Abstract
Epithelial tissues robustly respond to internal and external stressors via dynamic cellular rearrangements. Cell extrusion acts as a key regulator of epithelial homeostasis by removing apoptotic cells, orchestrating morphogenesis, and mediating competitive cellular battles during tumorigenesis. Here, we delineate the diverse functions of cell extrusion during development and disease. We emphasize the expanding role for apoptotic cell extrusion in exerting morphogenetic forces, as well as the strong intersection of cell extrusion with cell competition, a homeostatic mechanism that eliminates aberrant or unfit cells. While cell competition and extrusion can exert potent, tumor-suppressive effects, dysregulation of either critical homeostatic program can fuel cancer progression.
A Force for Good: Cell Extrusion in Development and Tissue Homeostasis
Complex cell rearrangements are required for shaping tissue and organ structures in normal development. During such processes, cell extrusion actively regulates development and enforces homeostasis by expelling cells from crowded regions, initiating cell differentiation and epithelial-mesenchymal transitions (EMTs), and exerting physical, morphogenetic forces.
Cell Extrusion Relieves Overcrowded Tissues
Epithelia can remove surplus cells from overcrowded regions by extruding live or dying cells. Crowding-induced cell extrusion occurs in diverse tissues and cell cultures, including human colon epithelia (Eisenhoffer et al., 2012), zebrafish epidermis (Eisenhoffer et al., 2012), the Drosophila pupal notum (Levayer et al., 2016; Marinari et al., 2012), and Madin-Darby canine kidney (MDCK) monolayers (Eisenhoffer et al., 2012). Importantly, modulating cell growth and density is sufficient to alter extrusion rates (Marinari et al., 2012), suggesting that overcrowding-induced mechanical forces trigger extrusion. In crowded regions, stochastic cell anisotropy may promote topological rearrangements of cell-cell boundaries to promote extrusion, as geometrically induced topological defects are sufficient to extrude MDCK cells (Saw et al., 2017).
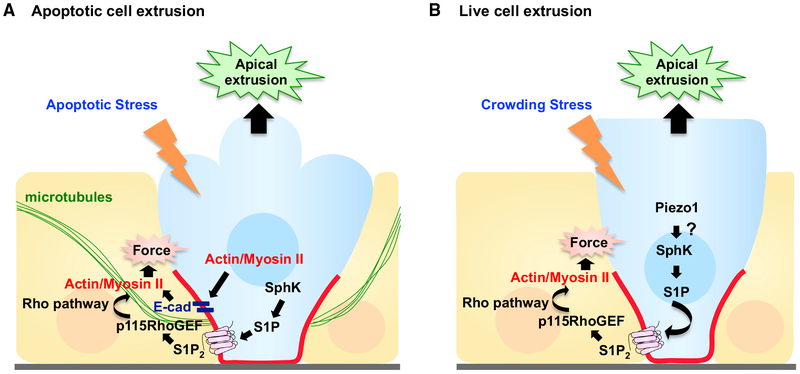
Live cell extrusion in MDCK monolayers or zebrafish epidermis requires the stretch-activated channel Piezo1 (Eisenhoffer et al., 2012; Gudipaty et al., 2017). Interestingly, Piezo1 also regulates cell division, as mechanically stretching MDCK cells at low cell density triggers Piezo1-dependent mitosis (Gudipaty et al., 2017). Thus, Piezo1 acts as a mechanosensor and master regulator of epithelial homeostasis by balancing cell extrusion with proliferation. Crowding-induced extrusion also requires sphingosine kinase to produce the bioactive lipid sphingosine-1-phosphate (S1P) in extruding cells, which signals to neighbors through S1P2 and p115 RhoGEF to form and contract a multicellular actomyosin ring (Gu et al., 2011; Rosenblatt et al., 2001; Slattum et al., 2009) (Figure 1).
Figure 1. Model for Apoptotic and Live Cell Extrusion.
(A) In response to apoptotic stress, cells undergoing apoptosis produce sphingosine-1-phosphate (S1P) via sphingosine kinase (SphK), which binds to the S1P receptor (S1P2) in neighboring cells. S1P2 activates Rho signaling through p115 RhoGEF recruited basally by microtubules, triggering basal actomyosin contraction and subsequent apical extrusion of the dying cell. For simplicity, the autonomous actomyosin force also required for apoptotic cell extrusion has been omitted (see text).
(B) In response to crowding stress, Piezo1 is activated, which triggers live cell extrusion. S1P-Rho signaling is again required for extrusion, but how and if Piezo1 cooperates with S1P-Rho signaling remains unclear.
Notably, although blocking crowding-induced extrusion can cause atypical cellular accumulations (such as in S1P2-deficient zebrafish; Gu et al., 2015), organismal-wide consequences of preventing crowding-induced extrusion reported thus far appear relatively mild; for example, blocking midline notum extrusion merely caused wider adult thorax midlines (Levayer et al., 2016). The most compelling case for a key role for live cell extrusion in maintaining organismal homeostasis is in mouse secondary palate development and fusion (Kim et al., 2015): here, cell extrusion and death were observed in vivo and in explants, and blocking cationic mechanosensitive channels with gadolinium prevented palatal shelf fusion in explants (although a direct role for Piezo1 and cell extrusion in vivo remains to be demonstrated). Nevertheless, the conservation of crowding-induced extrusion between diverse tissues and cell cultures implies that such extrusion is important for stressed epithelia and thus likely confers organismal benefits.
Cell Extrusion Couples Cell Location with Cell Fate
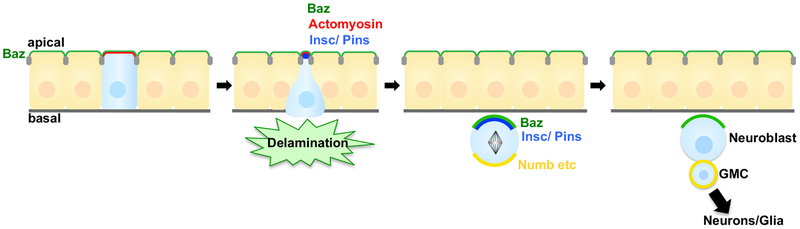
Cell extrusion impacts development not only by altering cell position but also by determining cell fate. This is best illustrated by Drosophila neurogenesis, where neural precursor cells delami-nate from an epithelium as neuroblasts (NBs) prior to initiating neurogenic divisions (Figure 2) (reviewed in Doe, 2017; Homem and Knoblich, 2012). Here, NB gene expression is linked to extrusion timing (Skeath and Carroll, 1992); for example, the key determinant Inscuteable becomes apically localized during the delamination process (Schaefer et al., 2000). Blocking neuroepithelial cell delamination by overexpressing the Notch intracellular domain compromises neurogenesis and is lethal (Lieber et al., 1993; Struhl et al., 1993). Critically, the NB derives its polarity from the original epithelia, an inheritance that determines NB daughter cell fate via asymmetric segregation of polarized protein determinants (Schober et al., 1999; Wodarz et al., 1999); uncoordinated NB extrusion would presumably alter NB polarity and derail subsequent neurogenesis. Thus, proper NB extrusion and polarity inheritance enables a cascade of polarized NB divisions and proper fate decisions.
Figure 2. Cell Extrusion Coupled to Drosophila Neuroblast Division and Differentiation.
Proper extrusion of Drosophila neuroblasts (NBs) enables asymmetric NB divisions, daughter cell fate decision, and correct neurogenesis. Bazooka (PAR3, Baz) localizes apically in the mother epithelium, a polarity that critically is inherited by extruding NBs. During NB delamination, Inscuteable (Insc) is expressed, localizes apically, and recruits Pins. During mitotic division, several proteins (including Numb) localize to the basal cortex. This correct, asymmetric segregation of key proteins governs daughter cell fates and generates a new neuroblast and a ganglion mother cell (GMC), which produces neurons and glia.
NB delamination is driven by a pulsatile, actomyosin network localized apically in NB precursors, with Rho signaling important for medial myosin accumulation (An et al., 2017). This is in contrast to the removal of apoptotic or live cells by non-autonomous Rho signaling and multicellular actomyosin ring assembly (Figure 1). However, apoptotic cells also require autonomous actomyosin force, and a cortical, actomyosin ring formed in dying cells is required for neighboring cells to form rosettes around apoptotic cells prior to assembling the multicellular actomyosin ring (Kuipers et al., 2014; Rosenblatt et al., 2001).
Conceptually similar to NB delamination, embryonic neural crest cell (NCC) morphogenesis in vertebrates involves NCC delamination from the dorsal neural epithelia followed by a characteristic EMT (reviewed in Thiery et al., 2009); cell extrusion failure is thus predicted to block neural crest dispersal, colonization of distal organs, and development. Actomyosin and Rho signaling are again important for orchestrating extrusion, with RhoB required in chick NCC delamination (Liu and Jessell, 1998) and Rho-associated protein kinase (ROCK) and myosin required for zebrafish NCC delamination (Berndt et al., 2008).
Lung development provides a final, intriguing example of extrusion influencing cell fate. Lung neuroendocrine progenitors are selected from bronchial epithelia by an extrusive event, with random cell subpopulations extruding and undertaking an EMT-like migration before settling at lung branch points as committed neuroendocrine progenitors (Kuo and Krasnow, 2015). Cell extrusion thus appears situated as a key mechanism for initiating migration and differentiation during development.
Cell Extrusion Generates Morphogenetic Forces
Although developmental apoptosis is best known for its role in sculpting tissues, as in digit separation, the extrusion of apoptotic cells itself can exert mechanical, morphogenetic forces. Below we outline three examples from Drosophila: dorsal closure, leg folding, and epidermal cell replacement during metamorphosis.
Apoptotic, extrusive morphogenetic forces were first reported in vivo for Drosophila dorsal closure. During dorsal closure, the amnioserosa contracts to promote fusion of adjacent lateral epidermal layers. Here, 10%–20% of amnioserosa cells undergo apoptosis and extrude basally prior to closure completion (Reed et al., 2004; Toyama et al., 2008). Interestingly, genetically inhibiting or enhancing apoptosis delays or accelerates dorsal closure, respectively, with one-third of mechanical forces attributed to apoptosis (Toyama et al., 2008). In a morphologically similar scenario, mouse neural tube closure requires apoptosis, although causal roles for apoptotic force transmission and cell extrusion remain unproven (Yamaguchi et al., 2011). Intriguingly, in addition to observing caspase-activated dying neural tube cells, the authors also observed caspase-activated cells dynamically moving about prior to extruding; these “active” extruded cells would be likely candidates for transmitting force to the closing neural tube (Yamaguchi et al., 2011).
Extruding apoptotic cells also facilitate epithelial folding in Drosophila leg-joint development (Monier et al., 2015). Here, sharp discontinuities in Decapentaplegic (Dpp, a transforming growth factor β [TGF-β]/BMP homolog) activity trigger apoptosis and extrusion, with Dpplow cells killed and extruded (Adachi-Yamada et al., 1999; Manjon et al., 2007). Crucially, apoptotic cell extrusion occurs at the center of the epithelial fold corresponding to the presumptive leg-joint regions. Live imaging confirmed that apoptotic cells exert a transient, pulling force on the apical surface of surrounding cells to cause epithelial folding (Figure 3) (Monier et al., 2015). Interestingly, sharp boundaries of Dpp are also sufficient to trigger live cell extrusion in the Drosophila wing disc (Gibson and Perrimon, 2005; Shen and Dahmann, 2005).
Figure 3. Leg Folding by Extruding, Apoptotic Cell Forces.
During Drosophila leg-joint development, extruding apoptotic cells (blue) pull their neighbors toward the basal side at the presumptive joint area by accumulating myosin II along their apical-basal axis. Neighboring cells accumulate myosin II at their apical surface, which triggers apical constriction and subsequent fold formation.
A final example of apoptotic cell extrusion driving morphogenesis occurs during Drosophila metamorphosis, where pupal epidermal cells termed histoblasts replace larval epidermal cells (LECs) (Madhavan and Madhavan, 1980). LECs predominantly extrude at the interface with histoblasts via an apical actomyosin ring and by reducing E-cadherin (E-cad); basally extruded LECs are subsequently removed by hemocytes (Drosophila macrophages) (Nakajima et al., 2011; Ninov et al., 2007; Teng et al., 2017). LECs and histoblasts closely cooperate during tissue remodeling, as histoblast proliferation is required for LEC extrusion, and LEC extrusion is necessary for histoblast proliferation (Nakajima et al., 2011; Ninov et al., 2007). LEC extrusion may drive histoblast expansion mechanically, as extruding LECs deform surrounding histoblasts (Teng et al., 2017). Unexpectedly, blocking LEC death delayed LEC extrusion and also caused histoblast extrusion and death (Ninov et al., 2007), suggestive of competitive interactions between LECs and histo-blasts. This complex interplay of death, extrusion, and proliferation between separate cell populations underscores the key role of cell-cell communication and competitive interactions in tissue homeostasis.
Cell Competition and the Active Extrusion of Aberrant Cells
Cell competition is a conserved cell-elimination mechanism that functions through short-range cell-cell interactions whereby “winner” cells eliminate neighboring “loser” cells defective in some capacity, such as protein biogenesis, growth potential, or aberrant polarity (Amoyel and Bach, 2014; Baker, 2017; Claveria and Torres, 2016; de Beco et al., 2012; Maruyama and Fujita, 2017; Merino et al., 2016; Morata and Ballesteros-Arias, 2015; Tamori and Deng, 2011). Cell competition enables epithelia to actively eliminate emergent oncogenic cells as an endogenous tumor-suppression mechanism. Notably, loser cell extrusion occurs across diverse genetic contexts that trigger competitive interactions, including cells expressing oncogenic Src, Ras, or Myc, or in cells suffering from altered apicobasal polarity. Fly, vertebrate, and cell culture studies indicate key roles for junctional remodeling and autonomous actomyosin accumulation in competition-mediated cell extrusion, as well as the unexpected adaptation of axon-guidance signaling systems upstream of cytoskeletal and junctional changes in extruding epithelial cells.
Competition-Mediated Cell Extrusion in Drosophila
Loser cell extrusion was initially considered to be a passive consequence of apoptosis following deficient Dpp transduction (Moreno et al., 2002). It has been shown that cell extrusion is important for cell competition-based elimination of Minute (ribosomal protein mutants) or Myclow losers, with extrusion following apoptosis and preceding corpse clearance by hemocytes (Lolo et al., 2012; Moreno et al., 2002), although cell engulfment also contributes to Minute elimination (Li and Baker, 2007). As in many cases of competition-driven cell elimination, c-Jun N-terminal kinase (JNK) signaling is paramount (Eisenhoffer et al., 2012; Igaki et al., 2009; Moreno and Basler, 2004; Moreno et al., 2002; Ohsawa et al., 2011; Vaughen and Igaki, 2016; Yamamoto et al., 2017); for example, dMyc loser extrusion is blocked by impairing JNK (Moreno and Basler, 2004), as is apoptotic MDCK cell extrusion (Eisenhoffer et al., 2012).
However, not all competition-mediated cell extrusion is passive, as first suggested by studies of the Src inhibitor C-terminal Src kinase (dCsk). While mutating dCsk disinhibits Src and causes hyperproliferation (Read et al., 2004; Stewart et al., 2003), clones of cells depleted for dCsk are exterminated and extruded basally, selectively at the interface with wild-type (WT) cells (Vidal et al., 2006, 2010). dCsk mutant cells alter junctional components such as E-cad and p120-catenin and upregulate JNK signaling and matrix metalloproteinases at the boundary with WT cells, boundary phenomena the authors also found in human squamous cell carcinoma (Vidal et al., 2010). Intriguingly, universally halving E-cad or p120-catenin blocked dCsk cell extrusion and migration, suggesting that differential levels of junctional components (high in WT cells, low in dCsk) are essential for competition-driven extrusion. This is striking given E-cad’s critical role in transmitting force during apoptotic cell extrusion (Grieve and Rabouille, 2014; Lubkov and Bar-Sagi, 2014), in part by recruiting the actin-binding protein Coronin 1B to zonula adherens to generate an autonomous, actomyosin force (Michael et al., 2016). Alongside force transmission, cell-junction rearrangements following altered E-cad expression or localization can also alter epithelial cell morphology (reviewed in Gumbiner, 1996), potentially leading to extrusion and EMT, as later discussed.
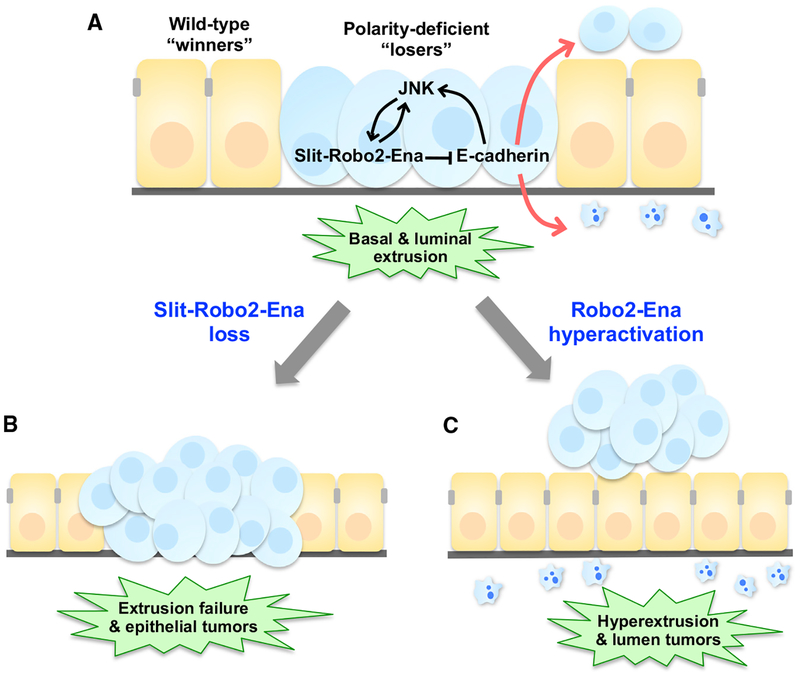
Could cell extrusion be a double-edged sword wielded by cell competition, capable of both helping or harming the host? An insightful example is the competitive elimination of cells mutant for scribble (scrib), a conserved apicobasal polarity gene. While tissues entirely mutant for scrib develop into massive tumors (Bilder et al., 2000), the coexistence of WT cells in the tissue restrains scrib proliferation and eliminates scrib cells via autonomous and non-autonomous JNK activity (Brumby and Richardson, 2003; Igaki et al., 2009; Ohsawa et al., 2011). Like dCsk cells, live scrib cells in eye disc epithelia are mainly extruded basally and subsequently undergo apoptosis, as scrib cells accumulate basally following cell-autonomous apoptosis inhibition (Nakajima et al., 2013; Vaughen and Igaki, 2016). Importantly, blocking scrib cell extrusion resulted in intraepithelial tumors (Figure 4b). While scrib basal extrusion is tumor suppressive, hyperactivating scrib cell extrusion drove aberrant apical extrusion and lethal, luminal tumors (Figure 4c) (Vaughen and Igaki, 2016), underscoring that balanced extrusion rates are critical for homeostasis. This strongly parallels recent findings from scrib-RNAi experiments in wing discs, where scribKD cells selectively overgrow when extruded apically at tumor “hotspots” characterized by dense, basal extracellular matrix (Tamori et al., 2016). Thus, luminal scrib tumors can avoid engulfment by neighboring cells (Ohsawa et al., 2011) or circulating hemocytes (Pastor-Pareja et al., 2008) while simultaneously capitalizing on endogenous JAK/STAT signaling at hotspots (Tamori et al., 2016). In turn, extruded cells may fuel non-autonomous growth of local tissue, as in Wingless (a Wnt homolog) produced by extruded cells harboring unstable genomes (Dekanty et al., 2012).
Figure 4. Tumor Cell Extrusion through Slit-Robo2-Ena Signaling in Cell Competition.
(A) Polarity-deficient loser scrib cells (blue loser cells) are predominantly extruded basally from Drosophila epithelial tissue when confronted with wild-type, winner cells (orange winner cells). In polarity-deficient cells, JNK signaling activates Slit-Robo2-Ena signaling, which dysregulates E-cadherin to drive basal extrusion.
(B) In the absence of Slit-Robo2-Ena signaling, polarity-deficient loser cells (blue) escape from extrusion and overgrow in the epithelial tissue.
(C) Hyperactivation of Slit-Robo2-Ena signaling results in hyperextrusion and luminal tumors.
Surprisingly, Slit-Roundabout (Robo) signaling executes scrib cell extrusion downstream of JNK signaling, echoing Slit-Robo’s established role in cell-cell repulsion during axon guidance. Here, autocrine Slit ligands bind to Robo2 receptors presented on scrib cells, triggering autonomous upregulation of the actin-nucleator Enabled/Vasp (Ena), actomyosin accumulation, E-cad disruption, and then cell extrusion (Figure 4) (Vaughen and Igaki, 2016). Intriguingly, a recent screen identified different “axon-guidance” molecules, Sas-PTP10D, as critical mediators of scrib cell recognition and elimination (Yamamoto et al., 2017). Cell-cell communication mechanisms may therefore be broadly conserved between neuroepithelial progeny, with typically repulsive axon-guidance genes functioning in epithelia to extrude aberrant cells. Indeed, alongside Slit-Robo, ephrin-A-EphA2 signaling repels and extrudes RasV12 cells in MDCK monolayers and wing discs (Porazinski et al., 2016), and repulsive Semaphorin-Plexin signaling removes damaged cells by basal extrusion during JNK-dependent wound healing in wing discs (Yoo et al., 2016). As JNK signaling is required for diverse phenomena that include cell extrusion, such as cell competition and wound healing, downstream signals like Slit-Robo and subsequent junctional dissolution may more broadly mediate cell extrusion and regulate epithelial homeostasis.
Competition-Mediated Cell Extrusion in Vertebrates and Cell Cultures
Competitive cell elimination also occurs in vertebrates in many contexts, including mouse hematopoietic stem cell competition (Bondar and Medzhitov, 2010) and mouse epiblast development (Claveria et al., 2013). However, the bulk of cell competition studies extending fly findings have been undertaken in MDCK monolayers or in zebrafish epidermis.
Striking parallels to Drosophila cell competition emerged from analysis of scribKD MDCK cells: only when surrounded by normal, WT cells do scribKD cells undergo apoptosis and extrude apically (Norman et al., 2012). scribKD MDCK cells also mislocalized E-cad, similar to scrib mutant cells in Drosophila epithelia (Vaughen and Igaki, 2016). In addition to altered E-cad, scribKD cell extrusion and death was dependent on ROCK’s activation of p38 and p53 (Wagstaff et al., 2016); notably, ROCK is also involved in apoptotic cell extrusion (Rosenblatt et al., 2001). Together, these studies indicate that extrusion plays an important role in scrib cell competition in both flies and mammalian cell cultures. Unfortunately, in vivo analysis of scrib cell competition in other organisms is missing.
Interestingly, scribKD MDCK cells are also sensitive to mechanical compaction (Wagstaff et al., 2016), hinting that mechanical state or sensitivity may be an index for cellular fitness. Growth differences can generate mechanical stress in and around fast-growing winners, leading to cell competition (Lee and Morishita, 2017; Mao et al., 2013; Shraiman, 2005; Vincent et al., 2013). Cell mixing and mechanical competition play key roles in Myc-based competition in the Drosophila pupal notum (Levayer et al., 2015). Moreover, notum clones expressing oncogenic RasV12 can compress neighboring WT tissue and induce ectopic extrusion up to three cell diameters away (Levayer et al., 2016), further supporting a role for mechanical stress in cell competition and extrusion.
Alongside scribKD, cell competition triggered by oncogenic RasV12 occurs in MDCK cocultures with WT cells (Hogan et al., 2009). RasV12 cells apically extrude (while alive) when surrounded by WT cells (Hogan et al., 2009) in a process dependent on autonomous ROCK activation and actomyosin accumulation. E-cad again plays a critical role in mediating RasV12 cell extrusion, with E-cad required in neighbor cells to extrude RasV12 cells (Hogan et al., 2009). Remarkably, RasV12 cell competition also involves the S1P-S1P2 extrusion pathway, and S1P2 is required in WT cells to extrude RasV12 cells. While S1P is also required, it is not produced by WT epithelial cells or extruding cells, as in apoptotic or live cell extrusion (Figure 1); instead, S1P is required in culture serum for RasV12 cell extrusion, and would thus presumably be derived from some other source in vivo, such as endothelial cells or erythrocytes (Yamamoto et al., 2016). Importantly, ~80% of RasV12 cell clones are apically extruded/extruding in mouse intestine (Kon et al., 2017), suggesting that competition-mediated extrusion may play an important role in vivo. Supporting this, in the mouse hair follicle stem cell niche, activation of oncogenic HRas in specific clones triggers a competition-like interaction wherein WT cells ultimately surround and expel oncogenic outgrowths into the dermis (Brown et al., 2017).
Last, like oncogenic Ras, oncogenic Src cells are apically extruded independent of cell death in MDCK monolayers and the zebrafish embryonic enveloping layer (Kajita et al., 2010, 2014). Notably, β-catenin, which complexes with E-cad, was mislocalized basally in Src-activated MDCK cells in a myosin-dependent manner (Kajita et al., 2010), suggesting that junctional changes occur downstream of autonomous actomyosin activity in loser, extruding cells. During oncogenic Src cell extrusion, WT cells also accumulate the actin-binding protein filaminA and, interestingly, the intermediate filament vimentin (Kajita et al., 2014), which is often used as a marker for EMT.
A Force for Evil: Cell Extrusion in Disease
Just as cell competition can suppress or fuel cancers (Enomoto et al., 2015; Moreno, 2008; Ohsawa et al., 2014; Slattum and Rosenblatt, 2014), cell extrusion can exert positive or negative effects on organismal health. Dysregulated cell extrusion adversely impacts epithelia homeostasis through two main pathologies, tumorigenesis and bacterial invasion. As recent reviews have highlighted the role of cell extrusion in barrier function against pathogens (Gu and Rosenblatt, 2012; Gudipaty and Rosenblatt, 2017), we will focus more on the intersection of competition-mediated cell extrusion with cancer, including the critical role for the direction of cell extrusion as well as overlap between extrusion genes and signaling pathways with anoikis resistance, EMT, and human cancers.
Pathogenic Hijacking of Intestinal Cell Extrusion
Intestinal epithelia undergo extensive cell extrusion at villi tips (“shedding”) (Creamer et al., 1961) yet still provide critical barriers against external pathogens. As noted recently (Williams et al., 2015), remarkably little has been uncovered about homeo-static intestinal extrusion, including whether apoptosis precedes or follows extrusion. Nonetheless, most extruding human small intestine cells are caspase positive, and neighboring cells upregulate phosphorylated myosin light chain (Bullen et al., 2006), reminiscent of the multicellular actomyosin ring that accumulates around extruding apoptotic cells (Rosenblatt et al., 2001). Intriguingly, small intestine cell extrusion at the villi tip triggered neighboring cell extrusion in 15% of cases, suggesting non-autonomous mechanical forces may also be at play in gut homeostasis (Guan et al., 2011).
Bacterial infection stimulates gut shedding and stem cell proliferation from flies to mammals, indicating that intestinal cell extrusion is also a defense mechanism (Jiang et al., 2009; Vodovar et al., 2005). For example, in flies challenged with infection, JNK-activated enterocytes rapidly delaminate and undergo anoikis (Buchon et al., 2010). However, pathogenic bacteria can hijack intestinal extrusion to promote their dissemination and invasion into underlying tissues. For example, Listeria monocytogenes specifically invades epithelia by attaching to E-cad that is transiently exposed during cell extrusion (Pentecost et al., 2006). Salmonella hijack cell extrusion differently, with an invasive Salmonella subtype actually stimulating apical extrusion, host cell apoptosis, and release of the bacteria into the lumen (Knodler et al., 2010), which was proposed to license secondary-site infection and host-to-host transmission. Conversely, Neisseria gonorrhoeae actively suppresses intestinal cell extrusion to prevent the removal of infected cells by gut shedding (Muenzner et al., 2005). Thus, for intestinal homeostasis and pathogen defense, failure to extrude and excess extrusion are equally problematic; the same is true for a different stressor, cancer.
Competition-Mediated Cell Extrusion and Cancer
How does cell extrusion intersect with cell competition and cancer? On the one hand, extrusion of a tumor cell can facilitate dissemination and metastasis, which is a primary cause of death in cancer. On the other hand, failure to properly dispose of oncogenic cells is potentially fatal, from adversely impacting the host tissue and/or from triggering a systemic, pathological muscle wasting termed cachexia, which accounts for a substantial (~20%) amount of cancer-associated deaths (Fearon et al., 2013). For example, in Drosophila, transplanted scrib (Figueroa-Clarevega and Bilder, 2015) or gut-activated Yorkie (Yap/Taz homolog) (Kwon et al., 2015) tumors systemically impair insulin signaling to drive organ ismal wasting in a non-autonomous, cachexia-like process. Persistence of extrusion-defective cancer cells may thus adversely impact distant organs and health via a non-autonomous and systemic cachexic wasting. Alternatively, extrusion failure could also permit unremoved dying cells to act as “oncogenic niches” (Enomoto et al., 2015), fueling non-autonomous tissue overgrowth analogous to “undead” cells, which secrete cytokines and growth factors and disrupt epithelial homeostasis (Huh et al., 2004; Pérez-Garijo et al., 2004; Ryoo et al., 2004).
Notably, although tumorigenesis stemming from altered competition-mediated cell extrusion has been demonstrated in Drosophila (Tamori et al., 2016; Vaughen and Igaki, 2016), other evidence linking competition-mediated cell extrusion to tumorigenesis remains sparse. Given cancer’s heterogeneous nature (Marusyk et al., 2012), cell competition and cell extrusion are expected to play important roles. Indeed, cell competition by itself has been linked to human tumorigenesis: human cancer cell lines undergo Myc-based cell competition (Di Giacomo et al., 2017), and cell competition was proposed to explain human skin cancer size and mutational distribution (Lynch et al., 2017). Moreover, in mice, Notch-inhibited esophageal progenitor cells outcompete neighboring cells through cell divisions favoring winner renewal and loser differentiation (Alcolea et al., 2014). Importantly, although the resulting esophagi composed entirely of Notch-inhibited winner cells are morphologically normal, they are primed for tumorigenesis and permit p53-stabilized overgrowths (Alcolea et al., 2014). Although these examples did not monitor cell extrusion, observations of cell extrusion during the competitive removal of oncogenic cells in mouse hair follicles (Brown et al., 2017) and intestines (Kon et al., 2017) make more plausible a role for vertebrate competition-mediated cell extrusion in tumorigenesis.
Direction of Cell Extrusion Determines Cancer Cell Fate
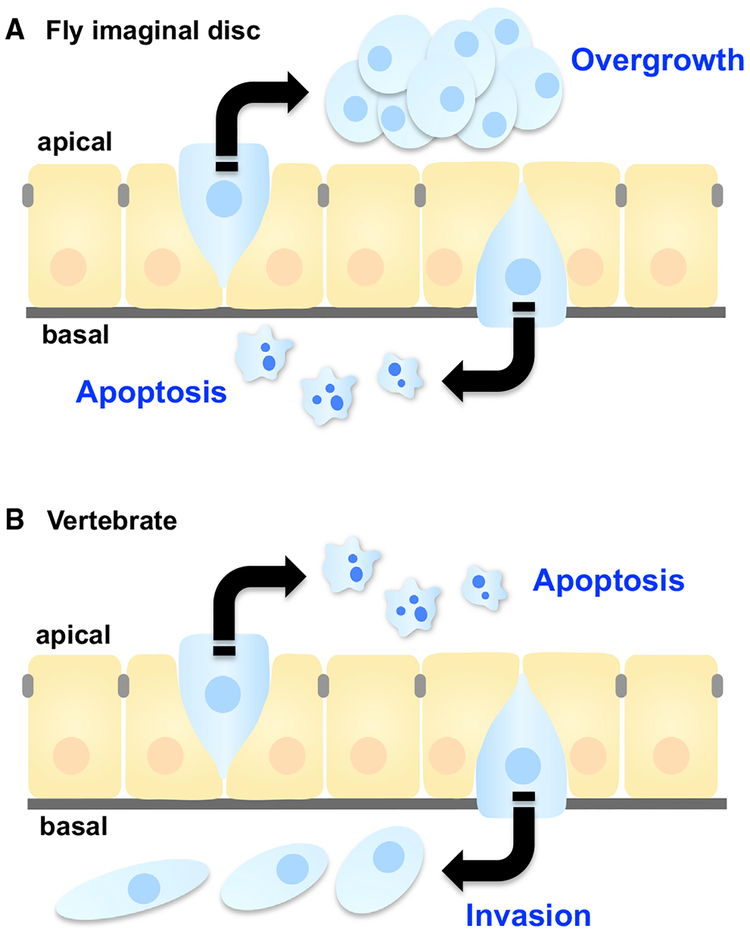
While the active extrusion of scrib cells by Slit-Robo signaling was essential for preventing tumorigenesis (Vaughen and Igaki, 2016), hyperactivating Slit-Robo signaling was similarly fatal due to excessive, apical extrusion into the lumen (Figure 4), where scrib cells evade engulfment (Ohsawa et al., 2011) and can survive on endogenous cytokine signaling (Tamori et al., 2016). Thus, the direction of cell extrusion can determine tumor fate, as was shown for RasV12 cell competition in MDCK monolayers: here, apical extrusion promotes cell death, while basal extrusion is characterized by cellular protrusions that could facilitate metastatic invasion (reviewed in Slattum and Rosenblatt, 2014). Notably, the directionality of tumor-suppressive cell extrusion is reversed between Drosophila imaginal discs and vertebrate epithelia (Figure 5). In Drosophila imaginal epithelia (which form monolayer sacs), basally extruded cells undergo apoptosis and/or are removed by hemocytes, but apically extruded cells can survive as luminal tumorous masses. Conversely, in vertebrate epithelia such as intestines, apical extrusion is thought to sequester and eliminate cells, while basal extrusion may promote invasion of underlying tissue and then secondary metastases. In either case, cells lacking proper “directionality” (such as polarity-defective scrib cells) may be more susceptible to erroneously extruding in a direction beneficial to the cancer and detrimental to the host.
Figure 5. Extrusion Direction Impacts Cancer Cell Outcome.
Epithelial tissues (orange) remove either live or dying cells (blue) in response to crowding or apoptotic stimuli, respectively. Epithelial tissues also eliminate oncogenic cells (blue) through cell competition. Although in most cases, apically extruded cells eventually die by anoikis, anoikis-resistant cells can cause luminal overgrowths in Drosophila. (top). In Drosophila, basally extruded cells undergo apoptotic cell death (A), while basally extruded cells in vertebrates can cause invasion and secondary metastasis (B).
Future work will likely illuminate clearer roles for extrusion direction in other, in vivo cancer models. For example, it was recently demonstrated that human pancreatic cancer cells lack S1P2 and are incapable of apical extrusion, instead utilizing basal extrusion to proliferate and metastasize; notably, rescuing S1P2 expression in pancreatic tumor cells prevented orthotopically transplanted metastasis (and decreased tumor size) in nude mice (Gu et al., 2015).
Could distinct molecular mechanisms govern the direction and outcome of tumor cell extrusion? In MDCK and zebrafish epidermis, p115 RhoGEF has an essential role in determining apical versus basal extrusion (Slattum et al., 2009). When targeted to the basolateral cell surface by microtubules, p115 RhoGEF recruits and contracts the actomyosin ring at baso-lateral intracellular junctions, leading to apical extrusion of apoptotic cells (Figure 1). Disrupting p115 RhoGEF’s basolateral targeting results in actomyosin contraction at the apical surface and erroneous, basal extrusion. Notably, basolateral targeting of microtubules is controlled by adenomatous polyposis coli, a conserved tumor suppressor (Marshall et al., 2011). Intriguingly, the Drosophila homolog of p115 RhoGEF, RhoGEF2, also controls extrusion direction in vivo: scrib cell elimination via basal extrusion in wing discs depends on RhoGEF2, with RhoGEF2KD causing aberrant apical extrusion and tumor survival in permissive microenvironments (Tamori et al., 2016).
Alongside microenvironments rich in survival factors, other mechanisms can permit extruded tumor cells to survive anoikis (reviewed in Buchheit et al., 2014). One intriguing escape route from anoikis is by activating autophagy to survive via cellular self-cannibalization (Fung et al., 2008). Notably, autophagy biases RasV12 cell extrusion basally in MDCK monolayers through direct degradation of S1P (Slattum et al., 2014), hinting that cell-extrusion machinery and anoikis survival are likely coupled. Coupling extrusion to anoikis survival is plausible given the role of cell extrusion in developmental processes such as NB delamination or neural crest EMT, where cells must necessarily avoid anoikis to generate daughter cells or migrate, respectively. Indeed, another common feature of cell extrusion, dysregulated E-cad or p120-catenin, is linked to anoikis resistance in mouse tumor models of mammary carcinomas (Derksen et al., 2006; Schackmann et al., 2013). A last, notable example linking extrusion to anoikis survival is the oncogenic upregulation of ERBB2 (EGFR family member), which promotes anoikis resistance (Haenssen et al., 2010) but also apical extrusion and luminal overgrowth in three-dimensional organotypic human mammary cultures (Leung and Brugge, 2012). Certain cancers may thus hijack these developmental mechanisms, coupling cell extrusion with anoikis resistance to permit survival and then invasion.
Cell Extrusion May Facilitate EMT
An intriguing hypothesis linking extrusion to cancer is that extrusion may facilitate EMT, as suggested by similar players involved in both dynamic processes (Table 1). Conceptually, for an epithelial cell to become mesenchymal and migratory, coupling of extrusion and EMT machinery could be a parsimonious biological solution to the challenge of leaving an epithelium and switching to mesenchymal fate. While evidence directly linking cell extrusion to EMT is currently sparse, overlap between signaling pathways (TGF-β/JNK), junctional remodeling (E-cad downregulation), and cytoskeletal changes (actomyosin contractility) suggests that cell extrusion could prime a cell toward EMT, provided that anoikis is avoided.
Table 1.
Similarities between Cell Extrusion and Epithelial-Mesenchymal Transition
| Cell Extrusion | Epithelial-Mesenchymal Transition | |
|---|---|---|
| TGF-β signaling | Dpp boundaries promote cell extrusion in wing discs and in leg morphogenesis; Dpp required for amnioserosa cell apical constriction and extrusion during dorsal closure; Dpp regulates LEC extrusion | TGF-β is a key regulator of developmental and tumorigenic EMT; exogenous TGF-β is sufficient to trigger EMT in many epithelial cell lines; TGF-β activates a TF network important for EMT |
| JNK signaling | JNK required for apoptotic extrusion of MDCK cells; JNK is essential for tumorigenic scrib or dCsk cell extrusion; JNK acts upstream of Dpp in dorsal closure | JNK required for TGF-β-driven EMT in mouse tracheal epithelia and keratinocytes; JNK required for EMT of A549 human lung cells; JNK required for scrib/RasV12 cell invasion; TRAF6 directly links JNK to TGF-β and EMT |
| Axon-guidance signaling | Slit-Robo2 extrudes scrib cells; ephrin-A-EphA2 extrudes RasV12 MDCK cells; PlexA-Sema1a/1b extrudes wounded cells from epithelia | Slit2-Robo1 signaling downregulates E-cad in colorectal carcinomas and HEK293 cells to drive EMT, cancer growth, and metastasis |
| Junctional rearrangements | E-cad is downregulated or mislocalized (scrib, dCsk); extracellular E-cad is cleaved in MDCK cell extrusion; Coronin 1B recruitment to zonula adherens mediates extrusion and contractility in Caco-2 cells | E-cad cleavage/degradation are EMT hallmarks; apicobasal polarity complexes are disassembled and relocalized to the leading edge; scribKD MDCK cells upregulate Snail, lose E-cad, and increase their motility |
| Cytoskeleton | non-autonomous/autonomous actomyosin drives MDCK cell extrusion; autonomous actomyosin via Ena extrudes scrib cells; autonomous ROCK/MLC extrudes RasV12 cells; p115 RhoGEF/RhoGEF2 determines extrusion direction | increased cell contractility and actin stress fibers via autonomous actomyosin are regulated by Rho/ROCKand MLC during EMT; actin and integrinsare rearranged to promote directional migration; Mena isoforms regulate invadopodia during EMT |
| Twist/Snail TFs | Twist/Snail are required for apical constriction and pulsed actomyosin networks in Drosophila gastrulation, but are not yet directly linked to developmental/oncogenic cell extrusion | Twist/Snail are core TFs in an EMT gene network that represses epithelial (i.e., E-cad) and activates mesenchymal (i.e., vimentin) genes; other key EMT TFs include zinc-finger E-box binding proteins |
| Mechanical force | Piezo1 mediates mechanical cell extrusion in cell cultures and zebrafish epidermis | none reported, although mechanical force can induce Twist (Farge, 2003) |
| S1P | S1P-S1P2 signaling extrudes apoptotic/live and oncogenic cells in culture and zebrafish epidermis | isolated cases of S1P-driven EMT in human ATII/A549 (Milara et al., 2012) and HepG2 lines (Zeng et al., 2016) |
See Cell Extrusion May Facilitate EMT for details and references therein. EMT, epithelial-mesenchymal transition; HEK293, human embryonic kidney cells; MDCK, Madin-Darby canine kidney cells; MLC, myosin light chain; E-cad, E-cadherin; ROCK, Rho-associated protein kinase; TF, transcription factor; S1P, sphingosine-1-phosphate; ATII, epithelial alveolar cell line; A549, human lung cancer cell line; HepG2, human liver hepatocellular carcinoma; Caco-2 cells, human epithelial colorectal adenocarcinoma line.
One key inducer of EMT during development and disease is TGF-β (reviewed in Thiery et al., 2009; Xu et al., 2009), a signaling pathway that also features prominently during Drosophila developmental to orchestrate cell movements, including cell extrusion. Along with Dpp boundaries triggering apoptotic cell-extrusive forces during leg folding or extrusion from wing discs, Dpp also regulates timely dorsal closure and epidermal cell replacement, morphogenetic events dependent on cell extrusion. In epidermal cell replacement, LEC-produced Dpp prevents LECs’ premature extrusion while facilitating histoblast migration by reducing E-cad (Ninov et al., 2010). During dorsal closure, Dpp is required for amnioserosa cell apical constriction and extrusion (Fernandez et al., 2007).
Alongside TGF-β, JNK signaling plays a key role in cell motility and is required in certain instances of cell extrusion and EMT. For example, JNK is required for apoptotic cell extrusion (Eisenhoffer et al., 2012), tumorigenic dCsk (Vidal et al., 2006) and scrib cell extrusion (Vaughen and Igaki, 2016), and also acts upstream of TGF-β/Dpp in extrusion-mediated dorsal closure (Riesgo-Escovar et al., 1996) (Riesgo-Escovar and Hafen, 1997). JNK signaling is also strongly linked to regulating TGF-β-induced EMT and cell motility (Xu et al., 2009). For example, the EMT triggered by TGF-β requires JNK signaling in mouse tracheal epithelial cells (Velden et al., 2011) and in mouse keratinocytes (Santibanez, 2006), with TGF-β1 activating JNK upstream of E-cad mislocalization. Notably, TGF-β can directly activate JNK through TRAF6 binding to TGF-β receptors (Sorrentino et al., 2008; Yamashita et al., 2008), and TRAF6KD blocked EMT in mouse mammary cell lines (Yamashita et al., 2008). Moreover, in human A549 lung cancer cell lines, EMT following radiation (Jung et al., 2007) or TGF-β1 stimulation is JNK dependent (Chen et al., 2013). Lastly, JNK is essential for the cooperative metastasis of scrib/RasV12 tumors in Drosophila (Igaki et al., 2006). Thus, JNK is a versatile regulator of cell movement that broadly regulates extrusion and EMT through diverse mechanisms. However, depending on the context, JNK may instead promote anoikis and death rather than metastasis, as recently demonstrated for murine kidney epithelial cells in vitro and removal of mammary duct epithelia in vivo (Girnius and Davis, 2017).
JNK activation and downstream cell-junction rearrangements such as E-cad mislocalization occur both during EMT and during cell extrusion. In scrib cell extrusion, JNK-activated Slit-Robo2-Ena signaling mislocalized E-cad, and ectopic Robo2-Ena was sufficient to disrupt E-cad and extrude cells (Vaughen and Igaki, 2016; J.V. and T.I., unpublished data). In a notable parallel, Slit2-Robo1 activation in both human colorectal carcinoma lines and human embryonic kidney cells downregulated E-cad to license EMT, and higher Slit2-Robo1 protein levels were associated with worse metastasis and poorer survival in colorectal patients (Zhou et al., 2011). Moreover, in cell culture, scribKD itself triggered EMT-like behaviors by disrupting E-cad (Qin et al., 2005) and by upregulating Snail, a TGF-β target and key EMT gene (Zhou et al., 2016). Alongside junctional remodeling, EMT and cell extrusion necessitate cytoskeletal rearrangements, which are both broadly characterized by increased contractility (Thiery et al., 2009; Xu et al., 2009), such as in the cases of oncogenic scrib (Vaughen and Igaki, 2016) or RasV12 cell extrusion (Hogan et al., 2009).
One apparent distinction between EMT and cell extrusion is that the networks of transcription factors (TFs) that are central to most EMTs are not yet clearly implicated in extrusion. During EMT, the basic-helix-loop-helix TF Twist and the zingfinger TF Snail constitute the core EMT machinery that directly represses epithelial genes (i.e., Snail binding to E-cad’s promoter) while promoting mesenchymal gene transcription (Thiery et al., 2009; Xu et al., 2009). Tantalizingly, these canonical EMT factors also play roles in extrusion-like processes. Apical constriction of ventral cells during Drosophila gastrulation is driven by Twist and Snail, with Snail acting to initiate pulsatile contractions driven by an actomyosin network, and Twist acting to stabilize the network in its constricted state (Martin et al., 2009) while also recruiting RhoGEF2 via its target T48 (Kolsch et al., 2007). Rho signaling, which determined extrusion direction in flies (Tamori et al., 2016) and MDCK cells (Slattum et al., 2009), also governs actin dynamics during EMT migration (Thiery et al., 2009; Xu et al., 2009). Moreover, apical constriction and invagination in gastrulation precede a conserved EMT that gives rise to mesoderm in a Snail-dependent manner (Carver et al., 2001; Kalluri and Wein berg, 2009; Thiery et al., 2009; Xu et al., 2009), suggestive of coupling between extrusive-like forces and EMT during development.
This tight relationship between extrusion and developmental programs such as differentiation (NB extrusion and division) or EMT (neural crest extrusion and migration) implies that cancers could recapitulate extrusion-initiated developmental mechanisms to potentiate invasion and proliferation. Indeed, the recent discovery that neuroendocrine lung progenitors extrude and migrate during development provides a new hypothesis for why small-cell lung carcinomas initiated by these cells are highly metastatic and thus particularly deadly: reactivation of a developmental extrusion and subsequent EMT-like migration program is one of the plausible mechanisms underlying this carcinoma’s metastatic tendencies (Kuo and Krasnow, 2015). If extruded cells can indeed undergo EMT, then dysregulated cell extrusion has the potential to trigger the manifold consequences of EMT, including metastasis (Thiery et al., 2009), chemotherapy resistance (Fischer et al., 2015), and a reversion to stem cell-like fates (Mani et al., 2008) or the generation of cancer stem cells (Morel et al., 2008).
Cell-Extrusion Genes Are Strongly Implicated in Human Cancers
Cell-extrusion genes maintain epithelial homeostasis and could enable EMT, and there is accordingly mounting evidence linking dysregulation of these genes to cancers. For example, Slit-Robo signaling, which ievicts tumorigenic cells from epithelia but can also generate lumen tumors upon hyperactivation and excess cell extrusion (Figure 4) (Vaughen and Igaki, 2016), is both inactivated or activated in many human cancers, including epithelial lung and colon cancers (reviewed in Ballard and Hinck, 2012). Though increasing evidence implicates axon guidance genes in human cancers, the current explanation for this predominantly focuses on angiogenic axon-guidance signaling nurturing tumor survival (Chédotal et al., 2005; Surawska et al., 2004). However, the loss or gain of extrusion capability may also contribute to axon-guidance genes’ complex roles in cancer etiology. Indeed, other axon-guidance genes have been linked to metastasis, including Sema3E-Plexin-D1 facilitating metastasis in cancer line xenografts (Casazza et al., 2010), mammalian Ena isoforms mediating invadopodia formation and basement membrane degradation (Philippar et al., 2008), and Plexin-B1 enabling breast cancer metastasis upon ERBB2 activation (Worzfeld et al., 2012), which is of particular interest given ERBB2’s role in extrusion (Leung and Brugge, 2012) and anoikis resistance (Haenssen et al., 2010).
Other cell-extrusion genes intersect with cancer. For example, S1P, the key ligand mediating apoptotic and live cell extrusion, is strongly linked to cancers (reviewed in Ogretmen and Hannun, 2004; Pyne and Pyne, 2010). Though S1P’s role in cancer is multifaceted, the cancer field has mainly explored roles for cer-amide signaling and an inflammatory response; we suggest that dysregulated cell extrusion should also be considered, especially considering the elegant report demonstrating the S1P receptor’s key role in cancer cell extrusion and metastasis (Gu et al., 2015). Intriguingly, S1P is reported to trigger EMT in certain human hepatocellular carcinoma (Zeng et al., 2016) and lung cancer lines (Milara et al., 2012), and S1P was upregulated in patients with idiopathic pulmonary fibrosis, a disease characterized by frequent EMT (Milara et al., 2012); this further hints at the possibility of extrusion-EMT coupling in cancer. Last, Piezo1, the master regulator of live cell extrusion, is mutated or misex-pressed in colorectal, gastric, and thyroid cancers (Gudipaty et al., 2017), and Piezo1 promoted the invasion of gastric cancer cells in vitro (Yang et al., 2014). Thus, genes regulating developmental and homeostatic cell extrusion may broadly be important players in pathological cell extrusion during tumorigenesis.
Conclusions and Outstanding Questions
Cell extrusion is a potent mechanism for maintaining epithelial homeostasis when confronted with internal or external stressors. Both live and apoptotic cell extrusion can sculpt tissues during morphogenesis, and cell extrusion protects tissues from emergent oncogenic cells by eliminating them into the extracellular void. Despite intensive study, many fascinating questions concerning cell extrusion remain. First, although key molecular mechanisms of cell extrusion have been identified, it is unclear how cadherin-based mechanotransduction and/or junctional rearrangements, autonomous actomyosin activity, and/or Piezo1 cooperate with the S1P-S1P2 pathway and non-autonomous actomyosin ring assembly. Moreover, whether extrusion mechanisms found in cell culture are fully relevant to in vivo scenarios awaits further corroboration, although the general conservation of S1P-S1P2-Rho and Piezo1 signaling appears promising. Second, for mechanical competition and subsequent cell extrusion, it would be interesting to test if loser cell extrusion relays mechanical signals back to the epithelia to influence winner cell behavior, as in the mechanical influence of apoptotic extrusion on tissue development. Conversely, during crowding-induced extrusion, what prevents extruding cells from transmitting force to the underlying tissue and affecting tissue morphology? Third, organismal defects associated with blocking crowding-induced extrusion or apoptotic cell extrusion are relatively subtle; are there more consequential developmental or long-term defects that occur when these homeostatic programs are perturbed, as strongly hinted at by explant studies of palate fusion (Kim et al., 2015)?
Cancers often exploit developmental mechanisms such as junctional rearrangements, EMT, or proliferation to enable tumor growth or metastasis. Joining cancer’s developmental-hijacking toolkit is cell extrusion, another key mechanism for cell rearrangement that can eliminate, or disseminate, dangerous oncogenic cells. Balanced, properly directed extrusion is critical for cancer cell outcomes and organismal fate, as demonstrated by cell competition studies of conserved tumor suppressors in Drosophila. Moreover, cancers may spread by extrusion-initiated programs that couple cell extrusion with anoikis resistance and EMT, as in development. While studies in cell culture systems have largely supported these findings, evidence for competition-mediated cell extrusion regulating cancers has not yet been reported outside of Drosophila. Although mouse colon RasV12 cells are apically extruded, and MDCK RasV12 cells undergo competition-based extrusion, the link between such extrusion and tumorigenesis remains to be demonstrated in vivo. Nonetheless, we have every expectation that, as in Drosophila, cell extrusion will continue to be at the fore of epithelial cell homeostasis, a simple but powerful force for combatting the cellular stressors of the dynamic biological world.
ACKNOWLEDGMENTS
We apologize to those whose work we could not cite due to space constraints. The work in the Igaki laboratory was supported in part by grants from the MEXT/JSPS KAKENHI (grant nos. 26114002, 16H02505, 17H03673, and 15H05862), the Takeda Science Foundation, and the Naito Foundation to S.O. and T.I., and Japan Agency for Medical Research and Development (Project for Elucidating and Controlling Mechanisms of Aging and Longevity) to T.I.
REFERENCES
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, and Matsumoto K (1999). Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400, 166–169. [DOI] [PubMed] [Google Scholar]
- Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, and Jones PH (2014). Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat. Cell Biol. 16, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, and Bach EA (2014). Cell competition: how to eliminate your neighbours. Development 141, 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Xue G, Shaobo Y, Mingxi D, Zhou X, Yu W, Ishibashi T, Zhang L, and Yan Y (2017). Apical constriction is driven by a pulsatile apical myosin network in delaminating Drosophila neuroblasts. Development 144, 2153–2164. [DOI] [PubMed] [Google Scholar]
- Baker NE (2017). Mechanisms of cell competition emerging from Drosophila studies. Curr. Opin. Cell Biol. 48, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard MS, and Hinck L (2012). A roundabout way to cancer. Adv. Cancer Res. 114, 187–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt JD, Clay MR, Langenberg T, and Halloran MC (2008). Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev. Biol 324, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Li M, and Perrimon N (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116. [DOI] [PubMed] [Google Scholar]
- Bondar T, and Medzhitov R (2010). p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Pineda CM, Xin T, Boucher J, Suozzi KC, Park S, Matte-Martone C, Gonzalez DG, Rytlewski J, Beronja S, et al. (2017). Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, and Richardson HE (2003). Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J 22, 5769–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit CL, Weigel KJ, and Schafer ZT (2014). Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat. Rev. Cancer 14, 632–641. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, and Lemaitre B (2010). Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, and Watson AJ (2006). Characterization of epithelial cell shedding from human small intestine. Lab. Invest 86, 1052–1063. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, and Gridley T (2001). The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol 21, 8184–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, et al. (2010). Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J. Clin. Invest 120, 2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A, Kerjan G, and Moreau-Fauvarque C (2005). The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ 12, 1044–1056. [DOI] [PubMed] [Google Scholar]
- Chen HH, Zhou XL, Shi YL, and Yang J (2013). Roles of p38 MAPK and JNK in TGF-beta1-induced human alveolar epithelial to mesenchymal transition. Arch. Med. Res 44, 93–98. [DOI] [PubMed] [Google Scholar]
- Claveria C, Giovinazzo G, Sierra R, and Torres M (2013). Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44. [DOI] [PubMed] [Google Scholar]
- Claveria C, and Torres M (2016). Cell competition: mechanisms and physiological roles. Annu. Rev. Cell Dev. Biol 32, 411–439. [DOI] [PubMed] [Google Scholar]
- Creamer B, Shorter RG, and Bamforth J (1961). The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut 2, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beco S, Ziosi M, and Johnston LA (2012). New frontiers in cell competition. Dev. Dyn 241, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekanty A, Barrio L, Muzzopappa M, Auer H, and Milan M (2012). Aneuploidy-induced delaminating cells drive tumorigenesis in Drosophila epithelia. Proc. Natl. Acad. Sci. USA 109, 20549–20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, et al. (2006). Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449. [DOI] [PubMed] [Google Scholar]
- Di Giacomo S, Sollazzo M, de Biase D, Ragazzi M, Bellosta P, Pession A, and Grifoni D (2017). Human cancer cells signal their competitive fitness through MYC activity. Sci. Rep 7, 12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ (2017). Temporal patterning in the Drosophila CNS. Annu. Rev. Cell Dev. Biol 33, 219–240. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, and Rosenblatt J (2012). Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M, Vaughen J, and Igaki T (2015). Non-autonomous overgrowth by oncogenic niche cells: cellular cooperation and competition in tumorigenesis. Cancer Sci 106, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E (2003). Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol 13, 1365–1377. [DOI] [PubMed] [Google Scholar]
- Fearon K, Arends J, and Baracos V (2013). Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol 10, 90–99. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, and Jacinto A (2007). Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech. Dev 124, 884–897. [DOI] [PubMed] [Google Scholar]
- Figueroa-Clarevega A, and Bilder D (2015). Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 33, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al. (2015). Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Lock R, Gao S, Salas E, and Debnath J (2008). Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, and Perrimon N (2005). Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science 307, 1785–1789. [DOI] [PubMed] [Google Scholar]
- Girnius N, and Davis RJ (2017). JNK promotes epithelial cell anoikis by transcriptional and post-translational regulation of BH3-only proteins. Cell Rep 21, 1910–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve AG, and Rabouille C (2014). Extracellular cleavage of E-cadherin promotes epithelial cell extrusion. J. Cell Sci 127, 3331–3346. [DOI] [PubMed] [Google Scholar]
- Gu Y, Forostyan T, Sabbadini R, and Rosenblatt J (2011). Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J. Cell Biol 193, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, and Rosenblatt J (2012). New emerging roles for epithelial cell extrusion. Curr. Opin. Cell Biol 24, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Shea J, Slattum G, Firpo MA, Alexander M, Mulvihill SJ, Golubovskaya VM, and Rosenblatt J (2015). Defective apical extrusion signaling contributes to aggressive tumor hallmarks. Elife 4, e04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Watson AJ, Marchiando AM, Bradford E, Shen L, Turner JR, and Montrose MH (2011). Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am. J. Physiol. Cell Physiol 300, C1404–C1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, Krishnegowda V, and Rosenblatt J (2017). Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudipaty SA, and Rosenblatt J (2017). Epithelial cell extrusion: pathways and pathologies. Semin. Cell Dev. Biol 67, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Haenssen KK, Caldwell SA, Shahriari KS, Jackson SR, Whelan KA, Klein-Szanto AJ, and Reginato MJ (2010). ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. J. Cell Sci 123, 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C, Dupré-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-López LA, Vincent JP, Itoh Y, et al. (2009). Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol 11, 460–467. [DOI] [PubMed] [Google Scholar]
- Homem CC, and Knoblich JA (2012). Drosophila neuroblasts: a model for stem cell biology. Development 139, 4297–4310. [DOI] [PubMed] [Google Scholar]
- Huh JR, Guo M, and Hay BA (2004). Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr. Biol 14, 1262–1266. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, and Xu T (2006). Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol 16, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, and Xu T (2009). Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, and Edgar BA (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JW, Hwang SY, Hwang JS, Oh ES, Park S, and Han IO (2007). Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. Eur. J. Cancer 43, 1214–1224. [DOI] [PubMed] [Google Scholar]
- Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, and Fujita Y (2010). Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J. Cell Sci 123, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M, Sugimura K, Ohoka A, Burden J, Suganuma H, Ikegawa M, Shimada T, Kitamura T, Shindoh M, Ishikawa S, et al. (2014). Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun 5, 4428. [DOI] [PubMed] [Google Scholar]
- Kalluri R, and Weinberg RA (2009). The basics of epithelial-mesenchymal transition. J. Clin. Invest 119, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lewis AE, Singh V, Ma X, Adelstein R, and Bush JO (2015). Convergence and extrusion are required for normal fusion of the mammalian secondary palate. PLoS Biol 13, e1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, and Steele-Mortimer O (2010). Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. USA 107, 17733–17738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, and Leptin M (2007). Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386. [DOI] [PubMed] [Google Scholar]
- Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, Kajita M, Ishikawa S, Yamauchi H, Yako Y, et al. (2017). Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat. Cell Biol 19, 530–541. [DOI] [PubMed] [Google Scholar]
- Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, and Gale JE (2014). Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J. Cell Sci 127, 1229–1241. [DOI] [PubMed] [Google Scholar]
- Kuo CS, and Krasnow MA (2015). Formation of a neurosensory organ by epithelial cell slithering. Cell 163, 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, and Perrimon N (2015). Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev. Cell 33, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, and Morishita Y (2017). Possible roles of mechanical cell elimination intrinsic to growing tissues from the perspective of tissue growth efficiency and homeostasis. PLoS Comput. Biol 13, e1005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, and Brugge JS (2012). Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levayer R, Dupont C, and Moreno E (2016). Tissue crowding induces caspase-dependent competition for space. Curr. Biol 26, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levayer R, Hauert B, and Moreno E (2015). Cell mixing induced by myc is required for competitive tissue invasion and destruction. Nature 524, 476–480. [DOI] [PubMed] [Google Scholar]
- Li W, and Baker NE (2007). Engulfment is required for cell competition. Cell 129, 1215–1225. [DOI] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Alcamo E, Corbin V, and Young MW (1993). Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev 7, 1949–1965. [DOI] [PubMed] [Google Scholar]
- Liu JP, and Jessell TM (1998). A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125, 5055–5067. [DOI] [PubMed] [Google Scholar]
- Lolo FN, Casas-Tinto S, and Moreno E (2012). Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep 2, 526–539. [DOI] [PubMed] [Google Scholar]
- Lubkov V, and Bar-Sagi D (2014). E-cadherin-mediated cell coupling is required for apoptotic cell extrusion. Curr. Biol 24, 868–874. [DOI] [PubMed] [Google Scholar]
- Lynch MD, Lynch CNS, Craythorne E, Liakath-Ali K, Mallipeddi R, Barker JN, and Watt FM (2017). Spatial constraints govern competition of mutant clones in human epidermis. Nat. Commun 8, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan MM, and Madhavan K (1980). Morphogenesis of the epidermis of adult abdomen of Drosophila. J. Embryol. Exp. Morphol 60, 1–31. [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjon C, Sanchez-Herrero E, and Suzanne M (2007). Sharp boundaries of Dpp signalling trigger local cell death required for Drosophila leg morpho-genesis. Nat. Cell Biol 9, 57–63. [DOI] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Hoppe A, Kester L, Thompson BJ, and Tapon N (2013). Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J 32, 2790–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, and Baum B (2012). Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545. [DOI] [PubMed] [Google Scholar]
- Marshall TW, Lloyd IE, Delalande JM, Nathke I, and Rosenblatt J (2011). The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol. Biol. Cell 22, 3962–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, and Wieschaus EF (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Almendro V, and Polyak K (2012). Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334. [DOI] [PubMed] [Google Scholar]
- Maruyama T, and Fujita Y (2017). Cell competition in mammals - novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol 48, 106–112. [DOI] [PubMed] [Google Scholar]
- Merino MM, Levayer R, and Moreno E (2016). Survival of the fittest: essential roles of cell competition in development, aging, and cancer. Trends Cell Biol 26, 776–788. [DOI] [PubMed] [Google Scholar]
- Michael M, Meiring JCM, Acharya BR, Matthews DR, Verma S, Han SP, Hill MM, Parton RG, Gomez GA, and Yap AS (2016). Coronin 1B reorganizes the architecture of F-Actin networks for contractility at steady-state and apoptotic adherens junctions. Dev. Cell 37, 58–71. [DOI] [PubMed] [Google Scholar]
- Milara J, Navarro R, Juan G, Peiro T, Serrano A, Ramon M, Morcillo E, and Cortijo J (2012). Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax 67, 147–156. [DOI] [PubMed] [Google Scholar]
- Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A, and Suzanne M (2015). Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature 518, 245–248. [DOI] [PubMed] [Google Scholar]
- Morata G, and Ballesteros-Arias L (2015). Cell competition, apoptosis and tumour development. Int. J. Dev. Biol 59, 79–86. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, and Puisieux A (2008). Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 3, e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E (2008). Is cell competition relevant to cancer? Nat. Rev. Cancer 8, 141–147. [DOI] [PubMed] [Google Scholar]
- Moreno E, and Basler K (2004). dMyc transforms cells into super-competitors. Cell 117, 117–129. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, and Morata G (2002). Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759. [DOI] [PubMed] [Google Scholar]
- Muenzner P, Rohde M, Kneitz S, and Hauck CR (2005). CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J. Cell Biol 170, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Kuranaga E, Sugimura K, Miyawaki A, and Miura M (2011). Nonautonomous apoptosis is triggered by local cell cycle progression during epithelial replacement in Drosophila. Mol. Cell. Biol 31, 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Meyer EJ, Kroesen A, McKinney SA, and Gibson MC (2013). Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature 500, 359–362. [DOI] [PubMed] [Google Scholar]
- Ninov N, Chiarelli DA, and Martin-Blanco E (2007). Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development 134, 367–379. [DOI] [PubMed] [Google Scholar]
- Ninov N, Menezes-Cabral S, Prat-Rojo C, Manjon C, Weiss A, Pyrowolakis G, Affolter M, and Martin-Blanco E (2010). Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Curr. Biol 20, 513–520. [DOI] [PubMed] [Google Scholar]
- Norman M, Wisniewska KA, Lawrenson K, Garcia-Miranda P, Tada M, Kajita M, Mano H, Ishikawa S, Ikegawa M, Shimada T, et al. (2012). Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci 125, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, and Hannun YA (2004). Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 4, 604–616. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, and Igaki T (2011). Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315–328. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Takemoto D, and Igaki T (2014). Dissecting tumour heterogeneity in flies: genetic basis of interclonal oncogenic cooperation. J. Biochem 156, 129–136. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, and Xu T (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Model. Mech 1, 144–154, discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost M, Otto G, Theriot JA, and Amieva MR (2006). Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog 2, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Garijo A, Martin FA, and Morata G (2004). Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development 131, 5591–5598. [DOI] [PubMed] [Google Scholar]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. (2008). A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15, 813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porazinski S, de Navascués J, Yako Y, Hill W, Jones MR, Maddison R, Fujita Y, and Hogan C (2016). EphA2 drives the segregation of Ras-transformed epithelial cells from normal neighbors. Curr. Biol 26, 3220–3229. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, and Pyne S (2010). Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 10, 489–503. [DOI] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, and Macara IG (2005). The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol 171, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RD, Bach EA, and Cagan RL (2004). Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol. Cell. Biol 24, 6676–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BH, Wilk R, Schock F, and Lipshitz HD (2004). Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr. Biol 14, 372–380. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, and Hafen E (1997). Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev 11, 1717–1727. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Jenni M, Fritz A, and Hafen E (1996). The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev 10, 2759–2768. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, and Cramer LP (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol 11, 1847–1857. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, and Steller H (2004). Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 7, 491–501. [DOI] [PubMed] [Google Scholar]
- Santibanez JF (2006). JNK mediates TGF-beta1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett 580, 5385–5391. [DOI] [PubMed] [Google Scholar]
- Saw TB, Doostmohammadi A, Nier V, Kocgozlu L, Thampi S, Toyama Y, Marcq P, Lim CT, Yeomans JM, and Ladoux B (2017). Topological defects in epithelia govern cell death and extrusion. Nature 544, 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackmann RC, Klarenbeek S, Vlug EJ, Stelloo S, van Amersfoort M,Tenhagen M, Braumuller TM, Vermeulen JF, van der Groep P, Peeters T, et al. (2013). Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer Res 73, 4937–4949. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Shevchenko A, Shevchenko A, and Knoblich JA (2000). A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol 10, 353–362. [DOI] [PubMed] [Google Scholar]
- Schober M, Schaefer M, and Knoblich JA (1999). Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature 402, 548–551. [DOI] [PubMed] [Google Scholar]
- Shen J, and Dahmann C (2005). Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science 307, 1789–1790. [DOI] [PubMed] [Google Scholar]
- Shraiman BI (2005). Mechanical feedback as a possible regulator of tissue growth. Proc. Natl. Acad. Sci. USA 102, 3318–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB, and Carroll SB (1992). Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development 114, 939–946. [DOI] [PubMed] [Google Scholar]
- Slattum G, Gu Y, Sabbadini R, and Rosenblatt J (2014). Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Curr. Biol 24, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattum G, McGee KM, and Rosenblatt J (2009). P115 RhoGEF and micro-tubules decide the direction apoptotic cells extrude from an epithelium. J. Cell Biol 186, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattum GM, and Rosenblatt J (2014). Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat. Rev. Cancer 14, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, and Landstrom M (2008). The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol 10, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Li DM, Huang H, and Xu T (2003). A genetic screen for modifiers of the lats tumor suppressor gene identifies C-terminal Src kinase as a regulator of cell proliferation in Drosophila. Oncogene 22, 6436–6444. [DOI] [PubMed] [Google Scholar]
- Struhl G, Fitzgerald K, and Greenwald I (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331–345. [DOI] [PubMed] [Google Scholar]
- Surawska H, Ma PC, and Salgia R (2004). The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev 15, 419–433. [DOI] [PubMed] [Google Scholar]
- Tamori Y, and Deng WM (2011). Cell competition and its implications for development and cancer. J. Genet. Genomics 38, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Suzuki E, and Deng WM (2016). Epithelial tumors originate in tumor hotspots, a tissue-intrinsic microenvironment. PLoS Biol 14, e1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Qin L, Le Borgne R, and Toyama Y (2017). Remodeling of adhesion and modulation of mechanical tensile forces during apoptosis in Drosophila epithelium. Development 144, 95–105. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, and Nieto MA (2009). Epithelialmesenchymal transitions in development and disease. Cell 139, 871–890. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Peralta XG, Wells AR, Kiehart DP, and Edwards GS (2008). Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughen J, and Igaki T (2016). Slit-robo repulsive signaling extrudes tumor-igenic cells from epithelia. Dev. Cell 39, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velden JL, Alcorn JF, Guala AS, Badura EC, and Janssen-Heininger YM (2011). c-Jun N-terminal kinase 1 promotes transforming growth factor-beta1-induced epithelial-to-mesenchymal transition via control of linker phosphorylation and transcriptional activity of Smad3. Am. J. Respir. Cell Mol. Biol 44, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Larson DE, and Cagan RL (2006). Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev. Cell 10, 33–44. [DOI] [PubMed] [Google Scholar]
- Vidal M, Salavaggione L, Ylagan L, Wilkins M, Watson M, Weilbaecher K, and Cagan R (2010). A role for the epithelial microenvironment at tumor boundaries: evidence from Drosophila and human squamous cell carcinomas. Am. J. Pathol 176, 3007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Fletcher AG, and Baena-Lopez LA (2013). Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell Biol 14, 581–591. [DOI] [PubMed] [Google Scholar]
- Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boc-card F, and Lemaitre B (2005). Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. USA 102, 11414–11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff L, Goschorska M, Kozyrska K, Duclos G, Kucinski I, Chessel A, Hampton-O’Neil L, Bradshaw CR, Allen GE, Rawlins EL, et al. (2016). Mechanical cell competition kills cells via induction of lethal p53 levels. Nat. Commun 7, 11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Duckworth CA, Burkitt MD, Watson AJ, Campbell BJ, and Pritchard DM (2015). Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet. Pathol 52, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, and Knust E (1999). Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402, 544–547. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, and Offer-manns S (2012). ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J. Clin. Invest 122, 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lamouille S, and Derynck R (2009). TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19, 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, and Miura M (2011). Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J. Cell Biol 195, 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ohsawa S, Kunimasa K, and Igaki T (2017). The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature 542, 246–250. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yako Y, Fujioka Y, Kajita M, Kameyama T, Kon S, Ishikawa S, Ohba Y, Ohno Y, Kihara A, et al. (2016). A role of the sphingo-sine-1-phosphate (S1P)-S1P receptor 2 pathway in epithelial defense against cancer (EDAC). Mol. Biol. Cell 27, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, and Zhang YE (2008). TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol. Cell 31, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XN, Lu YP, Liu JJ, Huang JK, Liu YP, Xiao CX, Jazag A, Ren JL, and Guleng B (2014). Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig. Dis. Sci 59, 1428–1435. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Pascoe HG, Pereira T, Kondo S, Jacinto A, Zhang X, and Hariharan IK (2016). Plexins function in epithelial repair in both Drosophila and zebrafish. Nat. Commun 7, 12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang Y, Feng T, Wu J, and Liu X (2016). Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/ syndecan-1/TGF-beta auto-crine loop. Oncotarget 7, 63324–63337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF, Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, et al. (2011). Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res 21, 609–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chang R, Ji W, Wang N, Qi M, Xu Y, Guo J, and Zhan L (2016). Loss of scribble promotes snail translation through translocation of HuR and enhances cancer drug resistance. J. Biol. Chem 291, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]