Abstract
Obesity-mediated inflammation is a major cause of insulin resistance, and macrophages play an important role in this process. The 78-kDa glucose-regulated protein (GRP78) is a major endoplasmic reticulum chaperone that modulates unfolded protein response (UPR), and mice with GRP78 heterozygosity were resistant to diet-induced obesity. Here, we show that mice with macrophage-selective ablation of GRP78 (Lyz-GRP78−/−) are protected from skeletal muscle insulin resistance without changes in obesity compared with wild-type mice after 9 wk of high-fat diet. GRP78-deficient macrophages demonstrated adapted UPR with up-regulation of activating transcription factor (ATF)-4 and M2-polarization markers. Diet-induced adipose tissue inflammation was reduced, and bone marrow–derived macrophages from Lyz-GRP78−/− mice demonstrated a selective increase in IL-6 expression. Serum IL-13 levels were elevated by >4-fold in Lyz-GRP78−/− mice, and IL-6 stimulated the myocyte expression of IL-13 and IL-13 receptor. Lastly, recombinant IL-13 acutely increased glucose metabolism in Lyz-GRP78−/− mice. Taken together, our data indicate that GRP78 deficiency activates UPR by increasing ATF-4, and promotes M2-polarization of macrophages with a selective increase in IL-6 secretion. Macrophage-derived IL-6 stimulates the myocyte expression of IL-13 and regulates muscle glucose metabolism in a paracrine manner. Thus, our findings identify a novel crosstalk between macrophages and skeletal muscle in the modulation of obesity-mediated insulin resistance.—Kim, J. H., Lee, E., Friedline, R. H., Suk, S., Jung, D. Y., Dagdeviren, S., Hu, X., Inashima, K., Noh, H. L., Kwon, J. Y., Nambu, A., Huh, J. R., Han, M. S., Davis, R. J., Lee, A. S., Lee, K. W., Kim, J. K. Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity.
Keywords: glucose metabolism, inflammation, unfolded protein response
Obesity is a major cause of type 2 diabetes and is characterized by macrophage infiltration into metabolic organs, such as adipose tissue, skeletal muscle, and liver (1–8). During this obesity-mediated inflammatory process, there is evidence of endoplasmic reticulum (ER) stress in macrophages that actively secrete inflammatory cytokines (9). Altered macrophage polarization is also found in obesity and modulates the effects of macrophages on glucose metabolism (3–5, 10–12). In this regard, we have recently found that mice that lack stress-responsive JNK signaling in macrophages were protected from insulin resistance, which indicates an important role for macrophages and ER stress in obesity-mediated insulin resistance (4).
When ER stress results from an imbalance between protein load and folding capacity in cells, the adaptive process that involves unfolded protein response (UPR) is activated to maintain ER homeostasis (13). Among the UPR effectors, 78-kDa glucose-regulated protein (GRP78)/BiP is a central regulator of ER homeostasis (14, 15). Under nonstressed conditions, GRP78 binds to and inactivates ER stress transducers, such as protein kinase R-like ER kinase, activating transcription factor (ATF), and X-box binding protein (XBP)-1. When misfolded proteins accumulate, the equilibrium for the association of GRP78 with misfolded proteins shifts to insufficient GRP78 to associate with and suppress protein complexes of the UPR, thereby activating UPR as part of the protective mechanism of the cell (14, 15). In our recent study, mice with GRP78 heterozygosity were protected from diet-induced obesity and insulin resistance (16). This was largely a result of the compensatory up-regulation of UPR and other ER chaperones, such as GRP94, and improved energy expenditure in adipose tissue (16). The current study examines the role of GRP78 on macrophage function in diet-induced obesity, inflammation, and insulin resistance.
MATERIALS AND METHODS
Animals
Floxed-GRP78 (GRP78f/f) mice were generated as previously described (17). GRP78f/f and Lyz2-Cre mice were crossbred to generate Lyz2-Cre+-GRP78f/f (Lyz-GRP78−/−) mice that were used in the current experiments. Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Effects of high-fat feeding on body composition and energy balance
Male mice (12–16 wk old) were fed a high-fat diet (HFD; 55% kcal from fat, 24% kcal from carbohydrate, and 21% kcal from protein; TD 93075; Harlan Teklad, Frederick, MD, USA) ad libitum for 9 wk to induce obesity. Effects of HFD on body composition were monitored by noninvasively measuring whole-body fat mass and lean mass using 1HMRS (Echo Medical Systems, Houston, TX, USA). Indirect calorimetry and energy balance parameters, including food intake, physical activity, and energy expenditure, were noninvasively assessed for 3 d using metabolic cages (TSE Systems, Chesterfield, MO, USA). All mice were housed under controlled temperature (23°C) and light/dark cycle.
Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity
After feeding a standard chow diet (Prolab Isopro RMH3000 5P75/5P76; LabDiet, St. Louis, MO, USA) (14% kcal from fat, 60% kcal from carbohydrate, and 26% kcal from protein) or HFD, a survival surgery was performed at 5–6 d before clamp experiments to establish an indwelling catheter in the jugular vein. The food was withdrawn overnight (∼15 h) prior to the clamp experiment. A 2-h hyperinsulinemic-euglycemic clamp was conducted in awake mice with a primed and continuous infusion of human insulin (150 mU/kg body weight priming followed by 2.5 mU/kg per minute, Novolin; Novo Nordisk, Plainsboro, NJ, USA) (Table 1) (18). To maintain euglycemia, 20% glucose was infused at variable rates during clamp experiments. Whole-body glucose turnover was assessed with a continuous infusion of [3-3H]glucose (PerkinElmer, Waltham, MA, USA), and 2-deoxy- d-[1-14C]glucose (2-[14C]DG) was administered as a bolus (10 μCi) at 75 min after the start of clamp experiments to measure insulin-stimulated glucose uptake in skeletal muscle (gastrocnemius) and white adipose tissue (epidydimal). At the end of the clamp experiments, mice were anesthetized and tissues were taken for biochemical analysis (18). Acute IL-13 infusion study was performed by using 50 ng/g per hour of recombinant murine IL-13 (PeproTech, Rocky Hill, NJ, USA) infused for 4 h before and during the 2-h hyperinsulinemic-euglycemic clamp.
TABLE 1.
Plasma glucose and insulin concentrations in HFD-fed Lyz-GRP78−/− and WT mice at basal (unfed overnight) and during hyperinsulinemic-euglycemic clamp experiments
| Mouse | Basal | Clamp | ||
|---|---|---|---|---|
| Glucose (mg/dl) | Insulin (ng/ml) | Glucose (mg/dl) | Insulin (ng/ml) | |
| WT | 248 ± 30 | 0.87 ± 0.08 | 121 ± 9 | 1.40 ± 0.12 |
| Lyz-GRP78−/− | 202 ± 19 | 1.09 ± 0.35 | 130 ± 3 | 1.72 ± 0.24 |
Biochemical analysis of glucose metabolism
Glucose concentrations during clamp experiments were analyzed by using 5 μl of plasma by a glucose oxidase method on an Analox GM9 Analyzer (Analox Instruments, Hammersmith, London, United Kingdom). Plasma concentrations of [3-3H]glucose, 2-[14C]DG, and 3H2O were determined after the deproteinization of plasma samples as previously described (18). Whole-body glucose turnover, glycolysis, and hepatic insulin action were calculated as previously described (18). For the determination of tissue 2-[14C]DG-6-phosphate (2-[14C]DG-6-P) content, tissue samples were homogenized and supernatants were subjected to an ion exchange column to separate 2-[14C]DG-6-P from 2-[14C]DG. Insulin-stimulated glucose uptake in individual tissues was assessed by determining the tissue content of 2-[14C]DG-6-P and plasma 2-[14C]DG profile. We used a Plasma Triglyceride Determination Kit (Sigma-Aldrich, St. Louis, MO, USA) to measure triglyceride content in frozen skeletal muscle samples.
Bone marrow–derived macrophages and mouse myoblast cell line
Bone marrow–derived macrophages (BMDMs) were prepared and cultured in DMEM that was supplemented with 75 ng/ml recombinant murine M-CSF (PeproTech), 20% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Polarization studies were performed using macrophages (7–10 d in culture) that were incubated with 100 ng/ml recombinant murine IFN-γ (PeproTech) or 10 ng/ml recombinant murine IL-4 (PeproTech). C2C12 cells were cultured in DMEM that was supplemented with 25 ng/ml IL-6, 2% horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Medium was replaced every 24 h until harvesting.
Molecular analysis using real-time quantitative PCR
RNA was isolated by using Trizol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. cDNA was synthesized with an Omniscript Reverse Transcription Kit (Qiagen, Valencia, CA, USA). Quantitative PCR was run on a CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA) using SYBR Green (Bio-Rad). Relative mRNA expression was normalized to TATA-box binding protein, ribosomal protein L32, or β-2 microglobulin. Primer sequences are shown in Supplemental Table 1. In addition, the mRNA expression of a disintegrin and metalloprotease 10 (ADAM10), ADAM17, and suppressor of cytokine signling-3 (SOCS3) was examined by quantitative PCR analysis using a 7500 Fast Real-Time PCR machine and Taqman assays (both from Thermo Fisher Scientific). Relative mRNA expression was normalized by measuring the amount of 18S RNA in each sample using Taqman assays.
Immunoblot analysis
Protein lysates from BMDM and skeletal muscle (gastrocnemius) samples were prepared for Western blot analysis (19). After blotting, proteins on the membrane were detected with primary Abs against GRP94 (1:1000; Cell Signaling Technology, Danvers, MA, USA), ATF-6A (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), ATF-4 (1:1000; Santa Cruz Biotechnology), phospho-Akt (Ser473; 1:1000; Cell Signaling Technology), Akt (1:1000; Cell Signaling Technology), GLUT4 (1:1000; Cell Signaling Technology), phospho-insulin receptor substrate (IRS)-1 (Tyr608; 1:1000; EMD Millipore, Billerica, MA, USA), and IRS-1 (1:125; EMD Millipore). Glyceraldehyde-3-phosphate dehydrogenase (1:1000; Cell Signaling Technology) and α-tubulin (1:1000; Cell Signaling Technology) were used as loading controls. Immunocomplexes were visualized by using a SuperSignal West Femto Substrate (Thermo Fisher Scientific) and ECL Prime Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, United Kingdom).
Multiplexed ELISA of cytokines and chemokines
Cytokines and chemokines in the culture media supernatant of BMDMs and in serum samples that were collected from the submandibular vein of mice were analyzed by using a Bio-Plex 200 system utilizing Luminex technology (Bio-Rad) with Milliplex Map Kits (EMD Millipore).
Flow cytometry
Monocytes and macrophages in the spleen and lymph nodes were analyzed by flow cytometry (20). Single-cell suspensions were preincubated in 2.4G2 hybridoma supernatant to block Fc-gamma receptor binding for 15 min, then incubated with the following conjugated Abs for 25 min on ice: B220-FITC, CD4-FITC, CD8-FITC, CD11b-PE, CD4-PerCP Cy5.5, NK1.1-PECy7, CD8α-Pacific Blue, Ly6G-eFluor450, CD11c-APC, and Ly6C-APC eFluor780. Abs used for flow cytometric analysis were purchased from eBioscience (San Diego, CA, USA) and BD Biosciences (San Jose, CA, USA). Cells were washed, fixed in 4% paraformaldehyde for 8 min, washed again, and resuspended in fluorescence-activated cell sorting buffer (1× PBS/1.5% fetal calf serum/0.05% NaN3).
For T-cell cytokine analysis, mononuclear cells that were isolated from the spleen and lymph nodes were incubated in RPMI-based medium [Roswell Park Memorial Institute (RPMI) 1640; Thermo Fisher Scientific] that was supplemented with 10% (v/v) heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, UT, USA), 50 U penicillin–streptomycin (Thermo Fisher Scientific), 2 mM glutamine, and 50 μM 2-ME) for 3 h in the presence of phorbol 12-myristate 13-acetate (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and GolgiStop (BD Biosciences). Intracellular cytokine staining was performed according to the manufacturer’s protocol [Cytofix/Cytoperm buffer set from BD Biosciences with TCRβ-APC eFluor780, TCRβ-FITC, CD4-PerCP Cy5.5, IL13-eFluor660 Abs (eBioscience)]. LSR II (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA) were used for flow cytometry and analysis. Dead cells were excluded by using the Live/Dead Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific).
Statistical analysis
All data are expressed as means ± se, and differences between groups were examined for statistical significance using ANOVA with Fisher’s exact test. Values of P < 0.05 were considered statistically significant.
RESULTS
GRP78 deficiency promotes adaptive UPR in macrophages
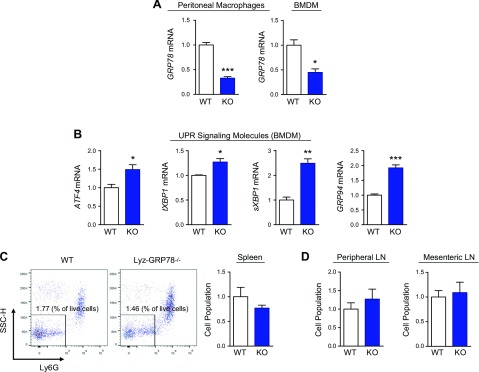
Mice with GRP78-deficient macrophages (Lyz-GRP78−/−) were generated as described in Materials and Methods (Supplemental Fig. 1). Lyz-GRP78−/− mice were born at a normal Mendelian ratio without obvious anomalies. Lyz-GRP78−/− mice showed normal growth and body weight that was comparable to LysM-Cre mice [herein referred to as wild-type (WT) mice]. Our data from the real-time quantitative PCR indicated a ∼70% reduction in GRP78 mRNA levels in peritoneal macrophages and BMDMs that were isolated from Lyz-GRP78−/− mice (Fig. 1A). By using BMDMs from Lyz-GRP78−/− mice, we found significant increases in mRNA levels of ATF4, total and spliced form of XBP1, and GRP94 compared with WT mice (Fig. 1B). These data indicate that GRP78 deficiency activated adaptive UPR in macrophages, which is consistent with our previous findings in adipocytes (16). Despite significant up-regulation of UPR signaling, monocyte and macrophage populations in the spleen (Fig. 1C) and in peripheral and mesenteric lymph nodes (Fig. 1D) were not affected in Lyz-GRP78−/− mice, which suggests that GRP78 deficiency did not affect cell death.
Figure 1.
GRP78 deficiency promotes adaptive UPR in macrophages without affecting cell population. A, B) Total RNA was prepared from peritoneal macrophages and BMDMs that were isolated from WT and Lyz-GRP78−/− mice that were fed chow diet (n = 3–4/group). The relative expression of GRP78 mRNA (A) and UPR signaling molecules (B) were measured by quantitative RT-PCR analysis. C, D) The number of monocytes and macrophages in chow-fed 10-mo-old mice (n = 4 mice/group) were examined by flow cytometry. Gating strategy: live > singlets > CD11b+ Lineage− > NK1.1− > CD11b+ CD11c− > Ly6G−. Representative flow cytometry data and statistical quantification of monocytes and macrophages in the spleen (C). Statistical quantification of monocytes and macrophages in peripheral lymph nodes (pulled from popliteal, brachial, and axillary lymph nodes) and mesenteric lymph nodes (D). *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT mice.
Lyz-GRP78−/− mice are protected from obesity-mediated insulin resistance
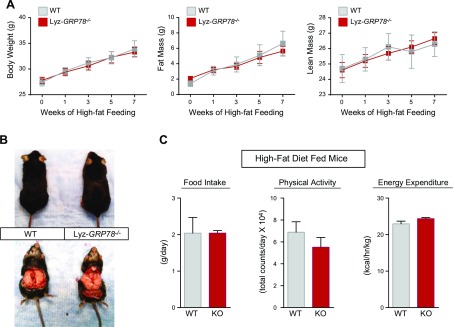
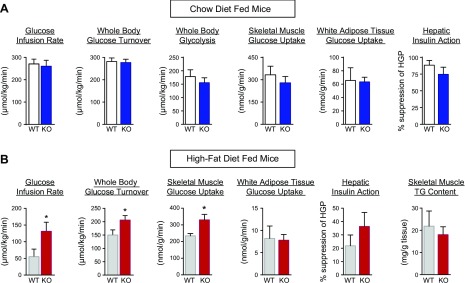
We next examined the effects of chronic HFD on glucose metabolism in mice. Throughout the 9 wk of HFD, both groups of Lyz-GRP78−/− and WT mice became obese with comparable increases in body weight and whole-body fat mass and lean mass (Fig. 2A, B). A 3-day analysis of energy balance using metabolic cages demonstrated no significant differences in food intake, physical activity, and energy expenditure between HFD-fed groups (Fig. 2C). Insulin action and glucose metabolism were assessed by using a 2-h hyperinsulinemic-euglycemic clamp in awake Lyz-GRP78−/− and WT mice that were fed chow or HFD for 9 wk (Table 1). On chow diet, Lyz-GRP78−/− mice demonstrated normal insulin sensitivity as there were no significant differences in glucose infusion rates during clamps, whole-body glucose turnover and glycolysis, hepatic insulin action (i.e., insulin-mediated suppression of hepatic glucose production), and insulin-stimulated glucose uptake in skeletal muscle and white adipose tissue between Lyz-GRP78−/− and WT mice (Fig. 3A).
Figure 2.
Lyz-GRP78−/− mice become obese during 9 wk of high-fat feeding without alteration in energy balance. A) Total body weight and whole-body fat mass and lean mass of WT and Lyz-GRP78−/− mice that were fed HFD for 9 wk (n = 5–8/group). B) Representative image of epidydimal fat pad in WT and Lyz-GRP78−/− mice that were fed HFD for 16 wk. C) Food intake, physical activity, and energy expenditure of WT and Lyz-GRP78−/− mice that were fed HFD for 10 wk measured by metabolic cages (n = 6 mice/group).
Figure 3.
Lyz-GRP78−/− mice are protected from obesity-mediated insulin resistance in skeletal muscle. A, B) Insulin action and glucose metabolism were measured during hyperinsulinemic-euglycemic clamp in WT and Lyz-GRP78−/− mice that were fed chow (A) and HFD (B) for 9 wk (n = 5–8/group). *P < 0.05 vs. HFD-fed WT mice.
After 9 wk of HFD, obese WT mice developed systemic insulin resistance as indicated by profound decreases in glucose infusion rates (57 ± 21 vs. 273 ± 19 μmol/kg/min in chow-fed WT mice) and whole-body glucose turnover (152 ± 17 vs. 282 ± 16 μmol/kg/min in chow-fed WT mice; Fig. 3B). In contrast and despite similar obesity, HFD-fed Lyz-GRP78−/− mice were more insulin sensitive as indicated by a 3-fold increase in glucose infusion rates during the clamp compared with HFD-fed WT mice (Fig. 3B). Whole-body glucose turnover was significantly increased in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice, and this was a result of a 40% increase in insulin-stimulated glucose uptake in the skeletal muscle of HFD-fed Lyz-GRP78−/− mice (Fig. 3B). This protective effect was selective to skeletal muscle as glucose uptake in white adipose tissue and hepatic insulin action were not affected in HFD-fed Lyz-GRP78−/− mice (Fig. 3B). Ectopic lipid accumulation is causally associated with insulin resistance (21), but muscle triglyceride levels were not affected in HFD-fed Lyz-GRP78−/− mice (Fig. 3B). Taken together, these data indicate that Lyz-GRP78−/− mice were protected from obesity-mediated insulin resistance in skeletal muscle.
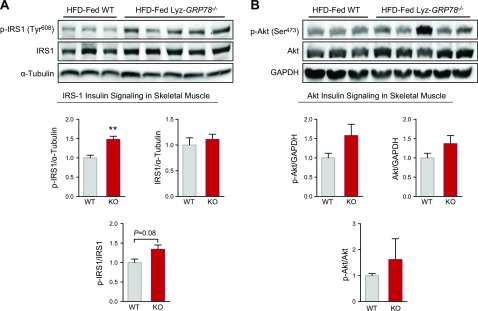
Furthermore, we performed Western blot analysis to assess skeletal muscle insulin signaling in HFD-fed mice. Insulin-stimulated tyrosine phosphorylation of IRS-1 was significantly increased (150%) in the gastrocnemius muscle of Lyz-GRP78−/− mice compared with WT mice (Fig. 4A). Total IRS-1 protein levels did not differ between groups, but the ratio of IRS-1 tyrosine phosphorylation and total IRS-1 demonstrated a strong tendency to be elevated in Lyz-GRP78−/− mice (1.34 ± 0.11 vs. 1.00 ± 0.09 arbitrary units in WT mice; P = 0.081; Fig. 4A). We also determined the Ser473 phosphorylation of Akt and phosphor-Akt/Akt ratio, which tended to be higher in Lyz-GRP78−/− mice compared with WT mice, but this difference did not reach statistical significance (Fig. 4B). Total GLUT4 protein levels did not differ between groups (Supplemental Fig. 2).
Figure 4.
Increased skeletal muscle insulin signaling in HFD-fed Lyz-GRP78−/− mice. Western blot analysis was performed to assess insulin signaling using skeletal muscle (gastrocnemius) samples that were obtained at the end of hyperinsulinemic-euglycemic clamp in HFD-fed Lyz-GRP78−/− and WT mice (n = 3–5/group). A) Tyr608 phosphorylation of IRS-1 and total IRS-1 protein levels. B) Ser473 phosphorylation of Akt and total AKT protein levels. **P < 0.01 vs. HFD-fed WT mice.
Adipose tissue macrophages are reduced in HFD-fed Lyz-GRP78−/− mice
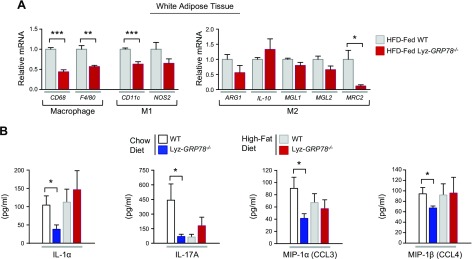
Adipose tissue infiltration of macrophages plays a major role in obesity-mediated insulin resistance (2). To determine the effects of GRP78 deficiency on macrophage function in diet-induced obesity, quantitative RT-PCR was performed for macrophage markers in adipose tissue samples that were obtained from HFD-fed mice. Adipose tissue expression of CD68 and F4/80 were markedly reduced in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice (Fig. 5A). There were concomitant decreases in CD11c (macrophage M1 marker) and MRC2 (macrophage M2 marker) in HFD-fed Lyz-GRP78−/− mice (Fig. 5A). These data suggest that adipose tissue macrophage and inflammatory response are reduced in HFD-fed Lyz-GRP78−/− mice.
Figure 5.
Reduced adipose tissue macrophages in HFD-fed Lyz-GRP78−/− mice. A) Local inflammation profile was determined by using total RNA that was isolated from white adipose tissue samples obtained from WT and Lyz-GRP78−/− mice that were fed HFD for 9 wk (n = 5–8/group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. HFD-fed WT mice. B) Multiplexed Luminex analysis was performed to analyze serum cytokine levels [IL-1α, IL-17A, macrophage inflammatory protein (MIP)-1α, and MIP-1β] in WT and Lyz-GRP78−/− mice that were fed chow or HFD for 9 wk (n = 5–8 mice/group). *P < 0.05 vs. WT mice.
We performed multiplexed Luminex analysis in mouse serum samples to determine the effects of GRP78-deficient macrophages on cytokine profiles. Circulating levels of inflammatory cytokines, including IL-1α, IL-17A, macrophage inflammatory protein (MIP)-1α (also known as CCL3), and MIP-1β (also known as CCL4), were all significantly reduced in Lyz-GRP78−/− mice (Fig. 5B), and these results are consistent with reduced adipose tissue inflammation in these mice.
GRP78 deficiency activates UPR and alters macrophage polarization in skeletal muscle
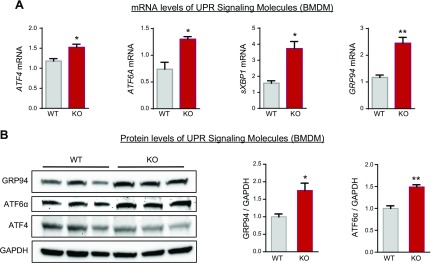
Molecular analysis of UPR signaling in BMDMs that were isolated from HFD-fed mice indicated that mRNA levels of ATF4 and ATF6A were markedly elevated in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice (Fig. 6A). Macrophage expression of the spliced form of XBP1 and GRP94 were also significantly increased by 2- to 3-fold in HFD-fed Lyz-GRP78−/− mice (Fig. 6A). In addition, we performed Western blot analysis of UPR signaling proteins in BMDM samples that were obtained from HFD-fed mice and found significant increases in GRP94 and ATF-6α protein levels in Lyz-GRP78−/− mice compared with WT mice (Fig. 6B). Thus, activated UPR signaling in GRP78-deficient macrophages under the chow-fed condition, as shown in Fig. 1B, was additionally enhanced under the HFD-fed condition.
Figure 6.
GRP78 deficiency activates UPR in HFD-fed mice. Total RNA and protein lysates were prepared from BMDM samples that were isolated from WT and Lyz-GRP78−/− mice fed HFD for 9 wk (n = 3/group). A) Relative expression of mRNA (to chow-fed WT) was measured by quantitative RT-PCR analysis. B) Western blot analysis of UPR signaling proteins (GRP94, ATF-6α, and ATF-4) normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in BMDM samples. *P < 0.05; **P < 0.01 vs. HFD-fed WT mice.
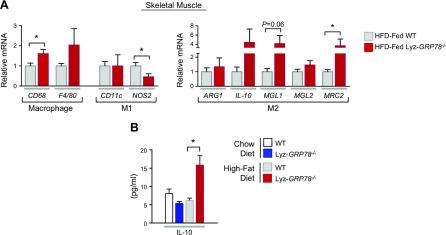
Local macrophages and cytokines are known to regulate skeletal muscle glucose metabolism (22). In this regard, we were initially surprised to find an increased level of macrophages (CD68 as marker) in the skeletal muscle of HFD-fed Lyz-GRP78−/− mice (Fig. 7A); however, additional analysis of macrophages for M1 and M2 markers demonstrated a significant decrease in the level of NOS2 (M1 marker) and a significant increase in the level of MRC2 (M2 marker) in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice (Fig. 7A). Another M2 marker, MGL1, was also increased by >4-fold in HFD-fed Lyz-GRP78−/− mice, although this difference did not reach statistical significance (P = 0.06 vs. HFD-fed WT mice). Consistent with these observations, circulating levels of IL-10—a major anti-inflammatory cytokine secreted by M2-polarized macrophages—were significantly elevated in HFD-fed Lyz-GRP78−/− mice (Fig. 7B). These results collectively indicate that GRP78-deficient macrophages with activated UPR are polarized to an alternatively activated state in skeletal muscle, and this was associated with improved muscle glucose metabolism in HFD-fed Lyz-GRP78−/− mice.
Figure 7.
GRP78 deficiency alters macrophage polarization in the skeletal muscle of HFD-fed mice. A) Relative expression of mRNA was measured by quantitative RT-PCR analysis. Local inflammation profile was determined by using total RNA isolated from skeletal muscle samples that were obtained from WT and Lyz-GRP78−/− mice fed HFD for 9 wk (n = 5–8/group). B) Serum IL-10 levels were measured by using multiplexed Luminex in chow- and HFD-fed WT and Lyz-GRP78−/− mice. *P < 0.05 vs. HFD-fed WT mice.
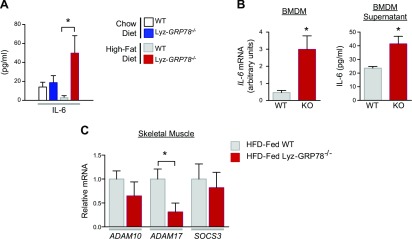
GRP78-deficient macrophages increase expression and secretion of IL-6
IL-6 is a multifunctional cytokine produced and released by a wide variety of cell types, including macrophages, adipocytes, and skeletal muscle. In this regard, our multiplexed Luminex analysis demonstrated significantly elevated serum IL-6 levels in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice (Fig. 8A). By using quantitative RT-PCR in BMDM samples that were obtained from HFD-fed mice, this increase in circulating IL-6 was a result, in part, of a 6-fold increase in IL-6 mRNA levels in HFD-fed Lyz-GRP78−/− mice (Fig. 8B). In addition, we observed a 2-fold increase in IL-6 protein levels in BMDM supernatant from Lyz-GRP78−/− mice compared with WT mice, which suggests increased IL-6 secretion by GRP78-deficient macrophages (Fig. 8B). We next examined IL-6 trans-signaling in skeletal muscle and found significantly reduced expression of ADAM17 in HFD-fed Lyz-GRP78−/− mice (Fig. 8C). In contrast, skeletal muscle mRNA levels of ADAM10 and suppressor of cytokine signling-3 (SOCS3) were not significantly altered in either group of HFD-fed mice.
Figure 8.
Increased IL-6 expression and secretion by GRP78-deficient macrophages. A) Serum IL-6 levels were measured by using multiplexed Luminex in chow- and HFD-fed WT and Lyz-GRP78−/− mice. B) IL-6 expression and secretion by BMDMs that were isolated from HFD-fed Lyz-GRP78−/− and WT mice after a 36-h incubation with IL-4 (10 ng/ml; n = 3/group). Relative expression of IL-6 mRNA was measured by quantitative RT-PCR analysis, and the secretion of IL-6 was analyzed by multiplexed Luminex analysis. C) IL-6 trans-signaling molecules were measured by quantitative RT-PCR analysis using total RNA that was isolated from skeletal muscle samples obtained from WT and Lyz-GRP78−/− mice that were fed HFD for 9 wk (n = 5–8/group). *P < 0.05 vs. HFD-fed WT mice.
GRP78-deficient macrophages mediate IL-6 regulation of IL-13 signaling and action in skeletal muscle
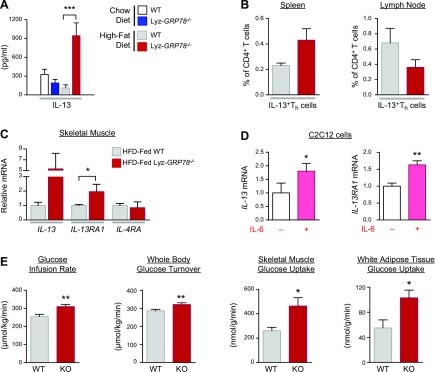
IL-13, a T-helper 2 cytokine largely derived from lymphocytes, is another cytokine that was markedly elevated in serum samples by >7-fold in HFD-fed Lyz-GRP78−/− mice (Fig. 9A). We performed flow cytometric analysis with gating strategy for selective lymphocytes and found that the percentage of IL-13–expressing CD4+ T-helper cells was not significantly altered in HFD-fed Lyz-GRP78−/− mice (Fig. 9B), which suggests that other cell types are responsible for increased IL-13 levels in these mice. In this regard, IL-13 has recently been shown to be secreted by peripheral metabolic organs, such as skeletal muscle and adipose tissue (10, 23), and, therefore, we performed quantitative RT-PCR analysis of skeletal muscle samples from HFD-fed mice. Strikingly, there was a 5-fold increase in IL-13 mRNA levels in HFD-fed Lyz-GRP78−/− mice, although this difference did not reach statistical significance (P = 0.10 vs. HFD-fed WT mice; Fig. 9C). Skeletal muscle expression of the IL-13 receptor (IL-13RA1) was significantly elevated (2-fold) in HFD-fed Lyz-GRP78−/− mice without alteration in IL-4RA mRNA levels (Fig. 9C).
Figure 9.
IL-6 promotes myocyte IL-13 signaling and increases glucose metabolism in Lyz-GRP78−/− mice. A) Serum IL-13 levels were measured by using multiplexed Luminex in chow- and HFD-fed WT and Lyz-GRP78−/− mice. B) Comparison of the number of IL-13–expressing T-helper (Th) cells in HFD-fed WT and Lyz-GRP78−/− mice. Percentages of IL-13–expressing Th cells in the spleen and lymph nodes of HFD-fed WT and Lyz-GRP78−/− mice were examined by using flow cytometry (n = 3–5 mice/group). C) IL-13 signaling was measured by quantitative RT-PCR analysis. Relative mRNA levels of IL-13, IL-13RA1, and IL-4RA in skeletal muscle samples that were obtained from HFD-fed Lyz-GRP78−/− and WT mice (n = 5–8/group). D) Relative mRNA levels of IL-13 and IL-13RA1 in C2C12 mouse myoblast cells that were treated with or without IL-6 (25 ng/ml) for 96 h (n = 4/group). E) Hyperinsulinemic-euglycemic clamp in chow-fed Lyz-GRP78−/− and WT mice during a 4-h intravenous infusion of recombinant murine IL-13 (50 ng/g/h; n = 5/group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT mice or vs. controls (without IL-6 treatment).
As IL-6 has pleiotropic effects that modulate other cytokines, we examined whether IL-6 may affect myocyte expression of IL-13 by incubating mouse myoblast C2C12 cells with or without IL-6 under a myotube differentiation condition in vitro. Remarkably, IL-6–treated C2C12 cells demonstrated ∼2-fold increases in IL-13 and IL-13RA1 mRNA levels compared with untreated cells (Fig. 9D). To our knowledge, this is the first demonstration of the IL-6 induction of myocyte expression of IL-13, and, together, our data suggest that IL-6 derived from GRP78-deficient macrophages might be involved in the increased myocyte expression of IL-13 and IL-13RA1 in Lyz-GRP78−/− mice.
To determine the potential role of IL-13 in Lyz-GRP78−/− mice, we performed a 4-h intravenous infusion of recombinant murine IL-13 and measured insulin action in chow-fed Lyz-GRP78−/− and WT mice. Acute IL-13 infusion increased insulin sensitivity, with significant increases in glucose infusion rates and whole-body glucose turnover in Lyz-GRP78−/− mice (Fig. 9E). This was largely a result of a 2-fold increase in insulin-stimulated glucose uptake in the skeletal muscle and white adipose tissue of Lyz-GRP78−/− mice after IL-13 infusion (Fig. 9E). There were no effects of IL-13 infusion on hepatic insulin action in either group of mice (data not shown). These results indicate that an acute elevation of serum IL-13 levels increased glucose metabolism in chow-fed Lyz-GRP78−/− mice, which is consistent with increased insulin action in HFD-fed Lyz-GRP78−/− mice.
DISCUSSION
Macrophages secrete a multitude of cytokines that can either promote or suppress inflammation via different polarization states, and macrophages exhibit plasticity that changes their function in response to environmental cues. We have recently shown that JNK promotes M1 polarization of macrophages, and that mice with JNK-deficient macrophages were rescued from diet-induced insulin resistance (4). The results of our study indicate that GRP78 deficiency activates UPR and induces M2 polarization of macrophages. This is the first demonstration of UPR involvement in the regulation of macrophage function in obesity. In addition, our findings that GRP78-deficient macrophages that confer an alternatively activated state regulate myocyte IL-13 signaling identify a potential physiologic role for macrophages in skeletal muscle.
One of the most interesting observations in current study is that GRP78-deficient macrophages demonstrated a significant increase in IL-6 expression. Recent studies have found that pro- and anti-inflammatory effects of IL-6 are mediated by different intracellular signaling pathways (24). IL-6 stimulation of classic signaling that involves gp130 and Janus kinases mediates anti-inflammatory effects, whereas IL-6 stimulation of trans-signaling that involves ADAM10 and ADAM17 mediates the proinflammatory effects of IL-6 (25). Furthermore, Mauer et al. and others have recently shown that IL-6 signaling, in fact, promotes alternative activation of macrophages (26, 27). Indeed, nuclear transcription factors are known to interact with the IL-6 promoter to initiate mRNA synthesis (28–30). ATF-6 and XBP1 are bZIP-domain containing transcription factors that bind to the ATF/cAMP response element, and ATF-6 initiates the induction of XBP1 mRNA, which is necessary to produce the active form of spliced XBP1 (31). In our Lyz-GRP78−/− mice, we observed significant increases in macrophage mRNA levels of ATF4, ATF6, and spliced XBP1 (Figs. 1B and 6A). In this regard, ATF-4 was recently shown to directly activate the IL-6 promoter in macrophages (32), and small interfering RNA–mediated knockdown of ATF6 in peritoneal macrophages inhibited the IL-6 expression induced by deoxynivalenol (33). Thus, these results indicate that the activation of UPR—in particular, ATF-4 and ATF-6—in response to GRP78 deficiency may be responsible for the increased IL-6 expression by GRP78-deficient macrophages.
Alterations in macrophage function and stress kinase signaling, such as JNK, have been shown to affect energy balance during chronic high-fat feeding (34, 35). In the case of our Lyz-GRP78−/− mice, despite dramatic changes in multiple macrophage genes that are associated with ER stress and UPR, GRP78-deficient macrophages did not affect feeding behavior, physical activity, and energy expenditure during chow or high-fat feeding. As a result, mice with GRP78-deficient macrophages became obese and gained significant fat mass that was comparable to WT mice during chronic high-fat feeding. This is in stark contrast to global GRP78-haploinsufficient mice (GRP78+/− mice) that have been previously shown to be resistant to diet-induced obesity, and, of importance, this suggests that the previous phenotypes in GRP78+/− mice were not a result of altered GRP78 expression in macrophages (16). In addition, our results indicate that macrophage function can be altered without affecting energy balance and diet-induced obesity.
Obesity is a major cause of insulin resistance, and whole-body fat mass is closely correlated with insulin resistance in mice (36, 37). Although both Lyz-GRP78−/− and WT mice became equally obese after 9 wk of HFD, Lyz-GRP78−/− mice were profoundly more insulin sensitive compared with WT mice, as indicated by an almost 3-fold increase in glucose infusion rates during hyperinsulinemic-euglycemic clamp. This was largely a result of a significant increase in skeletal muscle glucose metabolism in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice. This is consistent with the fact that skeletal muscle is a major organ of glucose disposal during an insulin-stimulated state, and skeletal muscle insulin resistance is a major characteristic of type 2 diabetes (38). As ectopic fat accumulation is causally associated with insulin resistance (21), muscle triglyceride levels were measured in HFD-fed mice and showed no significant differences between Lyz-GRP78−/− and WT mice (Fig. 3B). These results indicate that the protective effects of GRP78-deficient macrophages against obesity-mediated insulin resistance were not associated with changes in tissue lipid distribution.
It is well established that obese humans and animals are characterized by adipose infiltration of proinflammatory M1-polarized macrophages, and this is causally associated with obesity-mediated insulin resistance (4, 5, 10–12). In this regard, HFD-fed Lyz-GRP78−/− mice demonstrated a significant reduction in adipose tissue infiltration of macrophages compared with HFD-fed WT mice. Thus, the effects of GRP78-deficient macrophages to blunt obesity-mediated adipose inflammation may be responsible, in part, for improved insulin sensitivity in HFD-fed Lyz-GRP78−/− mice. This is consistent with the previously reported effects of CD11c+ cell depletion that reversed obesity-mediated insulin resistance in mice (5); however, it is important to note that improved insulin sensitivity in HFD-fed Lyz-GRP78−/− mice was selective to skeletal muscle, as both hepatic insulin action and adipose tissue glucose uptake did not differ between HFD-fed Lyz-GRP78−/− and WT mice.
Increasing evidence indicates that obesity is a chronic state of systemic inflammation, and local inflammation has been detected in multiple organs, including skeletal muscle and the liver (5–8). Of importance, we and others have shown that obesity-mediated inflammation and locally secreted cytokines in skeletal muscle may be directly and causally associated with insulin resistance (5, 6). In this regard, HFD-fed Lyz-GRP78−/− mice demonstrated significant increases in M2-polarized macrophages in skeletal muscle and circulating IL-10 levels compared with HFD-fed WT mice. These results are consistent with previous findings that alternatively activated M2-polarized macrophages confer protection against diet-induced insulin resistance (3, 10–12). Moreover, the current results are also consistent with our previous findings that transgenic mice with muscle-specific overexpression of IL-10 were protected from obesity-mediated insulin resistance in skeletal muscle (19). Thus, the anti-inflammatory effects of GRP78-deficient macrophages in skeletal muscle may be responsible for the muscle-specific improvement in insulin sensitivity in HFD-fed Lyz-GRP78−/− mice.
Furthermore, other potential mechanisms by which GRP78-deficient macrophages increase muscle glucose metabolism may involve IL-13 signaling. Circulating IL-13 levels were increased by 7-fold, and muscle expression of IL-13RA1 was increased by >2-fold in HFD-fed Lyz-GRP78−/− mice compared with HFD-fed WT mice. In this regard, recent studies have identified important endocrine and paracrine roles for IL-13 that affect systemic glucose metabolism (23, 39–41). Jiang et al. (23) identified the autocrine role for IL-13 in the regulation of muscle glucose metabolism, possibly via the IL-13Rα1/STAT/Akt axis. Stanya et al. (39) also found decreased phosphorylated-Akt (Ser473) in the skeletal muscle of IL-13 knockout mice and suggested that the IL-13Rα1/STAT3 axis directs IL-13 signaling toward metabolic responses. Consistent with these findings, the results of our current study show that acute IL-13 infusion increased skeletal muscle glucose metabolism in chow-fed Lyz-GRP78−/− mice compared with WT mice. In addition, our results indicate that IL-6 secreted by GRP78-deficient macrophages may be responsible for stimulating IL-13 expression in skeletal muscle as well as for up-regulating IL-13RA1 expression and signaling in skeletal muscle. Lastly, reduced adipose tissue macrophages and inflammation may also be responsible for increased insulin sensitivity in HFD-fed Lyz-GRP78−/− mice.
In summary, our findings identify a novel role for GRP78 in the modulation of macrophage function and glucose metabolism in diet-induced obesity. GRP78 deficiency promotes adaptive UPR by up-regulating GRP94 and ATF-4, which, in turn, increases IL-6 expression and secretion by M2-polarized macrophages. Macrophage-derived IL-6 stimulates myocyte IL-13 signaling via up-regulating IL-13Rα1 and increases glucose metabolism in skeletal muscle. Overall, GRP78 regulates macrophage function and insulin resistance in diet-induced obesity, and pharmacologic targeting of macrophage UPR signaling may be a potential therapeutic approach with which to treat skeletal muscle insulin resistance.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Payal Patel, Duy Tran, Kevin Hsu, Marilia Loubato, and Sung-Yong Hwang (all from University of Massachusetts Medical School) for technical support. This study was supported by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grants R01-DK080756 and 5U2C-DK093000 to J.K.K.; R01-DK079999 to A.S.L. and J.K.K.; and R24-DK090963 to R.J.D. and J.K.K.), and the Mid-Career Researcher Program (Grant 2015R1A2A1A10053567 to K.W.L.) of the National Research Foundation, funded by the Korean Ministry of Science, Information and Communications Technology (ICT), and Future Planning. R.J.D. is an investigator of the Howard Hughes Medical Institute. The authors declare no conflicts of interest.
Glossary
- 2-[14C]DG
2-deoxy-d-[1-14C]glucose
- 2-[14C]DG-6-P
2-deoxy-d-[1-14C]glucose-6-phosphate
- ADAM
a disintegrin and metalloprotease
- ATF
activating transcription factor
- BMDM
bone marrow–derived macrophage
- ER
endoplasmic reticulum
- GRP78
78-kDa glucose-regulated protein
- HFD
high-fat diet
- IRS
insulin receptor substrate
- MIP
macrophage inflammatory protein
- SOCS3
suppressor of cytokine signaling-3
- UPR
unfolded protein response
- WT
wild-type
- XBP
X-box binding protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. H. Kim, E. Lee, R. H. Friedline, D. Y. Jung, and J. K. Kim designed the study; J. H. Kim, E. Lee, R. H. Friedline, S. Suk, D. Y. Jung, S. Dagdeviren, X. Hu, K. Inashima, H. L. Noh, J. Y. Kwon, A. Nambu, J. R. Huh, M. S. Han, R. J. Davis, A. S. Lee, K. W. Lee, and J. K. Kim performed experiments and analyzed data; and J. H. Kim, E. Lee, S. Suk, J. Y. Kwon, R. J. Davis, A. S. Lee, K. W. Lee, and J. K. Kim wrote the manuscript.
REFERENCES
- 1.Hotamisligil G. S. (2006) Inflammation and metabolic disorders. Nature 444, 860–867 [DOI] [PubMed] [Google Scholar]
- 2.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han M. S., Jung D. Y., Morel C., Lakhani S. A., Kim J. K., Flavell R. A., Davis R. J. (2013) JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patsouris D., Li P. P., Thapar D., Chapman J., Olefsky J. M., Neels J. G. (2008) Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong E. G., Ko H. J., Cho Y. R., Kim H. J., Ma Z., Yu T. Y., Friedline R. H., Kurt-Jones E., Finberg R., Fischer M. A., Granger E. L., Norbury C. C., Hauschka S. D., Philbrick W. M., Lee C. G., Elias J. A., Kim J. K. (2009) Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 58, 2525–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng C. N., Saltiel A. R. (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai D., Yuan M., Frantz D. F., Melendez P. A., Hansen L., Lee J., Shoelson S. E. (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil G. S. (2010) Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang K., Reilly S. M., Karabacak V., Gangl M. R., Fitzgerald K., Hatano B., Lee C. H. (2008) Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odegaard J. I., Ricardo-Gonzalez R. R., Red Eagle A., Vats D., Morel C. R., Goforth M. H., Subramanian V., Mukundan L., Ferrante A. W., Chawla A. (2008) Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman R. J., Scheuner D., Schröder M., Shen X., Lee K., Liu C. Y., Arnold S. M. (2002) The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3, 411–421 [DOI] [PubMed] [Google Scholar]
- 14.Luo B., Lee A. S. (2013) The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32, 805–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A. S. (2014) Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 14, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye R., Jung D. Y., Jun J. Y., Li J., Luo S., Ko H. J., Kim J. K., Lee A. S. (2010) Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes 59, 6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y., Wey S., Wang M., Ye R., Liao C. P., Roy-Burman P., Lee A. S. (2008) Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc. Natl. Acad. Sci. USA 105, 19444–19449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J. K. (2009) Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol. Biol. 560, 221–238 [DOI] [PubMed] [Google Scholar]
- 19.Dagdeviren S., Jung D. Y., Lee E., Friedline R. H., Noh H. L., Kim J. H., Patel P. R., Tsitsilianos N., Tsitsilianos A. V., Tran D. A., Tsougranis G. H., Kearns C. C., Uong C. P., Kwon J. Y., Muller W., Lee K. W., Kim J. K. (2016) Altered interleukin-10 signaling in skeletal muscle regulates obesity-mediated inflammation and insulin resistance. Mol. Cell. Biol. 36, 2956–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose S., Misharin A., Perlman H. (2012) A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J. K., Fillmore J. J., Chen Y., Yu C., Moore I. K., Pypaert M., Lutz E. P., Kako Y., Velez-Carrasco W., Goldberg I. J., Breslow J. L., Shulman G. I. (2001) Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA 98, 7522–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kewalramani G., Bilan P. J., Klip A. (2010) Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr. Opin. Clin. Nutr. Metab. Care 13, 382–390 [DOI] [PubMed] [Google Scholar]
- 23.Jiang L. Q., Franck N., Egan B., Sjögren R. J., Katayama M., Duque-Guimaraes D., Arner P., Zierath J. R., Krook A. (2013) Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. Am. J. Physiol. Endocrinol. Metab. 305, E1359–E1366 [DOI] [PubMed] [Google Scholar]
- 24.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 [DOI] [PubMed] [Google Scholar]
- 25.Garbers C., Jänner N., Chalaris A., Moss M. L., Floss D. M., Meyer D., Koch-Nolte F., Rose-John S., Scheller J. (2011) Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J. Biol. Chem. 286, 14804–14811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steensberg A., Fischer C. P., Keller C., Møller K., Pedersen B. K. (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433–E437 [DOI] [PubMed] [Google Scholar]
- 27.Mauer J., Chaurasia B., Goldau J., Vogt M. C., Ruud J., Nguyen K. D., Theurich S., Hausen A. C., Schmitz J., Brönneke H. S., Estevez E., Allen T. L., Mesaros A., Partridge L., Febbraio M. A., Chawla A., Wunderlich F. T., Brüning J. C. (2014) Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R. J., Prywes R. (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275, 27013–27020 [DOI] [PubMed] [Google Scholar]
- 29.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liou H. C., Boothby M. R., Finn P. W., Davidon R., Nabavi N., Zeleznik-Le N. J., Ting J. P., Glimcher L. H. (1990) A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science 247, 1581–1584 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki Y., Suganami T., Hachiya R., Shirakawa I., Kim-Saijo M., Tanaka M., Hamaguchi M., Takai-Igarashi T., Nakai M., Miyamoto Y., Ogawa Y. (2014) Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes 63, 152–161 [DOI] [PubMed] [Google Scholar]
- 33.Shi Y., Porter K., Parameswaran N., Bae H. K., Pestka J. J. (2009) Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol. Sci. 109, 247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 35.Sabio G., Davis R. J. (2010) cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 35, 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boden G., Shulman G. I. (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur. J. Clin. Invest. 32(Suppl 3), 14–23 [DOI] [PubMed] [Google Scholar]
- 37.Park S. Y., Cho Y. R., Kim H. J., Higashimori T., Danton C., Lee M. K., Dey A., Rothermel B., Kim Y. B., Kalinowski A., Russell K. S., Kim J. K. (2005) Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54, 3530–3540 [DOI] [PubMed] [Google Scholar]
- 38.Kahn C. R. (1994) Banting lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43, 1066–1084 [DOI] [PubMed] [Google Scholar]
- 39.Stanya K. J., Jacobi D., Liu S., Bhargava P., Dai L., Gangl M. R., Inouye K., Barlow J. L., Ji Y., Mizgerd J. P., Qi L., Shi H., McKenzie A. N., Lee C. H. (2013) Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Invest. 123, 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon H., Laurent S., Tang Y., Zong H., Vemulapalli P., Pessin J. E. (2014) Adipocyte-specific IKKβ signaling suppresses adipose tissue inflammation through an IL-13-dependent paracrine feedback pathway. Cell Rep. 9, 1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darkhal P., Gao M., Ma Y., Liu D. (2015) Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. Int. J. Obes. 39, 1292–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.