Abstract
Although gas adsorption properties of extended three-dimensional metal−organic materials have been widely studied, they remain relatively unexplored in porous molecular systems. This is particularly the case for porous coordination cages for which surface areas are typically not reported. Herein, we report the synthesis, characterization, activation, and gas adsorption properties of a family of carbazole-based cages. The chromium analog displays a coordination cage record BET (Brunauer−Emmett−Teller) surface area of 1235 m2/g. With precise synthesis and activation procedures, two previously reported cages similarly display high surface areas. The materials exhibit high methane adsorption capacities at 65 bar with the chromium (II) cage displaying CH4 capacities of 194 cm3/g and 148 cm3/cm3. This high uptake is a result of optimal pore design, which was confirmed via powder neutron diffraction experiments.
As a result of continually increasing global reserves,1 natural gas has been touted as a cleaner alternative to gasoline and coal as a fuel in the transportation and power generation sectors, respectively.2,3 Because natural gas primarily consists of methane, which is the upper limit of H:C ratio, it is the most gravimetrically energy dense hydrocarbon fuel. Consequently, its combustion releases considerably less pollutants on a per energy basis.4 Indeed, U.S. CO2 emissions from power generation are near a 30 year low resulting from a transition to natural gas.5 In the transportation sector, however, the low volumetric energy density of methane at STP has limited its utility.6 Proposed solutions to this have included liquefaction or compression. Both of these have generally been viewed as not viable in passenger vehicles.7 Adsorbed natural gas systems have shown promise in this regard.8 The challenge here is the development of an adsorbent with appropriate methane capacity. Zeo-lites,9,10 activated carbons,11 metal−organic frameworks (MOFs),12–18 and a variety of other porous materials have been investigated for high pressure natural gas storage. MOFs currently display the most promising uptakes19–22 with reported CH4 adsorption capacities as high as 259 cm3/cm3.23 Additionally, a number of interesting adsorption properties have been observed in these materials, including flexibility for thermal management and temperature-dependent negative gas adsorption.22,24 However, advancements in this area remain short of Department of Energy targets.25
Porous molecular adsorbents may show promise in this regard as they can be thought of as soluble adsorbents with designer pores. This could potentially endow them with favorable properties such as tailored syntheses, flexibility upon adsorption, cooperative binding, and increased ease of processability. These molecules, which can be either all-organic26–28 or metal−organic,29–31 are discrete zero-dimensional materials that display permanent porosity in the solid state. Although the underlying chemistry of porous organic cages and porous coordination cages has been established for decades, permanent porosity in these materials is a relatively recent phenomena when compared to zeolites and MOFs. As a result, they have remained almost completely unexplored for high-pressure gas storage applications. This is somewhat surprising given their conceptually analogous nature to porous extended solid materials. In fact, many metal−organic frameworks contain cages as their building units.32,33 In an effort to expand the library of carboxylate-based porous cages, we have thoroughly investigated the synthesis of novel isophthalic acid and carbazole-dicarboxylic acid materials.34 These latter molecules are particularly interesting for gas storage applications as the M12(cdc)12 (cdc2− = carbazoledi-carboxylic acid) octahedral cage is present as one of three cages in DUT-4920 and PCN-82,35 two MOFs with high methane and hydrogen storage capacities, respectively. Given the high surface area of recently reported cuboctahedral M24(R-bdc)24 cages based on chromium(II),30,36 we sought to expand the synthesis of M12(cdc)12 to this metal as a porous cage of this type is expected to show promising high-pressure adsorption properties.
Analogous to the synthesis of the Cu- and Mo-based M12(cdc)12 materials, the air-free reaction of anhydrous Cr2(OAc)4 with H2cdc in a dimethylformamide (DMF)/methanol (MeOH) mixture affords Cr12(cdc)12·nDMF as large red/purple crystals. Single crystal X-ray diffraction confirms the material is isostructural to the previously reported Cu2+ and Mo2+ cages.37,38 This structure is comprised of six bimetallic paddlewheel units in an octahedral arrangement coordinated to 12 cdc2− ligands (Figure 1). The cages consist of 8 triangular windows with corner−corner distances of approximately 12 Å. The molecules pack in the solid state with cage center to center distances of approximately 22 Å. The structure of Cr12(cdc)12 contains solvent accessible voids in excess of 60%, which rivals the values displayed by many MOFs. Accordingly, thermogravimetric analysis (TGA) of a DMF-exchanged sample reveals a mass loss of 40% at 250 °C (Figure S4). Precise solvent exchange protocols must be implemented to achieve high surface areas for molecular materials as cages are potentially soluble in a variety of solvents. For Cr12(cdc)12, room temperature solvent exchanges with DMF and methanol were used. Powder X-ray diffraction confirms the as-synthesized material retains high crystallinity upon exchange with DMF and methanol (Figure 2). However, given the lack of three-dimensional connectivity and in contrast to most MOFs, there is significant structural rearrangement upon initial solvent exchange. Activation at 70 °C affords a BET surface area of 1235 m2/g. To the best of our knowledge, this is the highest surface area reported for a molecular metal−organic material.
Figure 1.
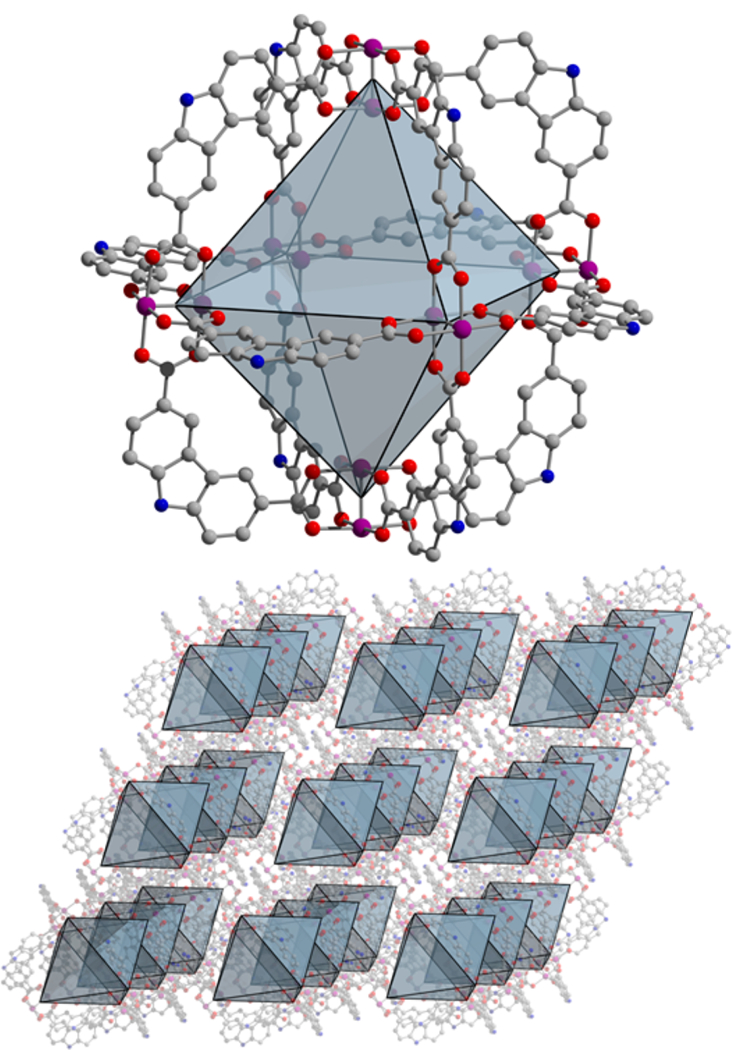
Structure of Cr12(cdc)12 as determined by single-crystal X-ray diffraction. Purple, gray, red, and blue represent chromium, carbon, oxygen, and nitrogen, respectively. Hydrogen atoms and solvent molecules have been omitted.
Figure 2.
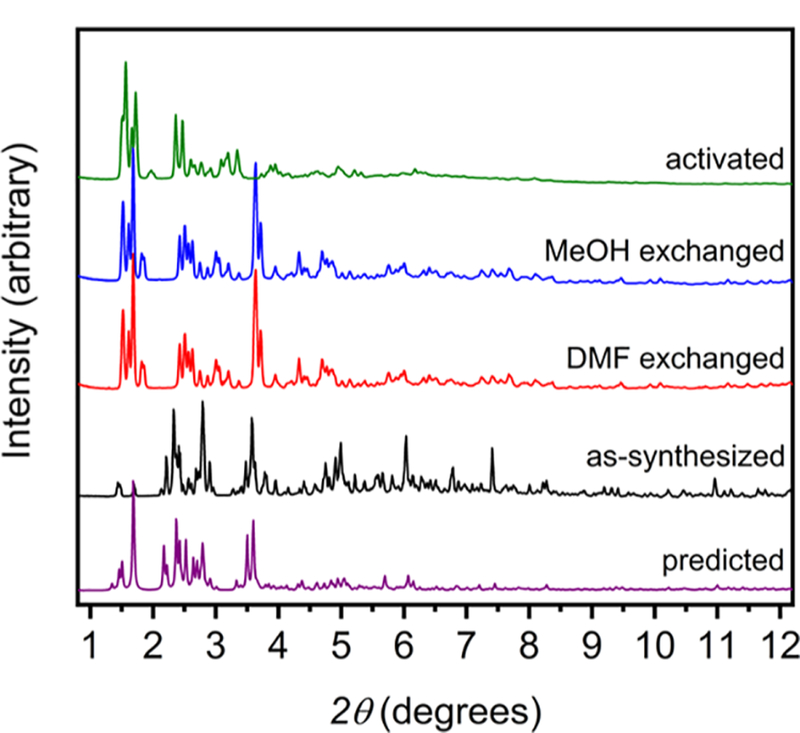
Powder X-ray diffraction patterns of as-synthesized (black), DMF exchanged (red), MeOH exchanged (blue), and desolvated (green) Cr12(cdc)12 vs predicted (purple).
In order to achieve comparable surface areas for Cu12(cdc)12 and Mo12(cdc)12, we surveyed a range of synthesis, solvent exchange, and activation procedures. In contrast to metal− organic frameworks where identical surface areas for a material should be obtained regardless of synthesis conditions (assuming phase purity and full activation), a porous coordination cage can deposit in different crystalline phases depending on synthetic conditions. Although the individual cage structures remain in these various analogs, their three-dimensional packing, stability, and porosity can potentially vary. For Mo12(cdc)12, a DMPU-based synthesis was reported affording a solid that crystallizes in .38 Despite our best efforts, we were only able to obtain an activated material with a surface area of approximately 550 m2/g. Synthesis of the cage in DEF affords the same cage that instead crystallizes in P21/n. Methanol exchange of this material followed by activation at 50 °C affords a solid with a BET surface area of 1108 m2/g. In an analogous manner, Cu12(cdc)12 can be synthesized and crystallized from a variety of amide-based solvents. The highest surface area that was observed (657 m2/g) was for a material synthesized in DMF/MeOH, washed in MeOH, and activated at 50 °C.
To evaluate the methane storage potential of the three porous cages, high-pressure adsorption isotherms were collected from 0 to 65 bar at 298 K. At 35 bar the total gravimetric uptakes follow the surface area trends with capacities of 148, 135, and 81 cm3/g for Cr12(cdc)12, Mo12(cdc)12, and Cu12(cdc)12, respectively (Figure 3). These values are not only significantly higher than those for previously reported coordination cages,39 they are consistent with the capacities displayed by metal−organic frameworks with similar surface areas.15 Methane uptake increases up to at least 65 bar with the Cr12(cdc)12 displaying the highest gravimetric capacity of 194 cm3/g while Mo12(cdc)12 has the highest volumetric capacity (150 cm3/cm3) (Table 1) (Figures S30, S31). To the best of our knowledge, both the gravimetric and volumetric capacities are the highest observed for a porous molecular assembly. For all three materials, moderate hysteresis is seen upon desorption, likely a result of the lack of three-dimensional connectivity between the cages.
Figure 3.
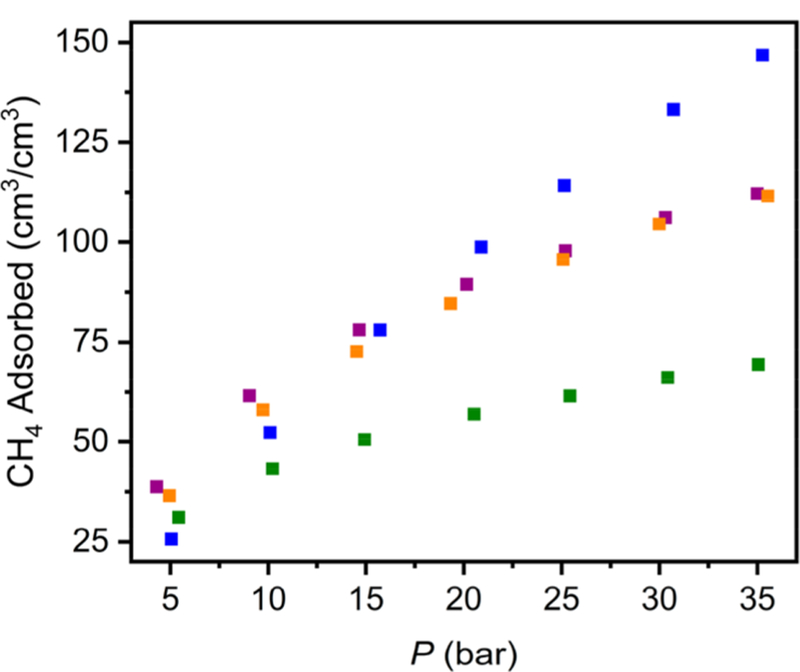
Total high-pressure CH4 adsorption at 298 K in Cr12(cdc)12 (purple), Mo12(cdc)12 (orange), Cu12(cdc)12 (green), and PCN-81 (blue).
Table 1.
Methane Storage Properties of M12(cdc)12 Cages in Comparison to PCN-81, HKUST-1, and IRMOP-51a
| Material | BET Surface Area m2/g |
35 bar Capacity cm3/g [cm3/cm3] |
65 bar Capacity cm3/g [cm3/cm3] |
|---|---|---|---|
| IRMOP-5139 | 480 | 48.4 [25] | — |
| Cu12(cdc)12 | 657 | 81.3 [69.4] | 111.8 [95.4] |
| Cr12(cdc)12 | 1235 | 147.5 [112.1] | 193.9 [147.8] |
| Mo12(cdc)12 | 1108 | 135.0 [111.5] | 181.1 [150.0] |
| PCN-81 | 4050 | 305.3 [146.8] | 448.7 [215.8] |
| HKUST-115 | 1850 | 255.24 [224.9] | 298.5 [263.0] |
All values are total uptake.
To directly compare the CH4 storage capacities of M12(cdc)12 cages with MOFs containing similar methane binding environments, we targeted previously reported materials with M12(cdc)12 as their building units. DUT-49 has been thoroughly investigated for its high CH4 uptake at high pressure, however the flexibility of the material complicates adsorption studies.24 Two analogous materials, PCN-81 and PCN-82, are similarly based on M12(cdc)12 cages, although the former was shown to lack permanent porosity.35 However, synthesis of PCN-81 via the reported route followed by thorough room temperature DMF and MeOH exchanges and activation at 100 °C afforded a highly crystalline material with a BET surface area of 4050 m2/g. Consistent with its high gravimetric surface area, PCN-81 displays an incredibly high CH4 uptake of 305 cm3/g at 35 bar and 298 K. At 65 bar this value reaches 449 cm3/g. Given the low density of the material, its volumetric capacity at 35 bar is significantly lower at 147 cm3/cm3. As a result of the shallow nature of its adsorption isotherm (Figure 3), PCN-81 has a deliverable CH4 capacity of 190 cm3/cm3 for a pressure swing of 65 to 5 bar, which is on par with the highest value reported for a porous material. Low temperature isotherms collected over a range of temperatures indicate methane adsorption enthalpies of −16 to −18.5 kJ/ mol for all four materials.
We turned to powder neutron diffraction to gain insight into the nature of CH4 adsorption in these materials. Although the porous coordination cages retain crystallinity upon solvent removal, it is decreased during activation to the point of precluding the use of diffraction studies to interrogate binding sites. PCN-81, however, remains highly crystalline upon degassing. Refinement of data collected on an activated sample reveals it is significantly less distorted upon evacuation (Figure 4) compared to its solvated state.35 At a CD4:Cu loading of 1:1, three main adsorption sites are apparent. Although the framework is composed of three types of pores, the methane is exclusively adsorbed in the M12(cdc)12 cage. A primary adsorption site is the open copper(II) center with a Cu−C distance of 2.777(5) Å and an occupancy of 0.63(3). A site with similar occupancy (0.64(3)) is at the edge of a carbazole ligand with Cmethane−Cligand distances of 3.27 and 3.69 Å. This methane molecule is only present in six of the eight triangular windows of the Cu12(cdc)12 building unit. Together, these two adsorption sites form the basis for the lowest occupancy site (0.28(3)) in a triangular pocket between two of the metal-bound CD4 and a carbazole-bound CD4 with methane−methane distances of 2.79−4.13 Å (Figure 4). At double the methane loading, a number of additional sites are populated. The occupancy of the first two sites are essentially unchanged while the occupancy of the third site increases to 0.37(3). At this loading, the final two triangular windows of the cage are populated at their three corners. An additional adsorption site on the inside of the cage and the copper on the exterior bind methane at this loading. Increasing the methane dose to 4.0/ Cu reveals no additional CD4 binding sites, although the overall occupancy increases. Interestingly, at this loading the small cage in PCN-81 remains the only one that is occupied. Furthermore, the concurrent population of multiple adsorption sites in PCN-81 is consistent with the shallow nature of the adsorption isotherm, which indicates a lack of very strong methane binding sites. Eight methane adsorption sites are related by symmetry to afford a total methane capacity of 49 molecules per cage. This corresponds to a methane adsorption capacity of approximately 260 cm3/g, a value PCN-81 reaches by 35 bar. At this pressure, the two additional pores in the material remain unoccupied but would allow for additional storage at higher pressures. At lower pressures, the two other pores are essentially empty space that is detrimental to the density, and thus volumetric capacity of the material. This is consistent with the nearly equal volumetric adsorption capacities of PCN-81, Cr12(cdc)12, and Mo12(cdc)12 at 35 bar and 298 K.
Figure 4.
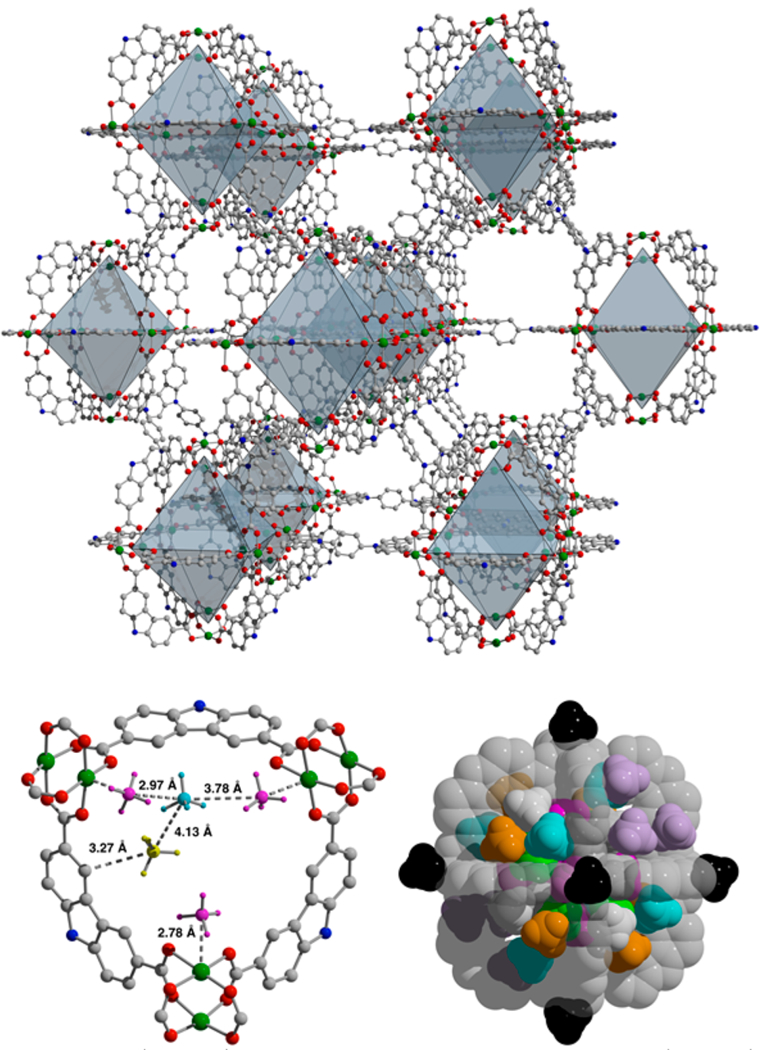
(Upper) Structure of activated PCN-81. (Lower) CD4 adsorption in PCN-81 as determined by powder neutron diffraction. (Left) At low loading three sites in the Cu12(cdc)12 portion of the structure are populated. (Right) Up to 4.0 CD4/Cu, methane exclusively resides in the octahedral cage. With 12 Cu2+ ions per cage, this results in a cage capacity of 49 methane molecules.
In conclusion, we have shown that with precise synthesis, solvent exchange, and activation procedures, high surface areas are attainable for porous coordination assemblies. Although the surface areas displayed by these materials currently fall short of the record values displayed by metal−organic frameworks, the materials discussed here show the potential utility of porous coordination cages for high-pressure gas storage. Neutron diffraction experiments suggest the M12(cdc)12 cage that is also present in PCN-81 and a number of related MOFs may be the optimal pore environment for high-pressure methane storage. An ideal methane storage material may consist nearly entirely of M12(cdc)12 cages, whether it is molecular or an extended solid. Future work along these lines will involve tailoring cage stability, solubility, and gas uptake via ligand functionalization.
Supplementary Material
Acknowledgments
Funding
We are grateful to the University of Delaware for start-up funds. This paper was prepared under cooperative agreement no. 70NANB17H302 from NIST, U.S. Department of Commerce. We acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce, in providing the neutron facilities used in this work. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. We thank the staff of 17-BM for help with synchrotron X-ray data collection. B.A.T. recognizes the National Academies/National Research Council for his Postdoctoral Fellowship. A portion of this work was supported by the National Institutes of Health under award number P20GM104316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Detailed experimental procedures, powder X-ray and neutron diffraction data, gas adsorption isotherms and fits, and spectroscopic data (PDF)Single-crystal data for C204H176Cu12N24O66 (CIF)Single-crystal data for C210H178Cr12N26O68 (CIF)Single-crystal data for C168H84Mo12N12O68 (CIF)Single-crystal data for C17.00CuH8.00NO4 (CIF)Single-crystal data for C17.79H8.00CuD3.17NO4 (CIF)Single-crystal data for C18.89H8.00CuD7.56NO4 (CIF)Single-crystal data for C20.25H8.00CuD13.00NO4 (CIF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Xu C; Bell L Oil 2017, 115, 18–19. [Google Scholar]
- (2).Khan MI Energy Policy 2017, 110, 126–136. [Google Scholar]
- (3).Pratson LF; Haerer D; Patino-Echeverri D Environ. Sci. Technol 2013, 47, 4926–4933. [DOI] [PubMed] [Google Scholar]
- (4).Celzard A; Fierro V Energy Fuels 2005, 19, 573–583. [Google Scholar]
- (5).Annual Energy Outlook 2018 U.S. Energy Information Administration, www.eia.gov/aeo (accessed 05/31/2018).
- (6).Alternative Fuels Data Center − Fuel Properties Comparison, 2013, http://www.afdc.energy.gov/fuels/fuel_comparison_chart.pdf. (accessed 05/31/2018).
- (7).Rios-Mercado RZ; Borraz-Sanchez C Appl. Energy 2015, 147, 536–555. [Google Scholar]
- (8).Vasiliev LL; Kanonckik LE; Mishkinis DA; Rabetsky MI Int. J. Therm. Sci 2000, 39, 1047–1055. [Google Scholar]
- (9).Duren T; Sarkisov L; Yaghi OM; Snurr RQ Langmuir 2004, 20, 2683–2689. [DOI] [PubMed] [Google Scholar]
- (10).Menon VC; Komarneni SJ Porous Mater 1998, 5, 43–58. [Google Scholar]
- (11).Lozano-Castello D; Alcaniz-Monge J; de la Casa-Lillo MA;Cazorla-Amoros D; Linares-Solano A Fuel 2002, 81, 1777–1803. [Google Scholar]
- (12).Furukawa H; Yaghi OM J. Am. Chem. Soc 2009, 131, 8875– 8883. [DOI] [PubMed] [Google Scholar]
- (13).Ma S; Zhou HC Chem. Commun 2010, 46, 44–53. [DOI] [PubMed] [Google Scholar]
- (14).Makal TA; Li J-R; Lu W; Zhou H-C Chem. Soc. Rev 2012, 41, 7761–7779. [DOI] [PubMed] [Google Scholar]
- (15).Mason JA; Veenstra M; Long JR Chem. Sci 2014, 5, 32– 51. [Google Scholar]
- (16).He Y; Qian G; Chen B; Zhou W Chem. Soc. Rev 2014, 43, 5657–5678. [DOI] [PubMed] [Google Scholar]
- (17).Li B; Wen H-M; Zhou W; Xu JQ; Chen C Chem 2016,1, 557–580. [Google Scholar]
- (18).Lin Y; Kong C; Zhang Q; Chen L Adv. Energy Mater 2017,7, 1601296. [Google Scholar]
- (19).Ma S; Sun D; Simmons JM; Collier CD; Yuan D; Zhou H-CJ Am. Chem. Soc 2008, 130, 1012–1016. [DOI] [PubMed] [Google Scholar]
- (20).Stoeck U; Krause S; Bon V; Senkovska I; Kaskel S Chem. Commun 2012, 48, 10841–10843. [DOI] [PubMed] [Google Scholar]
- (21).Gandara F; Furukawa H; Lee S; Yaghi OM J. Am. Chem. Soc 2014, 136, 5271–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mason JA; Oktawiec J; Taylor MK; Hudson MR;Rodriguez J; Bachman JE; Gonzalez MI; Cervellino A; Guagliardi A; Brown CM; Llewellyn PL; Masciocchi N; Long JR Nature 2015, 527, 357–361. [DOI] [PubMed] [Google Scholar]
- (23).Tian T; Zeng Z; Vulpe D; Casco ME; Divitini G;Midgley PA; Silvestre-Albero J; Tan J-C; Moghadam PZ; Fairen-Jimenez D Nat. Mater 2018, 17, 174–179. [DOI] [PubMed] [Google Scholar]
- (24).Krause S; Bon V; Senkovska I; Stoeck U; Wallacher D;Tobbens DM; Zander S; Pillai RS; Maurin G; Coudert F-X; Kaskel S Nature 2016, 532, 348–352. [DOI] [PubMed] [Google Scholar]
- (25).Methane Opportunities for Vehicular Energy; Funding Opportunity no. DE-FOA-0000672; Advanced Research Project Agency− Energy, U.S. Department of Energy, 2012. [Google Scholar]
- (26).Tozawa T; Jones JTA; Swamy SI; Jiang S; Adams DJ; Shakespeare S; Clowes R; Bradshaw D; Hasell T; Chong SY; Tang C; Thompson S; Parker J; Trewin A; Bacsa J; Slawin AMZ; Steiner A; Cooper AI Nat. Mater 2009, 8, 973–978. [DOI] [PubMed] [Google Scholar]
- (27).Chen L; Reiss PS; Chong SY; Holden D; Jelfs KE;Hasell T; Little MA; Kewley A; Briggs ME; Stephenson A; Thomas KM; Armstrong JA; Bell J; Busto J; Noel R; Liu J; Strachan DM; Thallapally BK; Cooper AI Nat. Mater 2014, 13, 954–960. [DOI] [PubMed] [Google Scholar]
- (28).Hasell T; Cooper AI Nat. Rev. Mater 2016, 1, 16053. [Google Scholar]
- (29).Chen T-H; Wang L; Trueblood JV; Grassian VH; Cohen SM J. Am. Chem. Soc 2016, 138, 9646–9654. [DOI] [PubMed] [Google Scholar]
- (30).Lorzing GR; Trump BA; Brown CM; Bloch ED Chem.Mater 2017, 29, 8583–8587. [Google Scholar]
- (31).Fang Y; Xiao Z; Li J; Lollar C; Liu L; Lian X; Yuan S;Banerjee S; Zhang P; Zhou H-C Angew. Chem., Int. Ed 2018, 57, 5283–5287. [DOI] [PubMed] [Google Scholar]
- (32).Yan Y; Lin X; Yang S; Blake AJ; Dailly A; Champness NR; Hubberstey P; Schroder M Chem. Commun 2009, 0, 1025– 1027. [DOI] [PubMed] [Google Scholar]
- (33).Wu H; Simmons JM; Liu Y; Brown CM; Wang X-S; Ma S; Peterson VK; Southon PD; Kepert CJ; Zhou H-C; Yildirim T; Zhou W Chem. Eur. J 2010, 16, 5205–5214. [DOI] [PubMed] [Google Scholar]
- (34).Gosselin EJ; Rowland CA; Balto KP; Yap GPA;Bloch ED Design and Synthesis of Porous Nickel(II) and Cobalt(II) Cages. Inorg. Chem 2018, DOI: DOI: 10.1021/acs.inorgchem.8b01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lu W; Yuan D; Makal TA; Wei Z; Li J-R; Zhou H-C Dalton Trans 2013, 42, 1708–1714. [DOI] [PubMed] [Google Scholar]
- (36).Park J; Perry Z; Chen Y-P; Bae J; Zhou H-C ACS Appl. Mater. Interfaces 2017, 9, 28064–28068. [DOI] [PubMed] [Google Scholar]
- (37).Li J-R; Timmons DJ; Zhou H-CJ Am. Chem. Soc 2009,131, 6368–6369. [DOI] [PubMed] [Google Scholar]
- (38).Li J-R; Yakovenko AA; Lu W; Timmons DJ; Zhuang W; Yuan D; Zhou H-CJ Am. Chem. Soc 2010, 132, 17599– 17610. [DOI] [PubMed] [Google Scholar]
- (39).Sudik AC; Millward AR; Ockwig NW; Cote AP; Kim J;Yaghi OM J. Am. Chem. Soc 2005, 127, 7110–7118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


