Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy characterized by fibrofatty replacement of RV myocardium resulting in re-entrant ventricular tachycardia (VT). Cardiac magnetic resonance imaging (CMR) can non-invasively measure regional abnormalities using tissue-tracking strain as well as late-gadolinium enhancement (LGE). In this study, we examine arrhythmogenic substrate using regional CMR-strain, LGE and electro-anatomical mapping (EAM) in ARVC patients presenting for VT ablation.
Methods & Results
Twenty-one patients underwent RV endocardial EAM, while 17 underwent epicardial-EAM, to detect dense-scar (<0.5mV) as well as CMR study within 12 months. Quantitative regional strain analysis was performed in all 21 patients, while the presence of LGE was visually examined in 17 patients. Strain was lower in segments with dense-scar on endocardial and epicardial-EAM (−9.7±4.1 vs −7.3±4.0, and −9.8±2.8 vs −7.6±3.8; p<0.05), in segments with LGE-scar (−9.9±4.4 vs −6.0±3.6, p=0.001), and at VT culprit sites (−7.4±3.7 vs −10.1±4.1, p<0.001), compared to the rest of RV. On patient-clustered analysis, a unit-increase in strain was associated with 21% and 18%-decreased odds of scar on endocardial and epicardial-EAM respectively, 17%-decreased odds of co-localizing VT-culprit site, and 43%-decreased odds of scar on LGE-CMR (p<0.05 for all). LGE and EAM demonstrated poor agreement with kappa=0.18 (endocardial, n=17) and kappa=0.06 (epicardial, n=13). Only 8(15%) VT-termination sites exhibited LGE.
Conclusion
Regional myocardial strain on cine-CMR improves detection of arrhythmogenic VT-substrate compared to LGE. This may enhance diagnostic accuracy of CMR in ARVC without the need for invasive procedures and facilitate the planning of VT-ablation procedures.
Keywords: Arrhythmogenic Right Ventricular Cardiomyopathy, Magnetic Resonance Imaging, Late Gadolinium Enhancement, Feature Tracking, Strain, Electro-Anatomical Mapping, Electrophysiological Substrate, Voltage Mapping, Myocardial Scar, Endocardial Substrate, Epicardial Substrate
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy presenting with regional dysfunction and enlargement of the right ventricle (RV) 1. Histologically, there is fibrofatty replacement of the myocardium 2. Diagnosis is challenging and is currently based on the Task Force Criteria (TFC), including imaging, to detect RV global and regional dysfunction and/or enlargement 3. Ventricular arrhythmias characteristic of the disease range from premature ventricular beats to ventricular tachycardia (VT) and/or fibrillation 2,4. Indeed, fibrofatty replacement progresses from epicardium to endocardium and serves as a substrate for reentry 5. Electro-anatomic mapping (EAM) performed as part of an electrophysiological study (EPS) is an important diagnostic tool to rule out mimicking conditions like idiopathic-VT and a therapeutic tool to guide lesion placement during VT-ablation 6. Nonetheless, EPS is invasive, can only be done in an electrophysiological laboratory, and require anesthesia. Cardiac magnetic resonance imaging (CMR) is a promising technique for delineation of RV anatomy and function as well as for characterizing the composition of the RV wall, especially with regard to the presence of fibrosis and fatty tissue 7,8. Indeed, myocardial late gadolinium-enhancement (LGE) after intravenous administration of a gadolinium-based contrast agent has been shown to correlate with histopathology and predict inducible VT in patients with ARVC 8. In recently published work, we have shown the feasibility and reproducibility of feature tracking-based regional RV-strain analysis on cine-CMR for the diagnosis of ARVC 9. However, the correlation between these techniques in delineating arrhythmogenic substrate in RV is unknown.
In this study, we sought to examine and compare VT-substrate using different modalities (regional strain on cine-CMR, LGE imaging, and electroanatomical voltage mapping) in ARVC patients presenting for VT ablation.
METHODS
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
PATIENT POPULATION
Twenty-one patients with ARVC presenting to the Johns Hopkins Hospital for VT-ablation were retrospectively selected based on availability of CMR acquired no more than 12 months prior to the procedure. Patients with implanted devices at the time of CMR acquisition were excluded due to significant artifact which precludes proper strain analysis. All selected patients had undergone comprehensive clinical evaluation to confirm the presence of diagnostic TFC for ARVC 3. All subjects provided written informed consent to be included in research and the study was approved by our institutional review board.
MRI ACQUISITION
Cine images in the short axis (SA) planes were acquired at 1.5 Tesla (Avanto, Siemens Medical Imaging, Erlangen, Germany) using a balanced, steady-state free precession sequence (repetition time/echo time/flip angle [TR/TE/FA] 2.4/1.2/50–75 degrees, matrix 256–192, resolution 1.3×1.3×8 mm3, field of view 30–36 cm, temporal resolution ≤40 ms, slice thickness 6–8 mm). In-plane axial resolution ranged from 0.8–1.5mm per dimension. Delayed enhancement images were acquired at end-diastole during breath-holding using a segmented inversion-recovery gradient-echo turbo fast low angle shot sequence obtained 10–15 min after injection of 0.2 mmol/kilogram of gadopentetate dimeglumine (Magnevist, Bayer) contrast medium. Images were visually scored for the presence or absence of LGE by consensus of two experienced readers in ARVC imaging (SZ and IK).
STRAIN ANALYSIS
Cardiac MR analysis was done in a blinded fashion to the findings of the EP study. For global metrics, RV and left ventricular (LV) ejection-fraction (EF), end-systolic volume (ESV), and end-diastolic volume (EDV) were measured with CVI42 (Circle Cardiovascular Imaging; Client Version 248, Server Version 258; Calgary, Alberta, Canada). RV-ESV and EDV were indexed to body surface area (BSA) using the Dubois formula (BSA = body weight(kg)0.425 × height(cm)0.725 × 0.007184). For regional analysis, we measured peak circumferential strain using Multimodality Tissue Tracking (MTT) software (MTT Version 6.0.4725, Toshiba Medical Systems Corporation, Tokyo, Japan) 10. On SA views, the most basal slice without right atrium through-plane motion during systole, a mid-RV slice and an apical slice were selected for stain analysis. Endocardial and epicardial contours were drawn in a semi-automated manner from anterior to inferior RV insertion points. RV was segmented into outflow tract, free wall, angle, and inferior regions in basal and mid-RV cuts and into free-wall and inferior segments at the apex. The segmentation of the RV at various levels is illustrated in Figure 1. Contours were based on spline curves, the order of which is defined internally by the manufacturer. Points along the contour at end-diastole were used to define a 10×10 mm neighborhood, which was matched to a neighborhood of the subsequent frame within a search window by minimizing the sum of squared differences between pixel intensities. The quality of tracking was visually verified and manually adjusted if necessary. By convention for strain, segmental shortening is negative. Inter-observer reproducibility was measured using a randomly selected sample of 10 cases by two independent observers.
Figure 1-. Right Ventricular Segments.
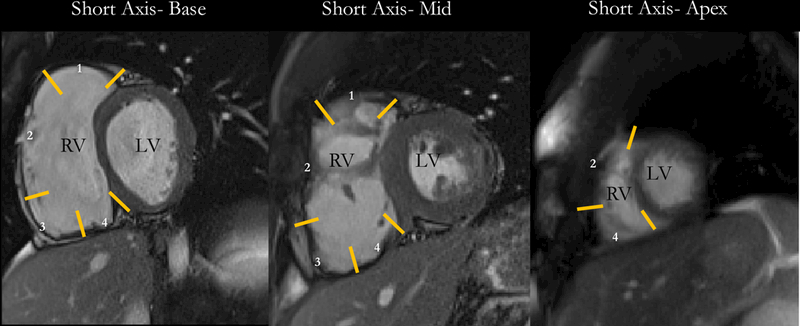
Illustration of the segmentation of the RV on various short-axis levels. Endocardial and epicardial contours were drawn in a semi-automated manner from the anterior to the inferior RV insertion points. RV was segmented into outflow tract (1), free wall (2), angle (3), and inferior regions (4) in basal and mid-RV cuts and into free wall and inferior segments at the apex. RV= Right Ventricle; LV= Left Ventricle.
MAPPING PROCEDURE
Informed written consent was obtained prior to all procedures. For all intra-cardiac electrophysiology studies, vascular access was obtained via femoral veins in a sterile fashion using the modified Seldinger technique. Detailed mapping of the RV endocardium was created in all patients in sinus rhythm using the CARTO system (Biosense Webster, Diamond Bar, CA) and either a 3.5-mm externally irrigated catheter (ThermoCool SF, Biosense Webster) or a 20-pole PentaRay NAV (Biosense Webster) catheter. The voltage data was reviewed during the procedure to ensure appropriate signal quality. Pericardial access was obtained anteriorly by marking 2 cm below the xyphoid process. An epidural needle was advanced under fluoroscopy in lateral and PA views ensuring to enter beneath the xiphoid process. 2–3cc of contrast was injected which outlined the pericardial space. The needle was further advanced until it traversed the pericardial space. A Bentson wire was advanced with circumferential looping of the wire to confirm its presence in the pericardial space. The wire was exchanged with a 0.035-floppy J-wire and a short steerable sheath was then advanced over the J-wire. Mapping catheter was advanced into the pericardial through the steerable sheath where high density mapping was repeated epicardially. Electrograms were band passed filtered at 2–240 Hz for unipolar recordings and at 16–500 Hz for bipolar recordings.
ABLATION STRATEGY
Our ablation strategy has been previously described 11. Briefly, target VTs were clearly defined at the beginning of the procedure by an electrophysiologic study including administration of high-dose Isoproterenol 12. Pace-maps were obtained from the regions of late-potentials and correlated with induced VTs. The arrhythmia was subsequently induced, and critical isthmus was defined with entrainment mapping. Ablation was performed either during VT (for stable VT) or during sinus rhythm in the case of unstable VTs. Radiofrequency energy output with the 3.5-mm irrigated-catheter was set to 20–30W in the epicardium and 40–50W in the endocardium. All regions of late potentials were systematically targeted and ablated. Ablation was guided by significant (>10 Ω) impedance drop during the ablation or by the inability to capture using 10V at 2ms after the ablation. During the waiting period of 30–90 minutes, programmed stimulation with up to 3 ventricular extra-stimuli was delivered from 2 different RV sites. Furthermore, isoproterenol was infused at a rate of 5 μg/min and incrementally increased to 30 μg/min at 2-minute intervals as tolerated. At peak tolerated doses, rapid burst pacing was performed. At the end of the procedure, 125 mg methylprednisolone sodium succinate was injected into the pericardial space, and all patients were given scheduled ketorolac for 1–2 days. Antiarrhythmic drugs were discontinued after catheter ablation unless the patient had frequent ventricular ectopy or recurrent VT.
STATISTICAL ANALYSIS
Continuous and discrete data are presented as mean(±SD), median[IQR] and n(%), respectively. Inter-observer reliability, or ICC and its 95% confidence intervals, was calculated using a 2-way mixed-effects model. The association between endocardial and epicardial dense scar (<0.5mV) on EAM as the dependent variables with clinical characteristics and regional strain absolute values as independent variables was examined using multi-level multivariable logistic regression models, clustered by patient. To avoid co-linearity, correlations between continuous variables by chamber (RV and LV) were tested using the Pearson’s Correlation Coefficient before fitting any 2 independent variables in the same model. Among the pool of co-linear variables, the parameters with the strongest association with the outcome were selected using Akaike’s information criterion (AIC) for inclusion in the multivariable model. Agreement between LGE and EAM-scar assessment was evaluated by kappa analysis whereas LGE vs srain analysis was evaluated using patient-clustered logistic regression. Two-sided P-value less than 0.05 were considered statistically significant. Statistical analysis was performed using STATA software (version 13, StataCorp, College Station, TX).
RESULTS
PATIENT CHARACTERISTICS
Clinical characteristics of the patients are shown in Table 1. Average age at ablation was 30.2±9.9 years, 57% of patients were female, mean BMI was 25.4±5.4 kg/m2. Ten patients (48%) had the desmosomal-PKP2 mutation while the remaining 11 patients were found to carry no pathologic mutation.
Table 1-.
Patient characteristics of the study cohort (all of whom underwent endocardial mapping), as well as characteristics of patients in whom epicardial mapping was performed.
| Endocardial Mapping (n=21) |
Epicardial Mapping (n=17) |
|
|---|---|---|
| Clinical Characteristics | ||
| Male Gender | 9 (43%) | 7 (41%) |
| Age at Ablation | 30.2±9.9 | 28.7±9.5 |
| Age at Diagnosis | 28.7±10.2 | 27.5±9.7 |
| Race | ||
| Caucasian | 18 (86%) | 14 (82%) |
| African-American | 2 (10%) | 2 (12%) |
| Asian | 1 (4%) | 1 (6%) |
| BMI (in kg/m2) | 25.4±5.4 | 24.4±3.6 |
| Congestive Heart Failure | 1 (4%) | 1 (6%) |
| Presentation | ||
| Symptomatic, Not Resuscitated | 18 (86%) | 14 (82%) |
| Sudden Cardiac Death, Resuscitated | 3 (14%) | 3 (18%) |
| Multiple VT Morphologies | 9 (43%) | 9 (53%) |
| VT Storm | 4 (19%) | 4 (24%) |
| Inducibility on EPS | 12 (57%) | 11 (65%) |
| Genotype | ||
| PKP2 (Desmosomal) | 10 (48%) | 9 (53%) |
| No Mutation Identified | 11 (52%) | 8 (47%) |
| Clinical Phenotype | ||
| # of TFC | 6 [5;7] | 6 [6;7] |
| Repolarization TFC | ||
| Major | 16 (76%) | 14 (82%) |
| Minor | 6 (29%) | 5 (29%) |
| Depolarization TFC | ||
| Major | 0 (0%) | 0 (0%) |
| Minor | 7 (33%) | 6 (35%) |
| Arrhythmia TFC | ||
| Major | 6 (29%) | 5 (29%) |
| Minor | 15 (71%) | 13 (76%) |
| Structural TFC | ||
| Major | 16 (76%) | 13 (76%) |
| Minor | 0 (0%) | 0 (%) |
| Family History TFC | ||
| Major | 11 (52%) | 8 (47%) |
| Minor | 1 (4%) | 0 (0%) |
BMI: Body Mass Index; VT: Ventricular Tachycardia; EPS: Electrophysiological Study; TFC: Task Force Criteria. Estimates are described as mean±SD, n (%), or median [inter-quartile range].
STRAIN ANALYSIS
On average, CMR study was performed 3.2[0; 8.1] months prior to the procedure. Mean RV end-diastolic volume index was 163.7±71.5ml/m2, mean RV ejection-fraction (EF) was 40.6±7.9% and LV-EF was 60.5±5.8%. Regional analysis included 210 ventricular segments (10 per patient) as described above. Mean circumferential strain by anatomical regions is summarized in Table 2. Overall, mean circumferential strain was −9.2±3.0% at the RV base, −7.3±3.4% at the mid-RV and −11.5±3.8% at the apex. Inter-observer reproducibility was found to be excellent using a two-way mixed-effects model (ICC=0.87 95%CI [0.81; 0.91], p<0.001). We previously showed excellent reproducibility of strain measurement within and between observers using the same methodology 9.
Table 2-.
Summary of mean longitudinal and circumferential strains stratified by anatomical regions.
| MRI Parameters | Overall ARVC Cohort (n=21 patients) |
|---|---|
| Anatomical | |
| RV EDVi (ml/m2) | 163.7±71.5 |
| RV ESVi (ml/m2) | 104.2±53.1 |
| LV EDVi (ml/m2) | 118.6±40.7 |
| LV ESVi (ml/m2) | 47.6±19.7 |
| Global Function | |
| RVEF (%) | 40.6±7.9 |
| LVEF (%) | 60.5±5.8 |
| Regional Function [n=210 segments (10 per patient)] | |
| Mean Segmental Circumferential Strain (SAX) | |
| Base - Outflow Track (%) | −7.9±3.5 |
| Base - Free Wall (%) | −10.8±3.5 |
| Base - Angle (%) | −11.5±4.6 |
| Base - Inferior (%) | −8.4±3.7 |
| Mid - Outflow Track (%) | −7.7±5.4 |
| Mid - Free Wall (%) | −8.3±4.3 |
| Mid - Angle (%) | −7.3±4.0 |
| Mid - Inferior (%) | −6.1±3.1 |
| Apex - Free Wall (%) | −11.4±6.6 |
| Apex - Angle (%) | −10.6±4.6 |
Measurements are reported as mean±SD. RV: Right Ventricle; LV: Left Ventricle; EDVi: Indexed-End Diastolic Volume; EDVi: Indexed-End Systolic Volume; EF: Ejection Fraction.
VOLTAGE MAPPING
Endocardial mapping was performed in all cases and epicardial mapping in 17 patients. Endocardial dense scar (defined as <0.5mV) was detected in 17(81%) patients and epicardial dense scar in 16(94%) patients. As seen in Table 3, scar was most commonly located at the RV base (angle, inferior aspect and outflow tract) on both endo- and epicardial mapping. Sixty-two percent of patients had inducible VT with 33 different morphologies. At the end of the procedure, VT was non-inducible in all cases. Distribution of VT-culprit sites (defined as ablation sites with VT termination) is included in Table 3. No major or minor complications were reported.
Table 3.
− Distribution of dense scar (<0.5mV) on endocardial and epicardial electro-anatomical mapping.
| Distribution of Scar by Anatomical Segment | Endocardial Dense Scar | Epicardial Dense Scar | Successful Ablation Sites |
|---|---|---|---|
| Base - Outflow Track | 9 (43%) | 12 (71%) | 16 (76%) |
| Base - Free Wall | 5 (24%) | 10 (59%) | 14 (67%) |
| Base - Angle | 7 (33%) | 14 (82%) | 10 (48%) |
| Base - Inferior | 7 (33%) | 12 (71%) | 12 (57%) |
| Mid - Outflow Track | 1 (5%) | 4 (24%) | 4 (19%) |
| Mid - Free Wall | 2 (10%) | 7 (41%) | 4 (19%) |
| Mid - Angle | 5 (24%) | 12 (71%) | 3 (14%) |
| Mid - Inferior | 5 (24%) | 11 (65%) | 5 (24%) |
| Apex - Free Wall | 3 (14%) | 3 (18%) | 0 (0%) |
| Apex - Angle | 2 (10%) | 8 (47%) | 0 (0%) |
| Total | 46 (22%) | 93 (55%) | 68 (32%) |
Measurements are reported as n(%). Endocardial Mapping was performed in 21 patients while epicardial mapping was performed in 17 patients. VT ablation was performed in all 21 patients.
STRAIN-EAM CORRELATION
Mean strain was lower in segments with compared to without EAM-scar both endocardially and epicardially (−9.7±4.1 [n=164 seg.] vs −7.3±4.0 [n=46 seg.], p<0.001 and −9.8±2.8 [n=77 seg.] vs −7.6±3.8 [n=93 seg.], p=0.002; respectively, Suppl. Table 1). On a patient-clustered logistic analysis shown in Table 4, each unit increase in CMR strain was associated with 21% decrease in odds of co-localized scar on endocardial-mapping (OR=0.79, p<0.001) and 18% decrease in odds of co-localized scar on epicardial mapping (OR=0.82, p=0.001), controlling for age at ablation, BMI, genotype, RV end-diastolic volume index, and LV ejection fractions. Furthermore, successful ablation sites were found to have lower mean strain compared to the rest of the RV (−7.4±3.7 vs −10.1±4.1, p<0.001, Suppl. Table 1) and each unit increase in strain was associated with 17% decrease in odds of co-localized VT-culprit site (OR=0.83, p<0.001).
Table 4.
– Summary of the multivariable patient-clustered logistic analysis performed to determine independent predictive value of regional strain for presence or absence of dense scar on endocardial (in 21 patients) and epicardial mapping (in 17 patients).
| Endocardial EAM Scar (n=21 patients, 210 segments) | Epicardial EAM Scar (n=17 patients, 170 segments) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis | Univariate Analysis | Multivariable Analysis | |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| MRI Strain (%) | 0.86 | [0.78; 0.96] | 0.008 | 0.79 | [0.70; 0.89] | <0.001 | 0.82 | [0.73; 0.91] | <0.001 | 0.82 | [0.74; 0.92] | 0.001 |
| Age at Ablation (years) | 0.98 | [0.92; 1.04] | 0.57 | 1.04 | [0.99; 1.11] | 0.10 | 0.98 | [0.94; 1.02] | 0.32 | 0.98 | [0.93; 1.03] | 0.46 |
| BMI (per 5 kg/m2) | 1.05 | [0.59; 1.76] | 0.97 | 1.54 | [0.95; 2.49] | 0.06 | 1.28 | [0.70; 2.19] | 0.44 | 1.10 | [0.59; 2.10] | 0.72 |
| Genotype | ||||||||||||
| Mutation Negative | 1 | − | − | 1 | − | − | 1 | − | − | 1 | − | − |
| PKP2 Positive | 1.18 | [0.38; 3.71] | 0.78 | 0.62 | [0.20; 1.97] | 0.42 | 0.79 | [0.36; 1.73] | 0.55 | 0.58 | [0.20; 1.66] | 0.31 |
| RV EDVi (per 10 ml/m2) | 1.10 | [1.02; 1.22] | 0.021 | 1.10 | [1.02; 1.22] | 0.010 | 1.10 | [1.02; 1.18] | 0.54 | 0.91 | [0.90; 1.10] | 0.89 |
| LV EF (per 5%) | 0.56 | [0.33; 0.95] | 0.045 | 0.53 | [0.31; 0.86] | 0.015 | 0.82 | [0.53; 1.16] | 0.22 | 0.70 | [0.47; 1.10] | 0.15 |
BMI: Body Mass Index; RV: Right Ventricle; LV: Left Ventricle; EDVi: Indexed-End Diastolic Volume; EF: Ejection Fr action; EAM: Electroanatomical Mapping.
Figure 2 exhibits endocardial and epicardial voltage maps in two patients with corresponding regional strain analysis. Interestingly, despite a normal endocardial voltage map, depressed regional myocardial strain correlated with dense-scar on epicardial mapping responsible for the observed regional dyskinesia and fueling of the arrhythmia.
Figure 2-. Illustrative cases of the correlation between CMR-strain and EAM-scar.
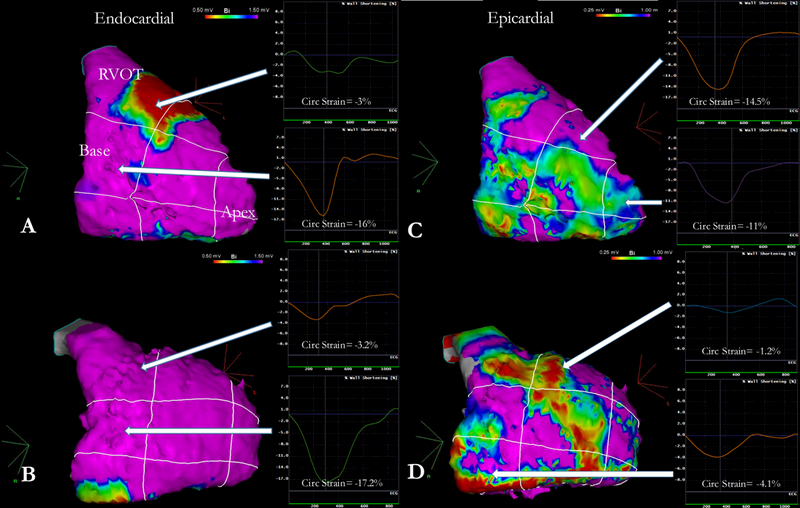
Two representative cases of endocardial and epicardial voltage maps with corresponding regional strain analysis. Panels A and C correspond to a 25-year old female patient, mutation negative genotype, presenting with resuscitated sudden cardiac death with an RV ejection fraction of 36% and RV end-diastolic volume index of 115.2 ml/m2. Strain at the RVOT was severely depressed (−3.0%) but normal otherwise. On endocardial mapping, dense scar at the RVOT was found; epicardial mapping revealed slight drop in bipolar voltage but no scar. Panels B and D correspond to a 35-year old female patient, PKP2 positive genotype, presenting with symptomatic, non-resuscitated VT with an RV ejection fraction of 51% and RV end-diastolic volume index of 108.4 ml/m2. Strain analysis revealed depressed function at the angle, the RVOT and the mid-lateral wall. Although endocardial mapping revealed no voltage abnormalities, further epicardial mapping revealed large areas of dense scar at the corresponding anatomical segments (RVOT, angle and mid-RV). RV= Right Ventricle; RVOT= Right Ventricular Outflow Tract.
LGE ANALYSIS
Late-gadolinium enhanced imaging was performed in 17(81%) participants. RV-LGE was found in 3(18%) of 17 patients, in a total of 15 segments: 1(7%) in the basal free wall, 4(27%) in the inferobasal region, 3(20%) in the mid-RVOT, 6(40%) in the mid-inferior region, and 1(7%) in the apex. Mean strain was significantly lower in LGE-areas (−9.9±4.4 vs −6.0±3.6, p=0.001) and each unit increase in strain was associated with a 43% decrease in odds of co-localized LGE-scar (OR=0.58, p=0.003).
There was modest agreement between endocardial-EAM and LGE on scar assessment (Kappa=0.18, n=21, p=0.004). Mismatch was found in 36(21%) segments, with 28 EAM-scars not confirmed by LGE and 8 LGE-scars undetected by endocardial-EAM. No association was found between epicardial-EAM and LGE scars (K=0.06, n=13, p=0.24). We have performed a multivariable analysis of the association between LGE-scar with endocardial and epicardial EAM-scar. As seen in Supplemental Table 2, presence of LGE-scar was significantly associated with presence of endocardial EAM-scar (OR=11.3, p=0.021, n=17 patients/170 segments) after adjusting for age at ablation, BMI, genotype, RV-EDVi and LV-EF. In a similar adjusted model, presence of LGE-scar was not associated with epicardial EAM-scar (OR=2.6, p=0.20, n=13 patients/130 segments). LGE poorly correlated with VT-culprit sites as 45(85%) of 53 VT-culprit sites were found to be LGE-free (p=0.06).
DISCUSSION
In this study, we examine the arrhythmogenic substrate in the RV of patients with ARVC presenting to our institution for VT ablation using a multi-modal approach: regional strain analysis on cine-CMR, late gadolinium-enhancement imaging, and invasive electroanatomical endocardial and epicardial voltage mapping. Main findings are (1) Strain was significantly lower in segments with dense scar compared to those without dense scar on endocardial and epicardial voltage mapping, and lower CMR-strain was associated with increased odds of harboring endocardial and epicardial dense scar as well as VT culprit sites; (2) Strain was significantly lower in segments with LGE compared to those without LGE, and lower CMR-strain was associated with increased odds of harboring LGE-scar; (3) LGE correlated poorly with EAM-scar on endocardial mapping, and did not correlate with epicardial-scar or successful ablation sites.
REGIONAL STRAIN ANALYSIS
Using quantitative metrics, we assessed regional RV myocardial strain through CMR feature-tracking. We have previously shown that global strain was significantly lower in ARVC compared with control subjects and that regional strain at the subtricuspid area showed the greatest differences between the two groups with good sensitivity and specificity 13. Prati et al. also found that ARVC patients had lower RV global longitudinal, circumferential and radial strain compared to patients with iodiopathic RV outflow tract VT and controls 14. Similarly, Heermann et al. found that global RV longitudinal strain rates and circumferential strain and strain rates at the basal level were significantly lower in ARVC patients compared to controls 15. To the best of our knowledge, we are the first to quantitatively demonstrate inverse association between regional RV myocardial strain with dense scar on endo- and epicardial EAM in ARVC.
REPRODUCIBILITY OF STRAIN ANALYSIS
Prior ARVC studies have measured RV strain using different software methods. Our group has recently assessed the reproducibility of global and regional strain using strain analysis in ARVC between various commercially available software packages 16. In 110 subjects (39 overt ARVC, 40 preclinical ARVC and 31 control), global and regional RV myocardial regional strain were analyzed using four softwares (including Multimodality Tissue Tracking – which we used in our present study). Bland-Altman analysis showed moderate agreement between the softwares but significant variability on the level of individual measurements given the different strain calculation algorithms. Despite this variability, all softwares were able to identify overt ARVC patients from controls by global and regional strain analysis. Therefore, although absolute strain values are not to be translated from one software to another, the use of feature-tracking to measure strain remains a robust method to diagnose regional myocardial dysfunction in ARVC.
LGE-MAPPING OF VT SUBSTRATE
Previous studies demonstrated that fibrofatty ventricular scar can be seen as LGE in ARVC patients 8,17,18. Indeed, Tandri et al. were the first to report RV-LGE in patients with ARVC and demonstrated its relation to fibrofatty myocardial changes 8. In our present study, RV segments with LGE demonstrated regional dyskinesia, with lower circumferential strain compared to areas without LGE. Although never reported in ARVC, the inverse relationship between CMR-regional strain and LGE was previously demonstrated in LV of patients with cardiac amyloidosis as well as hypertrophic cardiomyopathy 19,20.
Marra et al studied the correlation of LGE with endocardial-EAM in the characterization of ARVC-related scar 18. Consistent with our findings, the authors concluded that LGE scars modestly correlated with endocardial-EAM, with ~50% of electric scar in the RV not confirmed by LGE. We also found no agreement between LGE and epicardial-EAM scars. Of note, as seen in Suppl. Table 2, the odds ratio of LGE scar in prediction of endo/epicardial scar were associated with large confidence intervals demonstrating low precision in these estimates. The limited ability of LGE to characterize electrical abnormalities in the RV has been previously reported by Andrews et al. who showed that, in contrast to LV-LGE, RV-LGE did not correlate with electrogram markers of abnormal depolarization such as electrocardiogram amplitude (i.e bipolar voltage) and signal fractionation (p=0.76 and p=0.47, respectively)21. The modest correlation between invasive electro-anatomical substrate mapping and LGE has been attributed to the thin wall of the RV (~3–4mm) which complicates LGE imaging. The complex pathophysiology of ARVC (fibrofatty replacement of the myocardium) makes LGE imaging in the RV all-the-more challenging. Alternatively, it is possible that electrophysiological abnormalities in the RV manifest in the presence of minimal scar or scar that is diffuse and not easily resolved with current imaging techniques. Also, current LGE sequences are designed such that inversion time is inadequately set to null LV instead of RV myocardium 22.
RV STRAIN ANALYSIS FOR ARVC DIAGNOSIS
Our work in validating objective/semi-automatic techniques to analyze regional myocardial function is motivated by the known pitfalls of the 2010 TFC for ARVC diagnosis, which rely on visual inspection of RV and may be incorrectly applied in centers lacking experience in ARVC. In a study from a tertiary care center, misdiagnosis of ARVC was common with only 27% of 89 patients seeking a second opinion after receiving an ARVC diagnosis actually meeting TFC on secondary review 23. Moreover, RV free wall contraction can result in areas of subjective bulging near the moderator band insertion, even in normal patients, which can mimic a wall motion abnormality 24. Tethering of the RV anterior wall to the back of the sternum, frequently seen at the outflow tract, can mimic a wall motion abnormality 25. RV size can be increased in athletes and patients with intra-cardiac shunts, mimicking RV dilation 26. In all these cases, myocardial function is normal and regional strain analysis would be able to objectively rule out ARVC mimics. In recently published work, we have shown that quantitative strain analysis had higher sensitivity and accuracy than qualitative wall motion assessment done by visual inspection by two experts at our tertiary center 9.
RV STRAIN FOR VT SUBSTRATE MAPPING
Intra-cardiac electrophysiological testing has traditionally been used to assess the risk of ventricular arrhythmias, but the prognostic value of VT induced by programmed ventricular stimulation in patients with asymptomatic ARVC remains controversial, particularly in patients with benign-looking imaging 27,28. Although physicians still prefer to perform EPS to diagnose ARVC and/or rule out benign conditions like idiopathic RVOT tachycardia, the invasiveness of the procedure warrants investigating new modalities that do not carry the same inherent risks to the patient. Even in patients with more advanced forms of the disease, this study demonstrates that arrhythmia substrate can be assessed noninvasively using tissue-tracking on cine-CMR, allowing pre-procedural recognition of the spatial distribution of electrophysiologic scar and helping to focus mapping on the culprit region as successful ablation sites were found to have lower strain on CMR compared to the rest of the RV. In contrast, LGE-MRI displayed limited agreement with endocardial-EAM scar, and no agreement whatsoever with epicardial-EAM; thus, in its current state, LGE possesses limited capabilities in comprehensively mapping VT-substrate in RV of patients with ARVC.
LIMITATIONS
Although ARVC is a relatively common reason for heart evaluation by CMR, the disease is quite rare and the sample size is therefore small. This is a retrospective analysis of 21 patients who, as demonstrated by the number of TFC criteria, have advanced disease leading to clinically significant ventricular arrhythmias. Consequently, the results may not be extrapolated to all ARVC subjects with mild or subclinical disease. The small number of subjects included in the multivariable analyses could also lead to type I error or over-fitting. Given the significant artifact created by device leads which would theoretically affect feature-tracking capabilities, and the fact that strain analysis has not been validated as of yet in this setting, we decided to exclude patients with devices at the time of the MRI, which further limits generalizability of our findings. The temporal gap between scan and procedure (3.6±3.2 months) was not pre-defined. However, our recent report on ARVC progression suggests that RV fractional shortening changes minimally over a period of several years and is unlikely to have resulted in a significant change over a 3 month period 29. The thin wall of the RV (like that of the atria) makes analysis of wall strain challenging. However, the in-plane axial resolution of our CINE sequence ranged from 0.8–1.5mm per dimension and is thus able to capture and track the 3–4mm thick RV wall throughout the cardiac cycle. The challenges related to the resolution (spatial or temporal) constitute systematic error and would not affect the detection of inter-segment variability in wall strain. We relied solely on bipolar voltage values to define dense-scar on EAM. This may have led to an overestimation of reported substrate particularly on epicardial mapping due to fat, coronary vasculature and poor catheter contact.
CONCLUSION
In patients with ARVC and ventricular arrhythmias, regional wall-strain assessed on cine-CMR reliably predicts arrhythmogenic VT-substrate. This technique allows safe and improved diagnostic accuracy in ARVC, obviating the need for invasive procedures; and facilitates planning of VT-ablation procedures.
Supplementary Material
Clinical Perspective.
The 2010 TFC for ARVC diagnosis, which rely on visual inspection of RV abnormalities, may be incorrectly applied in centers lacking experience. Quantitative CMR-based strain analysis has higher sensitivity and accuracy than qualitative visual assessment to detect regional RV abnormalities and is thus better able to objectively rule out ARVC mimics. Electrophysiological study is performed to diagnose ARVC and map arrhythmogenic substrate in patients with ventricular arrhythmias but the invasiveness of the procedure warrants investigating new modalities that do not carry the same inherent risks to the patient. This study demonstrates that arrhythmia substrate can be assessed noninvasively using tissue-tracking on cine-CMR, allowing pre-procedural recognition of the spatial distribution of electrophysiologic scar and helping to focus mapping on the culprit regions. In contrast, LGE displays limited agreement with electro-anatomical mapping in RV, and thus possesses limited capabilities in detecting arrhythmogenic scar in patients with ARVC.
Acknowledgments
Sources of Funding
The Johns Hopkins ARVD/C program is supported by the Leyla Erkan Family Fund for ARVD research, the Cohen Family, Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bolge Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments. Dr. Nazarian was funded by the National Institutes of Health (grant nos. K23HL089333 and R01HL116280) as well as by a Biosense Webster.
Footnotes
Disclosures
Dr Nazarian is principal investigator for research funding to Johns Hopkins University from Biosense Webster and has served as a scientific advisor to Biosense Webster, CardioSolv, and St. Jude Medical, Inc. The other authors report no conflicts. Sponsors or funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
REFERENCES
- 1.Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med. 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right Ventricular Cardiomyopathy and Sudden Death in Young People. N Engl J Med. 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 3.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DMY, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of Clinicopathologic Manifestations of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: A Multicenter Study. J Am Coll Cardiol. 1997;30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 5.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial Substrate and Outcome With Epicardial Ablation of Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circulation. 2009;120:366–375. [DOI] [PubMed] [Google Scholar]
- 6.Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia H, Lin D, Nayak H, Russo A, Pulliam W. Electroanatomic Substrate and Outcome of Catheter Ablative Therapy for Ventricular Tachycardia in Setting of Right Ventricular Cardiomyopathy. Circulation. 2004;110. [DOI] [PubMed] [Google Scholar]
- 7.Ricci C, Longo R, Pagnan L, Dalla Palma L, Pinamonti B, Camerini F, Bussani R, Silvestri F. Magnetic resonance imaging in right ventricular dysplasia. Am J Cardiol. 1992;70:1589–95. [DOI] [PubMed] [Google Scholar]
- 8.Tandri H, Calkins H, Nasir K, Bomma C, Castillo E, Rutberg J, Tichnell C, Lima JAC, Bluemke DA. Magnetic Resonance Imaging Findings in Patients Meeting Task Force Criteria for Arrhythmogenic Right Ventricular Dysplasia. J Cardiovasc Electrophysiol. 2003;14:476–482. [DOI] [PubMed] [Google Scholar]
- 9.Vigneault DM, te Riele ASJM, James CA, Zimmerman SL, Selwaness M, Murray B, Tichnell C, Tee M, Noble JA, Calkins H, Tandri H, Bluemke DA Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging. 2016;43:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa K, Hozumi T, Sugioka K, Matsumura Y, Nishiura M, Kanda R, Abe Y, Takemoto Y, Yoshiyama M, Yoshikawa J. Usefulness of Automated Quantitation of Regional Left Ventricular Wall Motion by a Novel Method of Two-Dimensional Echocardiographic Tracking. Am J Cardiol. 2006;98:1531–1537. [DOI] [PubMed] [Google Scholar]
- 11.Philips B, te Riele ASJM, Sawant A, Kareddy V, James CA, Murray B, Tichnell C, Kassamali B, Nazarian S, Judge DP, Calkins H, Tandri H. Outcomes and ventricular tachycardia recurrence characteristics after epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Hear Rhythm. 2015;12:716–725. [DOI] [PubMed] [Google Scholar]
- 12.Philips B, Madhavan S, James C, Tichnell C, Murray B, Needleman M, Bhonsale A, Nazarian S, Laurita KR, Calkins H, Tandri H. High prevalence of catecholamine-facilitated focal ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2013;6:160–6. [DOI] [PubMed] [Google Scholar]
- 13.Vigneault DM, te Riele ASJM, James CA, Zimmerman SL, Selwaness M, Murray B, Tichnell C, Tee M, Noble JA, Calkins H, Tandri H, Bluemke DA Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging. 2016;43:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, Sinagra G, Nucifora G. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circ Cardiovasc Imaging. 2015;8:e003647; discussion e003647. [DOI] [PubMed] [Google Scholar]
- 15.Heermann P, Hedderich DM, Paul M, Schülke C, Kroeger JR, Baeßler B, Wichter T, Maintz D, Waltenberger J, Heindel W, Bunck AC. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2014;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourfiss M, Vigneault DM, Aliyari Ghasebeh M, Murray B, James CA, Tichnell C, Mohamed Hoesein FA, Zimmerman SL, Kamel IR, Calkins H, Tandri H, Velthuis BK, Bluemke DA, te Riele ASJM Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/ cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson. 2017;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen-Chowdhry S, Prasad SK, Syrris P, Wage R, Ward D, Merrifield R, Smith GC, Firmin DN, Pennell DJ, McKenna WJ. Cardiovascular Magnetic Resonance in Arrhythmogenic Right Ventricular Cardiomyopathy Revisited. J Am Coll Cardiol. 2006;48:2132–2140. [DOI] [PubMed] [Google Scholar]
- 18.Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, Silvano M, Rigato I, Tona F, Tarantini G, Cacciavillani L, Basso C, Buja G, Thiene G, Iliceto S, Corrado D. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012;5:91–100. [DOI] [PubMed] [Google Scholar]
- 19.Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, Wintersperger BJ, Thavendiranathan P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson. 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Chen J, Yang Z, Li R, Shi K, Zhang Q, Liu X, Xie L, Jiang L, Guo Y. Early marker of regional left ventricular deformation in patients with hypertrophic cardiomyopathy evaluated by MRI tissue tracking: The effects of myocardial hypertrophy and fibrosis. J Magn Reson Imaging. 2017;46:1368–1376. [DOI] [PubMed] [Google Scholar]
- 21.Andrews CM, Srinivasan NT, Rosmini S, Bulluck H, Orini M, Jenkins S, Pantazis A, McKenna WJ, Moon JC, Lambiase PD, Rudy Y. Electrical and Structural Substrate of Arrhythmogenic Right Ventricular Cardiomyopathy Determined Using Noninvasive Electrocardiographic Imaging and Late Gadolinium Magnetic Resonance Imaging. Circ Arrhythm Electrophysiol. 2017;10:e005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosse-Wortmann L, Macgowan CK, Vidarsson L, Yoo S- J. Late Gadolinium Enhancement of the right ventricular myocardium: Is it really different from the left ? J Cardiovasc Magn Reson. 2008;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bomma C, Rutberg J, Tandri H, Nasir K, Roguin A, Tichnell C, Rodriguez R, James C, Kasper E, Spevak P, Bluemke DA, Calkins H. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2004;15:300–306. [DOI] [PubMed] [Google Scholar]
- 24.Sievers B, Addo M, Franken U, Trappe H- J. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson. 2004;6:601–8. [DOI] [PubMed] [Google Scholar]
- 25.Rastegar N, Burt JR, Corona-Villalobos CP, Te Riele AS, James CA, Murray B, Calkins H, Tandri H, Bluemke DA, Zimmerman SL, Kamel IR. Cardiac MR findings and potential diagnostic pitfalls in patients evaluated for arrhythmogenic right ventricular cardiomyopathy. Radiographics. 2014;34:1553–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quarta G, Husain SI, Flett AS, Sado DM, Chao CY, Tomé Esteban MT, McKenna WJ, Pantazis A, Moon JC. Arrhythmogenic right ventricular cardiomyopathy mimics: role of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NAM. Ventricular Arrhythmias in the North American Multidisciplinary Study of ARVC: Predictors, Characteristics, and Treatment. J Am Coll Cardiol. 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NAM, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic Implantable Defibrillator in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia and No Prior Ventricular Fibrillation or Sustained Ventricular Tachycardia. Circulation. 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 29.Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, Loh P, Russell SD, Velthuis BK, Judge DP, Dooijes D, Tedford RJ, van der Heijden JF, Tandri H, Hauer RN, Abraham TP, Doevendans PA, Te Riele ASJM, Cramer MJ. Evaluation of Structural Progression in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. JAMA Cardiol. 2017;2:293–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


