκB (nuclear factor κ-light-chain-enhancer of activated B cells) pathway to exert an anti-inflammatory effect through repressing the release of proinflammatory cytokines from macrophages and other cell types (104, 114, 141).
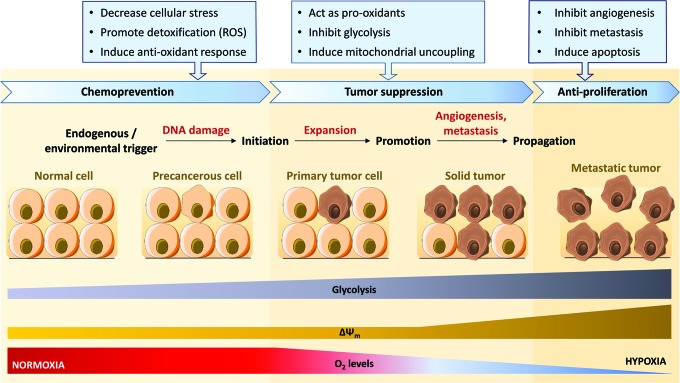
Polyphenols act as cancer chemopreventive and controlling agents on mitochondrial targets at all stages of carcinogenesis (Fig. 2). This review focuses on the differential effects of polyphenols on mitochondria of normal versus cancer cells. Only a limited number of clinical trials have been so far conducted that used polyphenols extracts and/or purified compounds in cancer prevention strategies or as adjunctive in conventional cancer treatment protocols. Those studies have been reviewed recently elsewhere (75, 76).
FIG. 2.
The multistep process of carcinogenesis. Cancer development, effect of malignant transition on glycolysis rate, mitochondrial membrane potential (ΔΨm) and dioxygen (O2) levels, and related actions of polyphenols at the different stages of carcinogenesis [adapted from Moga et al. (133) and Surh (190)]. ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Polyphenol Metabolism and Bioavailability
Polyphenols have been extensively studied for their health benefits related to cardiovascular diseases (14, 21) and cancer (123, 133, 160). Clinical trials testing efficacy of potential anticancer or chemopreventive phytochemicals have shown variable results [reviewed recently in Hosseini and Ghorbani (76), Kotecha et al. (97), Maru et al. (123), and Núñez-Sánchez et al. (146)]. While in vitro studies are crucial to decipher the mode of action of polyphenols at the cellular level, it is often difficult to translate the observations to in vivo bioactivity, because various factors influence the bioavailability of polyphenols. They include (i) water solubility (usually <50 μg/mL), (ii) route of administration, posology, and dosage formulation, and (iii) metabolic biotransformation [for a review, see Bohn (13)].
Polyphenol metabolism in vivo has long been considered as a major limitation for evaluation of clinical studies. Liver and gut metabolism generates a complex mixture of metabolites (methylated, hydroxylated, glucuronidated, sulfated, and hydrogenated forms), all of which contribute to the overall bioactivity of polyphenols. When comparing the physicochemical properties (lipophilicity, pKa) and reduction potentials of polyphenols (Table 1) and their gut microbial metabolites (Table 2), it appears that metabolites of polyphenols are likely to be bioactive.
Table 1.
Physicochemical Properties of Polyphenols Discussed in This Review
| Polyphenol | pKa1 (most acidic) | pKa2, pKa3 | LogP (logKOW) | One-electron reduction potential (mV) |
|---|---|---|---|---|
| 2,4 Dinitrophenol | 4.04 ± 0.22a | 1.715 ± 0.218a | — | |
| Apigenin | 6.53 ± 0.40a | 2.127 ± 0.452a | >500b (220) | |
| Catechin | 8.68 ± 0.23 (72) | 9.70 ± 0.24 (72) 11.55 ± 0.20 (72) | 0.610 ± 0.454a | 570 (83) |
| Curcumin | 8.38 ± 0.04 (10) | 9.88 ± 0.02 (10) 10.51 ± 0.01 (10) | 3.071 ± 0.444a | 770 (81) |
| Epigallocatechin-gallate | 7.69 (99) to 7.75 (80) | 8.80 (80) to 10.70 (99) | 0.639 ± 0.702a | 430 (80) |
| Honokiol | 9.89 ± 0.48a | 4.560 ± 0.352a | — | |
| Isoliquiritigenin | 7.50 ± 0.35a | 2.750 ± 0.340a | — | |
| Kaempferol | 6.96 ± 0.09 (72) | 8.78 ± 0.11 (72) 10.60 ± 0.12 (72) | 2.685 ± 0.812a | 950 (83) |
| Myricetin | 6.30 ± 0.40a | 1.206 ± 1.286a | 360 (9) | |
| Piceatannol | 9.17 ± 0.10a | 2.682 ± 0.359a | — | |
| Quercetin | 7.10 ± 0.12 (72) | 9.09 ± 0.11 (72) 11.02 ± 0.36 (72) | 1.989 ± 1.075a | 330 (82) |
| Resveratrol | 9.22 (27) | 10.55 (27) | 3.024 ± 0.267a | 653 (93) |
| 11.16 (27) | ||||
| Urolithin A | 9.07 ± 0.20a | 2.311 ± 0.793a | 332b (85) | |
| Urolithin M6 | 7.34 ± 0.20a | 0.832 ± 1.274a | — | |
| Xanthohumol | 7.59 ± 0.45a | 4.821 ± 0.416a | — |
Data obtained from SciFinder (calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2017 ACD/Labs).
Values given for anode peak voltage (Epa).
Table 2.
Physicochemical Properties of Metabolites of Polyphenols Biotransformed by the Gut Microbiome
| Metabolite | Example of precursor | pKa1 (most acidic) | LogP (logKOW) | One-electron reduction potential (mV) |
|---|---|---|---|---|
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone | Epicatechin/Epigallocatechin | 9.73 ± 0.10a | −0.002 ± 0.368a | — |
| Caffeic acid | P-coumaric acid | 4.58 ± 0.10a | 0.663 ± 0.286a | 534 (43) |
| Daidzein | Formononetin | 7.01 ± 0.20a | 2.632 ± 1.134a | 500 (220) |
| Dihydrocurcumin | Curcumin | 8.35 ± 0.60a | 3.244 ± 0.397a | — |
| Gallic acid | Malvidin | 4.33 ± 0.10a | 0.531 ± 0.325a | 550 (93) |
| Genistein | Biochanin A | 6.51 ± 0.20a | 3.114 ± 1.137a | 790b (42) |
| Hippuric acid | Caffeic acid | 3.71 ± 0.10a | 0.757 ± 0.533a | — |
| O-desmethylangolensin | Daidzein | 7.63 ± 0.35a | 2.587 ± 0.362a | — |
| Protocatechuic acid | Catechin | 4.45 ± 0.10a | 1.010 ± 0.237a | 298 (219) |
| (S)-equol | Daidzein | 9.94 ± 0.40a | 2.773 ± 0.319a | — |
| Urolithin A | Ellagitannins | 9.07 ± 0.20a | 2.311 ± 0.793a | 332b (85) |
| Urolithin M6 | Ellagitannins | 7.34 ± 0.20a | 0.832 ± 1.274a | — |
Some compounds (such as caffeic acid, genistein, or daidzein) are also polyphenols in their own right.
Data obtained from SciFinder (calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2017 ACD/Labs).
Values given for anode peak voltage (Epa).
A large number of studies have often observed antioxidant and antiproliferative activities of products of polyphenol metabolism (135), such as hydroxyphenyl-γ-valerolactones (58), protocatechuic acid (115, 224), and urolithins (165). Furthermore, conjugated metabolites accumulate in tissues, such as the liver (129), and form a metabolite pool for release of active metabolites. We observed that the magnitude of a biological effect correlates well with circulating levels of polyphenol glucuronides but poorly with circulating trace levels of free polyphenols (109), suggesting that plasma levels of glucuronides reflect tissue concentrations of bioactive forms better than circulating levels of free polyphenols.
Glucuronides are less lipophilic (lower partition coefficient octanol/water; logP) and need to be deconjugated by β-glucuronidase to accumulate in cells. β-Glucuronidase is present in the intestine (secreted by bacteria) and in other tissues confined in lysosomes and microsomes of healthy cells, but is extensively secreted by inflammatory cells surrounding tumor areas (16). Numerous anticancer prodrugs (glucuronide-conjugated drugs) have been designed with the aim to be delivered specifically to the site of the tumor (197).
Polyphenol glucuronides might act as natural prodrugs, being a reservoir of the bioactive aglycone (147). Many studies conducted by others have shown that phase II metabolites can contribute to the pharmacological efficacy of polyphenols (135, 215). Patel et al. demonstrated that sulfate conjugates of resveratrol contribute to the regeneration of the intracellular pool of resveratrol and to the beneficial effects against colorectal cancer (155, 156). Moreover, conjugated metabolites of resveratrol, especially resveratrol-3-O-sulfate, showed greater potential to inhibit proliferation of human colon carcinoma cells (SW480 and SW62220) compared to the parent polyphenol (1). The advantage here is that phase II metabolites excreted into the bile or generated in the intestine are readily available to colon cells.
In conclusion, the emerging knowledge of bioactive polyphenol metabolites argues in favor of including polyphenol metabolites in estimating overall bioavailability, in accordance with FDA recommendations (200). Pharmacokinetic studies conducted in recent years indicate that the combined bioavailability of polyphenols and their metabolites exceeds 30% (106a, 107, 151, 187). Nevertheless, phase II metabolism is a mechanism of detoxification aimed to remove phenolics from the body. A considerable fraction of the ingested polyphenols are excreted after conjugation. Oral consumption of polyphenols exerts beneficial effects against primary tumors in the gastrointestinal tract (156). Intravenous (IV) administration yields higher plasma levels at equal doses (107). Recently, IV administration has been proposed as the preferred route of administration of bioactive polyphenols for various oncotherapeutic applications (36).
The relatively high concentrations often used in cell culture studies raise the question whether in vitro findings bear relevance to the in vivo situation. Researchers usually perform cell assays after a single exposure of cells to treat compounds. A small fraction of the amount administered to cultured cells may actually be taken up by the cells due to polyphenol–protein binding in the growth medium surrounding the cells. Polyphenol–protein binding occurs in blood of animals and humans as well, but continuous in vivo dosing of a polyphenol or polyphenol mixtures may drive cellular uptake resulting in polyphenol accumulation in organs (129). These considerations suggest that comparisons between cell culture medium concentrations and plasma concentrations are not very meaningful to account for discrepancies between in vitro and in vivo bioactivity of polyphenols.
Many studies on the bioactivity of polyphenols involve mitochondria. For example, polyphenols extracted from pinecone of Pinus koraiensis inhibited tumor growth in sarcoma-bearing 180 mice (223), which the authors attributed to apoptotic pathways initiated by mitochondria. Although very few studies have verified mitochondrial uptake of polyphenols and their bioactive metabolites, their physicochemical properties (lipophilicity, pKa; Tables 1 and 2) actually favor their enrichment in mitochondria (142). Human T lymphocyte (Jurkat) cells accumulate quercetin inside the mitochondria, which led the authors to hypothesize that mitochondria represent a reservoir of biologically active quercetin (41). Taken together, the available evidence indicates that polyphenols have greater bioavailability than previously thought and they have the ability to reach potential sites of action in mitochondria. We now discuss the molecular mechanisms by which polyphenols affect mitochondrial function in cancer models (summarized in Table 3). Mechanistic information on the bioactivity of polyphenols primarily originated from in vitro studies.
Table 3.
Cancer-Related Bioactivities at the Mitochondria Level for Polyphenols Discussed in This Review
| Polyphenol | Type of cells | Effect described | References |
|---|---|---|---|
| 2,4 Dinitrophenol | Calu-6 human pulmonary adenocarcinoma | Proapoptotic; depolarizes the membrane, increases ROS, decreases GSH | Han et al. (64) |
| Apigenin | DU145 human prostate cells | Proapoptotic; inhibits ANT2 | Oishi et al. (148) |
| Catechin | B16F-10 melanoma cells | Anti-inflammatory, antiangiogenic; reduces mRNA levels of VEGF, reduces cytokines and nitric oxide | Guruvayoo-rappan and Kuttan (61) |
| Curcumin | Escherichia coli M15 bacteria | Inactivates PTP complex, induces VDAC closure | Tewari et al. (193) |
| SGC-7901 human adenocarcinoma cells | Proapoptotic; depolarizes the membrane | Eggler et al. (33) | |
| T-REx-293 cells | Proapoptotic; induces VDAC oligomerization | Keinan et al. (91) | |
| HCT116 and HT29 human colorectal cancer cells | Proapoptotic; downregulates expression of HK-II | Wang et al. (205) | |
| Epigallocatechin-gallate | MIA Paca-2 human pancreatic cells and human papillomavirus-18-(+) HeLa cells | Proapoptotic; depolarizes the membrane | Qanungo et al. (161); Kuo et al. (103) |
| Human malignant mesothelioma cells | Proapoptotic; impairs ETC | Balogun et al. (6) | |
| Honokiol | Neuro-2a neuroblastoma cells | Proapoptotic; depolarizes the membrane | Kensler and Wakabayashi (92) |
| Kaempferol | HeLa and HepG2 cells | Suppresses PDK2 and PDK4 mRNA expression | Wang et al. (204) |
| Myricetin | JAR and JEG-3 malignant trophoblast cells | Antiangiogenic, proapoptotic; increases ROS production, induces GSH depletion, depolarizes the membrane | Yang et al. (221) |
| Oroxylin A | MDA-MB-231 and MCF-7 human breast cancer | Enhances oxidative stress, activates SIRT3, deacetylates CypD, dissociates HK-II | Wei et al. (211, 212) |
| A549 lung carcinoma cells | Antimetastatic; dissociates HK-II | Wei et al. (211) | |
| Piceatannol | DU145 prostate cancer cells | Proapoptotic; increases MMP | Müller and Hengstermann (139) |
| Quercetin (and myricetin) | A549 adenocarcinoma human cells | Proapoptotic; increases ROS production, inhibits TrxR | Lu et al. (117) |
| Resveratrol | MDA-MB-231 and MCF-7 human breast cancer | Stimulates SIRT1, inhibits ETC complex I | Deus et al. (28) |
| SW620 colon cancer cells | Proapoptotic; downregulates UCP2 | Dinkova-Kostova and Abramov (30) | |
| Urolithin A | C2C12 myotubes | Decreases membrane potential | Waseem and Parvez (208) |
| Urolithin M6 | Raji cells | Antiproliferative; inhibits LDH, inhibits lactate production | Rupiani et al. (165) |
| Xanthohumol | C2C12 myotubes | Increases ROS, decreases respiration at concentration >25 μM | Kirkwood et al. (95) |
ANT, adenosine nucleotide translocase; CypD, cyclophilin D; ETC, electron transport chain; GSH, glutathione; HK, hexokinase; LDH, lactate dehydrogenase; MMP, mitochondrial membrane potential; mRNA, messenger RNA; PDK, pyruvate dehydrogenase kinase; PTP, permeability transition pore; ROS, reactive oxygen species; SIRT1, sirtuin 1; TrxR, thioredoxin reductase; UCP, uncoupling protein; VDAC, voltage-dependent anion channel; VEGF, vascular endothelial growth factor.
Polyphenols as Mild Mitochondrial Uncouplers
Beneficial effects of mitochondrial uncoupling in obesity
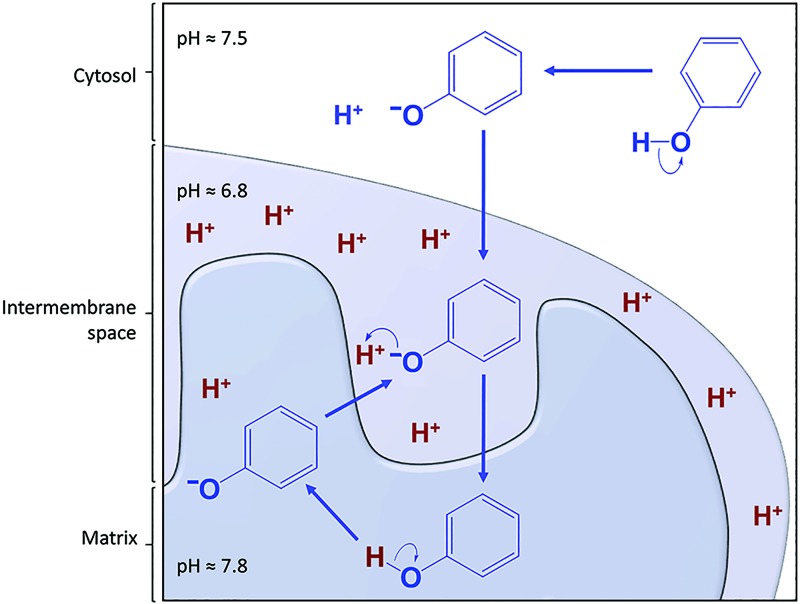
A mild mitochondrial uncoupling activity of dietary polyphenols represents one mechanism pertaining to their beneficial effects on dysfunctional glucose and lipid metabolism in type 2 diabetes, nonalcoholic fatty liver disease, and obesity. Uncoupling of the ETC from ATP production is achieved by dissipation of the electrochemical (proton) gradient across the inner mitochondrial membrane (180), referred to as the MMP, Δψm. When polyphenols move by diffusion in cells, they encounter different pH values. In the cytosol (pH 7.4), polyphenols exist as a mixture of phenolate anions and neutral phenols. The proportion of phenol anions and neutrals is dictated by the pKa of each phenolic group (Tables 1 and 2). Their propensity to cross the cell membrane and the outer and inner mitochondrial membranes depends on their lipophilicity.
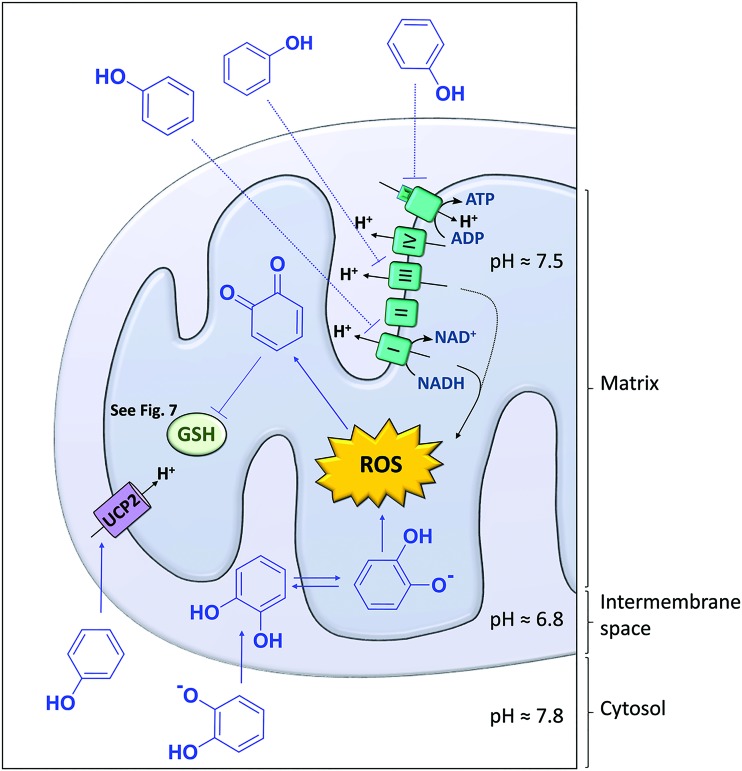
Because many polyphenols have favorable pKa values (pKa1 ranging between 6 and 9, i.e., close to the physiological pH of the cytosol and mitochondrial compartments), and distribution coefficients (Table 1) (71, 180), they have the ability to reach the mitochondrial matrix and release a proton in the relatively basic environment (pH 7.8) of the matrix (17). After dissociation, phenolate anions migrate back down the electrochemical gradient to the relatively acidic environment of the intermembrane space (pH 6.8). Back-and-forth diffusion of the phenolate–phenol species across the inner mitochondrial membrane results in transport of protons from the inner membrane space to the mitochondrial matrix (Fig. 3), leading to dissipation of Δψm (180, 185).
FIG. 3.
Polyphenols are protonophores. Polyphenols (represented by phenol for simplicity) dissociate to phenolate anions at cytosolic pH, diffuse through the outer mitochondrial membrane along the electrochemical gradient into the mitochondrial intermembrane space, where a more acidic environment facilitates their reprotonation and diffusion to the matrix. In the matrix, polyphenols deprotonate and the resulting phenolate anions diffuse back to the intermembrane space down the electrochemical gradient. They travel back and forth between the intermembrane space and the matrix, thereby transporting protons from the intermembrane space to the matrix. Dissipation of the mitochondrial membrane potential ensues. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To maintain Δψm, cells respond by increasing the activity of the ETC, which requires biofuel. Dissipation of Δψm will activate the nutrient sensor AMP-activated protein kinase (AMPK) to stimulate β-oxidation of fatty acids, glycolysis, and the tricarboxylic acid (TCA) cycle, which deliver the necessary reducing equivalents (FADH2 and NADH) to drive oxidative phosphorylation (150). The ultimate result is that mitochondria consume more fatty acid and glucose molecules to generate the same number of ATP molecules.
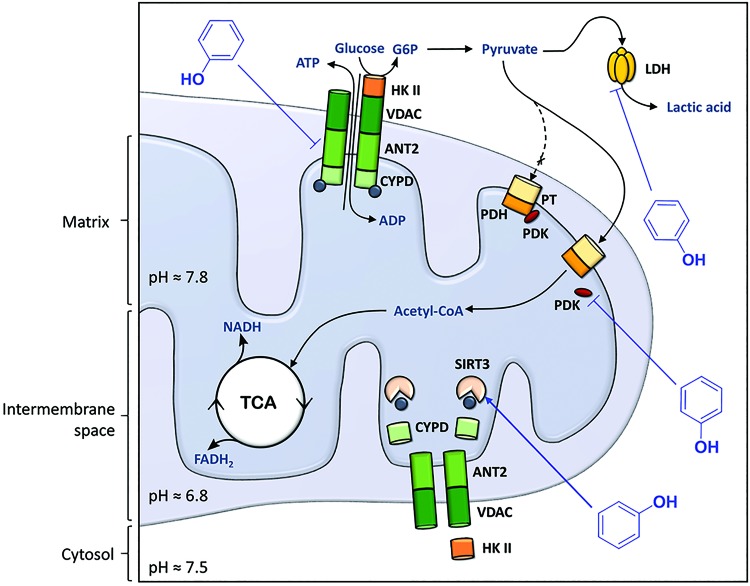
The capacity of polyphenols as proton carriers across the inner mitochondrial membrane is referred to as the protonophoric uncoupling or protonophore effect of polyphenols (17, 154, 162), to distinguish this mitochondrial uncoupling effect from effects of polyphenols on adenosine nucleotide translocases (ANTs) (Fig. 4) and uncoupling proteins (UCPs) (Fig. 5), both of which have the ability to leak protons across the inner mitochondrial membrane (18). The protonophore effect of polyphenols and the downstream signaling pathways (e.g., AMPK activation) often explains their improvement of obesity-related dysfunctional glucose and lipid metabolism. In cancer cells, which constantly fight oxidative stress under hypoxic conditions by keeping Δψm high, severe dissipation of the Δψm will drive the cells into apoptosis (66).
FIG. 4.
Polyphenols modulate mitochondrial metabolism. The PTP complex is composed of the VDAC in the mitochondrial outer membrane, the ANT in the mitochondrial inner membrane, and CypD in the matrix. Under physiological conditions, the PTP complex regulates exchange of ATP/ADP and small metabolites between the cytosol and the mitochondrial matrix, and it interacts with HK II in the cytosol that converts glucose to G6P, the first step of glycolysis. In cancer cells, HK II is overexpressed. Polyphenols downregulate ANT (and specifically the isoform 1) and activate the sirtuin 3 protein (SIRT3), responsible for deacetylation of CypD, resulting in detachment of CypD and HK II from the PTP complex. Pyruvate, the end product of glycolysis, is converted into acetyl-CoA by the complex pyruvate transporter (PT)/PDH to enter the TCA cycle. When PDK binds PDH, the conversion of pyruvate to acetyl-CoA is inhibited. In cancer cells, PDK is overexpressed, and pyruvate is converted into lactic acid by LDH in the cytosol. Polyphenols can inhibit PDK to allow conversion of pyruvate into acetyl-CoA by PDH, thereby limiting substrate availability for LDH, which results in inhibition of lactate production. Polyphenols are represented by phenol for simplicity. ADP, adenosine diphosphate; ANT, adenosine nucleotide translocase; ATP, adenosine triphosphate; CypD, cyclophilin D; G6P, glucose-6-phosphate; HK, hexokinase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PTP, permeability transition pore; TCA, tricarboxylic acid; VDAC, voltage-dependent anion channel. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 5.
Polyphenols modulate the mitochondrial redox status. Polyphenols target oxidative phosphorylation by interacting with the electron transport chain complexes (mainly complexes I and III, and ATP synthase) and by decreasing the membrane potential (acting as protonophores) or by activating the UCP2. ROS can convert polyphenols into their corresponding quinones, which can react with the thiol group of GSH. Polyphenols are presented as phenol or as catechol. GSH, glutathione; UCP2, uncoupling protein 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
2,4-Dinitrophenol (DNP) has attracted much attention as a potent mitochondrial uncoupler. It was a popular agent for inducing weight loss in the early 1900s, but in the late 1930s taken off the market in response to reports of severe morbidity and death (24). Its mechanism of action appears to be protonophoric in nature, but its acidic and hydrophilic properties (pKa = 4.09 and LogP = 1.7, Table 1) suggest a different mechanism. It is more likely that DNP forms an ion-pair with an endogenous amphiprotic molecule that enhances DNP's lipophilicity and increases the complex's pKa. McLaughlin has suggested that DNP binds to the lipid bilayer and forms a homodimeric complex consisting of one undissociated and one deprotonated molecule (DNP2−) (125). DNP has been considered for cancer therapy, but deemed too toxic due to its narrow therapeutic window (202). While it is possible to attenuate DNP's potency as a protonophore by improving its pharmacokinetic properties using a slow-release formulation (158), recent interest has shifted to the development of mild mitochondrial uncouplers with better physicochemical and therapeutic properties (202).
Attene-Ramos et al. (2) screened the Tox21 library of 10,000 compounds to identify chemicals and structural features that are associated with changes in the MMP in HepG2 cells. They found that 11% of the compounds decreased MMP after 1 h of treatment without affecting cell viability. By analysis of structure/activity relationships of MMP-decreasing compounds using a self-organizing map algorithm, the authors found that nitrobenzenes, thiazoles, flavonoids, and phenols emerged as enriched clusters (2). Many dietary polyphenols meet the requirements of protonophores (Table 1), but their development is hampered by their low bioavailability and rapid metabolism. On the contrary, many dietary polyphenols produce gut microbial or hepatic metabolites with (partial) retention of bioactivity (184).
The catabolic effects of mitochondrial uncouplers could indirectly suppress growth of metastatic tumor cells by depriving them of nutrients produced and supplied by surrounding cells. This “seed (cancer cell) and soil (tumor microenvironment)” theory offers a two-pronged approach to treat cancers by combination therapy of a mitochondrial uncoupler (e.g., DNP or a suitable polyphenol) to deplete macronutrients in the microenvironment together with a cancer chemotherapeutic to kill fewer and smaller metastatic cell colonies more effectively.
Mitochondrial uncoupling sensitizes cancer cells to apoptosis
Solid tumor cells are heterogeneous in terms of metabolic microenvironment and Δψm. Heerdt et al. (67) found, by characterizing single-cell subclones from the SW620 human colonic carcinoma cell line, that there are subtle, distinct, and stable differences in the cells' Δψm. While cell growth and proliferation were similar among cells with different Δψm, the differences in Δψm showed a significant effect on levels of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-7 levels. Both proteins play important roles in tumor progression, invasion, and metastasis. Therefore, there appears to be a positive relationship, associative or causative, between Δψm and metastatic potential of tumor cells. Polyphenols could conceivably repress the propensity for tumor progression through their protonophoric activity.
Few studies tested the hypothesis whether polyphenols have the ability to suppress the metastatic potential of cancer cells by lowering Δψm. Guruvayoorappan and Kuttan injected B16F-10 melanoma cells to induce capillary formation in C57BL/6 mice and subsequently monitored the effect of administration of (+)-catechin on angiogenesis (61). They found that the tumor-induced angiogenesis in control animals was accompanied by enhanced serum levels of proinflammatory cytokines and VEGF. Catechin-treated animals showed fewer tumor-directed capillaries, indicating an antiangiogenic effect of catechin. Using an in vitro system, they found that catechin reduced messenger RNA (mRNA) levels of VEGF in B16F-10 melanoma cells. The authors contributed the observed antiangiogenic effects of catechin to an anti-inflammatory effect by reducing the production of proinflammatory cytokines and nitric oxide. The authors did not investigate whether catechin exerted its effects through dissipation of Δψm.
Apigenin inhibits ANT-2
Tumor cells have a higher Δψm (≈−210 mV) compared with normal cells (≈−140 mV, more negative inside the mitochondrial matrix) (38). The Δψm is primarily regulated by the activity of the ETC, ATP synthase (complex V), UCPs, and ANTs. ANTs are embedded in the inner membrane of the mitochondria and are part of the ATP/adenosine diphosphate (ADP) exchange under physiological conditions (Fig. 4). The higher Δψm of cancer cells appears to be due, at least in part, to changes in the expression and activity of ANTs.
Tumor cells downregulate the expression of ANT1 (51, 122), which transports ATP from the matrix to the cytosol. Downregulation of ANT1 expression leads to a lower ATP concentration in the cytosol and to AMPK-mediated stimulation of glycolysis. In contrast, the expression of ANT2 is overexpressed in proliferating cells and upregulated in tumor cells (22, 51, 106). Unlike ANT1, ANT2 appears to export ADP from the mitochondrial matrix to the cytosol in exchange for an equivalent of ATP. The ensuing elevated concentration of ATP in the mitochondrial matrix switches ATP synthase in reverse mode: it hydrolyzes ATP and pumps protons across the inner membrane thereby creating a state of hyperpolarization (high Δψm) (22). In addition, the decreased cytosolic levels of ATP stimulate glycolysis.
Aside from stimulation of glycolysis, expression and activity of ANTs in tumor cells benefit from keeping Δψm high because relative inactivity of the ETC in combination with UCP/ANT-mediated proton leakage could dissipate Δψm to a threshold that triggers release of proapoptotic factors and initiation of apoptotic cell death (68, 70).
Thus, polyphenols that exert protonophoric activity or inhibit ANT2 would be expected to have cytotoxic (proapoptotic) potential that is more selective in cancer cells. Apigenin, a dietary flavonoid known to induce apoptosis, was recently identified as an inhibitor of ANT2 in cultured human prostate DU145 cells (148). The authors found that apigenin binds and inhibits ANT2, resulting in Apo 2 ligand (Apo2L)/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by upregulation of death receptor (DR)-5. Apo2L/TRAIL is a cytokine that belongs to the tumor necrosis factor family, binds to DR5 thereby inducing apoptosis in various malignant tumors but not in normal cells. Silencing ANT2 expression in DU145 cells led to repression of Apo2L/TRAIL-induced apoptosis by apigenin. Genistein also induced apoptosis in this study. However, it did not induce DR5 expression nor did it inhibit ANT2 (148).
Other polyphenols that have been shown to induce apoptosis in cancer cell in vitro through increasing TRAIL expression/activity include epigallocatechin gallate (EGCG; gastric cancer cells) (149), human prostate carcinoma LNCaP cells (176), resveratrol (xenograft mouse model of prostate cancer) (47), and the rice bran polyphenol cycloartenyl ferulate (human colorectal adenocarcinoma cells) (96), but none of the studies investigated the role of ANT2 inhibition. One study reported that the polyphenols, chrysin, apigenin, and acacetin, inhibited TRAIL-receptor 1 on activated RAW264.7 macrophages (207). Sensitization of TRAIL-mediated apoptosis is an important approach for cancer therapy because a number of tumors have developed resistance to TRAIL (226).
Polyphenols induce apoptosis and mitochondrial membrane depolarization
Many studies have demonstrated that polyphenols induce apoptosis and dissipate Δψm in cultured cancer cells. Curcumin induced apoptosis in human gastric adenocarcinoma SGC-7901 cells, which was associated with dissipation of Δψm (33). Honokiol, a neolignan-type biphenol from the bark of the Magnolia tree and available as a dietary supplement, induced apoptosis and decreased Δψm of neuroblastoma neuro-2a cells at 40 μM exposure (92).
The tea polyphenols, EGCG and theaflavins, caused a concentration- and time-dependent inhibition of proliferation of human papilloma virus-18-positive HeLa cervical cancer cells, apoptosis, and a decrease of Δψm (103). Qanungo et al. (161) observed that, in human pancreatic MIA PaCa-2 cells treated with 200 μM of EGCG, mitochondrial membrane depolarization preceded apoptosis.
Kim and coworkers examined the mechanism by which the grape stilbenoid, piceatannol, inhibited proliferation of cultured androgen-insensitive DU145 prostate cancer cells at concentration in the 1–10 μM range. Under these conditions, the cells exhibited increased mitochondrial membrane permeability and apoptosis (139).
These studies have in common that structurally unrelated polyphenols induced apoptosis and cause Δψm dissipation in various cultured cancer cells at low micromolar concentrations. We hypothesize that the protonophoric effect of these polyphenols forms a “universal” contributing mechanism for the polyphenol-induced dissipation of Δψm and apoptosis. The attractive aspect of the protonophore effect is that it is biophysical in nature and thus not sensitive to development of tolerance resulting from mutation-related changes of specific molecular targets.
Polyphenols as modulators of the ETC-complexes
Because cancer cells prefer glycolysis and circumvent oxidative phosphorylation, the inhibition of ETC complexes seems less promising as a cancer treatment strategy. However, cancer cells exhibit enhanced oxidative stress levels and production of ROS (compared with normal cells) due to their high energy demands (56, 196). Hence, enhanced generation of ROS by inhibiting the ETC complexes may overburden the antioxidant systems and make cancer cells more susceptible to cell death. Current reports are mostly limited to testing of polyphenols as ETC complex modulators in isolated mitochondria.
For instance, resveratrol was reported to inhibit complex I activity (136), while the isoflavone, genistein, inhibited complex III in isolated rat liver mitochondria (167). Flavonoids from a French maritime pine bark decreased activity of complexes I, II, and III and reduced cytochrome C reversibly in rat liver mitochondria (134). Interaction of polyphenols with ETC-complexes seems to stimulate generation of ROS (Fig. 5) (59, 168). Interestingly, resveratrol and its mitochondria tropic derivatives, that is, conjugates with (4-triphenyl-phosphonium)butyl group attached to either 4′- or 3-OH, in conjunction with capping of its free hydroxyl groups inhibited the F0F1-ATPase, accumulated in energized mitochondria as expected and showed cytotoxicity against fast growing but not against slower growing cells (11).
Valenti et al. (201) reported that EGCG negatively modulated oxidative phosphorylation by impairing ETC activity (particularly complex I, II, and ATP synthase) in human malignant pleural mesothelioma cells and induced growth arrest and apoptosis. EGCG did not inhibit the growth of normal mesothelial cells.
We postulate that inhibition of ETC constituents by polyphenols will lead to a nonfunctional ETC that is incapable of maintaining Δψm. Because cancer cell survival depends on high Δψm, any mechanism that leads to dissipation of Δψm will promote activation of apoptotic pathways.
Polyphenols affect UCP2 levels
Several cancer cell types have relatively high UCP2 expression compared with normal cells (Fig. 5) (74). Glycolysis-utilizing cells benefit from elevated UCP2 expression because the ensuing dissipation of Δψm triggers the reverse action of ATP synthase, which increases ATP hydrolysis and leads to a lower cytosolic ATP/ADP ratio (4), favoring glycolysis and production of NADPH by the pentose phosphate pathway (PPP) to keep oxidative stress low. Polyphenols have received little attention to target UCP2 in cancer cells: most studies investigating the effects of polyphenols on UCP2 expression support the hypothesis that polyphenols stimulate UCP2 to reduce ROS and confer protective effects against diabetes and cardiovascular disease.
In rats treated by intraperitoneal administration of resveratrol, Della-Morte et al. (26) found that resveratrol significantly decreased UCP2 levels in the hippocampus by 35% compared to sham-treated rats. They determined that the decrease was mediated by sirtuin 1 (SIRT1) based on the observation that treatment with the SIRT1-specific inhibitor, sirtinol, abolished the decrease in UCP2 levels. Deus et al. (28) found that resveratrol was toxic to breast cancer cells and that the cytotoxicity was similarly related to the stimulation of SIRT1 (the role of UCP2 was not examined). Furthermore, they observed that resveratrol decreased mitochondrial respiration, which they attributed to complex 1 inhibition. SW620 colon cancer cells responded to resveratrol treatment (10 μM) by downregulation of UCP2 expression, apoptosis, and cell growth inhibition (30).
By contrast, in rats fed a high-fat diet and treated orally with resveratrol, hepatic expression of UCP2 was significantly increased by 76% compared with rats fed the high-fat diet only (159). While resveratrol shows antiproliferative effects in cancer cells by inducing apoptosis (101, 192), the precise role of UCP2 in these studies remains to be determined. Stimulation of UCP2 activity or expression by polyphenols, if sufficiently potent, may result in antitumor effects by collapsing Δψm, thereby driving cells into apoptosis.
Polyphenols Reverse Metabolic Reprogramming in Cancer Cells
A significant hurdle in cancer control strategies is that tumors are heterogeneous, harboring different cell communities with different oncogenic genotypes. Therefore, treatment strategies that are designed for targeting a single gene or a distinct kinase cascade have been moderately successful. There is a growing body of work indicating that polyphenols display biological activities as metabolic modulators that have potential in cancer prevention and control.
In general, cancer prevention and chemotherapies aim to exploit differences between cancer cell phenotypes and their normal counterparts. Maybe, in part, stimulated by the renaissance of metabolomics, the fourth omics realm, there is a growing body of research that indicates that malignant cells exhibit a dependence on cytoplasmic glycolysis under aerobic conditions for covering their ATP demands, a phenomenon broadly known as the Warburg effect (54). Although aerobic glycolysis generates fewer ATP molecules than aerobic respiration (2 vs. 38 ATP molecules per molecule of glucose consumed), the lower efficiency of glycolysis is made up for by higher metabolic flux.
The metabolic advantages of the metabolic switch are multiple. Glycolysis does not require oxygen. Glycolysis produces fructose-6-phosphate and glyceraldehyde-3-phosphate that can be used as substrates in the PPP to generate building blocks for the biosynthesis of macromolecules that are needed to sustain cell division (e.g., ribose-5-phosphate and other C5 sugars for RNA and DNA synthesis). Glycolysis requires NAD+, which is provided by the conversion of pyruvate into lactate. The resultant extracellular acidosis creates a selection medium for growing cells, from which adapting cells with upregulated glycolysis and acid resistance emerge as a pool of cells that promote proliferation and invasion (48).
Polyphenols shift glycolysis to fatty acid β-oxidation
Urolithins are formed by gut microbial metabolism of ellagitannins (105), a group of bioavailable polyphenols that are abundantly present in walnuts and pomegranate (35, 127). The physicochemical characteristics of urolithins would give them potential protonophoric properties (Table 2). Urolithin A indeed acutely lowered Δψm in C2C12 myotubes, but the effect was not a result of inhibition of the ETC or mitochondrial uncoupling (166).
Instead, Ryu et al. (166) attributed the effect to a decrease in basal respiration. By challenging the urolithin A-treated myotubes with intermediates or precursors of glycolysis/TCA cycle, the researchers were able to reveal a urolithin-induced downregulation of aerobic glycolysis, resulting in indirect inhibition of complex I respiration. By exposing the cells to a medium containing palmitate that stimulates fatty acid β-oxidation (FAO), they demonstrated that the cells can partially compensate for this effect by shifting to FAO-driven respiration. Although the study was aimed at elucidating the effects of urolithin A on mitophagy in Caenorhabditis elegans and on muscle function in rodents, this reprogramming of energy metabolism would deprive tumor cells of using aerobic glycolysis for their energy needs.
del Mar Blanquer-Rosselló et al. (25) studied the effects of resveratrol in SW620 colon cancer cells. In these cells, resveratrol treatment (10 μM) resulted in decreased UCP2 levels, increased Δψm, increased H2O2 levels, and increased respiration with greater ATP production compared to vehicle-treated cells. These changes resulted in apoptosis and inhibition of cell proliferation, which the authors, substantiated with experimental data, attributed to a resveratrol-induced metabolic switch from glycolysis to FAO.
Polyphenols inhibit lactate dehydrogenase
The switch from aerobic respiration to glycolysis as the primary ATP-producing pathway makes tumor cells highly dependent on glucose. The lactate dehydrogenase (LDH)-mediated conversion of pyruvate into lactate is essential in tumor cells because it generates sufficient NAD+ equivalents to sustain glycolysis (38). The reliance on LDH makes it a potential target for starving tumor cells, especially in combination with cancer therapeutics that target other pathways to overcome resistance. Galloflavin and the gut microbial metabolite of ellagitannin, urolithin M6, inhibited human LDH-A with IC50 values of 70 and 77 μM in a biochemical assay (165). They inhibited proliferation of Raji cells (IC50 33 and 25 μM) and inhibited lactate production in these cells with IC50 values of 62 and 36 μM. While these IC50 are high, these ellagitannin derivatives provide lead compounds for further development and they could reach effective concentrations in the colon to kill colon cancer cells.
Polyphenols modulate pyruvate dehydrogenase
Pyruvate dehydrogenase (PDH) activity is a key determinant for metabolic flux from pyruvate to acetyl-CoA, an important entry metabolite of the TCA cycle. PDH activity is under control of PDH kinase (PDK) (98). PDK, which exists as multiple isoforms, has emerged as potential target in cancer therapy (191). PDK isoforms have been identified as molecular switches that downregulate the PDH complex by reversible phosphorylation in mitochondria (90). Phosphorylating the E1 subunit of PDH by PDK results in inhibition of PDH activity, thereby inhibiting oxidative decarboxylation of pyruvate to acetyl-CoA and thus facilitating cytosolic conversion of pyruvate into lactate (Fig. 4).
In cancer cells, PDK has been found activated and augmenting the glycolytic phenotype of cancer cells. Therefore, PDK is a potential therapeutic target because its inhibition would result in enhanced PDH activity, thereby facilitating the conversion of pyruvate into acetyl-CoA and reducing substrate availability for the conversion of pyruvate into lactate. The overall result is an increase in oxidative phosphorylation at the expense of aerobic glycolysis that underlies the Warburg phenotype.
Dichloroacetate (DCA) inhibits PDK by promoting local conformational changes that are communicated to both the nucleotide-binding and lipoyl-binding pockets of PDK1, leading to inactivation of kinase activity (90). This in turn increases the flux of pyruvate into the mitochondria, thereby stimulating oxidative phosphorylation over glycolysis. DCA has shown in preclinical work to reverse glycolysis-related suppression of mitochondrial apoptosis in cancer cells (20, 216) and boosted the effectiveness of hypoxia-specific chemotherapies in vitro (65, 164) and in animal models (19). Resveratrol was reported recently to enhance PDH activity, likely via activation of AMPK, which in turn led to a shift from glycolysis to oxidative phosphorylation in HTC116 colon cancer cells (171). Importantly, the resveratrol concentration (10 μM) used in the cell culture study to elicit the reversal of the Warburg effect was in the range that was detected in the serum of resveratrol-treated human subjects (8).
There is limited published work hinting toward a role of polyphenols as inhibitors of PDKs. Wang et al. (204) reported that kaempferol, a flavonol found in many plants and plant-derived foods, functions as an estrogen receptor (ER)-β agonist and as an estrogen-related receptor (ERR)-α and ERR-γ inverse agonist in HeLa and HepG2 cells. ERRs play important roles in oxidative phosphorylation, mitochondrial biogenesis, and energy metabolism (49). The authors attributed the kaempferol-induced inhibition of PDK to the existence of multiple isoforms of PDK whose expressions are regulated by ERRs. Noteworthy, ERR-α and Nrf (both isoforms 1 and 2) are targets of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) transcriptional coactivators (179). ERR-α is a recognized prognostic marker in breast, colon, and prostate cancers (182).
Polyphenols modulate voltage-dependent anion channel and hexokinase activity
In response to hypoxia, orchestrated, at least in part, by hypoxia-inducible factor-1 (HIF-1), mitochondrial ATP production shifts to glycolysis. Mitochondrial dysfunction has been linked to downregulation of the catalytic F1 subunit of mitochondrial ATP synthase in human carcinomas (116). Malignant cells display reduced mitochondrial vitality and compromised competency to orchestrate apoptosis. The mitochondrial phenotype of cancer cells and associated alteration of mitochondrial biology have been reviewed in Gogvadze et al. (52).
Highly malignant cancer cell phenotypes have acquired resistance to apoptotic cell death and have been associated with poor clinical prognosis. The intrinsic apoptotic pathway involves permeabilization of the outer mitochondrial membrane (OMM) and subsequent release of cytochrome c (and other proteins) from the intermembrane space of mitochondria. Cytosolic cytochrome c associates with apoptotic protease activating factor-1, Apaf-1, and initiates recruitment and activation of caspases leading to protein degradation and ultimately cell death. The permeabilization of the OMM is critically involved in initiating mitochondrial apoptosis (53).
There is a growing body of work that suggests that adaption to hypoxia associated with metabolic reprogramming is linked to enhanced resistance of malignant cells to apoptosis. Gogvadze et al. discussed the role of Bcl-2 family proteins in OMM permeabilization (52). In malignant cells, imbalance exists between proapoptotic (e.g., Bax and Bak) and antiapoptotic proteins (e.g., Bcl-2, Bcl-XL, and others). Gogvadze et al. argue that binding of Bcl-2 to the OMM-embedded voltage-dependent anion channel (VDAC) favors the open state preventing initiation of the apoptotic cell death program in cancer cells (54).
As an important gatekeeper of mitochondrial metabolic flux, VDAC interacts with ANT, forming a link between the outer and inner mitochondrial membranes (Fig. 4).
The VDAC family of pore-forming proteins consists of three isoforms, VDAC1-3. VDAC1 is overexpressed in many cancer types in line with the increased energy demands of malignant cells. VDAC is located in the OMM. VDAC1 controls the metabolic cross talk between mitochondria and the rest of the cell by the regulating the metabolite flux in the mitochondrion and thus cellular energy production. VDAC1 is also a key player in mitochondria-mediated apoptosis through its involvement in the release of apoptotic proteins and interaction with antiapoptotic proteins (175).
Curcumin, the main polyphenol of turmeric (Curcuma longa), has been reported to exert cancer preventive and anticancer activities (100). Human VDAC1, embedded in planar lipid bilayers, showed reduced conductance when treated with curcumin (193). Molecular docking in conjunction with site-directed mutagenesis experiments showed that curcumin is capable of interacting with residues in the N-terminal α-helical region and the channel wall of VDAC1. Based on the results, the authors proposed that curcumin stabilizes the closed state of VDAC1. There is an ongoing dispute as to which state of the channel, closed or open, is proapoptotic. However, stabilization of the closed state will presumably disrupt normal metabolite flux and hence likely induce apoptosis. VDAC1 closure by curcumin may therefore contribute to the chemopreventive and anticancer activity often attributed to this polyphenolic compound (193).
VDAC1 displays binding to multiple proteins, including proteins that regulate apoptosis, such as Bcl2-family members and hexokinase (HK) II, which catalyzes the ATP-dependent phosphorylation of glucose to glucose-6-phosphate (G6P), the rate-limiting step in glycolysis. HK-II binding to VDAC1 protects cancer cells against apoptosis and promotes cell survival (175). Curcumin (40 μM) also induces VDAC oligomerization and apoptosis in T-Rex293 cells (91). The effectiveness of curcumin to act as a proapoptotic agent is in agreement with earlier reports (32).
In many types of tumors, HK-II has been found to be upregulated and to promote enhanced aerobic glycolysis. Mitochondria-bound HK-II has been touted as both facilitator and gatekeeper of malignancy (124). Binding of HK-II to VDAC has been proposed to be critical for preventing induction of apoptosis in malignant cells. Gogvadez et al. suggested that HK-II antagonizes the binding of proapoptotic proteins and hence prevents induction of apoptotic pathways (54).
Oroxylin A, an O-methylated flavone, occurs in the root of the Chinese traditional medicinal plant Scutellaria baicalensis, and has been identified as a HK-II inhibitor. Oroxylin A treatment resulted in detachment of HK-II from VDAC and in redistribution of HK-II to the cytosol in the human breast cancer cell lines MDA-MB-231 and MCF-7 (211). Oroxylin A increased SIRT3 activity, which in turn caused deacetylation of cyclophilin D (CypD) and detachment from ANT2, which caused HK-II dissociation from the mitochondria and HK-II inhibition (211, 212). The same authors also reported that oroxylin A caused dissociation of HK-II from the mitochondria in human A549 lung carcinoma cells (210). These findings indicate that oroxylin A acts to enhance HIF-1α destabilization via SIRT3 activation, CypD deacetylation, and dissociation of HK-II from the mitochondria, resulting in an overall regain of apoptotic capacity in cancer cells (119).
Curcumin has been reported to downregulate the expression and activity HK-II thereby inhibiting aerobic glycolysis and inducing mitochondria-mediated apoptosis in human colorectal cell lines HCT116 and HT29 (205).
Antioxidant and Pro-oxidant Activities of Polyphenols
In vitro antioxidant and pro-oxidant chemistry of phenolic compounds
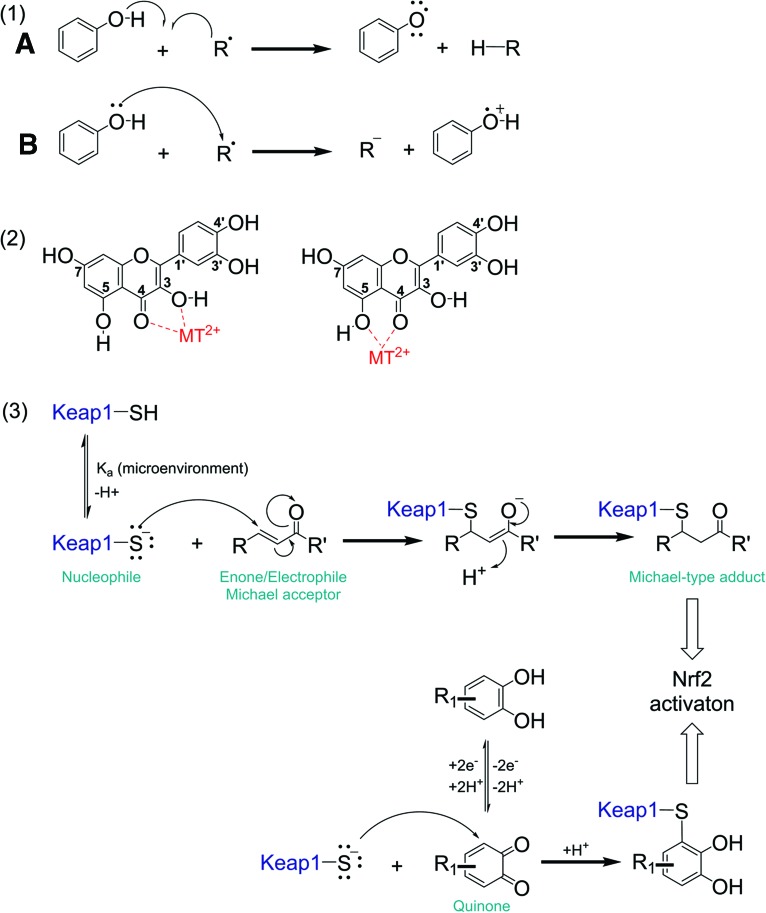
Radical scavenging, transition metal chelation, and activation of the antioxidant stress response represent diverse antioxidant mechanisms of polyphenols (Fig. 6). The radical scavenging properties and structure/function relationships have been reviewed previously (69, 163). Experimental in vitro studies often measure their antioxidant capacity. Gas-phase calculations indicate that radical scavenging by polyphenols can occur via two 1-electron mechanisms: (i) H-atom transfer from a polyphenol to a free radical R• and (ii) the free radical abstracts an electron from the polyphenol (110) [Fig. 6, schema (1)]. The reduction potential of a compound is another way to assess its ability to scavenge free radicals. It is calculated by voltammetry techniques and dependent on different parameters, including concentration.
FIG. 6.
Antioxidant mechanisms of polyphenols. (1) Hydrogen donation to a radical species R• to form RH (A), electron donation to a radical species R• (B), (2) transition metal chelation, (3) induction of the antioxidant Keap1 - Nrf2 pathway by Michael-type attack of Keap1 sulfhydryl groups at electrophilic moieties in polyphenol-derived quinones and, as shown, chalcone-type flavonoids. Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor (erythroid-derived 2)-like 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Published reduction potentials of polyphenols are given in Tables 1 and 2. The lower the reduction potential, the better hydrogen donor the polyphenol (antioxidant) is. The reduction potentials of GSH and vitamin C are −170 mV (128) and 282 mV (214), respectively. Polyphenols, with reduction potential values ranging between 300 and 900 mV, have the possibility to act as radical scavengers, but they are less potent compared to GSH and vitamin C. Moreover, in vivo direct radical scavenging by polyphenols is believed to be a minor antioxidant mechanism in the sense that the rate of this reaction is limited by the concentration of the antioxidant (45). Polyphenols could act as antioxidants in the intestine where polyphenol concentrations can reach millimolar concentrations after consumption of fruits and vegetables (172).
The antioxidant properties of polyphenols have also been associated with their capability of chelating metal ions, which would prevent Fenton-type reactions that produce highly reactive hydroxyl radicals. For instance, Leopoldini et al. (111) studied the iron(II) chelation mechanism of quercetin using density functional theory computation. These calculations indicate that neutral and deprotonated quercetin forms complexes with Fe2+ with 1:1 and 1:2 metal/ligand stoichiometry. Complex formation engages the oxygen atoms at the 3 and 4, and at the 5 and 4 carbons. The architecture of the chelator complex was also confirmed by electrospray ionization mass spectrometry studies (170). The chelating properties of polyphenols depend on the presence of a suitable chelation site, such as found in flavonoids (40).
In vitro pro-oxidant activity of polyphenolics is dependent on the total number of hydroxyl groups and their substitution patterns. Polyphenols with ortho or para substituted di- and trihydroxy groups display significantly increased production of hydroxyl radicals and readily undergo auto-oxidation, thereby forming conjugated dicarbonyl compounds (quinones) as reactive intermediates [Fig. 6, schema (2)].
The higher rate of production of ROS in cancer cells compared with normal cells puts cancer cells at a disadvantage to resist apoptosis when exposed to pro-oxidant polyphenols. Several studies have shown that polyphenols of the flavanol type (catechins and ECGC) and proanthocyanidin type, which are abundantly present in green tea and berries, induce apoptosis in colon cancer cells presumably by producing H2O2 in the gut lumen. Although oligomeric catechins are poorly taken up by cells, the H2O2 they produce extracellularly can diffuse into colon epithelial cells. This mechanism does not require uptake of polyphenols by cancer cells that face the gut lumen.
Chung et al. studied the effects of hop proanthocyanidins on human colon cancer HT-29 cells and found that the mixture of polyphenols induced apoptotic death in a dose-dependent manner (23). They attributed apoptosis to proanthocyanidin-mediated H2O2 production in the medium but noted that the increased ROS production could not fully account for the observed protein carbonylation. A satisfactory explanation for the observation that proanthocyandins promote protein carbonylation can be inferred from the work by Gosse et al. (57), who studied the effects of apple proanthocyanidins in the human adenocarcinoma SW620 cell line. They found that apple proanthocyanidins produced ROS, triggered apoptosis, and promoted polyamine catabolism, which produces H2O2 as well as the reactive aldehyde, acrolein (183). Weh et al. (209) reported that cranberry proanthocyanidins induced autophagic cell death in apoptotis-resistant esophageal adenocarcinoma (EAC) cells. They found that the cranberry proanthocyanidins increased ROS levels in EAC cells and also in a medium in the absence of cells, indicating that proanthocyanidins can produce ROS extracellularly.
Valenti et al. (201) addressed the EGCG-induced extracellular production of H2O2 by adding SOD and catalase, both cell impermeable, to the culture medium. Under these conditions, human malignant mesothelioma cells showed characteristics of cell growth arrest and apoptosis, which the authors explained by EGCG-induced impairment of ETC complexes, resulting in reduction of oxidative phosphorylation without a compensatory increase in glycolytic capacity. This study demonstrates that metabolic reprogramming may not be related to a pro-oxidant effect of EGCG.
Our laboratory investigated the role of intracellularly produced ROS by the hop prenylated polyphenol, xanthohumol, on oxidative phosphorylation. At low concentrations (<5 μM), xanthohumol increased respiration in C2C12 myotubes, whereas treatment at higher concentrations (>25 μM) resulted in a decrease. By pretreating the cells with the cell-permeable ROS scavengers, SOD/peroxynitrite dismutase mimetic MnTMPyP and the SOD/catalase mimetic EUK 134, we were able to demonstrate that ROS play a role in the xanthohumol-induced mitochondrial uncoupling (95).
Another prenylated flavonoid, artocarpin, appears to exert cytotoxic effects on nonsmall-cell lung carcinoma (NSCLC; A549), breast adenocarcinoma (MCF-7), ovarian carcinoma (1A9), and glioblastoma (U87-MG) cells (206). Tsai et al. found that the artocarpin-induced cytotoxicity (1–10 μM) in the A549 cell line was due to artocarpin's ability to produce ROS by activating NADPH oxidase via the Nox2/p47phox pathway, thereby triggering apoptosis (199).
Polyphenols act as inhibitors of the thiol redox system
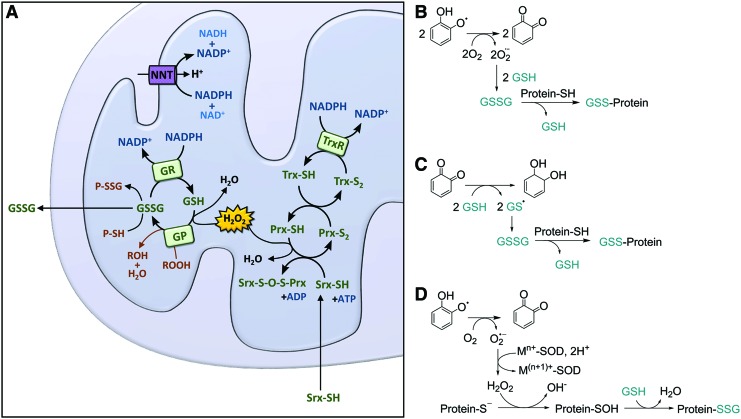
Cancer cells are forced to respond to increase in ROS production by increasing levels of antioxidant proteins to preserve redox homeostasis (Fig. 7). Thioredoxin (Trx), Trx reductase (TrxR), and NADP are the constituents of the Trx system, which are critical for maintenance and control of the redox homeostasis by removing ROS and replenishing antioxidants (Fig. 7A). In parallel to maintaining Trx redox homeostasis, the GSH system is active: the oxidized form of glutaredoxin is reduced by oxidation of GSH, which in turn is regenerated by GSH reductase (GR) (73).
FIG. 7.
Redox homeostasis in the mitochondrion. (A) ROS such as H2O2 can be metabolized in the conversion of GSH to GSSG by GP. GSSG can be reduced to GSH by reacting with protein sulfhydryl groups or by the action of GR. Under high oxidative stress, GSSG can also be excreted out of the mitochondria by GSH transporters, as stated (198). The antioxidant response can also be regulated by activating TrxR. Trx is oxidized by the action or Prx, and the oxidation of Prx depletes the H2O2 pool in the mitochondria to release H2O. Under high oxidative stress, Prx is inactivated. Srx is translocated to mitochondria to reactivate Prx (145). Protein glutathionylation by polyphenols can principally occur via the following mechanisms: (B) the pro-oxidant effect of polyphenols promotes depletion of GSH under formation of GSSG potentially promoting S-glutathionylation; (C) protein glutathionylation can also occur by free radical reactions. This assumes the formation of a thiyl radical (here on GSH, GS•, but this can also be a protein thiol, PS•) in agreement with the mechanisms suggested in Batinic-Haberle and Spasojevic (7) and (D) via protein sulfinylation. GP, glutathione peroxidase; GR, glutathione reductase; GS, glutathione synthesis; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; NNT, nicotinamide nucleotide transhydrogenase; Prx, peroxiredoxin; Srx, sulfiredoxin; Trx, thioredoxin; TrxR, thioredoxin reductase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In general, malignant cells with high levels of antioxidant enzymes display resistance to chemotherapies. For example, MCF-7 cells that overexpressed TrxR-1 were resistant to the proapoptotic synthetic flavonoid LW-214, a chrysin derivative (152). Gorlach et al. extensively reviewed polyphenols that act on the Trx system (55). In most reports, it is not specified which of the Trx systems, the cytosolic Trx1/TrxR-1 or the mitochondrial Trx2/TrxR-2 system, is inhibited. Mitochondrial targeting of polyphenols by mitochondriotropic derivatization has been used as an experimental strategy to determine their mitochondria-specific effects on the Trx and other redox systems (168, 169, 177).
Principally, Trx or TrxR can both be targeted by polyphenols. However, polyphenols have been most promising as inhibitors of the Trx system by targeting TrxR. TrxR activity is irreversibly inhibited on modification of the C-terminal redox active site by curcumin, thereby turning the modified enzyme into a pro-oxidant that stimulates ROS production via an acquired NADPH oxidase activity (37). Similarly, TrxR inhibition has been reported for myricetin and quercetin (117). Another mode of action of hijacking the thiol redox system to promote proapoptotic mechanisms in cancer cells is GSH depletion by polyphenols, shown in cell-free systems (3, 84) and in several cancer cell culture studies (84, 102).
The GSH/oxidized GSH (GSSG) system is tightly regulated (Fig. 7). In the mitochondrion, GSH plays a major role in the elimination of H2O2 in interplay with GSH peroxidase (GP) or peroxiredoxin (Prx) III (isoform III is specific to the mitochondrion). The conversion of GSSG back into GSH is facilitated by the NADPH-dependent GSSG reductase. However, under conditions of increased production of ROS, a shift to elevated concentration of GSSG is proposed, which may lead to mixed disulfide formation with protein thiols and GSSG is excreted out of the mitochondria by GSH transporters (Fig. 7A).
Mailloux and Willmore suggest that S-glutathionylation plays a role in the control of mitochondrial metabolism (121). It has also been proposed that the severity of GSH depletion and the extent and kinetics of distinct protein S-glutathionylation events may determine whether cells initiate apoptosis or undergo necrosis (29). Given the properties of polyphenols, different mechanisms of polyphenol-mediated glutathionylation can be considered (Fig. 7B–D). However, the ability of polyphenols to modulate protein glutathionylation levels has not yet been explored.
In normal cells, an increased level of GSH is associated with elevated rates of proliferation, an increase in detoxification capacity, and resistance to oxidative stress. This stress response, however, allows cancer cells to adapt to elevated levels of ROS generation under conditions of high proliferation. GSH synthesis is regulated by glutathione synthesis (GS) activity, and GS expression is regulated by Nrf2. In many cancer cells, Nrf2 is upregulated, which in turn endows cancer cells even further with enhanced capacity to counteract ROS. Enhanced Nrf2 activity and elevated levels of GSH promote malignancy, cancer cell aggressiveness, and resistance to many anticancer drugs. Targeting Nrf2 therefore promises new strategies in cancer prevention and adjuvant chemotherapies (87, 126).
In the context of exploiting redox modulation to combat cancer, Prxs have emerged as interesting targets (143). Prxs catalyze the reduction of H2O2 and alkylperoxides to water and the respective alcohols (88, 143). Prx levels are elevated in many human cancers in line with the concept that cancer cells need to adapt to elevated ROS production. Elevated levels of Prxs in cancer cells have been correlated with a more aggressive phenotype (94, 144, 157, 189, 213, 227).
Prx III is specific to the mitochondrion. Prx III together with Trx2 and TrxR2 plays a major role in the metabolism of H2O2 (Fig. 7A). Prx III is sensitive to hyperoxidation at elevated levels of H2O2 and hyperoxidation leads to inactivation. Inactivation can be reversed by sulfiredoxin (Srx), and mitochondrial translocation of Srx in response to oxidative stress results in resistance to apoptosis (145). Thus, targeting Prx III and the mitochondrion-specific Trx2, TrxR2, and Srx in combination with established anticancer drugs may potentially sensitize cells to radiation and established chemotherapeutic agents (178, 225).
Studies exploring potential roles of polyphenols in modulating ROS levels in cancer cells are emerging. For instance, quercetin enhanced radiosensitivity of HeLa and MCF-7 tumor cells, and sensitized tumors to irradiation in a DLD-1 colorectal tumor xenograft model (113). Similarly, isoliquiritigenin increased the radiosensitivity of HepG2 cells (188). On the contrary, polyphenols (and all other antioxidants) may positively or negatively affect the efficacy of chemotherapy or radiation treatment (138, 194, 222). Further research is required to establish beneficial effects of distinct polyphenols or well-characterized botanical extracts in cancer management and treatment strategies.
Polyphenols as modulators of the Keap1/Nrf2 system in cancer
Nrf2 activation is beneficial in cancer prevention
The Keap1–Nrf2 system is considered as the master redox regulator in mammalian cells and orchestrates transcription of genes associated with regulating mitochondrial biogenesis, oxidative phosphorylation, and genes whose products have been linked to oxidative and xenobiotic stress responses (30).
Keap1 is a highly sensitive redox sensor protein that assembles with the Cul3 protein to form a Cullin-RING E3 ligase complex to facilitate the degradation of Nrf2. Under basal conditions, the levels of Nrf2 protein are kept low by the E3 ubiquitin ligase Keap1, which ubiquitinates Nrf2 in the cytoplasm and targets it for proteosomal degradation. Under conditions of oxidative stress, or in the presence of electrophilic xenobiotics, the activity of Keap1 is diminished and Nrf2 can translocate to the nucleus, bind to the antioxidant response element (ARE), which in turn induces the expression of its target genes, including many that code for antioxidant and detoxification enzymes, stress response proteins, and ABC transporters. Nrf2 activity also regulates mitochondrial biogenesis via PGC-1α and mitochondrial transcription factor A, Tfam, activation (30, 173).
Hence, Nrf2-dependent gene expression may be beneficial in cancer prevention and control strategies targeting the early stage of cancer initiation and progression. However, in late stages of malignancy, Nrf2 activity is detrimental because Nrf2 activation increases chemoresistance (87). In this context, polyphenols seem to have efficacy as chemopreventive agents in normal cells or early stages of cancer development but seem less useful in late stages of cancer due to aberrant Nrf2/Keap1 signaling and redox homeostasis at elevated ROS levels (153).
Keap1 functions essentially as sensor of cellular oxidative or electrophile stress. Keap1 harbors a large number of cysteine residues, which display reactivity toward polyphenol-derived quinones and chalcone-type flavonoids. The cysteine modification patterns affect Keap1's structural conformation and function, and, in turn, affect inhibition of Nrf2 ubiquitination (174). Human Keap1 contains a total of 27 cysteine residues (87), seven of which have been identified as highly reactive toward ROS and electrophiles by in vitro proteomics studies using recombinant Keap1, namely Cys151, Cys257, Cys273, Cys288, Cys297, Cys434, and Cys613 (120, 174). However, one should keep in mind that in vitro adduction experiments using recombinant proteins are methodology dependent and do not necessarily reflect true in vivo behavior.
Adduct formation depends on (i) the nucleophilicity of the cysteine thiol, which is determined by the protein microenvironment, and (ii) by the electrophilicity of the xenobiotic (203). Many plant phenolics are Michael acceptors featuring α,β-unsaturated carbonyl functionalities and are capable of forming Michael-type adducts with distinct Keap1 cysteine residues (34, 131) [Fig. 6, schema (3)]. For instance, the electrophilic chalcones, isoliquiritigenin and xanthohumol, both feature an α,β-unsaturated ketone moiety that forms Michael-type adducts with cysteine thiols of human Keap1 in vitro. Distinct adduction sites have been reported for these chalcones (33, 118).
As mentioned above, polyphenolic compounds exert in vitro antioxidant and radical scavenging activities. In addition, polyphenolic compounds are able to induce the Keap1-Nrf2 signaling pathway and the expression of ARE-response genes after oxidation to their corresponding electrophilic quinones, which contain Michael-type acceptor moieties (31). Substituted 1,2- and 1,4-diphenols but not the mono- or 1,3-dihydroxy regio-isomers can be biotransformed to the corresponding quinonoids (34). For instance, quercetin will only exhibit ARE induction after oxidation to its quinone methide (78). Similarly, EGCG contains several aromatic 1,2-dihydroxy units that can form quinones capable of activation of the Keap1/NrF2 pathways.
In cancer patients, serum and tumor copper levels are elevated compared to healthy subjects. Copper levels positively correlate with tumor incidence, burden, malignant progression, and recurrence in many human and animal cancers (31,103). Copper promotes formation of hydroxyl radicals via Fenton-type chemistry, redox cycling, and formation of quinones. Dinkova-Kostova and Wang (31) hypothesized that endogenous copper levels are sufficient to enhance oxidation of polyphenols to quinone-type species that activate the Keap1/Nrf2/ARE pathway.
Aberrant Nrf2 activation promotes anabolic pathways in cancer
The Keap1-Nrf2 system in cancers might be considered a double-edged sword. In normal cells, Nrf2 activity protects cells and makes them resistant to oxidative and electrophilic stresses in line with the chemoprevention idea. However, in malignant cells, mutations and epigenetic modifications affecting the regulation and fate of Nrf2 can lead to constitutive, dominant hyperactivation of signaling pathways. They stimulate cancer cell survival and proliferation, enhance resistance to stresses, and render cancer therapies less effective (60, 92, 103, 139).
A recent study by Mitsuishi et al. (132) showed that, in human lung carcinoma A549 cells, Nrf2 modulated metabolic pathways that enhanced cell proliferation in the presence of active PI3K-Akt signaling. Nrf2 directly activates genes whose products are involved in the PPP and in NADPH production (namely, glucose-6-phosphate dehydrogenase, phosphogluconate dehydrogenase, transketolase, transalodase, and malic enzyme 1) and GS. Thus, in proliferating cells, Nrf2 promotes anabolic metabolism and reinforces metabolic reprogramming. This antioxidant mechanism explains why aberrant high expression of Nrf2 in many types of tumors is associated with a poor prognosis in the clinic (79).
There is currently a large interest in deciphering factors determining the balance between a chemopreventive and the protumorigenic role of Nrf2. In this context, research is needed to further clarify to what extent polyphenols or diets rich in polyphenols might exert beneficial health effects or produce opposite effects promoting tumor growth and progression, and how they impact chemotherapeutic efficacy and radiation treatments (126).
Conclusion and Perspective
Polyphenols interfere with the function of the ETC by mild mitochondrial uncoupling through a protonophoric or an ROS-producing effect, or both. Such uncoupling effect is beneficial to promote energy expenditure in conditions of obesity-related dysfunctional metabolism. Their ability to undergo oxidation and form electrophilic quinones that stimulate the antioxidant Keap1-Nrf2 pathway suppresses the cells' transition to malignancy. Indeed, the totality of the polyphenol research indicates that polyphenols hold promise as cancer chemopreventive agents to induce adaptive stress responses that provide cytoprotection and maintenance of normal metabolic homeostasis.
Cancer cells rely less on oxidative phosphorylation than normal cells, and therefore, cancer monotherapy with polyphenols will be moderately successful at best. However, polyphenol-induced mitochondrial uncoupling can promote β-oxidation of fatty acids and anaplerotic feeding of the TCA cycle, resulting in an overall catabolic effect to counteract the glycolytic, anabolic phenotype of cancer cells. Such metabolic reprogramming might have synergistic outcomes when used in combination with chemotherapy, targeting both the anabolic phenotype and mitotic division of proliferating tumor cells.
Many of the polyphenol effects discussed in this review relate to the physicochemical properties of polyphenols (protonophore and ROS-producing effects that decrease Δψm) rather than to their individual structure-specific pharmacophores targeting specific catalytic enzymes, channels, transporters, or receptors (with moderate potency). The general protonophoric and ROS-producing effects of polyphenols hold promise for combination chemotherapy, because tumor cells cannot develop resistance against these general MMP effects. Furthermore, the MMP effects of polyphenols reverse the antiapoptotic phenotype often seen in drug-resistant cancer cells (217).
Abbreviations Used
- ADP
adenosine diphosphate
- AMPK
AMP-activated protein kinase
- ANT
adenosine nucleotide translocase
- Apo2L
Apo 2 ligand
- ARE
antioxidant response element
- ATP
adenosine triphosphate
- CypD
cyclophilin D
- DCA
dichloroacetate
- DNP
2,4-dinitrophenol
- DR
death receptor
- EAC
esophageal adenocarcinoma
- EGCG
epigallocatechin gallate
- ERR
estrogen-related receptor
- ETC
electron transport chain
- FAO
fatty acid β-oxidation
- GP
glutathione peroxidase
- GR
glutathione reductase
- GS
glutathione synthesis
- GSH
glutathione
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HIF-1
hypoxia-inducible factor-1
- HK
hexokinase
- IV
intravenous
- Keap1
Kelch-like ECH-associated protein 1
- LDH
lactate dehydrogenase
- MMP
mitochondrial membrane potential
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- OMM
outer mitochondrial membrane
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1-α
- PPP
pentose phosphate pathway
- Prx
peroxiredoxin
- PTP
permeability transition pore
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- Srx
sulfiredoxin
- TCA
tricarboxylic acid
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- UCP
uncoupling protein
- VDAC
voltage-dependent anion channel
- VEGF
vascular endothelial growth factor
Acknowledgment
The authors received support from NIH grant R01AT009168.
References
- 1.Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, and Delmas D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res 57: 1170–1181, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Attene-Ramos MS, Huang R, Sam M, Witt KL, Richard A, Tice RR, Simeonov A, Austin CP, and Xia M. Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ Health Perspect 123: 49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babich H, Gottesman RT, Liebling EJ, and Schuck AG. Theaflavin‐3‐gallate and theaflavin‐3′‐gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol 103: 66–74, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bagkos G, Koufopoulos K, and Piperi C. A new model for mitochondrial membrane potential production and storage. Med Hypotheses 83: 175–181, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Baird L. and Dinkova-Kostova AT. The cytoprotective role of the Keap1–Nrf2 pathway. Arch Toxicol 85: 241–272, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Balogun E, Hoque M, Pengfei G, Killeen E, Green CJ, Foresti R, Jawed A, and Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371: 887–895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batinic-Haberle I. and Spasojevic I. Complex chemistry and biology of redox-active compounds, commonly known as SOD mimics, affect their therapeutic effects. Antioxid & Redox Signal 20: 2323–2325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baur JA. and Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bennett CJ, Caldwell ST, McPhail DB, Morrice PC, Duthie GG, and Hartley RC. Potential therapeutic antioxidants that combine the radical scavenging ability of myricetin and the lipophilic chain of vitamin E to effectively inhibit microsomal lipid peroxidation. Bioorg Med Chem 12: 2079–2098, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Bernabé-Pineda M, Ramírez-Silva MaT, Romero-Romo M, González-Vergara E, and Rojas-Hernández A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim Acta A Mol Biomol Spectrosc 60: 1091–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Biasutto L, Mattarei A, Marotta E, Bradaschia A, Sassi N, Garbisa S, Zoratti M, and Paradisi C. Development of mitochondria-targeted derivatives of resveratrol. Bioorg Med Chem Lett 18: 5594–5597, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, Van Duijnhoven FJ, Büchner FL, Key T, and Boeing H. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 102: 529–537, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev 72: 429–452, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Bondonno NP, Bondonno CP, Ward NC, Hodgson JM, and Croft KD. The cardiovascular health benefits of apples: whole fruit vs. isolated compounds. Trends Food Sci Technol 69 (Part B): 243–256, 2017 [Google Scholar]
- 15.Boots AW, Kubben N, Haenen GRMM, and Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun 308: 560–565, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Boyer MJ. and Tannock IF. Lysosomes, lysosomal enzymes, and cancer. Adv Cancer Res 60: 269–291, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Branca D, Roberti MS, Vincenti E, and Scutari G. Uncoupling effect of the general anesthetic 2, 6-diisopropylphenol in isolated rat liver mitochondria. Arch Biochem Biophys 290: 517–521, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, and Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392: 353–362, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cairns RA, Papandreou I, Sutphin PD, and Denko NC. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci U S A 104: 9445–9450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W, Yacoub S, Shiverick KT, Namiki K, Sakai Y, Porvasnik S, Urbanek C, and Rosser CJ. Dichloroacetate (DCA) sensitizes both wild‐type and over expressing Bcl‐2 prostate cancer cells in vitro to radiation. Prostate 68: 1223–1231, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol Aspects Med 2017 [DOI] [PubMed] [Google Scholar]
- 22.Chevrollier A, Loiseau D, Reynier P, and Stepien G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim Biophys Acta 1807: 562–567, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Chung WG, Miranda CL, Stevens JF, and Maier CS. Hop proanthocyanidins induce apoptosis, protein carbonylation, and cytoskeleton disorganization in human colorectal adenocarcinoma cells via reactive oxygen species. Food Chem Toxicol 47: 827–836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol 48: 115–117, 2007 [DOI] [PubMed] [Google Scholar]
- 25.del Mar Blanquer-Rosselló M, Hernández-López R, Roca P, Oliver J, and Valle A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim Biophys Acta 1861: 431–440, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, and Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159: 993–1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demianová Z, Sirén H, Kuldvee R, and Riekkola M-L. Nonaqueous capillary electrophoretic separation of polyphenolic compounds in wine using coated capillaries at high pH in methanol. Electrophoresis 24: 4264–4271, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Deus CM, Serafim TL, Magalhaes-Novais S, Vilaca A, Moreira AC, Sardao VA, Cardoso SM, and Oliveira PJ. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch Toxicol 91: 1261–1278, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Di Stefano A, Frosali S, Leonini A, Ettorre A, Priora R, Di Simplicio FC, and Di Simplicio P. GSH depletion, protein S-glutathionylation and mitochondrial transmembrane potential hyperpolarization are early events in initiation of cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta 1763: 214–225, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Dinkova-Kostova AT. and Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88: 179–188, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinkova-Kostova AT. and Wang XJ. Induction of the Keap1/Nrf2/ARE pathway by oxidizable diphenols. Chem Biol Interact 192: 101–106, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, and Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Eggler AL, Liu G, Pezzuto JM, Van Breemen RB, and Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A 102: 10070–10075, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggler AL. and Savinov SN. Chemical and biological mechanisms of phytochemical activation of Nrf2 and importance in disease prevention. In: 50 Years of Phytochemistry Research: Volume 43, edited by Gang D.R. Cham: Springer International Publishing, 2013, pp. 121–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espin JC, Larrosa M, Garcia-Conesa MT, and Tomas-Barberan F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med 2013: 270418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estrela JM, Mena S, Obrador E, Benlloch M, Castellano G, Salvador R, and Dellinger RW. Polyphenolic phytochemicals in cancer prevention and therapy: bioavailability versus bioefficacy. J Med Chem 2017 [DOI] [PubMed] [Google Scholar]
- 37.Fang J, Lu J, and Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin a novel molecular mechanism for its anticancer activity. J Biol Chem 280: 25284–25290, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fantin VR, St-Pierre J, and Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9: 425–434, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Farvid MS, Chen WY, Michels KB, Cho E, Willett WC, and Eliassen AH. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ 353: i2343, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez MT, Mira ML, Florencio MH, and Jennings KR. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J Inorg Biochem 92: 105–111, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Fiorani M, Guidarelli A, Blasa M, Azzolini C, Candiracci M, Piatti E, and Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J Nutr Biochem 21: 397–404, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Firuzi O, Lacanna A, Petrucci R, Marrosu G, and Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 1721: 174–184, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Foley S, Navaratnam S, McGarvey DJ, Land EJ, Truscott TG, and Rice-Evans CA. Singlet oxygen quenching and the redox properties of hydroxycinnamic acids. Free Radic Biol Med 26: 1202–1208, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Folkes LK, Trujillo M, Bartesaghi S, Radi R, and Wardman P. Kinetics of reduction of tyrosine phenoxyl radicals by glutathione. Arch Biochem Biophys 506: 242–249, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Forman HJ, Davies KJA, and Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66: 24–35, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forman HJ. and Ursini F. Para-hormesis: an innovative mechanism for the health protection brought by antioxidants in wine. Nutr Aging 2: 117–124, 2014 [Google Scholar]
- 47.Ganapathy S, Chen Q, Singh KP, Shankar S, and Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One 5: e15627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatenby RA. and Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891–899, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29: 677–696, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Giovannini C, Scazzocchio B, Varì R, Santangelo C, D'Archivio M, and Masella R. Apoptosis in cancer and atherosclerosis: polyphenol activities. Ann Ist Super Sanita 43: 406, 2007 [PubMed] [Google Scholar]
- 51.Giraud S, Bonod-Bidaud C, Wesolowski-Louvel M, and Stepien G. Expression of human ANT2 gene in highly proliferative cells: GRBOX, a new transcriptional element, is involved in the regulation of glycolytic ATP import into mitochondria. J Mol Biol 281: 409–418, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Gogvadze V, Orrenius S, and Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18: 165–173, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Gogvadze V, Orrenius S, and Zhivotovsky B. Mitochondria as targets for chemotherapy. Apoptosis 14: 624–640, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Gogvadze V, Zhivotovsky B, and Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med 31: 60–74, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Gorlach S, Fichna J, and Lewandowska U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett 366: 141–149, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Gorrini C, Harris IS, and Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12: 931, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Gosse F, Roussi S, Guyot S, Schoenfelder A, Mann A, Bergerat JP, Seiler N, and Raul F. Potentiation of apple procyanidin-triggered apoptosis by the polyamine oxidase inactivator MDL 72527 in human colon cancer-derived metastatic cells. Int J Oncol 29: 423–428, 2006 [PubMed] [Google Scholar]
- 58.Grimm T, Schäfer A, and Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic Biol Med 36: 811–822, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, Vessières E, Ayer A, Henrion D, Lenaers G, Reynier P, and Procaccio V. Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice. PLoS One 10: e0144290, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Y, Yu S, Zhang C, and Kong A-NT. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med 88: 337–349, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guruvayoorappan C. and Kuttan G. (+)-Catechin inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-α in LPS-stimulated macrophages. Innate Immun 14: 160–174, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476: 107–112, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Halliwell B. and Gutteridge J. Free Radicals in Biology and Medicine. New York: Oxford University Press, Inc., 1999 [Google Scholar]
- 64.Han YH, Kim SW, Kim SH, Kim SZ, and Park WH. 2,4-Dinitrophenol induces G1 phase arrest and apoptosis in human pulmonary adenocarcinoma Calu-6 cells. Toxicol In Vitro 22: 659–670, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Haugrud AB, Zhuang Y, Coppock JD, and Miskimins WK. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res Treat 147: 539–550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heerdt BG, Houston MA, Anthony GM, and Augenlicht LH. Mitochondrial membrane potential (ΔΨmt) in the coordination of p53-independent proliferation and apoptosis pathways in human colonic carcinoma cells. Cancer Res 58: 2869–2875, 1998 [PubMed] [Google Scholar]
- 67.Heerdt BG, Houston MA, and Augenlicht LH. The intrinsic mitochondrial membrane potential of colonic carcinoma cells is linked to the probability of tumor progression. Cancer Res 65: 9861–9867, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Heerdt BG, Houston MA, Wilson AJ, and Augenlicht LH. The intrinsic mitochondrial membrane potential (ΔΨm) is associated with steady-state mitochondrial activity and the extent to which colonic epithelial cells undergo butyrate-mediated growth arrest and apoptosis. Cancer Res 63: 6311–6319, 2003 [PubMed] [Google Scholar]
- 69.Heim KE, Tagliaferro AR, and Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13: 572–584, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Heiskanen KM, Bhat MB, Wang H-W, Ma J, and Nieminen A-L. Mitochondrial depolarization accompanies cytochrome crelease during apoptosis in PC6 cells. J Biol Chem 274: 5654–5658, 1999 [DOI] [PubMed] [Google Scholar]
- 71.Hemker H. Lipid solubility as a factor influencing the activity of uncoupling phenols. Biochim Biophys Acta 63: 46–54, 1962 [DOI] [PubMed] [Google Scholar]
- 72.Herrero-Martínez JM, Sanmartin M, Rosés M, Bosch E, and Ràfols C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 26: 1886–1895, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2: 811–820, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Horimoto M, Resnick MB, Konkin TA, Routhier J, Wands JR, and Baffy G. Expression of uncoupling protein-2 in human colon cancer. Clin Cancer Res 10: 6203–6207, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Hosein Farzaei M, Bahramsoltani R, and Rahimi R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr Pharm Des 22: 4201–4218, 2016 [DOI] [PubMed] [Google Scholar]
- 76.Hosseini A. and Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed 5: 84–97, 2015 [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii T, Ishikawa M, Miyoshi N, Yasunaga M, Akagawa M, Uchida K, and Nakamura Y. Catechol type polyphenol is a potential modifier of protein sulfhydryls: development and application of a new probe for understanding the dietary polyphenol actions. Chem Res Toxicol 22: 1689–1698, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Jacobs H, Moalin M, Bast A, van der Vijgh WJ, and Haenen GR. An essential difference between the flavonoids monoHER and quercetin in their interplay with the endogenous antioxidant network. PLoS One 5: e13880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaramillo MC. and Zhang DD. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev 27: 2179–2191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jovanovic SV, Hara Y, Steenken S, and Simic MG. Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J Am Chem Soc 117: 9881–9888, 1995 [Google Scholar]
- 81.Jovanovic SV, Steenken S, Boone CW, and Simic MG. H-atom transfer is a preferred antioxidant mechanism of curcumin. J Am Chem Soc 121: 9677–9681, 1999 [Google Scholar]
- 82.Jovanovic SV, Steenken S, Hara Y, and Simic MG. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J Chem Soc Perkin Trans 2 2497–2504, 1996 [Google Scholar]
- 83.Jovanovic SV, Steenken S, Tosic M, Marjanovic B, and Simic MG. Flavonoids as antioxidants. J Am Chem Soc 116: 4846–4851, 1994 [Google Scholar]
- 84.Kachadourian R. and Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med 41: 65–76, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kallio T, Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, and Virtanen V. Urolithins display both antioxidant and pro-oxidant activities depending on assay system and conditions. J Agric Food Chem 61: 10720–10729, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Kang NJ, Shin SH, Lee HJ, and Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther 130: 310–324, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Kansanen E, Kuosmanen SM, Leinonen H, and Levonen A-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1: 45–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karlenius TC. and Tonissen KF. Thioredoxin and cancer: a role for thioredoxin in all states of tumor oxygenation. Cancers 2: 209–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karunaweera N, Raju R, Gyengesi E, and Münch G. Plant polyphenols as inhibitors of NF-κB induced cytokine production—a potential anti-inflammatory treatment for Alzheimer's disease? Front Mol Neurosci 8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kato M, Li J, Chuang JL, and Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure 15: 992–1004, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keinan N, Tyomkin D, and Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol 30: 5698–5709, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kensler TW. and Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kilmartin PA. Electrochemical detection of natural antioxidants: principles and protocols. Antioxid Redox Signal 3: 941–955, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Kinnula V, Lehtonen S, Sormunen R, Kaarteenaho‐Wiik R, Kang S, Rhee S, and Soini Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol 196: 316–323, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Kirkwood JS, Legette LL, Miranda CL, Jiang Y, and Stevens JF. A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J Biol Chem 288: 19000–19013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong CK, Lam WS, Chiu LC, Ooi VE, Sun SS, and Wong YS. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem Pharmacol 77: 1487–1496, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Kotecha R, Takami A, and Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget 7: 52517–52529, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, and Harris AL; Tumor and Angiogenesis Research Group. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 7: 1–6, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumamoto M, Sonda T, Nagayama K, and Tabata M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci Biotechnol Biochem 65: 126–132, 2001 [DOI] [PubMed] [Google Scholar]
- 100.Kumar G, Mittal S, Sak K, and Tuli HS. Molecular mechanisms underlying chemopreventive potential of curcumin: current challenges and future perspectives. Life Sci 148: 313–328, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Kumar S, Eroglu E, Stokes JA, 3rd, Scissum-Gunn K, Saldanha SN, Singh UP, Manne U, Ponnazhagan S, and Mishra MK. Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells. Oncotarget 8: 20895–20908, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kunwar A, Jayakumar S, Srivastava A, and Priyadarsini K. Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch Toxicol 86: 603–614, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Kuo HW, Chen SF, Wu CC, Chen DR, and Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res 89: 1–11, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Lambert JD. and Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr 133: 3262S–3267S, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Landete JM, Arques J, Medina M, Gaya P, de Las Rivas B, and Munoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr 56: 1826–1843, 2016 [DOI] [PubMed] [Google Scholar]
- 106.Le Bras M, Borgne-Sanchez A, Touat Z, El Dein OS, Deniaud A, Maillier E, Lecellier G, Rebouillat D, Lemaire C, Kroemer G, Jacotot E, and Brenner C. Chemosensitization by knockdown of adenine nucleotide translocase-2. Cancer Res 66: 9143, 2006 [DOI] [PubMed] [Google Scholar]
- 106a.Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, Christensen JM, Rodriguez-Proteau R, Purnell JQ, and Stevens JF. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol Nutr Food Res 58: 248–255, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Legette L, Ma L, Reed RL, Miranda CL, Christensen JM, Rodriguez-Proteau R, and Stevens JF. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res 56: 466–474, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. This reference has been deleted
- 109.Legette LL, Luna AYM, Reed RL, Miranda CL, Bobe G, Proteau RR, and Stevens JF. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 91: 236–241, 2013 [DOI] [PubMed] [Google Scholar]
- 110.Leopoldini M, Marino T, Russo N, and Toscano M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108: 4916–4922, 2004 [Google Scholar]
- 111.Leopoldini M, Russo N, Chiodo S, and Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem 54: 6343–6351, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Lewandowska H, Kalinowska M, Lewandowski W, Stępkowski TM, and Brzóska K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J Nutr Biochem 32: 1–19, 2016 [DOI] [PubMed] [Google Scholar]
- 113.Lin C, Yu Y, Zhao H-G, Yang A, Yan H, and Cui Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother Oncol 104: 395–400, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Lin J-K. Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways. Arch Pharm Res 25: 561–571, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Liu Y-M, Jiang B, Bao Y-M, and An L-J. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol In Vitro 22: 430–437, 2008 [DOI] [PubMed] [Google Scholar]
- 116.López-Ríos F, Sánchez-Aragó M, García-García E, Ortega ÁD, Berrendero JR, Pozo-Rodríguez F, López-Encuentra Á, Ballestín C, and Cuezva JM. Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res 67: 9013–9017, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Lu J, Papp LV, Fang J, Rodriguez-Nieto S, Zhivotovsky B, and Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res 66: 4410, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, and van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom 18: 2226–2232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Machida K, Ohta Y, and Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J Biol Chem 281: 14314–14320, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Magesh S, Chen Y, and Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev 32: 687–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mailloux RJ. and Willmore WG. S-glutathionylation reactions in mitochondrial function and disease. Front Cell Dev Biol 2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maldonado EN, DeHart DN, Patnaik J, Klatt SC, Gooz MB, and Lemasters JJ. ATP/ADP turnover and import of glycolytic ATP into mitochondria in cancer cells is independent of the adenine nucleotide translocator. J Biol Chem 291: 19642–19650, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maru GB, Hudlikar RR, Kumar G, Gandhi K, and Mahimkar MB. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: from experimental models to clinical trials. World J Biol Chem 7: 88–99, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathupala S, Ko Ya, and Pedersen P. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25: 4777, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McLaughlin S. The mechanism of action of DNP on phospholipid bilayer membranes. J Membr Biol 9: 361–372, 1972 [PubMed] [Google Scholar]
- 126.Menegon S, Columbano A, and Giordano S. The dual roles of NRF2 in cancer. Trends Mol Med 22: 578–593, 2016 [DOI] [PubMed] [Google Scholar]
- 127.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, and Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem 54: 8956–8961, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Millis KK, Weaver KH, and Rabenstein DL. Oxidation/reduction potential of glutathione. J Org Chem 58: 4144–4146, 1993 [Google Scholar]
- 129.Miranda CL, Elias VD, Hay JJ, Choi J, Reed RL, and Stevens JF. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch Biochem Biophys 599: 22–30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miranda CL, Maier CS, and Stevens JF. Flavonoids. In eLS, John Wiley & Sons, Ltd: 2001 [Google Scholar]
- 131.Mitsuishi Y, Motohashi H, and Yamamoto M. The Keap1–Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2: 200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, and Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22: 66–79, 2012 [DOI] [PubMed] [Google Scholar]
- 133.Moga M, Dimienescu O, Arvatescu C, Mironescu A, Dracea L, and Ples L. The role of natural polyphenols in the prevention and treatment of cervical cancer—an overview. Molecules 21: 1055, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moini H, Arroyo A, Vaya J, and Packer L. Bioflavonoid effects on the mitochondrial respiratory electron transport chain and cytochrome c redox state. Redox Rep 4: 35–41, 1999 [DOI] [PubMed] [Google Scholar]
- 135.Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, Andres-Lacueva C, and Bartolomé B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct 1: 233–253, 2010 [DOI] [PubMed] [Google Scholar]
- 136.Moreira AC, Silva AM, Santos MS, and Sardão VA. Resveratrol affects differently rat liver and brain mitochondrial bioenergetics and oxidative stress in vitro: investigation of the role of gender. Food Chem Toxicol 53: 18–26, 2013 [DOI] [PubMed] [Google Scholar]
- 137.Moskaug JØ, Carlsen H, Myhrstad MC, and Blomhoff R. Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 81: 277S–283S, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Moss RW. Do antioxidants interfere with radiation therapy for cancer? Integr Cancer Ther 6: 281–292, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Müller T. and Hengstermann A. Nrf2: friend and foe in preventing cigarette smoking-dependent lung disease. Chem Res Toxicol 25: 1805–1824, 2012 [DOI] [PubMed] [Google Scholar]
- 140.Myhrstad MCW, Carlsen H, Nordström O, Blomhoff R, and Moskaug JØ. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med 32: 386–393, 2002 [DOI] [PubMed] [Google Scholar]
- 141.Nam N-H. Naturally occurring NF-κB inhibitors. Mini Rev Med Chem 6: 945–951, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Naven RT, Swiss R, Klug-Mcleod J, Will Y, and Greene N. The development of structure-activity relationships for mitochondrial dysfunction: uncoupling of oxidative phosphorylation. Toxicol Sci 131: 271–278, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Nicolussi A, D'inzeo S, Capalbo C, Giannini G, and Coppa A. The role of peroxiredoxins in cancer. Mol Clin Oncol 6: 139–153, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noh D-Y, Ahn S-J, Lee R-A, Kim S-W, Park I-A, and Chae H-Z. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res 21: 2085–2090, 2001 [PubMed] [Google Scholar]
- 145.Noh YH, Baek JY, Jeong W, Rhee SG, and Chang T-S. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. J Biol Chem 284: 8470–8477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Núñez-Sánchez MA, González-Sarrías A, Romo-Vaquero M, García-Villalba R, Selma MV, Tomás-Barberán FA, García-Conesa M-T, and Espín JC. Dietary phenolics against colorectal cancer—from promising preclinical results to poor translation into clinical trials: pitfalls and future needs. Mol Nutr Food Res 59: 1274–1291, 2015 [DOI] [PubMed] [Google Scholar]
- 147.O'Leary KA, Day AJ, Needs PW, Sly WS, O'Brien NM, and Williamson G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett 503: 103–106, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Oishi M, Iizumi Y, Taniguchi T, Goi W, Miki T, and Sakai T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS One 8: e55922, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Onoda C, Kuribayashi K, Nirasawa S, Tsuji N, Tanaka M, Kobayashi D, and Watanabe N. (-)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int J Oncol 38: 1403–1408, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Ost M, Keipert S, and Klaus S. Targeted mitochondrial uncoupling beyond UCP1–the fine line between death and metabolic health. Biochimie 134 (Supplement C) 77–85, 2017 [DOI] [PubMed] [Google Scholar]
- 151.Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, and Schroeter H. The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep 6: 29034, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pan D, Li W, Miao H, Yao J, Li Z, Wei L, Zhao L, and Guo Q. LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem Pharmacol 87: 598–610, 2014 [DOI] [PubMed] [Google Scholar]
- 153.Panieri E. and Santoro M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7: e2253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pardo-Andreu GL, Nuñez-Figueredo Y, Tudella VG, Cuesta-Rubio O, Rodrigues FP, Pestana CR, Uyemura SA, Leopoldino AM, Alberici LC, and Curti C. The anti-cancer agent nemorosone is a new potent protonophoric mitochondrial uncoupler. Mitochondrion 11: 255–263, 2011 [DOI] [PubMed] [Google Scholar]
- 155.Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, Brown VA, Brenner DE, Singh R, Steward WP, Gescher AJ, and Brown K. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med 5: 205ra133, 2013 [DOI] [PubMed] [Google Scholar]
- 156.Patel KR, Brown VA, Jones DJL, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, and Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 70: 7392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peng L, Wang R, Shang J, Xiong Y, and Fu Z. Peroxiredoxin 2 is associated with colorectal cancer progression and poor survival of patients. Oncotarget 8: 15057, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perry RJ, Zhang D, Zhang X-M, Boyer JL, and Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347: 1253–1256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poulsen MM, Larsen JO, Hamilton-Dutoit S, Clasen BF, Jessen N, Paulsen SK, Kjaer TN, Richelsen B, and Pedersen SB. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res 32: 701–708, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Qadir MI, Naqvi STQ, and Muhammad SA. Curcumin: a polyphenol with molecular targets for cancer control. Asian Pac J Cancer Prev 17: 2735–2739, 2016 [PubMed] [Google Scholar]
- 161.Qanungo S, Das M, Haldar S, and Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 26: 958–967, 2005 [DOI] [PubMed] [Google Scholar]
- 162.Reis FH, Pardo-Andreu GL, Nuñez-Figueredo Y, Cuesta-Rubio O, Marín-Prida J, Uyemura SA, Curti C, and Alberici LC. Clusianone, a naturally occurring nemorosone regioisomer, uncouples rat liver mitochondria and induces HepG2 cell death. Chem Biol Interact 212: 20–29, 2014 [DOI] [PubMed] [Google Scholar]
- 163.Rice-Evans CA, Miller NJ, and Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20: 933–956, 1996 [DOI] [PubMed] [Google Scholar]
- 164.Roh J-L, Park JY, Kim EH, Jang HJ, and Kwon M. Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer. Cancer Lett 371: 20–29, 2016 [DOI] [PubMed] [Google Scholar]
- 165.Rupiani S, Guidotti L, Manerba M, Ianni LD, Giacomini E, Falchi F, Di Stefano G, Roberti M, and Recanatini M. Synthesis of natural urolithin M6, a galloflavin mimetic, as a potential inhibitor of lactate dehydrogenase A. Org Biomol Chem 14: 10981–10987, 2016 [DOI] [PubMed] [Google Scholar]
- 166.Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-dit-Félix AA, Williams EG, Jha P, Sasso GL, and Huzard D. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22: 879–888, 2016 [DOI] [PubMed] [Google Scholar]
- 167.Salvi M, Brunati AM, Clari G, and Toninello A. Interaction of genistein with the mitochondrial electron transport chain results in opening of the membrane transition pore. Biochim Biophys Acta 1556: 187–196, 2002 [DOI] [PubMed] [Google Scholar]
- 168.Sassi N, Biasutto L, Mattarei A, Carraro M, Giorgio V, Citta A, Bernardi P, Garbisa S, Szabò I, Paradisi C, and Zoratti M. Cytotoxicity of a mitochondriotropic quercetin derivative: mechanisms. Biochim Biophys Acta 1817: 1095–1106, 2012 [DOI] [PubMed] [Google Scholar]
- 169.Sassi N, Mattarei A, Azzolini M, Bernardi P, Szabo I, Paradisi C, Zoratti M, and Biasutto L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr Pharm Des 20: 172–179, 2014 [DOI] [PubMed] [Google Scholar]
- 170.Satterfield M. and Brodbelt JS. Enhanced detection of flavonoids by metal complexation and electrospray ionization mass spectrometry. Anal Chem 72: 5898–5906, 2000 [DOI] [PubMed] [Google Scholar]
- 171.Saunier E, Antonio S, Regazzetti A, Auzeil N, Laprévote O, Shay JW, Coumoul X, Barouki R, Benelli C, and Huc L. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci Rep 7: 6945, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saura-Calixto F, Serrano J, and Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem 101: 492–501, 2007 [Google Scholar]
- 173.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611, 2008 [DOI] [PubMed] [Google Scholar]
- 174.Sekhar KR, Rachakonda G, and Freeman ML. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol Appl Pharmacol 244: 21–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shoshan-Barmatz V. and Mizrachi D. VDAC1: from structure to cancer therapy. Front Oncol 2: 164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, and Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 27: 2055–2063, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Skulachev VP. How to clean the dirtiest place in the cell: cationic antioxidants as intramitochondrial ROS scavengers. IUBMB Life 57: 305–310, 2005 [DOI] [PubMed] [Google Scholar]
- 178.Song I-S, Kim H-K, Jeong S-H, Lee S-R, Kim N, Rhee BD, Ko KS, and Han J. Mitochondrial peroxiredoxin III is a potential target for cancer therapy. Int J Mol Sci 12: 7163–7185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. In: Novartis Foundation Symposium. Chichester; New York; John Wiley, 1999; 2007, p. 60. [PubMed] [Google Scholar]
- 180.Spycher S, Smejtek P, Netzeva TI, and Escher BI. Toward a class-independent quantitative structure–activity relationship model for uncouplers of oxidative phosphorylation. Chem Res Toxicol 21: 911–927, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Stefanson AL. and Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients 6: 3777–3801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stein R. and McDonnell D. Estrogen-related receptor α as a therapeutic target in cancer. Endocr Relat Cancer 13: S25–S32, 2006 [DOI] [PubMed] [Google Scholar]
- 183.Stevens JF. and Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52: 7–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stevens JF. and Maier CS. The chemistry of gut microbial metabolism of polyphenols. Phytochem Rev 15: 425–444, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stockdale M. and Selwyn MJ. Effects of ring substituents on the activity of phenols as inhibitors and uncouplers of mitochondrial respiration. Eur J Biochem 21: 565–574, 1971 [DOI] [PubMed] [Google Scholar]
- 186.Stoner GD. and Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl 22: 169–180, 1995 [DOI] [PubMed] [Google Scholar]
- 187.Stoupi S, Williamson G, Viton F, Barron D, King LJ, Brown JE, and Clifford MN. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C] procyanidin B2 in male rats. Drug Metab Dispos 38: 287–291, 2010 [DOI] [PubMed] [Google Scholar]
- 188.Sun C, Zhang H, Ma X-F, Zhou X, Gan L, Liu Y-Y, and Wang Z-H. Isoliquiritigenin enhances radiosensitivity of HepG2 cells via disturbance of redox status. Cell Biochem Biophys 65: 433–444, 2013 [DOI] [PubMed] [Google Scholar]
- 189.Sun Y-L, Cai J-Q, Liu F, Bi X-Y, Zhou L-P, and Zhao X-H. Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World J Gastroenterol 21: 10840, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768–780, 2003 [DOI] [PubMed] [Google Scholar]
- 191.Sutendra G. and Michelakis ED. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front Oncol 3: 38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takashina M, Inoue S, Tomihara K, Tomita K, Hattori K, Zhao QL, Suzuki T, Noguchi M, Ohashi W, and Hattori Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int J Oncol 50: 787–797, 2017 [DOI] [PubMed] [Google Scholar]
- 193.Tewari D, Ahmed T, Chirasani VR, Singh PK, Maji SK, Senapati S, and Bera AK. Modulation of the mitochondrial voltage dependent anion channel (VDAC) by curcumin. Biochim Biophys Acta 1848: 151–158, 2015 [DOI] [PubMed] [Google Scholar]
- 194.Tong L, Chuang C-C, Wu S, and Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett 367: 18–25, 2015 [DOI] [PubMed] [Google Scholar]
- 195.Traber MG. and Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 51: 1000–1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Trachootham D, Alexandre J, and Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8: 579, 2009 [DOI] [PubMed] [Google Scholar]
- 197.Tranoy-Opalinski I, Legigan T, Barat R, Clarhaut J, Thomas M, Renoux B, and Papot S. β-Glucuronidase-responsive prodrugs for selective cancer chemotherapy: an update. Eur J Med Chem 74: 302–313, 2014 [DOI] [PubMed] [Google Scholar]
- 198.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, and Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013: article ID 972913, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tsai M-H, Liu J-F, Chiang Y-C, Hu SC-S, Hsu L-F, Lin Y-C, Lin Z-C, Lee H-C, Chen M-C, and Huang C-L. Artocarpin, an isoprenyl flavonoid, induces p53-dependent or independent apoptosis via ROS-mediated MAPKs and Akt activation in non-small cell lung cancer cells. Oncotarget 8: 28342, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.US Department of Health and Human Services. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Rockville, MD: Center for Drug Evaluation and Research, Food and Drug Administration, 2003 [Google Scholar]
- 201.Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, and Vacca RA. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta 1832: 2085–2096, 2013 [DOI] [PubMed] [Google Scholar]
- 202.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10: 671, 2011 [DOI] [PubMed] [Google Scholar]
- 203.Vasil'ev YV, Tzeng SC, Huang L, and Maier CS. Protein modifications by electrophilic lipoxidation products: adduct formation, chemical strategies and tandem mass spectrometry for their detection and identification. Mass Spectrom Rev 33: 157–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang J, Fang F, Huang Z, Wang Y, and Wong C. Kaempferol is an estrogen-related receptor α and γ inverse agonist. FEBS Lett 583: 643–647, 2009 [DOI] [PubMed] [Google Scholar]
- 205.Wang K, Fan H, Chen Q, Ma G, Zhu M, Zhang X, Zhang Y, and Yu J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 26: 15–24, 2015 [DOI] [PubMed] [Google Scholar]
- 206.Wang Y-H, Hou A-J, Chen L, Chen D-F, Sun H-D, Zhao Q-S, Bastow KF, Nakanish Y, Wang X-H, and Lee K-H. New isoprenylated flavones, artochamins A–E, and cytotoxic principles from Artocarpus chama. J Nat Prod 67: 757–761, 2004 [DOI] [PubMed] [Google Scholar]
- 207.Warat M, Szliszka E, Korzonek-Szlacheta I, Krol W, and Czuba ZP. Chrysin, apigenin and acacetin inhibit tumor necrosis factor-related apoptosis-inducing ligand receptor-1 (TRAIL-R1) on activated RAW264.7 macrophages. Int J Mol Sci 15: 11510–11522, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Waseem M. and Parvez S. Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma 253: 417–430, 2016 [DOI] [PubMed] [Google Scholar]
- 209.Weh KM, Aiyer HS, Howell AB, and Kresty LA. Cranberry proanthocyanidins modulate reactive oxygen species in Barrett's and esophageal adenocarcinoma cell lines. J Berry Res 6: 125–136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wei L, Dai Q, Zhou Y, Zou M, Li Z, Lu N, and Guo Q. Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: c-Src and hexokinase II. Biochim Biophys Acta 1830: 3835–3845, 2013 [DOI] [PubMed] [Google Scholar]
- 211.Wei L, Zhou Y, Dai Q, Qiao C, Zhao L, Hui H, Lu N, and Guo QL. Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis 4: e601, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wei L, Zhou Y, Qiao C, Ni T, Li Z, You Q, Guo Q, and Lu N. Oroxylin A inhibits glycolysis-dependent proliferation of human breast cancer via promoting SIRT3-mediated SOD2 transcription and HIF1α destabilization. Cell Death Dis 6: e1714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Whitaker H, Patel D, Howat W, Warren A, Kay J, Sangan T, Marioni J, Mitchell J, Aldridge S, and Luxton H. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer 109: 983, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Williams NH. and Yandell JK. Outer-sphere electron-transfer reactions of ascorbate anions. Aust J Chem 35: 1133–1144, 1982 [Google Scholar]
- 215.Williamson G. and Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr 104: S48–S66, 2010 [DOI] [PubMed] [Google Scholar]
- 216.Wong JY, Huggins GS, Debidda M, Munshi NC, and De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol 109: 394–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30: 87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu M, Chen YM, Huang J, Fang YJ, Huang WQ, Yan B, Lu MS, Pan ZZ, and Zhang CX. Flavonoid intake from vegetables and fruits is inversely associated with colorectal cancer risk: a case-control study in China. Br J Nutr 116: 1275–1287, 2016 [DOI] [PubMed] [Google Scholar]
- 219.Yakovleva K, Kurzeev S, Stepanova E, Fedorova T, Kuznetsov B, and Koroleva O. Characterization of plant phenolic compounds by cyclic voltammetry. Appl Biochem Microbiol 43: 661–668, 2007 [PubMed] [Google Scholar]
- 220.Yang B, Kotani A, Arai K, and Kusu F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 17: 599–604, 2001 [DOI] [PubMed] [Google Scholar]
- 221.Yang C, Lim W, Bazer FW, and Song G. Myricetin suppresses invasion and promotes cell death in human placental choriocarcinoma cells through induction of oxidative stress. Cancer Lett 399: 10–19, 2017 [DOI] [PubMed] [Google Scholar]
- 222.Yasueda A, Urushima H, and Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integr Cancer Ther 15: 17–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yi J, Qu H, Wu Y, Wang Z, and Wang L. Study on antitumor, antioxidant and immunoregulatory activities of the purified polyphenols from pinecone of Pinus koraiensis on tumor-bearing S180 mice in vivo. Int J Biol Macromol 94: 735–744, 2017 [DOI] [PubMed] [Google Scholar]
- 224.Yip E, Chan A, Pang H, Tam Y, and Wong Y. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol 22: 293–302, 2006 [DOI] [PubMed] [Google Scholar]
- 225.Zhang B, Wang Y, and Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett 286: 154–160, 2009 [DOI] [PubMed] [Google Scholar]
- 226.Zhang L. and Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12: 228–237, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Zhou J, Shen W, He X, Qian J, Liu S, and Yu G. Overexpression of Prdx1 in hilar cholangiocarcinoma: a predictor for recurrence and prognosis. Int J Clin Exp Pathol 8: 9863, 2015 [PMC free article] [PubMed] [Google Scholar]