Abstract
Significance: Head and neck squamous cell cancer (HNSCC) is a complex disease characterized by high genetic and metabolic heterogeneity. Radiation therapy (RT) alone or combined with systemic chemotherapy is widely used for treatment of HNSCC as definitive treatment or as adjuvant treatment after surgery. Antibodies against epidermal growth factor receptor are used in definitive or palliative treatment.
Recent Advances: Emerging targeted therapies against other proteins of interest as well as programmed cell death protein 1 and programmed death-ligand 1 immunotherapies are being explored in clinical trials.
Critical Issues: The disease heterogeneity, invasiveness, and resistance to standard of care RT or chemoradiation therapy continue to constitute significant roadblocks for treatment and patients' quality of life (QOL) despite improvements in treatment modality and the emergence of new therapies over the past two decades.
Future Directions: As reviewed here, alterations in redox metabolism occur at all stages of HNSCC management, providing opportunities for improved prevention, early detection, response to therapies, and QOL. Bioinformatics and computational systems biology approaches are key to integrate redox effects with multiomics data from cells and clinical specimens and to identify redox modifiers or modifiable target proteins to achieve improved clinical outcomes. Antioxid. Redox Signal.
Keywords: : redox, oxidative stress, head and neck, squamous cancer, HNSCC
Introduction
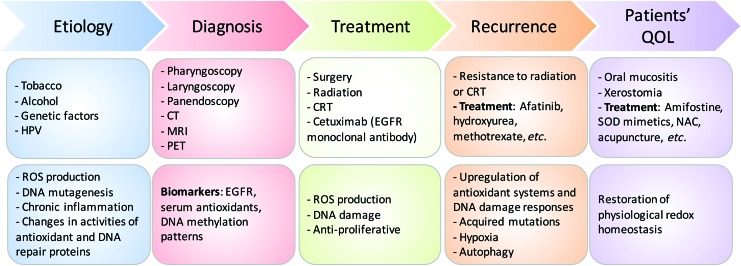
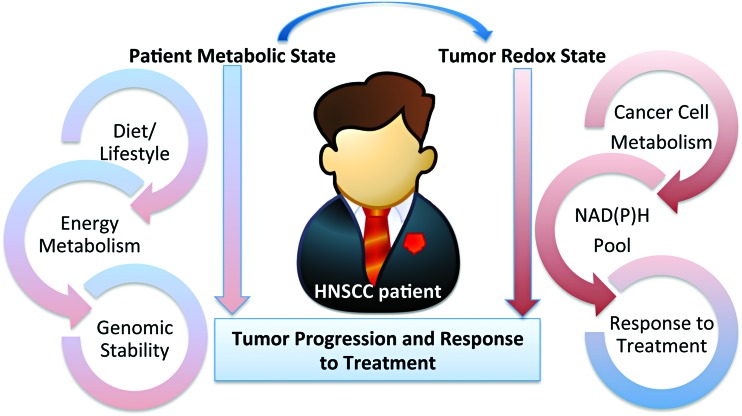
A systematic review of the literature is presented here outlining the role of redox metabolism and regulation in head and neck squamous cell cancer (HNSCC). HNSCC is a broad term encompassing malignancies of the squamous epithelium in the upper aerodigestive tract and is the eighth most common cause of cancer death worldwide (153). HNSCC management presents with multiple treatment challenges limiting overall survival (OS) rates and affecting patients' quality of life (QOL). Shifts in redox metabolism leading to accumulation or suppression of reactive oxygen species (ROS) are present at all stages of HNSCC underlying cancer etiology, progression, response to therapies, and QOL post-treatment (Fig. 1). Smoking, environmental pollutants, infection with viruses or pathogenic bacteria, chronic inflammation, and diet/microbiota generate ROS, and are well-established factors of malignant transformation (77). ROS further support tumor growth and metastasis by facilitating angiogenesis and cell invasion processes. The dependence of cancer on ROS and redox-regulated processes also enables the manipulation of these species as anticancer agents through the use of ROS-inducing ionizing radiation (IR), targeted agents, and systemic chemotherapies (77, 82, 141). Suppression of ROS and upregulation of DNA-damage responses have emerged as major mechanisms of resistance to standard of care not only in HNSCC but also across cancers. Dysregulation of redox metabolism is also responsible for the side effects of treatment impacting the QOL of HNSCC patients (e.g., oral mucositis and xerostomia), and treatments to alleviate these involve restoration of redox homeostasis.
FIG. 1.
Stages during HNSCC development and their relationship with altered redox homeostasis. ROS underlies cancer etiology, progression, and response to treatment, and is used for diagnosis and improving patient's QOL. CRT, chemoradiation therapy; CT, computed tomography; EGFR, epidermal growth factor receptor; HNSCC, head and neck squamous cell cancer; HPV, human papillomavirus; MRI, magnetic resonance imaging; NAC, N-acetylcysteine; PET, positron emission tomography; QOL, quality of life; ROS, reactive oxygen species; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Prevention: Redox Effects in HNSCC Etiology
Multifactorial interactions between environment and genetic mutations play a critical role in the development and complexities of staging HNSCC. Key etiologic factors associated with HNSCC are tobacco smoking, alcohol consumption, and genetics. More recently, human papillomavirus (HPV) emerged as an important etiologic factor, in particular for oropharyngeal HNSCC (91, 242, 306). These environmental stressors induce acute or chronic shifts in redox homeostasis promoting inflammation and neoplastic transformation.
Tobacco smoke
It is estimated that 42% of the HNSCC deaths worldwide can be attributed to smoking alone (153). Studies show that each puff of smoke contains 5000 carcinogenic compounds that produce 1015 free radical molecules in the gas phase (121). Once inhaled, these compounds are metabolized either by the cytochrome P450 (CYP) super family of proteins (CYP1A1, CYP1A2, CYP2E1, and CYP2A6) leading to DNA adduct formation or by glutathione S-transferases (GSTs; GSTM1, GSTT1, and GSTP1) leading to secretion (85). The activities of both CYP and GST families of enzymes depend on the availability of reducing equivalents controlled by the nicotinamide adenine dinucleotide phosphate [NAD(P)H]/NAD(P)+ or reduced/oxidized glutathione (GSH/GSSG) redox couples, respectively. Exposure to cigarette smoke increases oxidative damage rate by 30–50% (183), inducing either cell death by activation of ceramide-mediated apoptosis or cell proliferation by activation of epidermal growth factor receptor (EGFR) (120). Prolonged exposure to cigarette smoke also leads to increased GSSG, which has two consequences: (i) limits the availability of reduced GSH needed for the detoxification activity of GSTs and (ii) activates inflammatory pathways, including nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and nuclear factor-erythroid 2-related factor 2 (NRF2) (85, 111). These result in increased gene expression, release of cytokines (121), and onset of chronic inflammation. The shift to a more oxidative environment could potentially further enhance the activity of EGFR through oxidation at Cys797 in the active site of this enzyme as reported in recent studies (229) and further detailed hereunder.
Alcohol consumption
Alcohol consumption in conjunction with tobacco smoking increases the risk for HNSCC (91, 242, 306). Excessive alcohol consumption increases mucosa permeability to toxins and reduces epithelial thickness, leading to damage of vital structures and tumor formation (193). Ethanol, for example, can be metabolized to carcinogenic acetaldehyde by alcohol dehydrogenase in the mitochondria or NADPH-dependent CYP2E1 in the microsomal ethanol oxidizing system (187). Catalase (CAT), an antioxidant enzyme with the main function of catalyzing H2O2 dismutation into H2O and O2, can also oxidize ethanol to acetaldehyde in peroxisomes in the presence of H2O2. Similar to ROS species, acetaldehyde interacts with proteins and nucleic acids to form adducts, which interfere with critical cellular functions such as DNA synthesis and repair (43).
Genetic factors
The relationship between genetic factors and ROS metabolism is not inherently obvious, but a strong case can be made by the data summarized here. Variant alleles of genes involved in carcinogen metabolism, alcohol and folate metabolism, and DNA repair and cell cycle control are thought to play a role in development of HNSCCs. Meta-analyses have focused on carcinogen metabolism enzymes such as CYP super family of enzymes and the antioxidant enzymes GSTs, introduced previously (2, 169). For example, several studies demonstrated an increased risk of HNSCCs in individuals with GSTP1 I105V mutation associated with increased DNA oxidative products (156, 198, 225). Presence of the null GSTMμ1 genotype versus the positive genotype also increases the risk for HNSCC (225). Genetic variants of cell cycle control gene, TP53, are also common in HNSCCs and increase HNSCC risk by 75% (36, 185). The G to C polymorphism in codon 72 of exon 4 results in an arginine to proline substitution in TP53. Although both variants are wild-type (WT), the proline/proline genotype was shown to be less effective in suppressing cellular transformation and showed a higher risk for HNSCC than individuals with the arginine/arginine genotype (36, 185). Striking ethnic differences have been observed in the frequencies of these variants, with the proline allele showing a latitude gradient from 0.17 in a Swedish population to 0.63 in a Nigerian population (18, 274). The role of 1-C/folate metabolism in maintaining the reduced NADPH pool has been reported in several publications (102, 289). Relationships between polymorphisms in folate metabolism enzymes (e.g., methylenetetrahydrofolate reductase [MTHFR], serine hydroxymethyltransferase [SHMT]), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and immune response factors (e.g., interleukin [IL]-8, toll-like receptor 10 [TLR10]), and HNSCC risks have been investigated (115, 218, 246, 304). However, the number of such studies is limited, and it is difficult to draw definite conclusions.
Human papillomavirus
The incidence of HNSCCs caused by tobacco and excessive alcohol consumption is decreasing, whereas there is an increase in incidence of HNSCCs in younger white individuals and nonsmokers due to infection with “high-risk” HPVs (e.g., HPV subtype 16, which accounts for 87% of all HPV+ tumors) (51, 80). Of note, HPV− tumors do not necessarly imply virus-free HNSCCs and the tumors may be positive for other viruses such as EBV, HCV, HSV, or HIV. The HPV viral genes are divided into “early” genes (E1–7) and “late” genes (L1, L2). Two “early” genes (E6, E7) are considered tumorigenic. Mechanisms involve suppression of p53 and retinoblastoma protein (pRb) function and induction of oxidative stress (180, 196, 316) by activation of NADPH oxidase 2 (NOX2) (196) and by suppression of antioxidant proteins superoxide dismutase 2 (SOD2) and glutathione peroxidase 1/2 (GPX1/2) (316). Consequently, ROS accumulation in HPV+ cells contributes to DNA damage and genomic instability needed for HPV-induced tumorigenesis and synergizes with IR by forming clustered lesions that are highly lethal (196), in keeping with the clinical observation that patients with HPV+ head and neck tumors have a more favorable outcome after chemoradiation therapy (CRT) than patients with HPV− tumors (6, 101). In turn, a more oxidative environment promotes several stages of HPV infection, including viral entry, DNA replication, and integration into the host genome (83, 317). In the case of HNSCCs, it has been shown that HPV16 utilizes a tissue-spanning redox gradient to facilitate viral maturation, and a complete viral life cycle can occur in oral (tonsil) tissue (151). HPV vaccines such as Gardasil® and Cervarix®, which are currently being evaluated for prevention of HPV-related HNSCCs, (135) are expected to decrease the incidence of HPV+ HNSCCs though their efficacy under increased oxidative stress environments remains to be tested. Common diagnostic methods for HPV positivity in HNSCCs include detection of viral DNA with in situ hybridization or polymerase chain reaction (PCR), and detection of p16 (a surrogate marker for HPV infection) protein expression with immunohistochemistry [reviewed in Chai et al. (51), Mirghani et al. (206), and Venuti and Paolini (303)]. With each of these methods providing different information and having their own specific limitations, there is currently no consensus on the optimal way to identify HPV-related HNSCC.
Detection and Diagnosis: Redox Biomarkers in HNSCC Detection
HNSCC patients can present with various precancerous conditions and lesions depending on the tumor location within specific areas of the head and neck. The most common symptoms presented by HNSCC patients include chronic sore throat, difficulty swallowing, a change or hoarseness in the voice, and a lump or sore that does not heal. There are currently no biomarkers for early HNSCC detection. Although preclinical studies to identify possible markers for early cancer detection have been reported (e.g., EGFR, serum antioxidant CAT, and GSH levels), these remain exploratory (126). DNA methylation patterns appear gradually due to environmental influences (i.e., tobacco and alcohol use), and are thus considered as potential source of biomarkers. Epigenetic modifications are essential for regulation of cell cycle control (i.e., p16 and p14), DNA repair, and apoptosis. Longitudinal studies reported higher hypermethylation of p16 in precancerous lesions leading to increased tumor progression compared with hypomethylation of p16 in precancerous lesions leading to tumor regression (46, 131). Clearly, the GSH levels and epigenetic DNA methylation are mechanistically connected as S-adenosylmethionine (SAM), the substrate for DNA methyltransferases and other methyltransferase enzymes, is synthesized from methionine, which is also part of the GSH biosynthesis through the redox-regulated transsulfuration pathway (136). Given the redox shifts associated with the etiology of HNSCC supported by the value of utilizing CAT and GSH antioxidant biomarkers for early detection, a need for development of redox positron emission tomography (PET) imaging methods for HNSCC early diagnosis is emerging. This has not yet been explored in preclinical or clinical studies in relation to HNSCC. Such PET imaging probes may include [18F]fluorothymidine probe for H2O2 or other yet unexplored biomarker indicators of redox shifts (48).
Treatment: Redox Modulators of Standard of Care and Emerging Therapies for HNSCC
Standard of care
The treatment plan for patients with HNSCC is determined from three parameters: (i) location of tumor, (ii) stage of cancer, and (iii) person's age and overall performance status regardless of HPV status (89, 92). Surgery followed by fractionated radiotherapy is the standard of care for resectable primary and secondary malignancy with the goal of obtaining tumor-free surgical margins (132). However, negative surgical margins often result in removal of normal tissue causing impairment of critical functions, such as chewing and swallowing, and an adverse QOL (132). In many cases, due to presence of high risk of relapse factors such as positive margins and/or the presence of extracapsular invasion of the positive lymph nodes by cancer cells, surgery is followed up with aggressive CRT to kill remaining tumor cells. Patients generally undergo fractionated doses of 2 Gy each in 5 weekly sessions for 6–6½ weeks for a total dose of 60–66 Gy (27). Patients with unresectable tumors or on whom an organ sparing approach is possible receive radiation therapy (RT) or most often CRT with an even higher dose of RT of 70–72 Gy for 7 weeks (116).
Based on large randomized clinical trials and meta-analysis, cisplatin is considered the standard radiosensitizing agent for definitive or adjuvant RT. When used in combination with radiotherapy, cisplatin is given at 100 mg/m2 every 3 weeks during the course of RT (15). However, in recurrent tumors or for palliative care, other chemotherapeutics such as taxanes, hydroxyurea, and the antifolates methotrexate or pemetrexed have been utilized as well as radiosensitizers (129, 258). Antifolates, such as methotrexate and the newer drug pemetrexed, were reported to sensitize tumors to RT in both preclinical and clinical studies (149, 199, 257). These drugs inhibit the enzyme dihydrofolate reductase, which is essential for DNA synthesis and connects the 1-C/folate metabolism to NAD(P)H/NAD(P)+ balance, GSH biosynthesis, ROS and epigenetics through biosynthesis of SAM, a substrate for DNA methyltransferases, already described. Consistent with this notion, methotrexate also exhibits potent immunosuppressant activity through ROS accumulation and decreased GSH biosynthesis (234). Normal tissue toxicities associated with methotrexate or pemetrexed therapy are typically reduced in the clinic by coadministration of folate/B9 and vitamin B12 supplements, without compromising the efficacy of treatment (41, 69, 219). What all these chemotherapeutics have in common is the connection to the central redox metabolism network, which sets the balance between the intracellular ROS (E0 = approximately −0.3 to 2.5 V; e.g., H2O2 + 1.78 V) and the NAD(P)H/NAD(P)+ (E0 = −0.32 V) reserve (245). Depletion of the NAD(P)H pool and the ensuing increase in ROS kill cancer cells by DNA damage and induction of cell death pathways.
Therapies targeting EGFR
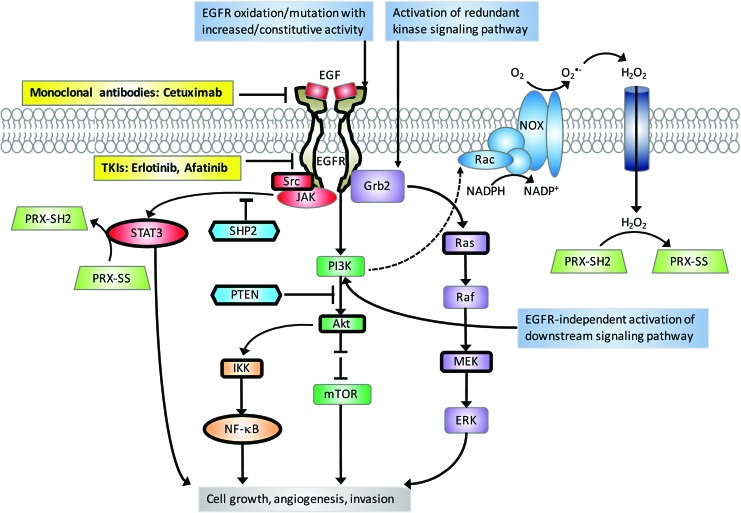
EGFR is overexpressed in 90% of HNSCC patients (19, 222), making EGFR a possible target to address locally advanced or metastatic HNSCCs (116). EGFR is a transmembrane glycoprotein with tyrosine kinase activity (91) serving as a critical signaling hub for tumor cell growth, angiogenesis, and invasion (Fig. 2). Currently, cetuximab (Erbitux®) is the only targeted therapy approved by the Food and Drug Administration (FDA) to be used in combination with RT for locally advanced HNSCC (38). Cetuximab is a recombinant immunoglobulin G1 (IgG1) monoclonal antibody directed at the extracellular domain of EGFR to block ligand-mediated activation of the EGFR pathway (288) (Fig. 2). It has been reported that cetuximab combined with RT improves progression-free survival (PFS) from 14.9 to 24.4 months and median OS rates from 20.3 to 54.0 months, but does not decrease rates of distant metastases in patients with locally advanced HNSCCs compared with RT alone (7, 15). Cetuximab is also FDA approved for use as a single agent or in combination with chemotherapy in metastatic HNSCCs. Cetuximab combined with platinum and 5-fluorouracil (5-FU) was tested in the EXTREME clinical trial. The study showed improved outcome in all end points including (i) increased PFS from 2.7 to 4.2 months, (ii) increased OS from 8.0 to 9.2 months, (iii) 1 -year survival rate from 31.7% to 38.6%, and (iv) locoregional control from 60% to 80% with no compromise in QOL (266). Other monoclonal antibodies, similar to cetuximab such as nimotuzumab (128, 186), zalutumumab, duligotuzumab (104), and panitumumab, have proved promising in preclinical research. One significant take away from the duligotuzumab study is that HPV− patients had higher response rates to both cetuximab and duligotuzumab than HPV+ patients (104). In general, newly developed EGFR monoclonal antibodies combined with radiotherapy or CRT have not improved oncologic outcomes or demonstrated superiority over CRT but promising attempts at enhancement continue.
FIG. 2.
EGFR signaling pathways. Activation of EGFR by ligand binding leads to a myriad of downstream signaling pathways (e.g., PI3K/Akt pathway, Ras/Raf/MEK/ERK pathway, JAK/STAT pathway, and NF-κB pathway) that ultimately drive tumor cell growth, angiogenesis, invasion, etc. Note that PI3K is required for membrane recruitment of Rac, a NOX subunit, and the subsequent ROS production. EGFR-targeted monoclonal antibodies block ligand binding to the extracellular domain of EGFR. EGFR-targeted TKIs inhibit the catalytic domain of EGFR. Mechanisms for resistance to EGFR-targeted therapies include EGFR oxidation/mutation with increased/constitutive activity, activation of redundant kinase signaling pathways (e.g., c-MET and HER2), and EGFR-independent activation of downstream pathways (e.g., PI3K/Akt). Bold borders indicate redox-regulated proteins. Akt, protein kinase B; c-MET, tyrosine-protein kinase Met; EGF, epidermal growth factor; ERK, extracellular signal-regulated kinase; Grb2, growth factor receptor-bound protein 2; HER2, human epidermal growth factor receptor 2; IKK, IκB kinase; JAK, Janus kinase; MEK, mitogen-activated protein kinase kinase; mTOR, mechanistic target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOX, NADPH oxidase; PI3K, phosphatidylinositide 3-kinase; PRX, peroxiredoxin; PTEN, phosphatase and tensin homologue; Rac, Ras-related C3 botulinum toxin substrate; SHP2, tyrosine-protein phosphatase nonreceptor type 11; STAT, signal transducer and activator of transcription factor; TKI, tyrosine kinase inhibitor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Emerging therapies for HNSCCs
Despite decades of research, the best treatment modality yielding significant efficacy with reduced side effects, improved organ function, and overall QOL has yet to be determined. Most research has focused on identifying radiation-sensitizing therapies with decreased side effects. There are many new emerging therapies on the horizon, including anti-EGFR monoclonal antibodies already described, tyrosine kinase inhibitors (TKIs), and other targeted drugs, as well as immunotherapies.
Tyrosine kinase inhibitors
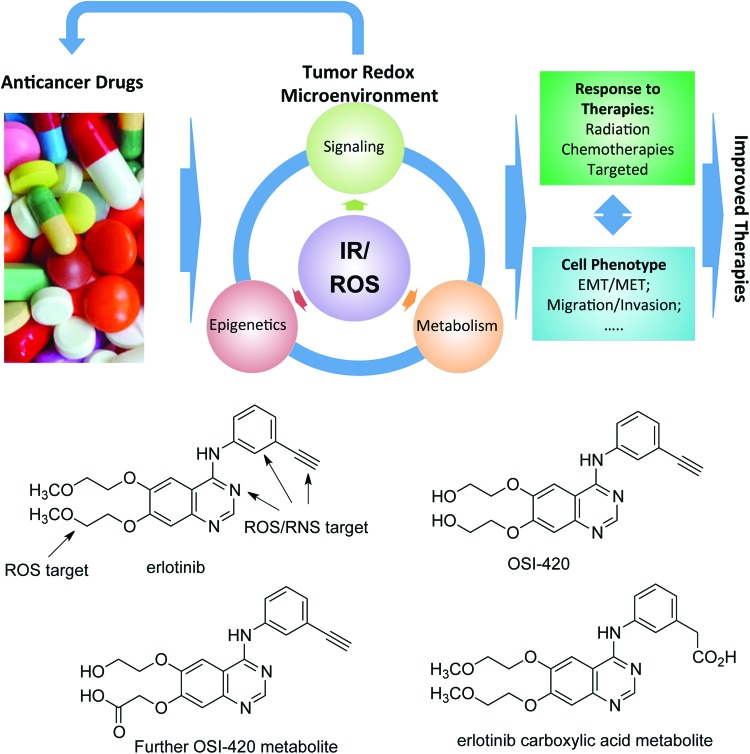
EGFR TKIs target the receptor catalytic domain (Fig. 2). Overall, lack of survival benefit for EGFR TKIs alone or in combination with radiotherapy or CRT in clinical trials has hindered their FDA approval for HNSCCs. For example, erlotinib (Tarceva®) and gefitinib (Iressa®) clinical trials have shown modest single agent response rate of only 4% and 10%, in recurrent or metastatic HNSCCs. However, in combination with cisplatin, the response improved to 21% but the combination of cisplatin, radiation, and erlotinib has not been well tolerated in HNSCC clinical trials (273, 281). Second generation EGFR TKIs, such as afatinib (Giotrif®), have shown increased cellular potency against EGFR (98, 204). Afatinib is an irreversible pan-EGFR TKI targeting Cys797 in the active site of EGFR. Studies have shown, however, that this cysteine site is also redox sensitive. Its oxidation to sulfenic acid state increases the activity of EGFR (229), and under chronic oxidative stress conditions induces resistance to afatinib (296). The impact of EGFR redox state on the response to afatinib and other targeted agents needs to be further investigated in the clinical context as RT or CRT may also induce oxidation of EGFR. There are at minimum three major redox mechanisms by which a drug could be rendered ineffective or result in unwanted toxicity when used in clinic (Fig. 3): (i) cancer drugs can be modified by tumor ROS (intrinsic or generated by cotherapies such as radiation or chemotherapeutics; e.g., erlotinib), (ii) molecular targeted drugs or the protein target itself may be modified by tumor ROS (e.g., EGFR), and (iii) drug-induced changes in tumor redox state can drive changes in the cellular phenotype (epithelial/mesenchymal, stem-cell like, etc.), which, in turn, would impact tumor progression and response to RT or CRT (14). Consideration of these effects along with others, which have been outlined recently (70), is expected to significantly improve the success rate of translating lead bench compounds into successful cancer therapeutics in clinic. Other TKIs targeting vascular endothelial growth factor (VEGF) and mechanistic target of rapamycin (mTOR) are being studied but have not provided any evidence of significant therapeutic efficacy in HNSCC patients. Tools to better predict patient responses to EGFR inhibitors will provide new opportunities for increased efficacy and newly identified mutations in the EGFR catalytic domain that confer sensitivity to EGFR TKIs promise to open new doors to use EGFR TKIs in the future.
FIG. 3.
Redox mechanisms for drug resistance. IR and/or intrinsic ROS generate an oxidative tumor microenvironment, which can modify the anticancer drug, rendering it less effective (e.g., erlotinib and its oxidized forms shown in the bottom) and the protein target itself (e.g., EGFR). IR and/or intrinsic ROS also affect tumor signaling, cell metabolism, and epigenetics in a cyclic way resulting in changes in the cellular phenotype, which, in turn, would impact tumor progression and response to therapies. EMT, epithelial-mesenchymal transition; IR, ionizing radiation; MET, mesenchymal-epithelial transition. To see this illustration in color, the reader is referred to the online version of this article at www.liebertpub.com/ars.
Exploiting NAD(P)H quinone oxidoreductase 1 bioactivatable drugs for radiosensitization of HNSCCs
The powerful potential of quinone oxidoreductase 1 (NQO1) bioactivatable drugs to sensitize NQO1 overexpressing human head and neck cancer cells in culture and xenografts in athymic nude mice to radiation was recently reported (177), consistent with findings in other NQO1 overexpressing human cancer cells (39, 40, 93, 177, 226). Importantly, synergy noted between radiation and NQO1 bioactivatable drugs avoids the only normal tissue toxicity noted with these drugs, methemoglobinemia (a condition characterized by elevated levels of hemoglobin that contains the ferric [Fe3+] form of iron). As these series of drugs are relatively new and are tightly connected to both ROS metabolism and NAD(P)H reserve, we present this class of compounds in more detail hereunder.
NQO1 expression specificity
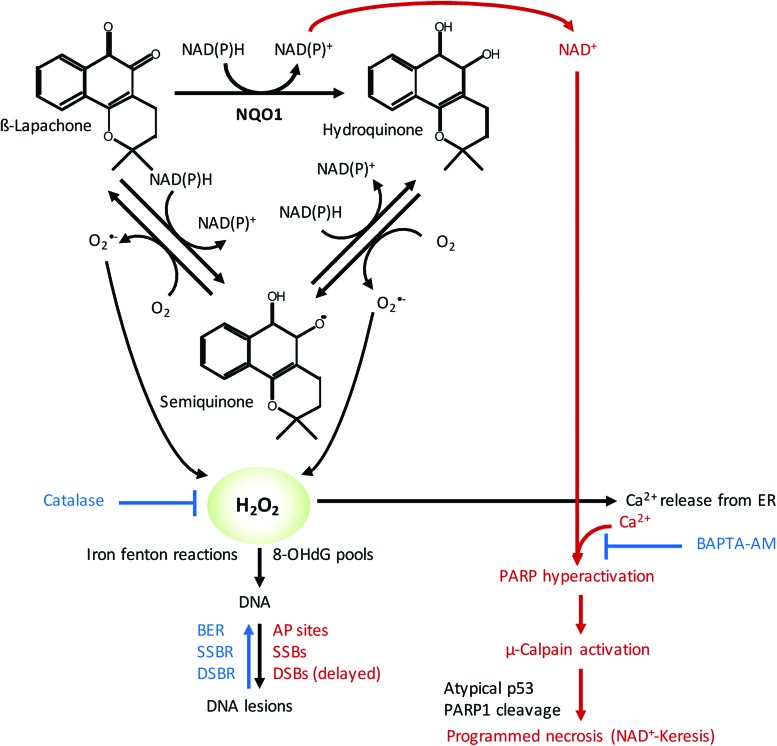
In normal tissue, NQO1 plays a role as a Phase 2 detoxification enzyme, performing two-electron oxidoreduction of most quinones. The enzyme converts quinones to their hydroquinone forms, which are then conjugated within the cell and exported. However, there are a few specific quinones that undergo a rapid futile cycle response, whereby NQO1 creates an unstable hydroquinone that spontaneously and rapidly converts back to its original compound using two oxygenation steps and creating two molecules of superoxides (Fig. 4) (235). For example, mitomycin C and streptonigrin can be converted to active drugs by NQO1, but these agents are not solely dependent on NQO1 and they are converted to active DNA-modifying agents through this reaction (267). There are currently three classes of futile cycle-bioactive quinones: (i) deoxynyboquinones (DNQ) (147), (ii) KP372 agents (328), and (iii) β-lapachone (235) with β-lapachone being the prototype drug (235). The term “NQO1 bioactivatable compounds” has been applied to these specific quinones (52–54, 147, 148, 209). In the NQO1 catalyzed reaction, β-lapachone treatment uses 60 moles NAD(P)H and creates 120 moles of superoxide within a 2 min period (Fig. 4). DNQ and its related derivatives cause five- to eightfold more superoxide and NAD(P)+ formation in the same timeframe (147). Measurements of superoxide formation and NAD(P)+ formation for KP372 have not been determined (328).
FIG. 4.
Exploiting NQO1 bioactivatable drugs for radiosensitization of HNSCCs. NQO1 metabolizes β-lapachone to an unstable hydroquinone (quinol) that spontaneously and rapidly converts back to its original compound using two oxygenation steps and creating two molecules of superoxides, which then damage DNA and cause cell death via programmed necrosis (NAD+-Keresis). This process is dependent on calcium release, and pretreatment with a specific calcium chelator BAPTA-AM inhibits NQO1-dependent cell death. AP, apurinic/apyrimidinic; BER, base excision repair; DSB, double-strand break; ER, endoplasmic reticulum; NQO1, quinone oxidoreductase 1; PARP, poly ADP ribose polymerase; SSB, single-strand break. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NQO1-dependent calcium release
A key feature of the cell death response caused by NQO1 bioactivatable drugs is that calcium release from otherwise inert central endoplasmic reticulum (ER) core stores is required for the specific programmed necrotic pathway referred to as NAD+-Keresis (177, 284, 285). Cell death does not require extensive formation or accumulation of poly ADP ribose (PAR) moieties generated by PAR glycohydrolase (PARG), since coadministration of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors and loss of NAD+ levels increased programmed necrosis induced by β-lapachone or isobutyl-deoxynyboquinone (IB-DNQ) (147, 177, 209). Instead, pretreatment with a specific calcium chelator, such as BAPTA-AM, caused the specific inhibition of NQO1-dependent cell death by these bioactivatable quinones (Fig. 4) (147, 177, 284, 285).
CAT detoxification
In HNSCCs, as well as in pancreatic, lung, prostate, and breast cancers, CAT levels were found to be lower in tumor than in associated normal tissue within human patients (52–54, 148, 177). This generates an extensive therapeutic index for these cancers that can be exploited using NQO1 bioactivatable drugs (52–54, 148, 177). Forced overexpression of CAT (>1000 U of cell membrane permeable polyethylene glycol [PEG]-CAT) can suppress NQO1-dependent DNQ or β-lapachone lethality in these cancers (Fig. 4); however, these levels are well beyond CAT expression typically seen in these cancers under endogenous conditions. This strongly suggests that NQO1 can generate more than “saturable” levels of H2O2 within human cancers that overwhelm the enzyme's capacity for degrading H2O2. The accumulated H2O2 can pass through membranes and easily damage neighboring NQO1-deficient cancer cells (29). In contrast, normal cells plated along side NQO1 overexpressing cancer cells are protected due to their elevated CAT levels. These findings supported by in vivo data suggest a potent NQO1-dependent bystander effect capable of suppressing the emergence of NQO1 cells growing from mixed cultures (47). Such bystander effects are likely to play a role in the radiosensitization responses observed in HNSCC xenografts (177).
DNA damage created by NQO1 futile recycling
DNA damage analyses of NQO1+ overexpressing cancer cells exposed to β-lapachone or IB-DNQ (or DNQ) revealed extensive DNA lesion formation by alkaline comet analyses, whereas little to no DNA lesions were noted after the same treated cells were examined using neutral comet assays (23, 24, 54, 93, 147). These data showed that the initial DNA damage created by NQO1 bioactivatable drugs is DNA single-strand breaks (SSBs) and apurinic/apyrimidinic (AP) site generation from incorporation of 8-oxo-deoxyguanine (8-OHdG), a result of H2O2-induced 8-oxoguanine-dGTP pools (54, 147, 177), as well as other altered bases. H2O2-induced Fenton reactions are also likely to ultimately induce DNA SSBs and double-strand breaks (DSBs). However, these lesions appear to be protected by hyperactivation of poly ADP ribose polymerase (PARP), which occurs immediately after exposure of cells to β-lapachone or IB-DNQ. PARP hyperactivation in this context is prevented by BAPTA-AM or dicoumarol (NQO1 inhibitor) and is not apparent in NQO1-deficient cells (e.g., resulting from NQO1 polymorphisms, *2 [C609T] and *3 [C465T]) (148, 177). Importantly, PARP1−/− cells lack these responses, but are not immune to overall cell death after exposure to NQO1 bioactivatable drugs. Instead, NQO1+/PARP1−/− cells treated with NQO1 bioactivatable drugs, or NQO1+ cancer cells exposed to PARP inhibitors, undergo a synergistic apoptotic cell death responses caused by DNA SSBs converted to DSBs due to inaccurate DNA replication, without a major loss in NAD+ or adenosine triphosphate (ATP) (148).
PARP hyperactivation and subsequent μ-calpain-dependent programmed necrosis
The massive level of DNA lesions, in concert with Ca2+ release from the ER, induces hyperactivation of PARP in NQO1+ cancer cells exposed to the NQO1 bioactivatable drugs. Increased NAD+ pools in these cells resulting from the oxidation of NADH in the futile cycle (Fig. 4) are rapidly degraded by the hyperactivated PARP. Such responses are not present in NQO1-deficient cells or in exposed NQO1+ cancer cells treated with dicoumarol, a fairly specific NQO1 inhibitor. NAD+ pools, aside from the sequestered mitochondrial NAD+ level, are degraded within 20–35 min in most NQO1+ cancer cells, leading to concomitant ATP losses within 30–40 min, μ-calpain activation with 8–24 h, terminal deoxynucleotidyl transferase dUTP nick end labeling positive staining within 24–48 h, and loss of attachment and programmed necrosis within 72 h of exposure to NQO1 bioactivatable drugs (Fig. 4) (23, 24, 28, 47, 52–54, 93, 176, 235, 236, 284, 285).
Combined radiation and NQO1 bioactivatable drugs
IR creates a spectrum of DNA lesions, including DNA DSBs, SSBs, AP sites, and DNA-protein crosslinks. DSBs are potentially the most lethal lesions, with only one DSB able to cause lethality (22, 50). The formation of DSBs among a range of SSBs and AP sites (also known as a multidamaged site) is enough to synergistically hyperactivate PARP when treated immediately with NQO1 bioactivatable drugs after exposure to IR. The formation of SSBs and AP sites after radiation, to which PARP binds with high affinity (239), synergistically interacts with the DNA lesions created by NQO1 bioactivatable drug exposure (exclusively SSBs and AP sites) to hyperactivate PARP activity (93, 148). Indeed, efficient synergy responses were noted with five doses of 1 Gy radiation and 5 mg/kg β-lapachone intravenous treatments (∼10-fold below the maximum tolerated dose of β-lapachone alone). As already mentioned, hyperactivation of PARP leads to massive NAD+ and ATP losses, which then prevent the repair of DSBs and greatly suppress NAD+, ATP, and, in theory, carbon metabolism (which has not been monitored to date). As a result, NQO1+ cancer cells, including from HNSCCs, can be efficiently killed by radiation and NQO1 bioactivatable drug combination therapy. The cell death pathway occurs specifically in NQO1+ cancer cells, independent of any oncogenic drivers, and causes a unique programmed necrotic death, NAD+-Keresis (93, 148, 177, 209). Similar to the treatment with the NQO1 bioactivatable drug alone, responses are only noted in NQO1 overexpressing cancer cells (177) with no responses in NQO1-deficient cells or normal tissue (93, 177). The therapeutic responses were also independent of the HPV or p53 status (177).
Future studies
The exact metabolic changes occurring in NQO1+ cancer cells after exposure to radiation and NQO1 bioactivatable drug treatments are not known. The role of released and circulating calcium in PARP hyperactivation, cell metabolism, and DNA repair has not been elucidated and is extremely important for radiation responses, since pretreatment with BAPTA-AM can suppress the synergistic response of combined radiation and NQO1 bioactivatable drug therapies (93, 177). Understanding the roles of genes that can suppress the responses is also unexplored, and genes involved in base excision repair (BER), DSB repair, ROS scavenging, and suppressing calcium ER release signaling are the most likely to strongly affect the response in NQO1+ cells. Currently, there are four clinical trials using a β-lapachone prodrug (as ARQ761) ongoing or planned. The monotherapy trial with ARQ761 is just about completed and responses to the agent were only noted in NQO1-expressing cancers, with no or little responses noted in NQO1-deficient cancers, most notably small cell lung cancers. Ongoing is a clinical trial treating NQO1+ pancreatic cancer patients in combination with abraxane and gemcitabine, wherein synergy was previously noted (162). Soon to open clinical trials are with sensitizing agents, including methoxyamine (54) and PARP inhibitors (148). Finally, another more potent NQO1 bioactivatable drug IB-DNQ should enter clinical trials within 1–2 years.
Immunotherapy
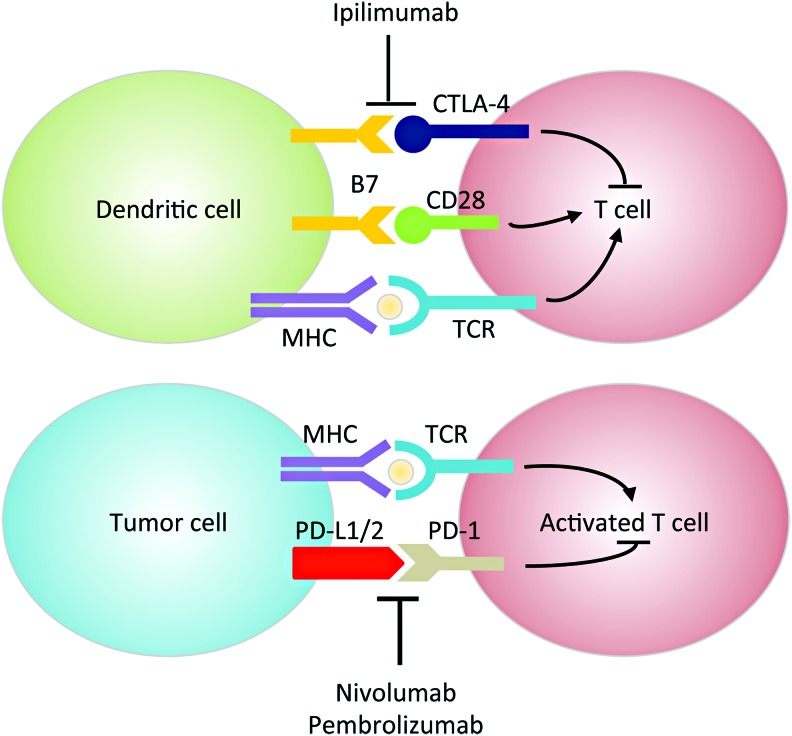
HNSCC is considered an immunosuppressive disease because of its ability to (i) dysregulate the cytokine profile (e.g., chemokine and VEGF overexpression), (ii) impair immune effector cell functions (e.g., upregulating IL-6), and (iii) cause abnormalities in tumor-associated antigen presentation (107). The interplay between the tumor and the host's immune system has become an increased area of research for HNSCC therapeutics. Tumor progression or relapse is believed to be associated with the inability of the immune system to eliminate cancer (255, 301). Immune checkpoints are critical in regulating T cell response, and coinhibitory molecules are new exciting targets of inhibition for HNSCC. The two most common targets include programmed cell death protein 1 (PD-1) or cluster of differentiation 279 (CD279), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4 or CD152) (Fig. 5). Since ROS affects many functions of T cells such as activation, differentiation, and apoptosis [reviewed in Belikov et al. (20), Chen et al. (59), and Nathan and Cunningham-Bussel (215)], modulation of ROS levels could potentially enhance or impede immunotherapy efficacy. For example, it has been shown that increasing ROS synergizes with the tumoricidal activity of PD-1 blockade by strongly activating mitochondrial function of tumor reactive cytotoxic T lymphocytes (56). This activation signals through mTOR, AMP-activated protein kinase (AMPK), and peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α). Small-molecule activators of AMPK and mTOR, or PGC-1α, also synergize with the PD-1 blockade therapy, whereas none of these activators alone has any effects on tumor growth. These findings provide novel combinatorial treatment strategies for patients who are less responsive to PD-1 immunotherapy alone. In contrast, as shown using multiple graft-versus-host-disease models, PD-1 signaling promotes apoptosis in alloreactive T cells by increasing ROS in a fatty acid oxidation-dependent manner, whereas PD-1 blockade, which decreases ROS, renders cells less susceptible to subsequent metabolic inhibition (292). Based on these findings, adjuvant therapies relying on increased ROS levels (e.g., glucocorticoids, methotrexate, and cyclophosphamide) will likely be undermined in T cells after PD-1 blockade. There are currently two promising PD-1 inhibitors for HNSCC, pembrolizumab (Keytruda®) and nivolumab (Opdivo®), which have shown efficacy irrespective of tumor programmed death-ligand 1 (PD-L1) expression (>1%) or HPV status (106). As of now, no CTLA-4 monoclonal antibodies have FDA approval for HNSCC treatment (95). However, there are several open-label dose escalation clinical trials with durvalumab (anti-PD-L1 monoclonal antibody) and ipilimumab or tremelimumab against CTLA-4. Preclinical data also suggest that there is increased efficacy when PD-1 and CTLA-4 immunotherapies are combined because they regulate T cell induction and maturation at different phases (95).
FIG. 5.
Mechanisms of PD-1 and CTLA-4 in immunosuppression. In the priming phase, dendritic cells present antigens to T cells via the interaction of MHC and TCR. B7 binding to CD28 generates a costimulatory signal. CTLA-4, upregulated shortly after activation, negatively regulates T cell activation by outcompeting CD28 for binding to B7. In the effector phase, PD-1 is expressed on activated T cells and binds to PD-L1/2 expressed on tumor cells, sending an inhibitory signal to T cells. Anti-CTLA-4 antibodies (e.g., ipilimumab) and anti-PD-1/PD-L1 antibodies (e.g., nivolumab and pembrolizumab) enhance T cell function by turning off the inhibitory signal. CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCR, T cell receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Resistance to Standard of Care Therapy: Redox Controlled Mechanisms of Resistance to HNSCC Therapies
Suppression of ROS and upregulation of DNA-damage response have emerged as major mechanisms of resistance to CRT across cancers. Thus, it is not surprising that a high number of emerging drugs directly target these pathways (e.g., PARP, DNA-dependent protein kinase [DNA-PK], and BER). PARP1, in particular, impacts both ROS and DNA-damage response by consuming intracellular NAD+ to modify critical nuclear proteins by PARylation. Inhibition of PARP increases intracellular NAD+ reserve while decreasing the NADH/NAD+ balance in cancer cells and contributing to the synergy of interaction with CRT. In addition, the effectiveness of PARP inhibitors in tumors defective in DNA-damage repair is well documented (240, 272). EGFR is overexpressed in HNSCCs and thus constitutes an important therapeutic target both because of its function in cell proliferation and because of its recently discovered role in DNA DSB repair with PARP (213). Surprisingly, the redox state of EGFR in the tumor environment is not currently being considered as a prognostic indicator of response to EGFR-targeted therapies when used alone or in combination with CRT. Studies have shown a significant role of ROS, driven by NAD(P)H/NAD(P)+ ratio and cholesterol/lipid rafts content, in the response to both radiation treatment and EGFR inhibitor erlotinib in vitro and clinical tumor specimens of HNSCCs (14, 205). These and other redox-dependent mechanisms of resistance to RT and chemotherapies are presented hereunder.
Resistance to radiation in HNSCC
The mechanisms of damage by IR were reviewed recently (243) and are only briefly summarized here. The major types of IR are alpha and beta particles, X-rays, and gamma rays, and all are capable of causing cellular damage and are thus used for therapeutic purposes (87). The adult human body is composed of 60% of H2O that absorbs energetic radiations leading to ROS production (Fig. 6). Gamma irradiation of cellular H2O rapidly generates ROS, including hydroxyl radical (•OH) and ionized water (H2O+) as well as hydrogen radical (H•) and hydrated electrons (e−aq). Within one picosecond, O2•− and H2O2 are formed as secondary ROS products and this chemical cascade generates cell-damaging molecules (271). These ROS and their progenies attack macromolecules (DNA, RNA, lipids, and proteins), induce mitochondrial ROS production, and upregulate NOX expression (11, 71, 94). Most IR-induced protein modifications are irreparable and critically affect protein stability. Reversible oxidation of cysteine amino acids in critical signaling molecules, such as kinases and phosphatases, can lead to amplification or dampening of signaling pathways and in some cases may synergize or antagonize the efficacy of targeted therapies when used in combination with RT. Under moderate oxidative stress, cysteine residues can be oxidized to relatively unstable sulfenic acid (-SOH), which can be further oxidized to -SO2H and -SO3H, or form mixed disulfides (S-thiolation) with other protein thiol groups (-SH) or low molecular mass thiols such as GSH (68). Disulfides are readily reduced to thiols by disulfide reductases in coupled reactions such as thioredoxin (TRX)/thioredoxin reductase (TR) or GPX/GSH/glutathione reductase (GR) with NADPH as ultimate provider of reducing equivalents (140) (Fig. 6). Loss or gain of function by disulfide formation is a regulatory mechanism by which proteins mediate the cellular response to oxidative stress. For example, phosphatase and tensin homologue (PTEN), which is mutated in HNSCC, has a catalytic Cys124 residue that when oxidized leads to disulfide bond formation with a neighboring Cys79 residue. The disulfide bond inactivates the phosphatase and protects the cysteine from hyperoxidation, enabling reactivation of PTEN by TRX (174). Redox-regulated proteins with signaling, metabolic, or epigenetic functions have been reviewed recently (86, 181, 224).
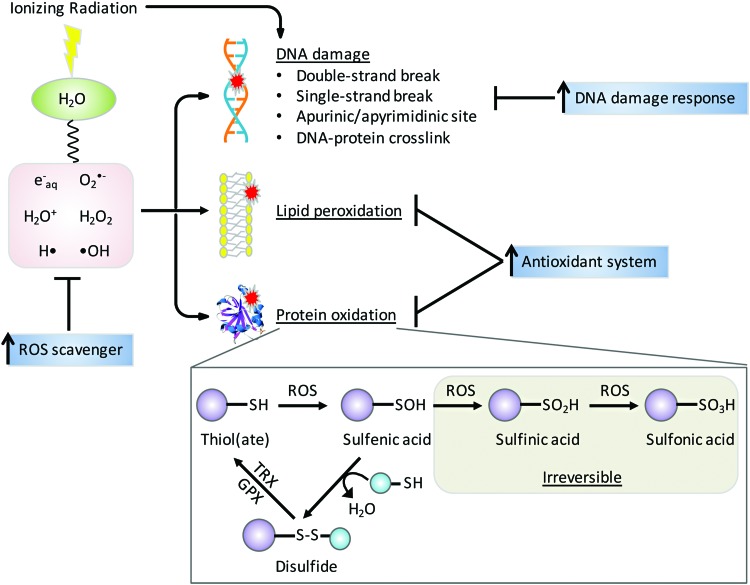
FIG. 6.
Mechanisms for radiation therapy. IR causes water radiolysis generating ROS that leads to DNA damage, lipid peroxidation, and protein oxidation. IR also directly damages DNA. ROS can oxidize specific protein cysteine residue to unstable sulfenic acid, which can be reversed back to thiol through mixed-disulfide formation. Further oxidation of sulfenic acid results in irreversible conversion to sulfinic and sulfonic acids. Upregulation of antioxidant systems and DNA damage responses can restore redox homeostasis and macromolecule integrity, underlying major mechanisms for radiation resistance. GPX, glutathione peroxidase; TRX, thioredoxin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Radiation resistance is a major clinical problem for HNSCC patients compounded by origin, location, and tumor grade that limits tumor control and ultimately affects patient QOL (232). A tumor is considered radioresistant if there is either lack of tumor response or partial response resulting in recurrence a few weeks after an initial complete response. Tumor size, location, grading, and stem-like cell population play a critical role in driving radiation resistance. For example, larger, more differentiated tumors have a worse overall prognosis due to hypoxic cores, and differentiated tumors have the ability to accelerate repopulation capacity during radiotherapy (268). Rapid repopulation depends on the stem-like cells ability to activate checkpoints and DNA repair to enhance self-renewal and results in differentiation into heterogeneous cells (100). There are several well-studied mechanisms of resistance to radiation involving redox processes associated with hypoxia, autophagy, intracellular pathway alteration, and metabolic reprogramming, as described hereunder.
Hypoxia
The cytotoxic effects of radiotherapy depend heavily on the presence of molecular O2 to react with free radicals to produce ROS and irreparable DNA damage. However, under hypoxic conditions, O2 availability is low due to either reduced perfusion or starvation of necessary O2 and nutrients (144). It is estimated that HNSCCs contain three zones where O2 tensions fluctuate between anoxic (0% O2), hypoxic (1%/7 mm Hg O2), and normoxic (8%/60 mm Hg O2) (302). A study measuring oxygen partial pressure (pO2) in 28 HNSCC patients reported an 80% postradiotherapy PFS correlation with a median oxygen tension of pO2 > 10 mm Hg (42). Transcription factors, such as hypoxia-inducible factors (HIFs), respond to changes in O2 availability in the cellular environment, making them attractive targets in cancer therapeutics. HIF-1α is considered most active during initial intense short period of hypoxia (i.e., <0.1% O2 and <24 h), whereas HIF-2α is active under longer more chronic periods of hypoxia (i.e., <5% O2 and >24 h) (259). Elevated expression of HIF-1α and HIF-2α is associated with radiation resistance in HNSCCs (166). Unfortunately, there are limitations to accurately detecting hypoxia as most exogenous markers can only detect chronic hypoxia, and endogenous markers may be upregulated due to stress factors that are not hypoxia related (17). However, PET studies using (18F)–fluoroazomycin arabinoside (FAZA) to measure hypoxia and predict PFS showed that hypoxic tumors had a lower PFS of 60% than nonhypoxic tumors PFS of 93% (261). The clinical trial attempts at increasing tumor oxygenation before and during radiotherapy have included red blood cell transfusion, erythropoietin administration, and hyperbaric oxygen treatment, but all have ended in conflicting or inconclusive results (223). Inhibitors against HIFs such as acriflavine, YC-1, endostatin, and TNP-470 have poor toxicity profiles and lack increased efficacy (173, 188).
Autophagy
Autophagy is the cellular recycling mechanism responsible for degrading dysfunctional cellular organelles in living cells to provide building blocks for metabolites and synthesis of macromolecules as a source of energy. Cell survival or death depends on the severity and length of autophagy: although cells utilize autophagy as a prosurvival mechanism for maintaining cellular homeostasis under starvation or other stress stimuli, extensive or prolonged autophagy can activate apoptosis or apoptosis-independent autophagic cell death (81, 191). Many lines of evidence support a role of ROS in both protective autophagy and autophagic cell death. The most direct demonstration of a redox-regulated mechanism in autophagy involves oxidation and inactivation of autophagy-related protein 4 (ATG4). Specifically, starvation-induced mitochondrial H2O2 oxidizes ATG4 at Cys81, which inactivates its delipidating activity on ATG8, thus allowing for autophagosome formation (251). Other possible mechanisms of redox regulation of autophagy include (i) S-glutathionylation of AMPK (332), which inhibits mTORC1 and activates unc-51-like autophagy activating kinase 1 (ULK1) to promote autophagy (96, 130, 163) and (ii) upregulation of proautophagic protein Beclin-1 (60, 90). ROS is involved in autophagic cell death, wherein it can be upstream of autophagy (60), or is accumulated as a result of autophagy-mediated specific degradation of CAT (325). In addition, autophagy can regulate ROS through NRF2/Kelch-like ECH-associated protein 1 (KEAP1)/p62 interactions, where degradation of KEAP1 by selective autophagy facilitated by autophagic adaptor p62 releases transcription factor NRF2 to enter the nucleus, leading to subsequent expression of antioxidant proteins (165, 171, 286). Given the dual role of autophagy in cell survival and death and the complex interplay between autophagy and ROS, it is not surprising that autophagy has been regarded as both a tumor promoter and a tumor suppressor, and its role in cancer cell response to therapies can be both protective and cytotoxic (10, 61, 119, 175, 283, 290, 315, 323). Nevetheless, one popular hypothesis suggests that during early tumor development, autophagy is anticarcinogenic through removal of damaged organelles and protein aggregates (195, 208). For example, short-term exposure to arecoline, similar to nicotine, increases ROS and inflammatory cytokines, leading to autophagy activation and removal of DNA damage or protein aggregates to prevent tumor formation. After tumor formation or radiation-induced oxidative stress, autophagy is upregulated and used by the tumor as a survival mechanism (143). However, the exact mechanism by which autophagy contributes to radiation resistance is unclear. Some studies suggest autophagy is regulated by HIFs to clear ROS-producing damaged organelles and protein aggregates too large for proteasomal removal (327). Overall, autophagy seems to have a dual role in cancer development and progression, making it a potential therapeutic target and biomarker to determine tumor stage. It is worth noting that mitophagy, which refers to the selective degradation of damaged or surplus mitochondria, also crosstalks with ROS and plays a role in cancer progression (62, 167, 184). On the one hand, ROS, produced by damaged mitochondria, RT, or chemotherapy, may trigger mitophagy to protect cells from oxidative stress and cell death (34, 112, 310). Suppression of mitophagy leads to overproduction of ROS and further damage to the mitochondria, which then produces more ROS in a vicious cycle (168). In this regard, mitophagy serves as a tumor suppressor to maintain the integrity of the mitochondrial pool and cellular homeostasis, supported by the observations that loss or downregulation of several mitophagy proteins promotes tumor growth (26, 63, 64, 105, 114, 238). On the other hand, mitophagy provides a survival mechanism for tumor cells, which already have higher ROS than normal cells and are likely more dependent on mitophagy to manage ROS and maintain functional mitochondria (62), suggesting that inhibition of mitophagy represents a promising antitumor strategy in combination with conventional cancer treatment (i.e., mitophagy inhibitors as radio- or chemosensitizers). However, studies showed conflicting results as to whether it is inhibition or induction of mitophagy that kills cancer cells (167). Just as autophagy, mitophagy is a double-edged sword in cancer biology and further research is needed to correctly target mitophagy for cancer intervention.
Reprogramming of cellular signaling and metabolism
Radiation-induced cell death is accompanied by alterations in intracellular pathways primarily involved in DNA repair and cell cycle, proliferation, apoptosis, and metabolic reprogramming. These insults can lead to radiation resistance over time. For example, p53 regulates cell cycle arrest through cyclin-dependent kinase inhibitor 1 (CDKN1A) stimulation and programmed cell death in response to environmental stimuli. In the presence of cellular stressors, p53 may undergo post-translational modifications, including phosphorylation, acetylation, and PARylation that lead to its activation. During cell cycle arrest in G1 phase, cells are able to repair DNA before its replication to avoid propagation of nucleic acid alterations (307). However, p53 may not be able to rescue a cell after repetitive insults, leading to damage and apoptosis induction through upregulation of Bcl-2-associated X protein (BAX) and p53-upregulated modulator of apoptosis (PUMA) (324). It is estimated that 50% of HNSCC patients have small mutations in the gene encoding TP53 (i.e., nonsense, missense, insertions, or deletions of nucleotides), leading to inactivation or absence of protein (278). These alterations impair the cells ability to arrest cell cycle and activate apoptosis, leading to more genetic mutations, genetic instability, and clonal selection of radiation-resistant cells. The origin and location of HNSCCs in combination with the presence of TP53 mutations further increase tumor heterogeneity, resulting in decreased survival and increased locoregional failure. As previously described, EGFR overexpression is correlated with poor prognosis in HNSCCs. Radiation can induce autophosphorylation of EGFR, causing protection from apoptosis (252), increased cell proliferation, and tumor repopulation after radiotherapy (230). EGFR and p53 expression and activity in HNSCCs are extremely important to therapeutic response. For example, HPV+ tumors are generally characterized by WT p53 and lower EGFR expression than HPV− tumors (49, 170), resulting in high chemoradiosensitivity. The metabolic reprogramming of cancer cells through the Warburg effect is also mediated by HIF-1α. Activation of glycolytic enzymes, pyruvate dehydrogenase kinase 1 (PDK1) (164) and pentose phosphate pathway (PPP) glucose-6-phosphate dehydrogenase (G6PD), by HIF-1α causes downregulation of aerobic respiration and upregulation of the PPP, leading to increased production of NADPH and ribonucleotides (231, 311) to protect cells against apoptosis (327) during radiation-induced hypoxia and oxidative stress. HIF-2α also regulates the expression of cytochrome c oxidase (COX) and SOD2 to increase mitochondrial electron transport chain efficiency and suppressing ROS generation (122, 123). Thus, tumor cell signaling and metabolism are connected by key enzymes and transcription factors to enhance resistance to RT and chemotherapy by regulating energy production, proliferation, and apoptosis.
Upregulation of antioxidant system
Human body has developed complex antioxidant defense mechanisms against endogenous- and exogenous-induced oxidative stress (Fig. 7). When ROS increases, cellular transcription factors, NRF2 and NF-κB, are activated to help increase production of antioxidants (12). Enzymatic (e.g., SOD, peroxiredoxin [PRX], and CAT) and nonenzymatic [e.g., GSH and NAD(P)H] antioxidants are some of the most well-studied intracellular defenses against oxidative damage. In addition to their antioxidant properties, several of these molecules are increasingly recognized as signaling molecules (262).
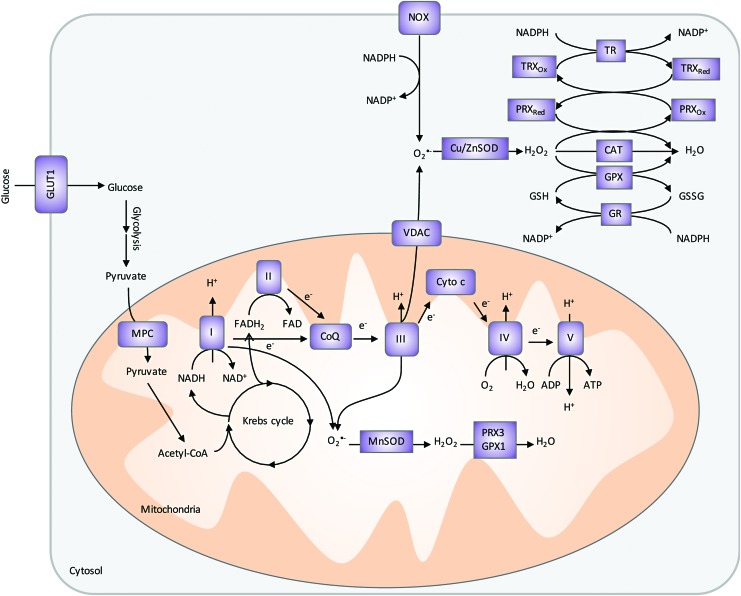
FIG. 7.
Cellular sources of ROS and the antioxidant systems. Two major cellular sources of ROS are produced by mitochondria ETC and NOX. In ETC, leaky electrons from complexes I and III reduce oxygen to superoxide. Cytosolic superoxide, generated by the NOX complex or transported by VDAC from mitochondria ETC, can be dismutated by Cu/ZnSOD to H2O2 and oxygen. Cytosolic H2O2 is then dismutated to water and oxygen by CAT, PRX/TRX/TR, or GPX/GSH/GR system using the reducing power of NADPH. Similarly, mitochondrial superoxide can be degraded by MnSOD and then PRX3 and GPX1 resided in the mitochondria. CAT, catalase; ETC, electron transport chain; GR, glutathione reductase; GSH, glutathione; GSSG, oxidized glutathione; MPC, mitochondria pyruvate carrier; TR, thioredoxin reductase; VDAC, voltage-dependent anion channel. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SODs are the major superoxide scavengers in the cells, catalyzing the dismutation of superoxide radicals to H2O2 and molecular oxygen. There are three forms of SODs, each encoded by a separate gene: CuZnSOD (SOD1) located mainly in the cytosol, MnSOD (SOD2) located in the mitochondrial matrix, and EcSOD (SOD3) located in the extracellular region of the cell. The role of SOD2 in cancer progression is controversial [reviewed in Holley et al. (137–139) and Miriyala et al. (207)]. Although many studies show a reduction in SOD2 expression in tumors compared with normal tissues suggesting a tumor suppressor activity (65, 78, 146, 221), there is also abundant evidence supporting that SOD2 promotes cancer progression to a more aggressive stage (e.g., increased SOD2 activity was detected in advanced HNSCCs) (66, 145, 152, 192, 249, 294). This dichotomy is reflected in a study using a chemically induced skin cancer mouse model, in which SOD2 expression, mediated by transcription factors Sp1 and p53, was decreased in the early stage but increased at late stages of skin cancer (88). To reconcile the opposing roles of SOD2 in cancer progression, one has to take into consideration that by catalyzing the dismutation reaction, SOD2 not only controls levels of superoxides but also contributes to H2O2 accumulation in cells (44). In other words, SOD2 is both an antioxidant and a pro-oxidant, whose role in cancer progression is likely a result from concerted efforts with other antioxidant enzymes. In fact, based on this dual role of SOD2, strategies aiming at increasing SOD2 expression and/or activity have proven to be important adjuvants to radiotherapy, sensitizing tumor cells to radiation while protecting normal tissues, as discussed in Survival and QOL: Improving HNSCC Patients' QOL with Redox Modifiers section.
PRXs are a family of thiol-dependent peroxidases that catalyze the reduction of H2O2, alkyl hydroperoxides, and peroxynitrite (ONOO−). PRXs also function as regulators in growth factor signaling and cell cycle progression by virtue of their inactivation, which enables localized build-up of H2O2 and protein oxidation. In this case, the transient inactivation of PRXs occurs by either hyperoxidation (PRX-SO2/3) (319, 320) or phosphorylation at key tyrosine or threonine residues (318). For example, cells stimulated by EGFR can cause PRX1 association with lipid rafts and subsequent inactivation through selective phosphorylation at tyrosine 194 by Src kinase (318). The inactivation allows H2O2 to accumulate near lipid rafts and mediate cell signaling. Therefore, PRXs play a critical role as a sensor and transducer of H2O2 signaling in cells. It has been shown that oxidized PRX2 forms a redox relay with transcription factor signal transducer and activator of transcription factor 3 (STAT3), resulting in STAT3 oxidation and attenuation of transcriptional activity (275). In HNSCC cells, PRX2 expression increases in cells after treatment with radiation and its overexpression blocks cells from radiation-induced cell death (227). Importantly, STAT3 is also expressed at high levels in HNSCCs and current therapeutics such as cetuximab lacks the ability to abrogate STAT3 activity. Combined inhibition of EGFR and control of redox metabolism could provide a path to improve cetuximab and other EGFR-targeted therapies.
GSH is synthesized in the cytosol by sequential reactions catalyzed by glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS). Cysteine availability is the rate-limiting factor in GSH biosynthesis and it can be either synthesized in cells from methionine through the activities of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE or GCL) or imported by the cystine/glutamate transporter system (xCT). GSH donates reducing equivalents (H++e−) to molecules such as cysteine disulfides in oxidized proteins to shift their redox state to reduced forms. As a result, GSH becomes oxidized to GSSG, which can be either excreted from the cell or reduced by GR in an NADPH-dependent manner. In addition, glutathione's protective roles against oxidative stress include (i) acting as a cofactor of detoxifying enzymes, (ii) participating in amino acid transport, (iii) scavenging hydroxyl radical and singlet oxygen, and (iv) regenerating other antioxidants back to their active forms (197). The ratio of reduced GSH versus oxidized GSSG is an indicative measure of oxidative stress in the cell (154).
TRX is a disulfide-containing redox protein with two redox active cysteines that can be oxidized to a disulfide and present in all subcellular organelles (159). TRX is reduced to its active state TRX-(SH)2 by TR in an NADPH-dependent manner (312). In addition to its function in the PRX catalytic cycle, TRX plays critical roles in controlling cell growth through redox regulation of DNA synthesis, cell signaling, and apoptosis by regulating transcription factors and kinase cascades. For example, TRX acts as a reducing cofactor for ribonucleotide reductase, the first unique step in DNA synthesis and methionine sulfoxide reductase, which reduces methionine sulfoxide to methionine (13). TRX also controls redox-sensitive points during apoptosis, including activating caspases by reducing cysteines (297) and by binding to apoptosis signal-regulated kinase 1 (ASK1) to create an inactive complex that when oxidized is activated and released from TRX (293). Overexpression of TRX has been shown to protect cancer cells from oxidative stress-induced apoptosis and provide a survival and growth advantage to tumors (298). Owing to the widespread redundancy between the TRX and GSH systems, strategies often target both of them to achieve powerful and synergistic antitumor effects (21, 133, 160, 194). In addition, dual inhibition of the TRX and GSH systems sensitizes cancer cells to chemotherapeutics (103, 250, 270). Remarkably, additional inhibition of NRF2 on top of GSH and TRX results in even more HNSCC cell death, particularly in cisplatin-resistant cells (247).
NAD(P)H is the ultimate donor of reducing equivalents in cells and a key substrate for TR and other antioxidant enzymes. In addition to its function in the maintenance of the redox homeostasis in cells, it is also necessary for synthesis of nucleic acids and lipids (300). NADPH is generated in cells via glucose and glutamine metabolism through the PPP enzymes G6PD and 6-phosphogluconate dehydrogenase (6PGD), conversion of pyruvate to malate by malic enzymes, conversion of isocitrate to α-ketoglutarate by isocitrate dehydrogenases 1 and 2 (IDH1 and 2), and through 1-C/folate metabolism. Transcription factors NRF2 and p53 modulate NADPH production by increasing transcription of NADPH-generating enzymes and upregulating TP53-induced glycolysis and apoptosis regulator (TIGAR), which inhibits glycolysis and promotes PPP (124). As previously described, NADPH is necessary to maintain reduced GSH and TRX during oxidative stress. Therefore, attenuation of the PPP or downregulation of NADPH production in tumors would reduce or inhibit the cells' ability to handle ROS and inhibit critical biosynthetic pathways.
Resistance to chemotherapy in HNSCC
Chemotherapy induces cell death primarily via DNA damage and apoptosis. Defective apoptotic initiation may cause drug resistance (190). Cisplatin is the most commonly used chemotherapy for HNSCC but has significant patient variability related to outcome, efficacy, and toxicity. To overcome disease resistance, cisplatin is used in combination with other chemotherapeutics, RT, or drug-targeted therapy. However, as the disease-free period shortens, the response to platinum-based therapy decreases, resulting in a platinum-resistant or a refractory disease state. The primary mechanism of resistance to cisplatin at low levels (i.e., 10- to 15-fold above baseline) is due to altered DNA repair. At intermediate and high levels of cisplatin resistance, the resistance is due to reduced cellular accumulation and cytosolic inactivation of cisplatin (3) (Fig. 8).
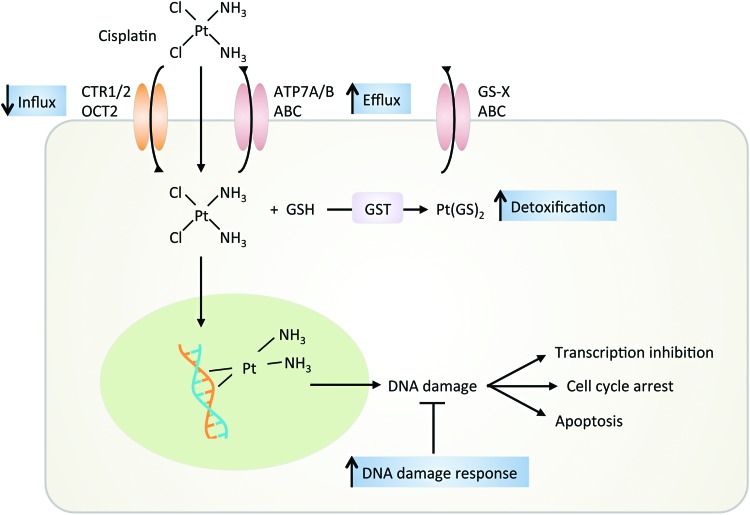
FIG. 8.
Mechanisms for cisplatin therapy. Cisplatin enters the cell through passive diffusion or active transport via CTR1/2 or OCT2. Binding of cisplatin to DNA results in platinum adducts, causing kinking of the DNA and inhibiting DNA transcription and cell proliferation until DNA damage is repaired or the cell dies. Resistance to cisplatin treatment can be due to (i) decreased influx and increased efflux, (ii) increased DNA repair, and (iii) neutralization with protein thiols. ABC, ATP-binding cassette; ATP, adenosine triphosphate; CTR, copper transporter protein; GST, glutathione S-transferase; OCT, organic cation transporter. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
DNA damage alteration
The balance of DNA damage versus DNA repair determines cancer cell death versus survival after cisplatin therapy (202). DNA excision repair protein, excision repair cross-complementation group 1 (ERCC1), complexed with xeroderma pigmentosum complementation group F (XPF), catalyzes the incision of the damaged DNA strand, the rate limiting step in the nucleotide excision repair pathway. High ERCC1 and XPF expression is indicative of increased removal of DNA platinum adducts and has a linear relationship with resistance to cisplatin in many cancers, including HNSCCs (322). Polymorphisms have been identified in ERCC1 and XPF but they have not correlated with increased sensitivity or resistance.
Platinum influx and efflux
Moderate platinum resistance is mediated by decreased uptake or increased export, thereby reducing platinum concentration (161). Cisplatin can passively diffuse the cell membrane or is transported by copper transporter proteins 1/2 (CTR1/2) and the organic cation transporter 2 (OCT2). Cisplatin-resistant cells show decreased levels of CTR1 messenger RNA (mRNA) and reduced levels of copper, but increased levels of CTR2, suggesting that CTR1 to CTR2 ratio may be a useful biomarker for determining cisplatin response (155). Cisplatin is exported out of the cell by P-type ATPase transporters (e.g., ATP7A and ATP7B) or by ATP-binding cassette (ABC) transporters. These transporters are trafficked to the cell membrane to remove excessive copper (3). As expected, increased ATP7A and ATP7B levels are associated with poor response to cisplatin (178).
Platinum conjugation
Cisplatin resistance can also be due to conjugation with protein thiols (e.g., metallothionein and GSTs such as GST-pi and GST-Mμ), leading to increased solubility, increased cellular export, and less DNA damage. In HNSCCs, there is a correlation between high expression of GST-pi and cisplatin resistance (217).
Other mechanisms of chemoresistance
HIFs have been implicated in increased resistance to chemotherapeutics by increasing drug efflux and reducing DNA damage. HIF-2's ability to inhibit apoptotic pathways and activate antiapoptotic signaling pathways results in enhanced cyclin D2 expression, causing improved growth and resistance to DNA damage (122, 123). Multidrug resistance 1 (ABCB1) is an ABC (ATP-binding cassette) transporter protein, which can traffic substances across cellular membranes and cause efflux of many xenobiotics (214). It was also shown in colon and gastric cancer that ABC transporter, ABCG2, mediates resistance to chemotherapies under hypoxic conditions (79). Acid ceramidase (AC) plays a role in ceramide metabolism by converting ceramide into sphingosine to prevent ceramide-induced apoptosis. AC is overexpressed in 70% of HNSCC malignant tissue and causes resistance to chemotherapeutic agents. An in vitro study demonstrated that AC silencing regulates expression of WT or mutant p53 by post-transcriptional processing and caspase-dependent mitochondrial apoptosis, increasing response to chemotherapy (210).
Resistance to EGFR-targeted therapies
Monoclonal antibodies and TKIs target EGFR at the ligand binding or the catalytic domain, respectively, causing their efficacy and cytotoxicity profiles to be different. Cetuximab resistance is associated with dysregulation of the internalization and degradation processing of EGFR. Forty-two percent of HNSCCs express an in-frame deletion in the ligand-binding portion of the receptor, resulting in a truncated EGFR-variant 3 (EGFRvIII) to be expressed (276). EGFRvIII is constitutively phosphorylated, independent of ligand binding causing resistance to monoclonal antibodies, which target the ligand binding domain (276) (Fig. 2). STAT3 is also expressed at higher levels in HNSCCs with EGFRvIII. Cetuximab lacks the efficacy to abrogate EGFRvIII constitutive activation and STAT3 activity. In contrast, resistance to TKIs is mostly attributed to (i) activation of redundant kinase signaling pathways such as tyrosine-protein kinase Met (c-MET) and other EGFR family members and (ii) EGFR-independent activation of downstream signaling pathways such as phosphatidylinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway (74, 182, 237). For example, recent studies have suggested that overexpression of other members of the EGFR family, such as human epidermal growth factor receptor 3 (HER3), is involved in resistance to EGFR TKIs (25). HER3 lacks tyrosine kinase activity but can be phosphorylated by c-MET or other receptor tyrosine kinases (75). c-MET is commonly overexpressed, mutated, or has increased number of gene copies in 60% of HNSCCs (182). Mesenchymal-epithelial transition (MET) amplification drives HER3-dependent activation of PI3K/Akt signaling bypassing the EGFR inhibition by TKI (263, 277). Similarly, inhibitory PTEN mutations seen in many cancers including HNSCC (263, 277) or persistent oxidation result in activation of Akt pathway and downstream effectors (237).
Survival and QOL: Improving HNSCC Patients' QOL with Redox Modifiers
Owing to the location of the tumor, HNSCC patients often suffer from impairments in swallowing, breathing, and speaking as side effects of RT or CRT, greatly affecting their QOL. Preventing and controlling these complications can not only improve the QOL of HNSCC patients but also allow them to continue or receive higher doses of treatment, achieving overall better cancer management. There are many aspects of preventing and treating oral complications associated with HNSCC treatment, including pain control, nutritional support, and oral hygiene, just to name a few. Here we highlight the use of ROS modifiers in managing oral mucositis and xerostomia.
Oral mucositis is a common side effect observed in HNSCC patients receiving RT or CRT. The pathogenesis of oral mucositis was defined to have five biological stages: (i) initiation, (ii) primary damage response, (iii) signal amplification, (iv) ulceration, and (v) healing (279). ROS, as a result of the chemotherapy and/or RT, is involved in the first two stages by causing direct mucosal tissue damage and activating NF-κB and ceramide pathways that further result in apoptosis and tissue injury. Management of oral mucositis has been largely palliative, although targeted therapeutic interventions exploiting the dependency of oral mucositis on ROS generation are emerging. Amifostine, originally developed by U.S. military to protect soldiers from radiation exposure, is thought to act as a thiol-based free radical scavenger to protect HNSCC patients against radiation- and chemoradiation-induced toxicity. However, due to conflicting results on its ability to reduce oral mucositis, insufficient evidence of benefit, and strong toxic side effects, a guideline regarding the use of this agent in oral mucositis in chemotherapy or RT patients could not be established (216, 241). Currently, FDA only approves amifostine for prevention of xerostomia in HNSCC radiotherapy and for prevention of nephrotoxicity in cisplatin-based chemotherapy in ovarian cancer.
Another group of ROS inhibitors with potential therapeutic benefit in treating oral mucositis involves SOD activity. Small-molecule SOD mimetic M40403 has shown efficacy in attenuating radiation-induced oral mucositis in a hamster model (212). Its enantiomer, GC4419, has completed a Phase 1b/2a clinical trial with positive results of reducing the incidence, severity, and duration of oral mucositis in HNSCC patients receiving CRT (5), and is currently being evaluated in a randomized Phase 2 clinical trial to assess its effect on radiation-induced oral mucositis in patients with HNSCC (NCT02508389). In addition, a novel manganese porphyrin SOD mimetic (MnBuOE-2-PyP5+, BMX-001) has shown radioprotective properties on normal tissue by reducing radiation-induced mucositis, xerostomia, and fibrosis, while acting as a radiosensitizer in a mouse model of HNSCCs (8, 35). Given its differential effect on normal tissue versus tumor (16, 313, 314), BMX-001 is currently under a Phase 1a/b clinical trial to test its ability to reduce radiation-induced mucositis and xerostomia in HNSCC patients receiving RT (NCT02990468). Along the same lines, overexpression of human SOD2 in the oral cavity mucosa by plasmid/liposome delivery prevented radiation-induced mucositis in a mouse HNSCC model (127). For details regarding different physical properties, distribution, and chemistry of these SOD mimetics, readers are referred to Batinic-Haberle et al.'s review (this Forum).
Finally, RK-0202, a proprietary matrix for topical application in the oral cavity, consists of an antioxidant N-acetylcysteine (NAC). In a Phase 2 clinical trial, RK-0202 significantly reduced the incidence of severe oral mucositis in HNSCC patients treated with radiotherapy (55). NAC also showed efficacy in reducing the incidence and total duration of oral mucositis in leukemia patients undergoing allogeneic hematopoietic stem cell transplant and high-dose chemotherapy (211). Other ROS inhibitors with promising antioral mucositis effects include benzydamine (99, 157, 264), vitamin E (97, 308), and allopurinol (321).
Xerostomia or dry mouth is a common side effect associated with RT or CRT. It is a consequence of the hypofunctioning salivary gland, which is damaged by these therapies. Standard treatment options for xerostomia include saliva substitutes and stimulating agents, such as pilocarpine hydrochloride, a cholinergic agonist. Recent treatment has included novel antioxidants, such as lecithinized SOD and Tempol, and has shown positive results in mouse models (76, 287). Interestingly, acupuncture has also been reported to alleviate xerostomia (117, 269). The underlying mechanism is unknown but believed to be mediated by decreasing ROS levels (265).
Bioinformatics and Computational Systems Biology Approaches to Integrate Redox Effects in HNSCC Management
The diverse tissue sources (e.g., laryngeal, oropharyngeal, and salivary gland) implicated in HNSCCs create challenges in the development of systems biology approaches for studying etiology of HNSCCs and prediction of effective treatment strategies. Compounding the tissue diversity is the distribution of genetic drivers in HNSCCs (45, 282, 309). Compared with other cancers, for example, genomic analysis of allelic mutations in HNSCCs indicates lesions across numerous genes rather than a small set of dysregulated drivers of carcinogenesis (282). Targeted deep sequencing of 51 oncogenes highlighted the diversity of mutations in HNSCCs that are related to the underlying patient population (tobacco users and HPV+) (118). Although the occurrence of shared gene mutations across samples analyzed by whole-exome sequencing was low, recurrent mutations of TP53, PI3KCA, PTEN, CASP8, and HRAS were enriched across the sample set (282). Strikingly, these proteins have been implicated in redox-regulated processes either as direct protein thiol targets or as accessories to redox enzyme regulation in other cell types and biological contexts (4, 73, 134, 256). The motivation for using computational models for precision medicine approaches for targeting of redox-dependent signaling pathways is further supported by the two examples given hereunder.
EGFR and ROS formation
Systems biology modeling of the EGFR receptor network has resulted in refined understanding of regulation and optimal drug targets in cancers in general and specific to breast cancer and nonsmall cell lung cancer (NSCLC) (37, 58, 113, 253, 254); however, these types of models have not been applied extensively to HNSCCs. Regulatory features gleaned from these models can potentially be generalized to HNSCCs. For example, a large-scale kinetic model of EGFR signaling identified HER3 (ErbB/EGFR3) as the optimal inhibitory target for monoclonal antibody modulation of PI3K signaling (254). In a computational and experimental analysis of HER2 overexpression in breast cancers, extracellular signal-regulated kinase (ERK) activity was more robust against variability in parameter values than Akt, which may reflect drug targeting of Akt to be more advantageous in heterogeneous tumors or across a broad patient population. Zhou et al. built upon these findings to include features of EGFR-induced NOX activation to examine regulatory features of ROS in oncogene addiction in NSCLC cells (329). This model predicted that crosstalk between Akt and ROS-activated ASK1 suppresses proapoptotic mechanisms during sustained EGFR signaling. Disruption of this crosstalk by the EGFR TKI gefitinib resulted in enhanced apoptosis that could be modulated by vitamin C (ascorbate); however, the matching of simulations to experiments was limited due to the lack of detail of additional redox-regulated mechanisms that would be impacted by the NOX activation. Interestingly, a large prospective study found an inverse correlation between the intake of vitamin C and the incidence of HNSCCs (84). A body of experimental evidence implicates EGFR-induced NOX activation in the thiol oxidation of multiple proteins within this receptor tyrosine kinase network, including EGFR itself, PTEN, protein-tyrosine phosphatase 1B (PTP1B), and tyrosine-protein phosphatase nonreceptor type 11 (SHP2) (203, 229, 296). Furthermore, proinflammatory cytokines such as IL-6 are reported to be modulated in a NOX4-dependent manner in HNSCC cells, and incorporation of tumor cell migration and invasion as additional phenotypic endpoints beyond cytoxicity in network simulations would provide insight into the role of ROS generated by NOX during different therapies (110). Despite these limitations, the authors propose from their systems-level analysis that oncogene-addicted cancers may require combinatorial treatment to target survival signals and enhance proapoptotic mechanisms, such as elevation of ROS. The applicability of these systems level features of cancer regulation to HNSCCs is unknown. For instance, isolation of the ERK or Akt module was reported to exhibit switch-like behavior that was very different from the behavior of the intact system, suggesting that parameter sensitivity and ligand thresholds are very context-specific (58). Thus, the nuances in expression levels of this complex network and the multiple nodes of cross-regulation via ROS may render cetuximab or erlotinib dose responses to regulate EGF signaling very differently from all of the other cancer modeling studies to date.
Transcriptomic analysis of 60 HNSCC patient samples identified 4 distinct subtypes of patients that were loosely categorized as EGFR-related, mesenchymal-enriched, normal epithelium-like, and high antioxidant defense (67). The latter subset of patients exhibited a gene signature of upregulated xenobiotic, pentose phosphate, and redox enzymes such as GPX2 and TR1. This profile corresponds to other transcriptional analyses of epithelium from cigarette smokers and clustered the furthest from the EGFR-related subset. Targeting EGF signaling may be suboptimal for HNSCC patients based upon history of tobacco use.
STAT3 as a redox sensor
A recent stratification of HNSCC patient outcomes using big-data approaches leveraged somatic mutation information with prior knowledge derived from protein–protein and immune signaling databases (179). This methodology resulted in three classes of patients that mapped into different Cox survival profiles, yet distributed smoking status, HPV status, and other clinical metrics similarly. Instead, the algorithm identified different protein subnetworks associated with mutations within the patient groups, with STAT3 being a key “hidden” linker between the network that associated with best prognosis. STAT3 activation and upregulation are associated with loss of growth control in early-stage HNSCC (125), and blockade of STAT3 signaling has demonstrated antitumor efficacy in preclinical models of HNSCCs (1, 30, 260). Notably, STAT3 has been identified as the first mammalian signaling protein capable of serving as a redox relay with PRX2, resulting in an attenuation of Janus kinase/STAT signaling due to either endogenous or exogenous H2O2 levels (275). Thus, transcriptional activity of STAT3 is dependent on the PRX/TRX system and indirectly coupled to NADPH for reducing equivalents. To date, no computational modeling has been done to explore the role of STAT3 in translating elevated tumor levels of ROS into transcriptional changes; implementing these effects in pre-existing three-dimensional computational models of tumor growth and hypoxia (189) would be insightful in the spatial contributions to chemotherapeutic resistance.
Models of redox-based drug mechanisms of action for HNSCC therapies
To date, very little computational modeling has been performed that incorporates redox-based mechanisms of action for the common chemotherapeutics used clinically for HNSCCs (e.g., cisplatin, 5-FU, and methotrexate). One study was able to determine a 10-gene signature to predict response of HNSCC patients to 5-FU induction chemotherapy by analyzing HNSCC biopsies with whole-genome microarrays and quantitative reverse transcriptase PCR (295). Some of the candidate genes identified play a role in response to redox stress such as heat shock protein 40 and TRX domain containing 9 (295). Another notable example is a computational network model, consisting of 44 ordinary differential equations with mass action kinetics, which incorporated cisplatin effects on apoptotic signaling pathways, DNA damage, and oxidative stress (142). Time and concentration-dependent effects of cisplatin are accounted for through explicit description of OCT2-mediated cellular uptake. The model provided insight into the three-way crosstalk between death receptor, mitochondrial, and ER stress contributions to apoptosis in a generic cell. The lack of literature-derived parameter values for a mechanistic model such as this precludes the use of this approach in HNSCCs unless concerted effort to quantify rates of caspase activation, Bcl-2 homologous antagonist/killer (BAK)/BAX binding, etc. is undertaken in a consistent experimental setting. Other examples of ROS production induced by chemotherapeutics, such as doxorubicin (108, 109), highlight the use of in vitro measurements for establishing cancer-specific parameters.
Models of HPV infection and progression
A spatial model of HPV infection and progression within the stratified squamous epithelium was developed to consider the role of stochastic division rates of stem-like progenitor cells in viral persistence/clearance within infected tissue (248). Although this model primarily focuses on the balance between immune responses and cellular dynamics for viral clearance, the framework provides an opportunity to couple progression from HPV infection to sustained neoplastic growth and account for viral-induced cellular oxidation (57, 200), which is spatially defined within a tumor mass. Model-based simulations that account for recent immunotherapeutic advances (280, 299) and susceptibility to redox-modulating therapeutics in HPV+ versus HPV− cancers could also feasibly incorporate T cell–HNSCC interactions, as recently developed in a computational analysis for HIV–HPV coinfection (305).
Bioinformatics analysis of the Cancer Genome Atlas and the Cancer Proteome Atlas HNSCC data
A growing number of studies are using data archived through NIH programs such as the Cancer Genome Atlas (TCGA) and the Cancer Proteome Atlas (TCPA). For example, a novel cascade propagation (CasP) subtyping approach, using TCGA data, was developed to investigate codependent immune signaling pathways in HNSCCs by using dynamic network modeling followed by stratifying somatic mutations. Using CasP subtyping, HNSCC patients were stratified into two distinct groups, including (i) patients with mutations in TLR signaling who have better OS and (ii) patients with mutations in T and B cell receptor signaling who have poorer survival (179). Also, another study identified genomic and/or epigenomic changes that lead to STAT3 activation in HNSCCs using data generated by the TCGA and the TCPA programs (233). The authors identified that STAT3 expression is associated with disease stage, nodal status, tumor size, PFS, and OS in early stage oral HNSCCs. These types of modeling techniques offer opportunities to better understand molecular signaling and identification of biomarkers as well as opportunities for therapeutic escalation/deescalation.
Concluding Remarks
The research reviewed here provides clear evidence of the critical role played by altered redox homeostasis at all stages of HNSCC from development and early detection to treatment and improvement of patients' QOL. There is an established connection between etiologic factors, ROS, and inflammation as driving mechanisms of HNSCCs. There remain, however, certain aspects of HNSCC etiology that are just starting to be investigated and are critical for disease prevention and early detection. These include (i) synergy of interaction between currently acknowledged drivers of HNSCCs (e.g., HPV and smoking), (ii) the possibility of other coinfections acting as cofactors with HPV [e.g., Chlamydia, another sexually transmitted infection with cofactor function in HPV+ cervical cancer (330) and with potential for upregulating host cell EGFR and ROS (228)], and (iii) the role of oral microbiota in HNSCC development. Composition of oral microbiota and associated biomarkers was shown relevant for HNSCC development and early detection (172) and for predicting severity of oral mucositis induced by RT (331). Detailed mechanisms of how bacterial metabolism interacts with current etiologic factors and standard of care HNSCC therapies require further investigation. Redox mechanisms remain relevant in this context as oral microbiota plays a key function in nitrate metabolism (e.g., high in cruciferous vegetables like broccoli), which ultimately contributes to blood nitric oxide levels and controls cancer-relevant processes such as neovascularization (150).
Accumulation of ROS as a mechanism to kill cancer was applied successfully over decades through the use of RT. Toxicities associated with treatment and damage to normal tissue have been, however, concerns from the very beginning. Earlier methods at the turn of the 20th century employed glass tubes placed in vicinity of the tumors to deliver radiation in a method known as interstitial brachytherapy (72). Current technologies such as hybrid imaging and image-guided treatment devices allow for better targeting of radiation to the tumor, largely avoiding damage of normal healthy tissue. Nevertheless, metabolic and signaling changes such as those enabling suppression of radiation-induced ROS and DNA damage impair efficacy of RT and CRT. As NADH and NADPH species are the ultimate donors of reducing equivalents fueling ROS degradation and energy metabolism, it is critical to learn how to manipulate these species with spatial and temporal resolution within tumors to increase sensitivity to treatment (Fig. 9). Although some tools to selectively detect and manipulate NADH, NADPH (31–33, 291, 328), and specific ROS species are currently available (in some cases with subcellular resolution) [e.g., Kelso et al. (158) and Robb et al. (244)], more are needed to enable basic and translational studies on this subject. NQO1 bioactivatable drugs are examples of how NAD(P)H-consuming and O2•−/H2O2-producing reactions can be manipulated to achieve tumor kill alone or in combination with RT. In particular, NQO1 substrates capable of futile cycling, like the β-lapachone described here, are exciting new leads for further development into therapeutics.
FIG. 9.
Key concepts connecting patient metabolic state and tumor redox state to tumor progression and response to treatment. Patients' diet and lifestyle underlie many aspects of HNSCC etiology by affecting energy metabolism and, therefore, genomic stability. At the same time, patient metabolic state affects tumor redox state, and together they drive tumor progression and response to treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Targeted therapies, such as EGFR kinase inhibitors and others, continue to remain of interest for treatment of HNSCCs. It is unfortunate to date that the impact of tumor redox microenvironment on the efficacy of response to small-molecule inhibitors is not being routinely considered. Similarly, more consideration needs to be given to physiologic redox modifiers such as aging, diet, and exercise on cancer development and response to treatment. Although a significant body of literature exists on the role of these factors in cancer development and incidence (9), less is known with regard to their impact on regulation of response to RT, CRT, targeted drugs or immunotherapies (220, 326), and QOL post-treatment (201).
There are currently two SOD mimetics under clinical trials as radioprotective agents in HNSCC treatment (GC4419 and BMX-001). This is the first time metal complexes are in clinical trials and target cellular redox environment. Although these drugs are indeed redox active, it is also likely that they act through functions other than SOD mimicking, such as inhibiting NF-κB and activating NRF2, which has been demonstrated for BMX-001 (16). For such functions and mitochondrial localization of BMX-001, readers are referred to Carroll and St Clair's review in this Forum.
In summary, there is strong evidence connecting redox metabolism with all stages of HNSCC management. The location of HNSCCs brings new opportunities for exploiting critical redox modifiers generated through activation of biologically active dietary molecules by oral microbiota to prevent or decrease HNSCC progression, improve response to therapies, and patients' QOL.
Abbreviations Used
- 5-FU
5-fluorouracil
- ABC
ATP-binding cassette
- AC
acid ceramidase
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- AP
apurinic/apyrimidinic
- ASK1
apoptosis signal-regulated kinase 1
- ATG
autophagy-related
- ATP
adenosine triphosphate
- BAX
Bcl-2-associated X protein
- BER
base excision repair
- CasP
cascade propagation
- CAT
catalase
- CD
cluster of differentiation
- c-MET
tyrosine-protein kinase Met
- COX
cytochrome c oxidase
- CRT
chemoradiation therapy
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CTR
copper transporter protein
- CYP
cytochrome P450
- DNQ
deoxynyboquinone
- DSB
double-strand break
- EGFR
epidermal growth factor receptor
- EGFRvIII
EGFR-variant 3
- ER
endoplasmic reticulum
- ERCC-1
excision repair cross-complementation group 1
- ERK
extracellular signal-regulated kinase
- FDA
Food and Drug Administration
- G6PD
glucose-6-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GPX
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- HER
human epidermal growth factor receptor
- HIF
hypoxia-inducible factor
- HNSCC
head and neck squamous cell cancer
- HPV
human papillomavirus
- IB-DNQ
isobutyl-deoxynyboquinone
- IL
interleukin
- IR
ionizing radiation
- KEAP1
Kelch-like ECH-associated protein 1
- MET
mesenchymal-epithelial transition
- mTOR
mechanistic target of rapamycin
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOX
NADPH oxidase
- NQO1
quinone oxidoreductase 1
- NRF2
nuclear factor-erythroid 2-related factor 2
- NSCLC
nonsmall cell lung cancer
- OCT
organic cation transporter
- OS
overall survival
- PAR
poly ADP ribose
- PARP
poly ADP ribose polymerase
- PCR
polymerase chain reaction
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PET
positron emission tomography
- PFS
progression-free survival
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1α
- PI3K
phosphatidylinositide 3-kinase
- pO2
oxygen partial pressure
- PPP
pentose phosphate pathway
- PRX
peroxiredoxin
- PTEN
phosphatase and tensin homologue
- QOL
quality of life
- ROS
reactive oxygen species
- RT
radiation therapy
- SAM
S-adenosylmethionine
- SOD
superoxide dismutase
- SSB
single-strand break
- STAT
signal transducer and activator of transcription factor
- TCGA
The Cancer Genome Atlas
- TCPA
The Cancer Proteome Atlas
- TKI
tyrosine kinase inhibitor
- TLR
toll-like receptor
- TR
thioredoxin reductase
- TRX
thioredoxin
- VEGF
vascular endothelial growth factor
- WT
wild-type
- XPF
xeroderma pigmentosum complementation group F
Acknowledgments
The authors would like to acknowledge the following funding support: NIH/NHLBI Training Grant T32 HL091797 (J.M.), NIH/NCI R33 CA1777461 (C.M.F.), and U01 CA215848 (M.L.K., C.M.F., and D.A.B.).
References
- 1.Adachi M, Cui C, Dodge CT, Bhayani MK, and Lai SY. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral Oncol 48: 1220–1226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albano E, Tomasi A, Persson J-O, Terelius Y, Goria-Gatti L, Ingelman-Sundberg M, and Dianzani MU. Role of ethanol-inducible cytochrome P450 (P450IIE1) in catalysing the free radical activation of aliphatic alcohols. Biochem Pharmacol 41: 1895–1902, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 106: 27–36, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, and Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol 184: 241–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CM, Allen BG, Sun W, Agarwala SS, Lee CM, Venigalla ML, Greenberg L, Adkins D, Chen Y, Zhen W, Mould DR, Holmlund J, Brill J, Sonis ST, and Buatti J. Phase 1b trial of superoxide (SO) dismutase (SOD) mimetic GC4419 to reduce chemoradiation therapy (CRT)-induced oral mucositis (OM) in patients (pts) with oral cavity or oropharyngeal carcinoma (OCC). Int J Radiat Oncol Biol Phys 96: S83–S84, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, and Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, and Axelrod RS. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 32: 2940–2950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashcraft KA, Boss MK, Tovmasyan A, Roy Choudhury K, Fontanella AN, Young KH, Palmer GM, Birer SR, Landon CD, Park W, Das SK, Weitner T, Sheng H, Warner DS, Brizel DM, Spasojevic I, Batinic-Haberle I, and Dewhirst MW. Novel manganese-porphyrin superoxide dismutase-mimetic widens the therapeutic margin in a preclinical head and neck cancer model. Int J Radiat Oncol Biol Phys 93: 892–900, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, and Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 76: 4032–4050, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad MB, Chen Y, and Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11: 777–790, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Azzam EI, Jay-Gerin J-P, and Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327: 48–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae SH, Woo HA, Sung SH, Lee HE, Lee SK, Kil IS, and Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal 11: 937–948, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Baker A, Payne CM, Briehl MM, and Powis G. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo. Cancer Res 57: 5162, 1997 [PubMed] [Google Scholar]
- 14.Bansal N, Mims J, Kuremsky JG, Olex AL, Zhao W, Yin L, Wani R, Qian J, Center B, Marrs GS, Porosnicu M, Fetrow JS, Tsang AW, and Furdui CM. Broad phenotypic changes associated with gain of radiation resistance in head and neck squamous cell cancer. Antioxid Redox Signal 21: 221–236, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-Ad V, Palmer J, Yang H, Cognetti D, Curry J, Luginbuhl A, Tuluc M, Campling B, and Axelrod R. Current management of locally advanced head and neck cancer: the combination of chemotherapy with locoregional treatments. Semin Oncol 41: 798–806, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, and Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal 20: 2372–2415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayer C, Shi K, Astner ST, Maftei CA, and Vaupel P. Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys 80: 965–968, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Beckman G, Birgander R, Sjalander A, Saha N, Holmberg PA, Kivela A, and Beckman L. Is p53 polymorphism maintained by natural selection? Hum Hered 44: 266–270, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Bei R, Budillon A, Masuelli L, Cereda V, Vitolo D, Di Gennaro E, Ripavecchia V, Palumbo C, Ionna F, Losito S, Modesti A, Kraus MH, and Muraro R. Frequent overexpression of multiple ErbB receptors by head and neck squamous cell carcinoma contrasts with rare antibody immunity in patients. J Pathol 204: 317–325, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Belikov AV, Schraven B, and Simeoni L. T cells and reactive oxygen species. J Biomed Sci 22: 85, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhar M, Shytaj IL, Stamler JS, and Savarino A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J Clin Invest 126: 1630–1639, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett CB, Lewis AL, Baldwin KK, and Resnick MA. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci U S A 90: 5613–5617, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentle MS, Reinicke KE, Bey EA, Spitz DR, and Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem 281: 33684–33696, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Bentle MS, Reinicke KE, Dong Y, Bey EA, and Boothman DA. Nonhomologous end joining is essential for cellular resistance to the novel antitumor agent, beta-lapachone. Cancer Res 67: 6936–6945, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Berghoff AS, Bartsch R, Preusser M, Ricken G, Steger GG, Bago-Horvath Z, Rudas M, Streubel B, Dubsky P, Gnant M, Fitzal F, Zielinski CC, and Birner P. Co-overexpression of HER2/HER3 is a predictor of impaired survival in breast cancer patients. Breast 23: 637–643, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Bernardini JP, Lazarou M, and Dewson G. Parkin and mitophagy in cancer. Oncogene 36: 1315–1327, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre J-L, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, and van Glabbeke M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350: 1945–1952, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J, and Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A 104: 11832–11837, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bey EA, Reinicke KE, Srougi MC, Varnes M, Anderson VE, Pink JJ, Li LS, Patel M, Cao L, Moore Z, Rommel A, Boatman M, Lewis C, Euhus DM, Bornmann WG, Buchsbaum DJ, Spitz DR, Gao J, and Boothman DA. Catalase abrogates beta-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Mol Cancer Ther 12: 2110–2120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bharadwaj U, Eckols TK, Xu X, Kasembeli MM, Chen Y, Adachi M, Song Y, Mo Q, Lai SY, and Tweardy DJ. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget 7: 26307–26330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilan DS. and Belousov VV. Genetically encoded probes for NAD+/NADH monitoring. Free Radic Biol Med 100: 32–42, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Bilan DS. and Belousov VV. HyPer family probes: state of the art. Antioxid Redox Signal 24: 731–751, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Bilan DS, Matlashov ME, Gorokhovatsky AY, Schultz C, Enikolopov G, and Belousov VV. Genetically encoded fluorescent indicator for imaging NAD(+)/NADH ratio changes in different cellular compartments. Biochim Biophys Acta 1840: 951–957, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bin-Umer MA, McLaughlin JE, Butterly MS, McCormick S, and Tumer NE. Elimination of damaged mitochondria through mitophagy reduces mitochondrial oxidative stress and increases tolerance to trichothecenes. Proc Natl Acad Sci U S A 111: 11798–11803, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birer SR, Lee CT, Choudhury KR, Young KH, Spasojevic I, Batinic-Haberle I, Crapo JD, Dewhirst MW, and Ashcraft KA. Inhibition of the continuum of radiation-induced normal tissue injury by a redox-active Mn porphyrin. Radiat Res 188: 94–104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birgander R SA, Zhou Z, Fan C, Beckman L, and Beckman G. p53 polymorphisms and haplotypes in nasopharyngeal cancer. Hum Hered 46: 49–54, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Birtwistle MR, Hatakeyama M, Yumoto N, Ogunnaike BA, Hoek JB, and Kholodenko BN. Ligand-dependent responses of the ErbB signaling network: experimental and modeling analyses. Mol Syst Biol 3: 144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, and Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Boothman DA, Greer S, and Pardee AB. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione), a novel DNA repair inhibitor. Cancer Res 47: 5361–5366, 1987 [PubMed] [Google Scholar]
- 40.Boothman DA, Trask DK, and Pardee AB. Inhibition of potentially lethal DNA damage repair in human tumor cells by beta-lapachone, an activator of topoisomerase I. Cancer Res 49: 605–612, 1989 [PubMed] [Google Scholar]
- 41.Boyer MJ, Rivory LP, and Clarke SJ. Pemetrexed: single-agent and combination phase I study overview. Semin Oncol 29: 18–23, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, and Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38: 285–289, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Brooks PJ. and Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol 35: 187–193, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Buettner GR, Ng CF, Wang M, Rodgers VG, and Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med 41: 1338–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517: 576–582, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J, Zhou J, Gao Y, Gu L, Meng H, Liu H, and Deng D. Methylation of p16 CpG island associated with malignant progression of oral epithelial dysplasia: a prospective cohort study. Clin Cancer Res 15: 5178–5183, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Cao L, Li LS, Spruell C, Xiao L, Chakrabarti G, Bey EA, Reinicke KE, Srougi MC, Moore Z, Dong Y, Vo P, Kabbani W, Yang CR, Wang X, Fattah F, Morales JC, Motea EA, Bornmann WG, Yordy JS, and Boothman DA. Tumor-selective, futile redox cycle-induced bystander effects elicited by NQO1 bioactivatable radiosensitizing drugs in triple-negative breast cancers. Antioxid Redox Signal 21: 237–250, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carroll V, Michel BW, Blecha J, VanBrocklin H, Keshari K, Wilson D, and Chang CJ. A boronate-caged [(1)(8)F]FLT probe for hydrogen peroxide detection using positron emission tomography. J Am Chem Soc 136: 14742–14745, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattani P, Hohaus S, Bellacosa A, Genuardi M, Cavallo S, Rovella V, Almadori G, Cadoni G, Galli J, Maurizi M, Fadda G, and Neri G. Association between cyclin D1 (CCND1) gene amplification and human papillomavirus infection in human laryngeal squamous cell carcinoma. Clin Cancer Res 4: 2585, 1998 [PubMed] [Google Scholar]
- 50.Ceccaldi R, Rondinelli B, and D'Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol 26: 52–64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai RC, Lambie D, Verma M, and Punyadeera C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med 4: 596–607, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakrabarti G, Gerber DE, and Boothman DA. Expanding antitumor therapeutic windows by targeting cancer-specific nicotinamide adenine dinucleotide phosphate-biogenesis pathways. Clin Pharmacol 7: 57–68, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakrabarti G, Moore ZR, Luo X, Ilcheva M, Ali A, Padanad M, Zhou Y, Xie Y, Burma S, Scaglioni PP, Cantley LC, DeBerardinis RJ, Kimmelman AC, Lyssiotis CA, and Boothman DA. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by β-lapachone. Cancer Metab 3: 12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakrabarti G, Silvers MA, Ilcheva M, Liu Y, Moore ZR, Luo X, Gao J, Anderson G, Liu L, Sarode V, Gerber DE, Burma S, DeBerardinis RJ, Gerson SL, and Boothman DA. Tumor-selective use of DNA base excision repair inhibition in pancreatic cancer using the NQO1 bioactivatable drug, beta-lapachone. Sci Rep 5: 17066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chambers MS, Welsh DV, Scrimger RA, Zehn W, Epstein JB, Troha J, and Sonis ST. RK-0202 for radiation-induced oral mucositis. J Clin Oncol 24: 285s, 2006 [Google Scholar]
- 56.Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, and Honjo T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci U S A 114: E761–E770, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chemes LB, Camporeale G, Sanchez IE, de Prat-Gay G, and Alonso LG. Cysteine-rich positions outside the structural zinc motif of human papillomavirus E7 provide conformational modulation and suggest functional redox roles. Biochemistry 53: 1680–1696, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Chen WW, Schoeberl B, Jasper PJ, Niepel M, Nielsen UB, Lauffenburger DA, and Sorger PK. Input-output behavior of ErbB signaling pathways as revealed by a mass action model trained against dynamic data. Mol Syst Biol 5: 239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Song M, Zhang B, and Zhang Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid Med Cell Longev 2016: 1580967, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, McMillan-Ward E, Kong J, Israels SJ, and Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15: 171–182, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Choi KS. Autophagy and cancer. Exp Mol Med 44: 109–120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chourasia AH, Boland ML, and Macleod KF. Mitophagy and cancer. Cancer Metab 3: 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chourasia AH. and Macleod KF. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy 11: 1937–1938, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chourasia AH, Tracy K, Frankenberger C, Boland ML, Sharifi MN, Drake LE, Sachleben JR, Asara JM, Locasale JW, Karczmar GS, and Macleod KF. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep 16: 1145–1163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chuang TC, Liu JY, Lin CT, Tang YT, Yeh MH, Chang SC, Li JW, and Kao MC. Human manganese superoxide dismutase suppresses HER2/neu-mediated breast cancer malignancy. FEBS Lett 581: 4443–4449, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Chung-man Ho J, Zheng S, Comhair SA, Farver C, and Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res 61: 8578–8585, 2001 [PubMed] [Google Scholar]
- 67.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X, Shockley WW, Weissler MC, Dressler LG, Shores CG, Yarbrough WG, and Perou CM. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5: 489–500, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Claiborne A, Miller H, Parsonage D, and Ross RP. Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. FASEB J 7: 1483–1490, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Cohen MH, Johnson JR, Wang YC, Sridhara R, and Pazdur R. FDA drug approval summary: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancer. Oncologist 10: 363–368, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, Willers H, Prasanna PG, Dewhirst MW, Bernhard EJ, and Ahmed MM. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res 22: 3138–3147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins-Underwood JR, Zhao W, Sharpe JG, and Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med 45: 929–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connell PP, Kron SJ, and Weichselbaum RR. Relevance and irrelevance of DNA damage response to radiotherapy. DNA Repair (Amst) 3: 1245–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, and Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem 280: 16916–16924, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Cooper JB. and Cohen EEW. Mechanisms of resistance to EGFR inhibitors in head and neck cancer. Head Neck 31: 1086–1094, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Cortot AB, Repellin CE, Shimamura T, Capelletti M, Zejnullahu K, Ercan D, Christensen JG, Wong K-K, Gray NS, and Jänne PA. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res 73: 834, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cotrim AP, Sowers AL, Lodde BM, Vitolo JM, Kingman A, Russo A, Mitchell JB, and Baum BJ. Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res 11: 7564–7568, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Cui X. Reactive oxygen species: the achilles' heel of cancer cells? Antioxid Redox Signal 16: 1212–1214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, and Oberley LW. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res 63: 1297–1303, 2003 [PubMed] [Google Scholar]
- 79.Cuvillier O, Ader I, Bouquerel P, Brizuela L, Gstalder C, and Malavaud B. Hypoxia, therapeutic resistance, and sphingosine 1-phosphate. Adv Cancer Res 117: 117–141, 2013 [DOI] [PubMed] [Google Scholar]
- 80.D'Souza G. and Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med 53 Suppl 1: S5–S11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalby KN, Tekedereli I, Lopez-Berestein G, and Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 6: 322–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Damia G. and Garattini S. The pharmacological point of view of resistance to therapy in tumors. Cancer Treat Rev 40: 909–916, 2014 [DOI] [PubMed] [Google Scholar]
- 83.De Marco F. Oxidative stress and HPV carcinogenesis. Viruses 5: 708–731, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Munter L, Maasland DH, van den Brandt PA, Kremer B, and Schouten LJ. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr 102: 420–432, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Derry MM, Raina K, Agarwal C, and Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Front Oncol 3: 119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devarie-Baez NO, Silva Lopez EI, and Furdui CM. Biological chemistry and functionality of protein sulfenic acids and related thiol modifications. Free Radic Res 50: 172–194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeVita VT, Lawrence TS, and Rosenberg SA. Cancer: Principles & Practice of Oncology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2011, xlvii, p. 2638 [Google Scholar]
- 88.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, and St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res 71: 6684–6695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dillon MT. and Harrington KJ. Human papillomavirus-negative pharyngeal cancer. J Clin Oncol 33: 3251–3261, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, and Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem 281: 30373–30382, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Döbróssy L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev 24: 9–17, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Dok R. and Nuyts S. HPV positive head and neck cancers: molecular pathogenesis and evolving treatment strategies. Cancers (Basel) 8: 41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong Y, Bey EA, Li LS, Kabbani W, Yan J, Xie XJ, Hsieh JT, Gao J, and Boothman DA. Prostate cancer radiosensitization through poly(ADP-ribose) polymerase-1 hyperactivation. Cancer Res 70: 8088–8096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du C, Gao Z, Venkatesha VA, Kalen AL, Chaudhuri L, Spitz DR, Cullen JJ, Oberley LW, and Goswami PC. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol Ther 8: 1962–1971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Economopoulou P, Perisanidis C, Giotakis EI, and Psyrri A. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): anti-tumor immunity and clinical applications. Ann Transl Med 4: 173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, and Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El-Housseiny AA, Saleh SM, El-Masry AA, and Allam AA. The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J Clin Pediatr Dent 31: 167–170, 2007 [PubMed] [Google Scholar]
- 98.Engelman JA, Zejnullahu K, Gale C-M, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong K-K, and Jänne PA. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67: 11924, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Epstein JB, Silverman S, Jr., Paggiarino DA, Crockett S, Schubert MM, Senzer NN, Lockhart PB, Gallagher MJ, Peterson DE, and Leveque FG. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 92: 875–885, 2001 [DOI] [PubMed] [Google Scholar]
- 100.Eyler CE. and Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 26: 2839–2845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, and Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261–269, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, and Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510: 298–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fath MA, Ahmad IM, Smith CJ, Spence J, and Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res 17: 6206–6217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fayette J, Wirth L, Oprean C, Udrea A, Jimeno A, Rischin D, Nutting C, Harari PM, Csoszi T, Cernea D, O'Brien P, Hanley WD, Kapp AV, Anderson M, Penuel E, McCall B, Pirzkall A, and Vermorken JB. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN study). Front Oncol 6: 232, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fei P, Wang W, Kim SH, Wang S, Burns TF, Sax JK, Buzzai M, Dicker DT, McKenna WG, Bernhard EJ, and El-Deiry WS. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6: 597–609, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, and Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375: 1856–1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferris RL, Whiteside TL, and Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 12: 3890, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Finn NA, Findley HW, and Kemp ML. A switching mechanism in doxorubicin bioactivation can be exploited to control doxorubicin toxicity. PLoS Comput Biol 7: e1002151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Finn NA. and Kemp ML. Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Mol Biosyst 8: 650–662, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fletcher EV, Love-Homan L, Sobhakumari A, Feddersen CR, Koch AT, Goel A, and Simons AL. EGFR inhibition induces proinflammatory cytokines via NOX4 in HNSCC. Mol Cancer Res 11: 1574–1584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flora SJMM. and Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128: 501–523, 2008 [PubMed] [Google Scholar]
- 112.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, and Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 1823: 2297–2310, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Fujita KA, Toyoshima Y, Uda S, Ozaki Y, Kubota H, and Kuroda S. Decoupling of receptor and downstream signals in the Akt pathway by its low-pass filter characteristics. Sci Signal 3: ra56, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara M, Marusawa H, Wang HQ, Iwai A, Ikeuchi K, Imai Y, Kataoka A, Nukina N, Takahashi R, and Chiba T. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene 27: 6002–6011, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Galbiatti AL, da Silva LM, Ruiz-Cintra MT, Raposo LS, Maniglia JV, Pavarino EC, and Goloni-Bertollo EM. Association between 11 genetic polymorphisms in folate-metabolising genes and head and neck cancer risk. Eur J Cancer 48: 1525–1531, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Galbiatti ALS, Padovani-Junior JA, Maníglia JV, Rodrigues CDS, Pavarino ÉC, and Goloni-Bertollo EM. Head and neck cancer: causes, prevention and treatment. Braz J Otorhinolaryngol 79: 239–247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia MK, Chiang JS, Cohen L, Liu M, Palmer JL, Rosenthal DI, Wei Q, Tung S, Wang C, Rahlfs T, and Chambers MS. Acupuncture for radiation-induced xerostomia in patients with cancer: a pilot study. Head Neck 31: 1360–1368, 2009 [DOI] [PubMed] [Google Scholar]
- 118.Gaykalova DA, Mambo E, Choudhary A, Houghton J, Buddavarapu K, Sanford T, Darden W, Adai A, Hadd A, Latham G, Danilova LV, Bishop J, Li RJ, Westra WH, Hennessey P, Koch WM, Ochs MF, Califano JA, and Sun W. Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLoS One 9: e93102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gewirtz DA. The autophagic response to radiation: relevance for radiation sensitization in cancer therapy. Radiat Res 182: 363–367, 2014 [DOI] [PubMed] [Google Scholar]
- 120.Goldkorn T. and Filosto S. Lung Injury and cancer: mechanistic insights into ceramide and EGFR signaling under cigarette smoke. Am J Respir Cell Mol Biol 43: 259–268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldkorn T, Filosto S, and Chung S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid Redox Signal 21: 2149–2174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gordan JD, Bertovrt JA, Hu C-J, Diehl JA, and Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell 11: 335–347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gordan JD, Thompson CB, and Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12: 108–113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gorrini C, Harris IS, and Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12: 931–947, 2013 [DOI] [PubMed] [Google Scholar]
- 125.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, and Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A 97: 4227–4232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guerra ENS, Rêgo DF, Elias ST, Coletta RD, Mezzomo LAM, Gozal D, and De Luca Canto G. Diagnostic accuracy of serum biomarkers for head and neck cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 101: 93–118, 2016 [DOI] [PubMed] [Google Scholar]
- 127.Guo H, Seixas-Silva JA, Jr., Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, Archer H, and Greenberger JS. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res 159: 361–370, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Guo J-H, Chen M-Q, Chen C, Lu H-J, and Xu B-H. Efficacy and toxicity of nimotuzumab combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Mol Clin Oncol 3: 1135–1138, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta NK, Pointon RC, and Wilkinson PM. A randomised clinical trial to contrast radiotherapy with radiotherapy and methotrexate given synchronously in head and neck cancer. Clin Radiol 38: 575–581, 1987 [DOI] [PubMed] [Google Scholar]
- 130.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, and Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hall GL, Shaw RJ, Field EA, Rogers SN, Sutton DN, Woolgar JA, Lowe D, Liloglou T, Field JK, and Risk JM. p16 promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev 17: 2174, 2008 [DOI] [PubMed] [Google Scholar]
- 132.Haque R, Contreras R, McNicoll MP, Eckberg EC, and Petitti DB. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord 6: 2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, Elia A, Berger T, Cescon DW, Adeoye A, Brustle A, Molyneux SD, Mason JM, Li WY, Yamamoto K, Wakeham A, Berman HK, Khokha R, Done SJ, Kavanagh TJ, Lam CW, and Mak TW. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27: 211–222, 2015 [DOI] [PubMed] [Google Scholar]
- 134.Heo J. and Campbell SL. Ras regulation by reactive oxygen and nitrogen species. Biochemistry 45: 2200–2210, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, Jimenez S, Schiller JT, Lowy DR, van Doorn L-J, Wacholder S, and Kreimer AR; CVT Vaccine Group. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 8: e68329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hitchler MJ. and Domann FE. Redox regulation of the epigenetic landscape in cancer: a role for metabolic reprogramming in remodeling the epigenome. Free Radic Biol Med 53: 2178–2187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holley AK, Dhar SK, and St Clair DK. Curbing cancer's sweet tooth: is there a role for MnSOD in regulation of the Warburg effect? Mitochondrion 13: 170–188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holley AK, Dhar SK, Xu Y, and St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids 42: 139–158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holley AK, Miao L, St Clair DK, and St Clair WH. Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxid Redox Signal 20: 1567–1589, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem 264: 13963–13966, 1989 [PubMed] [Google Scholar]
- 141.Holohan C, Van Schaeybroeck S, Longley DB, and Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13: 714–726, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Hong JY, Kim GH, Kim JW, Kwon SS, Sato EF, Cho KH, and Shim EB. Computational modeling of apoptotic signaling pathways induced by cisplatin. BMC Syst Biol 6: 122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hönscheid P, Datta K, and Muders MH. Autophagy: detection, regulation and its role in cancer and therapy response. Int J Radiat Biol 90: 628–635, 2014 [DOI] [PubMed] [Google Scholar]
- 144.Horsman MR. and Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res 57 Suppl 1: i90–i98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu H, Luo ML, Du XL, Feng YB, Zhang Y, Shen XM, Xu X, Cai Y, Han YL, and Wang MR. Up-regulated manganese superoxide dismutase expression increases apoptosis resistance in human esophageal squamous cell carcinomas. Chin Med J (Engl) 120: 2092–2098, 2007 [PubMed] [Google Scholar]
- 146.Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, and Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem 280: 39485–39492, 2005 [DOI] [PubMed] [Google Scholar]
- 147.Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS, Patel M, Parkinson EI, Wang Y, Williams NS, Gao J, Hergenrother PJ, and Boothman DA. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res 72: 3038–3047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang X, Motea EA, Moore ZR, Yao J, Dong Y, Chakrabarti G, Kilgore JA, Silvers MA, Patidar PL, Cholka A, Fattah F, Cha Y, Anderson GG, Kusko R, Peyton M, Yan J, Xie XJ, Sarode V, Williams NS, Minna JD, Beg M, Gerber DE, Bey EA, and Boothman DA. Leveraging an NQO1 bioactivatable drug for tumor-selective use of poly(ADP-ribose) polymerase inhibitors. Cancer Cell 30: 940–952, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huber PE, Bischof M, Jenne J, Heiland S, Peschke P, Saffrich R, Grone HJ, Debus J, Lipson KE, and Abdollahi A. Trimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapy. Cancer Res 65: 3643–3655, 2005 [DOI] [PubMed] [Google Scholar]
- 150.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, and Bryan NS. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One 9: e88645, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Israr M, Biryukov J, Ryndock EJ, Alam S, and Meyers C. Comparison of human papillomavirus type 16 replication in tonsil and foreskin epithelia. Virology 499: 82–90, 2016 [DOI] [PubMed] [Google Scholar]
- 152.Janssen AM, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, and Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res 6: 3183–3192, 2000 [PubMed] [Google Scholar]
- 153.Jemal A, Bray F, Center MM, Ferlay J, Ward E, and Forman D. Global cancer statistics. CA Cancer J Clin 61: 69–90, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Jones DP, Carlson JL, Mody VC, Jr., Cai J, Lynn MJ, and Sternberg P., Jr. Redox state of glutathione in human plasma. Free Radic Biol Med 28: 625–635, 2000 [DOI] [PubMed] [Google Scholar]
- 155.Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo Y-M, Rochdi M, and Howell SB. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res 62: 6559, 2002 [PubMed] [Google Scholar]
- 156.Katoh T KS, Takasawa S, Nagata N, Inatomi H, Ikemura K, Itoh H, Matsumoto T, Kawamoto T, and Bell DA. Human glutathione S-transferase P1 polymorphism and susceptibility to smoking related epithelial cancer; oral, lung, gastric, colorectal and urothelial cancer. Pharmacogenetics 9: 165–169, 1999 [PubMed] [Google Scholar]
- 157.Kazemian A, Kamian S, Aghili M, Hashemi FA, and Haddad P. Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: a double-blind placebo-controlled randomized clinical trial. Eur J Cancer Care (Engl) 18: 174–178, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, and Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 159.Kern JC. and Kehrer JP. Free radicals and apoptosis: relationships with glutathione, thioredoxin, and the BCL family of proteins. Front Biosci 10: 1727–1738, 2005 [DOI] [PubMed] [Google Scholar]
- 160.Kiebala M, Skalska J, Casulo C, Brookes PS, Peterson DR, Hilchey SP, Dai Y, Grant S, Maggirwar SB, and Bernstein SH. Dual targeting of the thioredoxin and glutathione antioxidant systems in malignant B cells: a novel synergistic therapeutic approach. Exp Hematol 43: 89–99, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim ES, Lee JJ, He G, Chow C-W, Fujimoto J, Kalhor N, Swisher SG, Wistuba II, Stewart DJ, and Siddik ZH. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol 30: 3345–3352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim H, Rigell CJ, Zhai G, Lee SK, Samuel SL, Martin A, Umphrey HR, Stockard CR, Beasley TM, Buchsbaum DJ, Li LS, Boothman DA, and Zinn KR. Antagonistic effects of anti-EMMPRIN antibody when combined with chemotherapy against hypovascular pancreatic cancers. Mol Imaging Biol 16: 85–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim J, Kundu M, Viollet B, and Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim J-W, Tchernyshyov I, Semenza GL, and Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 165.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, and Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223, 2010 [DOI] [PubMed] [Google Scholar]
- 166.Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E, and Harris AL. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol 24: 727–735, 2006 [DOI] [PubMed] [Google Scholar]
- 167.Kulikov AV, Luchkina EA, Gogvadze V, and Zhivotovsky B. Mitophagy: link to cancer development and therapy. Biochem Biophys Res Commun 482: 432–439, 2017 [DOI] [PubMed] [Google Scholar]
- 168.Kurihara Y, Kanki T, Aoki Y, Hirota Y, Saigusa T, Uchiumi T, and Kang D. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem 287: 3265–3272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Landi MT BP, Shields PG, Clark G, Lucier GW, Garte SJ, Cosma G, and Caporaso NE. Association between CYP1A1 genotype, mRNA expression and enzymatic activity in humans. Pharmacogenetics 4: 242–246, 1994 [DOI] [PubMed] [Google Scholar]
- 170.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, and Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27: 1992–1998, 2009 [DOI] [PubMed] [Google Scholar]
- 171.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, and Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 30: 3275–3285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G, Soueidan A, Jordana F, and de La Cochetiere MF. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can J Microbiol 63: 475–492, 2017 [DOI] [PubMed] [Google Scholar]
- 173.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, and Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A 106: 17910–17915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 174.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, and Downes C. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22: 5501–5510, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li L, Ishdorj G, and Gibson SB. Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic Biol Med 53: 1399–1410, 2012 [DOI] [PubMed] [Google Scholar]
- 176.Li LS, Bey EA, Dong Y, Meng J, Patra B, Yan J, Xie XJ, Brekken RA, Barnett CC, Bornmann WG, Gao J, and Boothman DA. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of beta-lapachone for pancreatic cancer therapy. Clin Cancer Res 17: 275–285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li LS, Reddy S, Lin ZH, Liu S, Park H, Chun SG, Bornmann WG, Thibodeaux J, Yan J, Chakrabarti G, Xie XJ, Sumer BD, Boothman DA, and Yordy JS. NQO1-mediated tumor-selective lethality and radiosensitization for head and neck cancer. Mol Cancer Ther 15: 1757–1767, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Z-H, Qiu M-Z, Zeng Z-L Luo H-Y, Wu W-J, Wang F, Wang Z-Q, Zhang D-S, Li Y-H, and Xu R-H. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC). J Transl Med 10: 21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu K, Chyr J, Zhao W, and Zhou X. Immune signaling-based cascade propagation approach re-stratifies HNSCC patients. Methods 111: 72–79, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Y, Tergaonkar V, Krishna S, and Androphy EJ. Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor alpha correlates with increased accumulation of reactive oxygen species. J Biol Chem 274: 24819–24827, 1999 [DOI] [PubMed] [Google Scholar]
- 181.Lo Conte M. and Carroll KS. The redox biochemistry of protein sulfenylation and sulfinylation. J Biol Chem 288: 26480–26488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lo Muzio L, Leonardi R, Mignogna MD, Pannone G, Rubini C, Pieramici T, Trevisiol L, Ferrari F, Serpico R, Testa N, De Rosa G, and Staibano S. Scatter factor receptor (c-MET) as possible prognostic factor in patients with oral squamous cell carcinoma. Anticancer Res 24: 1063–1070, 2004 [PubMed] [Google Scholar]
- 183.Loft SPH. Cancer risk and oxidative DNA damage in man. J Mol Med 74: 297–312, 1996 [DOI] [PubMed] [Google Scholar]
- 184.Lu H, Li G, Liu L, Feng L, Wang X, and Jin H. Regulation and function of mitophagy in development and cancer. Autophagy 9: 1720–1736, 2013 [DOI] [PubMed] [Google Scholar]
- 185.Lu J, Wang L-E, Xiong P, Sturgis EM, Spitz MR, and Wei Q. 172G>T variant in the 5′ untranslated region of DNA repair gene RAD51 reduces risk of squamous cell carcinoma of the head and neck and interacts with a p53 codon 72 variant. Carcinogenesis 28: 988–994, 2007 [DOI] [PubMed] [Google Scholar]
- 186.Lu M, Wang X, Shen L, Jia J, Gong J, Li J, Li J, Li Y, Zhang X, Lu Z, Zhou J, and Zhang X. Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: a single centre prospective phase II trial. Cancer Sci 107: 486–490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lu Y. and Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 44: 723–738, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lund EL, Bastholm L, and Kristjansen PEG. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res 6: 971, 2000 [PubMed] [Google Scholar]
- 189.Macklin P, Edgerton ME, Thompson AM, and Cristini V. Patient-calibrated agent-based modelling of ductal carcinoma in situ (DCIS): from microscopic measurements to macroscopic predictions of clinical progression. J Theor Biol 301: 122–140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, and Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat 10: 13–29, 2007 [DOI] [PubMed] [Google Scholar]
- 191.Maiuri MC, Zalckvar E, Kimchi A, and Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 192.Malafa M, Margenthaler J, Webb B, Neitzel L, and Christophersen M. MnSOD expression is increased in metastatic gastric cancer. J Surg Res 88: 130–134, 2000 [DOI] [PubMed] [Google Scholar]
- 193.Malik UU, Zarina S, and Pennington SR. Oral squamous cell carcinoma: key clinical questions, biomarker discovery, and the role of proteomics. Arch Oral Biol 63: 53–65, 2016 [DOI] [PubMed] [Google Scholar]
- 194.Mandal PK, Schneider M, Kolle P, Kuhlencordt P, Forster H, Beck H, Bornkamm GW, and Conrad M. Loss of thioredoxin reductase 1 renders tumors highly susceptible to pharmacologic glutathione deprivation. Cancer Res 70: 9505–9514, 2010 [DOI] [PubMed] [Google Scholar]
- 195.Mariño G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, and López-Otín C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem 282: 18573–18583, 2007 [DOI] [PubMed] [Google Scholar]
- 196.Marullo R, Werner E, Zhang H, Chen GZ, Shin DM, and Doetsch PW. HPV16 E6 and E7 proteins induce a chronic oxidative stress response via NOX2 that causes genomic instability and increased susceptibility to DNA damage in head and neck cancer cells. Carcinogenesis 36: 1397–1406, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Masella R, Di Benedetto R, Varì R, Filesi C, and Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16: 577–586, 2005 [DOI] [PubMed] [Google Scholar]
- 198.Matthias C, Bockmühl U, Jahnke V, Harries LW, Wolf CR, Jones PW, Alldersea J, Worrall SF, Hand P, Fryer AA, and Strange RC. The gluathione S-transferase GSTP1 polymorphism: effects on susceptibility to oral/pharyngeal and laryngeal carcinomas. Pharmacogenetics 8: 1–6, 1998 [DOI] [PubMed] [Google Scholar]
- 199.Mauceri HJ, Seetharam S, Salloum RM, Vokes EE, and Weichselbaum RR. Treatment of head and neck and esophageal xenografts employing Alimta and concurrent ionizing radiation. Int J Oncol 19: 833–835, 2001 [DOI] [PubMed] [Google Scholar]
- 200.Melle C, Ernst G, Winkler R, Schimmel B, Klussmann JP, Wittekindt C, Guntinas-Lichius O, and von Eggeling F. Proteomic analysis of human papillomavirus-related oral squamous cell carcinoma: identification of thioredoxin and epidermal-fatty acid binding protein as upregulated protein markers in microdissected tumor tissue. Proteomics 9: 2193–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 201.Menichetti J, Villa S, Magnani T, Avuzzi B, Bosetti D, Marenghi C, Morlino S, Rancati T, Van Poppel H, Salvioni R, Valdagni R, and Bellardita L. Lifestyle interventions to improve the quality of life of men with prostate cancer: a systematic review of randomized controlled trials. Crit Rev Oncol Hematol 108: 13–22, 2016 [DOI] [PubMed] [Google Scholar]
- 202.Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Konda B, and Leichman L. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16: 309–316, 1998 [DOI] [PubMed] [Google Scholar]
- 203.Miller EW, Tulyathan O, Isacoff EY, and Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol 3: 263–267, 2007 [DOI] [PubMed] [Google Scholar]
- 204.Miller VA, Hirsh V, Cadranel J, Chen Y-M, Park K, Kim S-W, Zhou C, Su W-C, Wang M, Sun Y, Heo DS, Crino L, Tan E-H, Chao T-Y, Shahidi M, Cong XJ, Lorence RM, and Yang JC-H. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 13: 528–538, 2012 [DOI] [PubMed] [Google Scholar]
- 205.Mims J, Bansal N, Bharadwaj MS, Chen X, Molina AJ, Tsang AW, and Furdui CM. Energy metabolism in a matched model of radiation resistance for head and neck squamous cell cancer. Radiat Res 183: 291–304, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mirghani H, Amen F, Moreau F, Guigay J, Ferchiou M, Melkane AE, Hartl DM, and Lacau St Guily J. Human papilloma virus testing in oropharyngeal squamous cell carcinoma: what the clinician should know. Oral Oncol 50: 1–9, 2014 [DOI] [PubMed] [Google Scholar]
- 207.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, and Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 1822: 794–814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mo N, Lu YK, Xie W, Liu Y, Zhou W, Wang H, Nong L, Jia Y, Tan A, Chen Y, Li S, and Luo B. Inhibition of autophagy enhances the radiosensitivity of nasopharyngeal carcinoma by reducing Rad51 expression. Oncol Rep 32: 1905–1912, 2014 [DOI] [PubMed] [Google Scholar]
- 209.Moore Z, Chakrabarti G, Luo X, Ali A, Hu Z, Fattah FJ, Vemireddy R, DeBerardinis RJ, Brekken RA, and Boothman DA. NAMPT inhibition sensitizes pancreatic adenocarcinoma cells to tumor-selective, PAR-independent metabolic catastrophe and cell death induced by beta-lapachone. Cell Death Dis 6: e1599, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Morad SAF. and Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13: 51–65, 2013 [DOI] [PubMed] [Google Scholar]
- 211.Moslehi A, Taghizadeh-Ghehi M, Gholami K, Hadjibabaie M, Jahangard-Rafsanjani Z, Sarayani A, Javadi M, Esfandbod M, and Ghavamzadeh A. N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Bone Marrow Transplant 49: 818–823, 2014 [DOI] [PubMed] [Google Scholar]
- 212.Murphy CK, Fey EG, Watkins BA, Wong V, Rothstein D, and Sonis ST. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res 14: 4292–4297, 2008 [DOI] [PubMed] [Google Scholar]
- 213.Myllynen L, Rieckmann T, Dahm-Daphi J, Kasten-Pisula U, Petersen C, Dikomey E, and Kriegs M. In tumor cells regulation of DNA double strand break repair through EGF receptor involves both NHEJ and HR and is independent of p53 and K-Ras status. Radiother Oncol 101: 147–151, 2011 [DOI] [PubMed] [Google Scholar]
- 214.Nakanishi T. and Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 31: 73–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nathan C. and Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 13: 349–361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, Riesenbeck D, Stokman M, Tissing W, Yeoh E, Elad S, and Lalla RV. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support Care Cancer 21: 357–364, 2013 [DOI] [PubMed] [Google Scholar]
- 217.Nishimura T, Newkirk K, Sessions RB, Andrews PA, Trock BJ, Rasmussen AA, Montgomery EA, Bischoff EK, and Cullen KJ. Immunohistochemical staining for glutathione S-transferase predicts response to platinum-based chemotherapy in head and neck cancer. Clin Cancer Res 2: 1859, 1996 [PubMed] [Google Scholar]
- 218.Niu YM, Deng MH, Chen W, Zeng XT, and Luo J. MTHFR C677T gene polymorphism and head and neck cancer risk: a meta-analysis based on 23 publications. Dis Markers 2015: 681313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Niyikiza C, Hanauske AR, Rusthoven JJ, Calvert AH, Allen R, Paoletti P, and Bunn PA., Jr. Pemetrexed safety and dosing strategy. Semin Oncol 29: 24–29, 2002 [DOI] [PubMed] [Google Scholar]
- 220.O'Flanagan CH, Smith LA, McDonell SB, and Hursting SD. When less may be more: calorie restriction and response to cancer therapy. BMC Med 15: 106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Oberley LW. and Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res 39: 1141–1149, 1979 [PubMed] [Google Scholar]
- 222.Ongkeko WM, Altuna X, Weisman RA, and Wang-Rodriguez J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol 124: 71–76, 2005 [DOI] [PubMed] [Google Scholar]
- 223.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck–a systematic review and meta-analysis. Radiother Oncol 100: 22–32, 2011 [DOI] [PubMed] [Google Scholar]
- 224.Pace NJ. and Weerapana E. Diverse functional roles of reactive cysteines. ACS Chem Biol 8: 283–296, 2013 [DOI] [PubMed] [Google Scholar]
- 225.Park JY, Schantz SP, Stern JC, Kaur T, and Lazarus P. Association between glutathione S-transferase pi genetic polymorphisms and oral cancer risk. Pharmacogenetics 9: 497–504, 1999 [PubMed] [Google Scholar]
- 226.Park MT, Song MJ, Lee H, Oh ET, Choi BH, Jeong SY, Choi EK, and Park HJ. Beta-lapachone significantly increases the effect of ionizing radiation to cause mitochondrial apoptosis via JNK activation in cancer cells. PLoS One 6: e25976, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Park S-H, Chung YM, Lee Y-S, Kim HJ, Kim JS, Chae HZ, and Yoo YD. Antisense of human peroxiredoxin II enhances radiation-induced cell death. Clin Cancer Res 6: 4915, 2000 [PubMed] [Google Scholar]
- 228.Patel AL, Chen X, Wood ST, Stuart ES, Arcaro KF, Molina DP, Petrovic S, Furdui CM, and Tsang AW. Activation of epidermal growth factor receptor is required for Chlamydia trachomatis development. BMC Microbiol 14: 277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, and Carroll KS. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol 8: 57–64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pedicini P, Nappi A, Strigari L, Alicia Jereczek-Fossa B, Alterio D, Cremonesi M, Botta F, Vischioni B, Caivano R, Fiorentino A, Improta G, Storto G, Benassi M, Orecchia R, and Salvatore M. Correlation between EGFR expression and accelerated proliferation during radiotherapy of head and neck squamous cell carcinoma. Radiat Oncol 7: 143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peña-Rico MA, Calvo-Vidal MN, Villalonga-Planells R, Martínez-Soler F, Giménez-Bonafé P, Navarro-Sabaté À, Tortosa A, Bartrons R, and Manzano A. TP53 induced glycolysis and apoptosis regulator (TIGAR) knockdown results in radiosensitization of glioma cells. Radiother Oncol 101: 132–139, 2011 [DOI] [PubMed] [Google Scholar]
- 232.Perri F, Pacelli R, Della Vittoria Scarpati G, Cella L, Giuliano M, Caponigro F, and Pepe S. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck 37: 763–770, 2015 [DOI] [PubMed] [Google Scholar]
- 233.Peyser ND, Pendleton K, Gooding WE, Lui VWY, Johnson DE, and Grandis JR. Genomic and transcriptomic alterations associated with STAT3 activation in head and neck cancer. PLoS One 11: e0166185, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Phillips DC, Woollard KJ, and Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol 138: 501–511, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, and Boothman DA. NAD(P)H:quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem 275: 5416–5424, 2000 [DOI] [PubMed] [Google Scholar]
- 236.Planchon SM, Pink JJ, Tagliarino C, Bornmann WG, Varnes ME, and Boothman DA. Beta-lapachone-induced apoptosis in human prostate cancer cells: involvement of NQO1/xip3. Exp Cell Res 267: 95–106, 2001 [DOI] [PubMed] [Google Scholar]
- 237.Poetsch M, Lorenz G, and Kleist B. Detection of new PTEN/MMAC1 mutations in head and neck squamous cell carcinomas with loss of chromosome 10. Cancer Genet Cytogenet 132: 20–24, 2002 [DOI] [PubMed] [Google Scholar]
- 238.Poulogiannis G, McIntyre RE, Dimitriadi M, Apps JR, Wilson CH, Ichimura K, Luo F, Cantley LC, Wyllie AH, Adams DJ, and Arends MJ. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci U S A 107: 15145–15150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Prasad R, Horton JK, Chastain PD, 2nd, Gassman NR, Freudenthal BD, Hou EW, and Wilson SH. Suicidal cross-linking of PARP-1 to AP site intermediates in cells undergoing base excision repair. Nucleic Acids Res 42: 6337–6351, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rabenau K. and Hofstatter E. DNA damage repair and the emerging role of poly(ADP-ribose) polymerase inhibition in cancer therapeutics. Clin Ther 38: 1577–1588, 2016 [DOI] [PubMed] [Google Scholar]
- 241.Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, and Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol 70: 261–264, 2004 [DOI] [PubMed] [Google Scholar]
- 242.Ragin CC, Modugno F, and Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res 86: 104–114, 2007 [DOI] [PubMed] [Google Scholar]
- 243.Reisz JA, Bansal N, Qian J, Zhao W, and Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 21: 260–292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Robb EL, Gawel JM, Aksentijevic D, Cocheme HM, Stewart TS, Shchepinova MM, Qiang H, Prime TA, Bright TP, James AM, Shattock MJ, Senn HM, Hartley RC, and Murphy MP. Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic Biol Med 89: 883–894, 2015 [DOI] [PubMed] [Google Scholar]
- 245.Rodgers MAJ. and Powers EL. Oxygen and Oxy-Radicals in Chemistry and Biology. Proceedings of the International Conference on Oxygen and Oxy-Radicals held at the University of Texas at Austin, May 1980 New York, NY: Academic Press, 1981, p. 808 [Google Scholar]
- 246.Rodrigues JO, Galbiatti AL, Ruiz MT, Raposo LS, Maniglia JV, Pavarino-Bertelli EC, and Goloni-Bertollo EM. Polymorphism of methylenetetrahydrofolate reductase (MTHFR) gene and risk of head and neck squamous cell carcinoma. Braz J Otorhinolaryngol 76: 776–782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Roh JL, Jang H, Kim EH, and Shin D. Targeting of the glutathione, thioredoxin, and Nrf2 antioxidant systems in head and neck cancer. Antioxid Redox Signal 27: 106–114, 2017 [DOI] [PubMed] [Google Scholar]
- 248.Ryser MD, Myers ER, and Durrett R. HPV clearance and the neglected role of stochasticity. PLoS Comput Biol 11: e1004113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salzman R, Kankova K, Pacal L, Tomandl J, Horakova Z, and Kostrica R. Increased activity of superoxide dismutase in advanced stages of head and neck squamous cell carcinoma with locoregional metastases. Neoplasma 54: 321–325, 2007 [PubMed] [Google Scholar]
- 250.Scarbrough PM, Mapuskar KA, Mattson DM, Gius D, Watson WH, and Spitz DR. Simultaneous inhibition of glutathione- and thioredoxin-dependent metabolism is necessary to potentiate 17AAG-induced cancer cell killing via oxidative stress. Free Radic Biol Med 52: 436–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, and Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26: 1749–1760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, and Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene 15: 1191–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 253.Schoeberl B, Eichler-Jonsson C, Gilles ED, and Muller G. Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotechnol 20: 370–375, 2002 [DOI] [PubMed] [Google Scholar]
- 254.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, Grantcharova V, Kohli N, West KA, Leszczyniecka M, Feldhaus MJ, Kudla AJ, and Nielsen UB. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal 2: ra31, 2009 [DOI] [PubMed] [Google Scholar]
- 255.Schoenfeld JD. Immunity in head and neck cancer. Cancer Immunol Res 3: 12, 2015 [DOI] [PubMed] [Google Scholar]
- 256.Seemann S. and Hainaut P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene 24: 3853–3863, 2005 [DOI] [PubMed] [Google Scholar]
- 257.Seiwert TY, Connell PP, Mauer AM, Hoffman PC, George CM, Szeto L, Salgia R, Posther KE, Nguyen B, Haraf DJ, and Vokes EE. A phase I study of pemetrexed, carboplatin, and concurrent radiotherapy in patients with locally advanced or metastatic non-small cell lung or esophageal cancer. Clin Cancer Res 13: 515–522, 2007 [DOI] [PubMed] [Google Scholar]
- 258.Seiwert TY, Salama JK, and Vokes EE. The concurrent chemoradiation paradigm—general principles. Nat Clin Pract Oncol 4: 86–100, 2007 [DOI] [PubMed] [Google Scholar]
- 259.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732, 2003 [DOI] [PubMed] [Google Scholar]
- 260.Sen M, Paul K, Freilino ML, Li H, Li C, Johnson DE, Wang L, Eiseman J, and Grandis JR. Systemic administration of a cyclic signal transducer and activator of transcription 3 (STAT3) decoy oligonucleotide inhibits tumor growth without inducing toxicological effects. Mol Med 20: 46–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Servagi-Vernat S, Differding S, Sterpin E, Hanin FX, Labar D, Bol A, Lee JA, and Gregoire V. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol 54: 1008–1016, 2015 [DOI] [PubMed] [Google Scholar]
- 262.Sharma PK. and Varshney R. 2-Deoxy-D-glucose and 6-aminonicotinamide-mediated Nrf2 down regulation leads to radiosensitization of malignant cells via abrogation of GSH-mediated defense. Free Radic Res 46: 1446–1457, 2012 [DOI] [PubMed] [Google Scholar]
- 263.She QB, Solit D, Basso A, and Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res 9: 4340–4346, 2003 [PubMed] [Google Scholar]
- 264.Sheibani KM, Mafi AR, Moghaddam S, Taslimi F, Amiran A, and Ameri A. Efficacy of benzydamine oral rinse in prevention and management of radiation-induced oral mucositis: a double-blind placebo-controlled randomized clinical trial. Asia Pac J Clin Oncol 11: 22–27, 2015 [DOI] [PubMed] [Google Scholar]
- 265.Shi GX, Wang XR, Yan CQ, He T, Yang JW, Zeng XH, Xu Q, Zhu W, Du SQ, and Liu CZ. Acupuncture elicits neuroprotective effect by inhibiting NAPDH oxidase-mediated reactive oxygen species production in cerebral ischaemia. Sci Rep 5: 17981, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 266.Shin DSM. and Khuri FR. Advances in the management of recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck 35: 443–453, 2013 [DOI] [PubMed] [Google Scholar]
- 267.Siegel D, Yan C, and Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem Pharmacol 83: 1033–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Silva P, Homer JJ, Slevin NJ, Musgrove BT, Sloan P, Price P, and West CML. Clinical and biological factors affecting response to radiotherapy in patients with head and neck cancer: a review. Clin Otolaryngol 32: 337–345, 2007 [DOI] [PubMed] [Google Scholar]
- 269.Simcock R, Fallowfield L, and Jenkins V. Group acupuncture to relieve radiation induced xerostomia: a feasibility study. Acupunct Med 27: 109–113, 2009 [DOI] [PubMed] [Google Scholar]
- 270.Simons AL, Parsons AD, Foster KA, Orcutt KP, Fath MA, and Spitz DR. Inhibition of glutathione and thioredoxin metabolism enhances sensitivity to perifosine in head and neck cancer cells. J Oncol 2009: 519563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Singh A. and Singh H. Time-scale and nature of radiation-biological damage: approaches to radiation protection and post-irradiation therapy. Prog Biophys Mol Biol 39: 69–107, 1982 [DOI] [PubMed] [Google Scholar]
- 272.Sistigu A, Manic G, Obrist F, and Vitale I. Trial watch—inhibiting PARP enzymes for anticancer therapy. Mol Cell Oncol 3: e1053594, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, Francis P, Harvey L, Klein M, Zhang W, Dancey J, Eisenhauer EA, and Winquist E. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol 25: 2178–2183, 2007 [DOI] [PubMed] [Google Scholar]
- 274.Sjalander A, Birgander R, Saha N, Beckman L, and Beckman G. p53 polymorphisms and haplotypes show distinct differences between major ethnic groups. Hum Hered 46: 41–48, 1996 [DOI] [PubMed] [Google Scholar]
- 275.Sobotta MC, Liou W, Stocker S, Talwar D, Oehler M, Ruppert T, Scharf AN, and Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11: 64–70, 2015 [DOI] [PubMed] [Google Scholar]
- 276.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, and Grandis JR. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res 12: 5064–5073, 2006 [DOI] [PubMed] [Google Scholar]
- 277.Soltoff SP, Carraway KL, Prigent SA, Gullick WG, and Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol 14: 3550–3558, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Somers KD, Merrick MA, Lopez ME, Incognito LS, Schechter GL, and Casey G. Frequent p53 mutations in head and neck cancer. Cancer Res 52: 5997, 1992 [PubMed] [Google Scholar]
- 279.Sonis ST. A biological approach to mucositis. J Support Oncol 2: 21–32; discussion 35–36, 2004 [PubMed] [Google Scholar]
- 280.Soto Chervin C. and Brockstein B. Current clinical immunotherapeutic approaches for head and neck cancer. F1000Res 5: 803, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, and Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22: 77–85, 2004 [DOI] [PubMed] [Google Scholar]
- 282.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, and Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science 333: 1157–1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, and Pan H. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4: e838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, and Boothman DA. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem 276: 19150–19159, 2001 [DOI] [PubMed] [Google Scholar]
- 285.Tagliarino C, Pink JJ, Reinicke KE, Simmers SM, Wuerzberger-Davis SM, and Boothman DA. Mu-calpain activation in beta-lapachone-mediated apoptosis. Cancer Biol Ther 2: 141–152, 2003 [DOI] [PubMed] [Google Scholar]
- 286.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, and Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A 109: 13561–13566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tai Y, Inoue H, Sakurai T, Yamada H, Morito M, Ide F, Mishima K, and Saito I. Protective effect of lecithinized SOD on reactive oxygen species-induced xerostomia. Radiat Res 172: 331–338, 2009 [DOI] [PubMed] [Google Scholar]
- 288.Taylor RJ, Chan S-L, Wood A, Voskens CJ, Wolf JS, Lin W, Chapoval A, Schulze DH, Tian G, and Strome SE. FcγRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 58: 997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tedeschi PM, Markert EK, Gounder M, Lin H, Dvorzhinski D, Dolfi SC, Chan LL, Qiu J, DiPaola RS, Hirshfield KM, Boros LG, Bertino JR, Oltvai ZN, and Vazquez A. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis 4: e877, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Thorburn A, Thamm DH, and Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol 85: 830–838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, and Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352: 231–235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tkachev V, Goodell S, Opipari AW, Hao LY, Franchi L, Glick GD, Ferrara JL, and Byersdorfer CA. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J Immunol 194: 5789–5800, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, and Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Toh Y, Kuninaka S, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Mori M, and Sugimachi K. Overexpression of manganese superoxide dismutase mRNA may correlate with aggressiveness in gastric and colorectal adenocarcinomas. Int J Oncol 17: 107–112, 2000 [DOI] [PubMed] [Google Scholar]
- 295.Tomkiewicz C, Hans S, Mucchielli MH, Agier N, Delacroix H, Marisa L, Brasnu D, Aggerbeck LP, Badoual C, Barouki R, and Aggerbeck M. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One 7: e47170, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Truong TH, Ung PM, Palde PB, Paulsen CE, Schlessinger A, and Carroll KS. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem Biol 23: 837–848, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ueda S, Nakamura H, Masutani H, Sasada T, Yonehara S, Takabayashi A, Yamaoka Y, and Yodoi J. Redox regulation of caspase-3(-like) protease activity: regulatory roles of thioredoxin and cytochrome c. J Immunol 161: 6689, 1998 [PubMed] [Google Scholar]
- 298.Valko M, Rhodes CJ, Moncol J, Izakovic M, and Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1–40, 2006 [DOI] [PubMed] [Google Scholar]
- 299.van Schalkwyk MC, Papa SE, Jeannon JP, Guerrero Urbano T, Spicer JF, and Maher J. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev 24: 134–142, 2013 [DOI] [PubMed] [Google Scholar]
- 300.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10: 671–684, 2011 [DOI] [PubMed] [Google Scholar]
- 301.Varilla V, Atienza J, and Dasanu CA. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin Biol Ther 13: 1241–1256, 2013 [DOI] [PubMed] [Google Scholar]
- 302.Vaupel P, Kallinowski F, and Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49: 6449, 1989 [PubMed] [Google Scholar]
- 303.Venuti A. and Paolini F. HPV detection methods in head and neck cancer. Head Neck Pathol 6 Suppl 1: S63–S74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Verim A, Turan S, Farooqi AA, Kahraman OT, Tepe-Karaca C, Yildiz Y, Naiboglu B, Ozkan NE, Ergen A, Isitmangil GA, and Yaylim I. Association between laryngeal squamous cell carcinoma and polymorphisms in tumor necrosis factor related apoptosis induce ligand (TRAIL), TRAIL receptor and sTRAIL levels. Asian Pac J Cancer Prev 15: 10697–10703, 2014 [DOI] [PubMed] [Google Scholar]
- 305.Verma M, Erwin S, Abedi V, Hontecillas R, Hoops S, Leber A, Bassaganya-Riera J, and Ciupe SM. Modeling the mechanisms by which HIV-associated immunosuppression influences HPV persistence at the oral mucosa. PLoS One 12: e0168133, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Vigneswaran N. and Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Maxillofac Surg Clin North Am 26: 123–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Vousden KH. and Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604, 2002 [DOI] [PubMed] [Google Scholar]
- 308.Wadleigh RG, Redman RS, Graham ML, Krasnow SH, Anderson A, and Cohen MH. Vitamin E in the treatment of chemotherapy-induced mucositis. Am J Med 92: 481–484, 1992 [DOI] [PubMed] [Google Scholar]
- 309.Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, Ang MK, Hayward MC, Salazar AH, Hoadley KA, Fritchie K, Sailey CJ, Weissler MC, Shockley WW, Zanation AM, Hackman T, Thorne LB, Funkhouser WD, Muldrew KL, Olshan AF, Randell SH, Wright FA, Shores CG, and Hayes DN. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One 8: e56823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wang Y, Nartiss Y, Steipe B, McQuibban GA, and Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 8: 1462–1476, 2012 [DOI] [PubMed] [Google Scholar]
- 311.Wanka C, Steinbach JP, and Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem 287: 33436–33446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Watson WH, Yang X, Choi YE, Jones DP, and Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci 78: 3–14, 2004 [DOI] [PubMed] [Google Scholar]
- 313.Weitzel DH, Tovmasyan A, Ashcraft KA, Boico A, Birer SR, Roy Choudhury K, Herndon J, 2nd, Rodriguiz RM, Wetsel WC, Peters KB, Spasojevic I, Batinic-Haberle I, and Dewhirst MW. Neurobehavioral radiation mitigation to standard brain cancer therapy regimens by Mn(III) n-butoxyethylpyridylporphyrin-based redox modifier. Environ Mol Mutagen 57: 372–381, 2016 [DOI] [PubMed] [Google Scholar]
- 314.Weitzel DH, Tovmasyan A, Ashcraft KA, Rajic Z, Weitner T, Liu C, Li W, Buckley AF, Prasad MR, Young KH, Rodriguiz RM, Wetsel WC, Peters KB, Spasojevic I, Herndon JE, 2nd, Batinic-Haberle I, and Dewhirst MW. Radioprotection of the brain white matter by Mn(III) n-Butoxyethylpyridylporphyrin-based superoxide dismutase mimic MnTnBuOE-2-PyP5+. Mol Cancer Ther 14: 70–79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.White E. The role for autophagy in cancer. J Clin Invest 125: 42–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Williams VM, Filippova M, Filippov V, Payne KJ, and Duerksen-Hughes P. Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J Virol 88: 6751–6761, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Williams VM, Filippova M, Soto U, and Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6: 45–57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Woo HA, Yim SH, Shin DH, Kang D, Yu D-Y, and Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140: 517–528, 2010 [DOI] [PubMed] [Google Scholar]
- 319.Wood ZA, Poole LB, and Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650, 2003 [DOI] [PubMed] [Google Scholar]
- 320.Wood ZA, Schröder E, Robin Harris J, and Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40, 2003 [DOI] [PubMed] [Google Scholar]
- 321.Worthington HV, Clarkson JE, and Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev CD001973, 2004 [DOI] [PubMed] [Google Scholar]
- 322.Xuelei M, Jingwen H, Wei D, Hongyu Z, Jing Z, Changle S, and Lei L. ERCC1 plays an important role in predicting survival outcomes and treatment response for patients with HNSCC: a meta-analysis. Oral Oncol 51: 483–492, 2015 [DOI] [PubMed] [Google Scholar]
- 323.Yang ZJ, Chee CE, Huang S, and Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10: 1533–1541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yu J. and Zhang L. PUMA, a potent killer with or without p53. Oncogene 27: S71–S83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, and Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A 103: 4952–4957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, Rodman SN, III, Snyders TP, Chenard CA, Eichenberger-Gilmore JM, Bodeker KL, Ahmann L, Smith BJ, Vollstedt SA, Brown HA, Hejleh TA, Clamon GH, Berg DJ, Szweda LI, Spitz DR, Buatti JM, and Allen BG. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of Iowa experience of two phase 1 clinical trials. Radiat Res 187: 743–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, and Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 328.Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou HM, Huang X, Yang K, Zhu Q, Wang X, Yi J, Zhu L, Qian X, Chen L, Tang Y, Loscalzo J, and Yang Y. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab 21: 777–789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhou JP, Chen X, Feng S, Luo SD, Pan YL, Zhong L, Ji P, Wang ZR, Ma S, Li LL, Wei YQ, and Yang SY. Systems biology modeling reveals a possible mechanism of the tumor cell death upon oncogene inactivation in EGFR addicted cancers. PLoS One 6: e28930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhu H, Shen Z, Luo H, Zhang W, and Zhu X. Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine (Baltimore) 95: e3077, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhu XX, Yang XJ, Chao YL, Zheng HM, Sheng HF, Liu HY, He Y, and Zhou HW. The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine 18: 23–31, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, and Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]