Abstract
Hypertension and inflammation during pregnancy are suggested to contribute to the development of post-partum depression and anxiety. Using a rat model of severe preeclampsia and Hemolysis Elevated Liver enzymes Low Platelet syndrome which displays both hypertension and inflammation during pregnancy we evaluated whether rats were prone to develop depression or anxiety in the post-partum period. On gestational day 12, mini-osmotic pumps infusing sFlt-1 and sEng were placed into rats, a subset of these rats were infused with 2mg/kg of Orencia (Abatacept) the following day to determine if immune suppression via T cell depletion prevented any changes in maternal depression and/or anxiety-like behavior. All rats including normal pregnant controls delivered between gestational days 21–22. Post-partum severe preeclamptic rats buried significantly more marbles compared to normal pregnant rats (p=0.002) and Orencia treated rats (p=0.05). Severe preeclamptic rats spent significantly more time in closed arms of the elevated plus maze compared to normal pregnant rats (p=0.009) and Orencia treated rats (p=0.05). Severe preeclamptic rats were hypertensive compared to normal pregnant (p=0.03) and Orencia treated rats (p=0.01). Finally, severe preeclamptic rats had increased blood brain barrier permeability compared to normal pregnant rats (p=0.03), which was reversed in Orencia treated rats (p=0.008). These results suggest that severe preeclampsia/Hemolysis Elevated Liver enzymes Low Platelet syndrome during pregnancy contributes to an increase in anxiety-like behavior, blood brain barrier permeability and hypertension in the post-partum. The current results suggest that T cell suppression during pregnancy can also help prevent chronic hypertension and increased anxiety in the post-partum period.
Keywords: anxiety, HELLP Syndrome, hypertension, inflammation, preeclampsia, post-partum anxiety, post-partum depression, T cells
INTRODUCTION
Hypertension affects 3–10% of pregnant women which is most commonly presented as either preeclampsia (PE) or Hemolysis Elevated Liver enzyme Low Platelet (HELLP) syndrome1, 2. HELLP occurs in 10–20% of women with PE and in 0.5% of women without PE3. There is increasing evidence demonstrating that women who suffered severe PE and/or HELLP are at an increased risk for the development of post-partum depression, anxiety, post-traumatic stress disorder (PTSD) and cognitive impairments later in life4–6. There is an increased risk for depression during the perinatal period in which 10–43% of pregnant women have been found to meet the criteria for depression7, 8. 5–20% of women are also affected by post-partum anxiety or increased levels of stress that is often linked to depression9, 10.
The etiology of depression and anxiety are unknown and are likely to vary among individuals, however obstetric complications, such as PE/HELLP along with activation of the immune system have been linked to depression and anxiety in the post-partum period11, 12. Although roles for inflammation, T cells and inflammatory cytokines have been suggested as contributors to hypertension13, 14, PE, HELLP15, 16 and mood disorders12, 17, the studies examining this relationship are scarce. In the current study we use an animal model of severe PE/HELLP (sPE/HELLP) to exam the relationship between inflammation and compromised BBB permeability during pregnancy and the advent of depressive and anxiety-like behavior in the post-partum period. To assess depressive-like behavior, or anhedonia, in rodents we used the two-bottle sucrose preference test18, and for anxiety-like behavior we used marble burying19, elevated plus maze (EPM)20 and novelty suppressed feeding (NSF)21. We have previously reported that 2mg/kg of Orencia (Abatacept; a fusion protein that binds to the extracellular domain of cytotoxic T-lymphocyte-associated protein 4 which inhibits the co-stimulation needed for T cell activation) infused on gestational day (GD)13 of pregnancy attenuates hypertension, the increase in T Cells, systemic inflammation and BBB permeability in pregnant HELLP rats22. As all of these factors also influence depression and anxiety, we also tested the hypothesis that decreasing hypertension, inflammation and BBB permeability during pregnancy, via attenuation of systemic T cell activation22, prevents any adverse behaviors in the post-partum period.
METHODS
The authors declare that all supporting data are available within the article and in the online-only Data Supplement; detailed data are available from the corresponding author on reasonable request.
All studies were performed in Sprague-Dawley female rats (Harlan, Indianapolis, IN). Animals were housed in a temperature controlled room with a 12:12 reverse light:dark cycle. All experimental procedures used in this study were in accordance with National Institutes of Health guidelines for use and care of animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Induction of sPE/HELLP
To induce sPE/HELLP, on GD12 normal pregnant (NP; n=32) rats were infused with of soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng; 4.7 and 7µg/kg respectively, R&D Systems, Minneapolis, MN) as previously described22–24. As we are not able to differentiate between sPE and HELLP during pregnancy in the current study all rats infused with sFlt-1 and sEng are grouped as sPE/HELLP (please see http://hyper.ahajournals.org). On GD13 a subset of rats infused with sFlt-1 and sEng (n=16) were infused with Orencia (2mg/kg) via the jugular veint22. Rats not infused with sFlt-1 or sEng served as NP controls (n=16). Dams gave birth on GD21–22, which was counted as post-partum day (PPD) 0 (Figure 1). 12–24hrs after birth mini-osmotic pumps were removed and all delivered pups were removed. After recovering from surgery, dams were group housed for the remainder of the study.
Figure 1.

Experimental design of the study: gestational day (GD), post-partum day (PPD).
Behavioral Protocols
Motor activity, anxiety and anhedonia were assessed with a series of well-established behavioral tests between post-partum weeks 2–4. All animals were submitted to behavioral testing under low-intensity lighting during the animal’s active cycle either in the Animal Behavioral Core or within the Center for Comparative Research.
Motor function was assessed by the total distance traveled and resting time in a novel environment. Post-partum rats were subjected to the marble burying as a high number of covered marbles is an indicator of anxiety19, the EPM, a pharmacologically validated test used to assess anxiety in rodents20 and NSF (measures of anxiety and depressive-like behaviors in rats)21. Sucrose preference, a reward-based test built on the rodent’s natural interest in sweet foods/solutions, was measured to determine anhedonia in post-partum rats. (please see http://hyper.ahajournals.org).
Hypertension, Inflammation and flow cytometry assays
Mean arterial pressure
Mean arterial pressure (MAP) was determined as previously described utilizing indwelling cartotid catheters23, 25, 26 (please see http://hyper.ahajournals.org). Immediately following MAP measurement, whole blood was collected to assess lactate dehydrogenase (hemolysis), aspartate aminotransferase (liver enzymes) and platelet counts23, 24. Whole blood, liver, spleens and kidneys were collected for flow cytometry and/or enzyme linked immunosorbent immunoassay (ELISA) experiments (please see http://hyper.ahajournals.org). Unless animals were being assessed for BBB permeability or flow cytometry, brains were dissected into frontal cortex, posterior cortex, cerebellum and brainstem sections, snap frozen and stored at −80°C for future studies.
Assessment of BBB permeability.
To evaluate Evan’s blue (EB) extravasation into the brain and maternal organs rats (n=5/group) were infused with Evan’s Blue (EB; MP Biomedical, Santa Ana, CA) as previously described22 (please see http://hyper.ahajournals.org).
Statistical Analysis.
All data are expressed as a mean + standard error of the mean when applicable. Differences between groups were analyzed via an one way analysis of variance with Tukey’s multiple comparisons test or Student’s T test for post-hoc analysis using GraphPad Prism 7.02. Data were considered statistically significant at P values <0.05. All reported p values are the results from post-hoc analysis conducted after the discovery of significant main effects in the ANOVA unless stated otherwise.
RESULTS
Immune suppression during pregnancy prevents anxiety-like behavior and anhedonia in post-partum sPE/HELLP rats
There was a significant increase in distance traveled among sPE/HELLP rats compared to NP rats (p=0.01; Figure 2A), which was prevented in sPE/HELLP+Orencia rats (p=0.02). This was accompanied by a subsequent decrease in resting time in sPE/HELLP rats (p=0.007, Figure 2B).
Figure 2. Post-partum sPE/HELLP rats have evidence of hyperactivity and anxiety-like behavior.

sPE/HELLP rats had a significant increase in distance traveled (A) and a significant decrease in resting time (B) compared to post-partum normal pregnant rats when assessed in the open field. Covered marbles were counted to assess anxiety and sPE/HELLP rats buried significantly more marbles compared to NP and Orencia treated rats (C). sPE/HELLP rats also displayed anxiety-like features indicated by decreased time spent in Open arms and increased time spent in closed arms of the EPM compared to both NP and sPE/HELLP+Orencia rats (D). sPE/HELLP rats had a statistically significant increased latency to approach the food in novelty suppressed feeding compared to post-partum NP rats (E). sPE/HELLP rats tended to consume less sucrose water compared to NP rats, however sPE/HELLP+Orenica rats drank significantly more sucrose water compared to untreated sPE/HELLP rats (F). ≠, ≠≠ denotes p<0.05, p<0.005 between NP and sPE/HELLP; * denotes p<0.05 between sPE/HELLP and sPE/HELLP+Orencia rats. N=16 rats/group.
Compared to sPE/HELLP dams both NP (p=0.002) and sPE/HELLP+Orencia (p=0.05) dams buried significantly less marbles (Figure 2C). In the EPM sPE/HELLP rats spent significantly less time in open arms (p=0.04) compared to NP rats which was not significantly affected in sPE/HELLP+Orencia rats (p=0.28; Figure 2D). sPE/HELLP rats spent significantly more time in the closed arms compared to NP rats (p=0.009) which was reversed in sPE/HELLP+Orencia rats (p=0.05; Figure 2D). There were no statistically significant differences between the 3 groups in the latency to feed in NSF when assessed by one-way ANOVA (p=0.58). However when the time taken to initially approach the food was assessed, sPE/HELLP rats took significantly longer to approach the food compared to NP rats (p=0.02; Figure 2E).
sPE/HELLP rats did not display a significant decrease in anhedonia compared to NP rats (p=0.12), however sPE/HELLP+Orencia rats drank significantly more sucrose water than untreated sPE/HELLP rats (p=0.03, Figure 2F).
Immune suppression during pregnancy prevents post-partum hypertension in sPE/HELLP rats.
Six weeks post-partum, rats with a history of sPE/HELLP were hypertensive compared to NP rats (p=0.03; Figure 3A). sPE/HELLP+Orencia rats were not hypertensive in the post-partum period compared to untreated sPE/HELLP rats (p=0.01; Figure 3A). There was no significant difference in hemolysis between NP and sPE/HELLP rats (p=0.63) however sPE/HELLP+Orencia rats had significantly less hemolysis than sPE/HELLP rats (p=0.03; Figure 3B). Liver enzymes were significantly increased in sPE/HELLP rats compared to NP rats (p=0.03). sPE/HELLP+Orencia rats had significantly less liver enzymes compared to both NP (p=0.003) and sPE/HELLP rats (p=0.002; Figure 3C). There were no significant differences in platelets between the groups (p=0.26; Figure 3D).
Figure 3. T-cell suppression during pregnancy prevents sPE/HELLP-induced hypertension in the post-partum period.

sPE/HELLP rats have significantly increased mean arterial pressure (MAP) (A), but not hemolysis (B) in the post-partum period compared to NP and sPE/HELLP+Orencia rats. Post-partum sPE/HELLP rats have significantly increased liver enzymes (C) compared to both post-partum NP and sPE/HELLP+Orencia rats, however there were no statistically significant changes in platelet levels between the groups (D). ≠ denotes p<0.05 between NP and sPE/HELLP; * , ** denotes p<0.05, p<0.005 between sPE/HELLP and sPE/HELLP+Orencia rats; a denotes p<0.05 between NP and sPE/HELLP+Orencia rats; n=16/group for MAP; n=11/group/biochemical measurements.
sPE/HELLP rats delivered significantly earlier than NP rats (p=0.04) and sPE/HELLP+Orencia rats (p=0.003). There was not a statistically significant difference in litter sizes between the groups (p=0.14) or in organs with the exception of the spleen (please see http://hyper.ahajournals.org).
sFlt-1 and sEng levels subside in the post-partum period
There were no significant differences in circulating levels of sFlt-1 between NP and sPE/HELLP rats when measured via Student’s T test (p=0.44; Figure 4A). Circulating levels of sFlt-1 were below levels of detection in sPE/HELLP+Orencia rats. There were no significant differences in circulating levels of sEng (p=0.89; Figure 4B). There was no significant difference in circulating levels of TNFα (p=0.88) between the NP and sPE/HELLP rats (Figure 4C). However, TNFα levels were significantly decreased in sPE/HELLP+Orencia rats compared to NP (p=0.006) and sPE/HELLP rats (p=0.001). Circulating IL-17 was not detectable via ELISA in any of the three groups of post-partum rats.
Figure 4. Orencia infusion decreases circulating sFlt-1 and TNFα levels in post-partum sPE/HELLP rats.
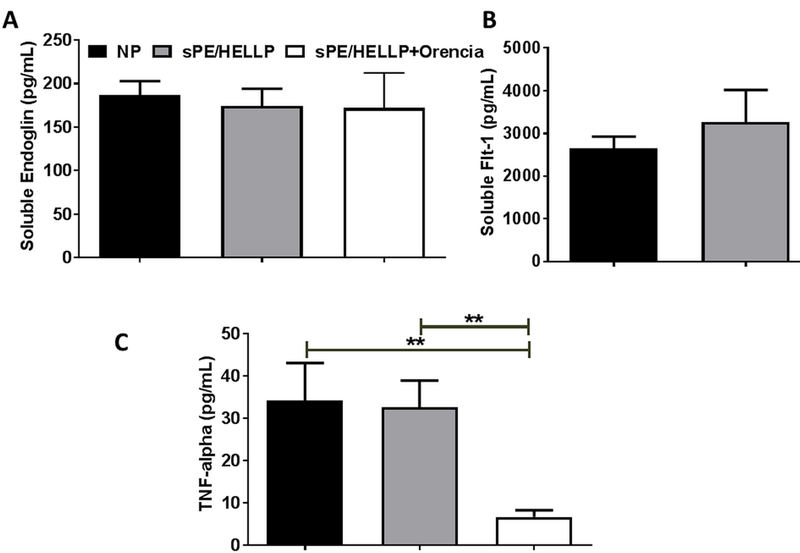
There were no significant changes in circulating levels of sEng (A), sFlt-1 (B), or TNFα (C) between post-partum NP and sPE/HELLP rats when measured via ELISA. Whereas sFlt-1 was undetectable in sPE/HELLP rats infused with Orencia. Orencia infusion did not affect circulating sEng levels and significantly decreased TNFα levels compared to both NP and sPE/HELLP rats. ** denotes p<0.005 between indicated groups; n=8 in NP and sPE/HELLP rats, n=6 in sPE/HELLP+Orencia.
CD4 and CD8 T Cells are suppressed in sPE/HELLP+Orencia rats
There was not a significant difference in circulating CD4+ T cells between NP and sPE/HELLP rats (p=0.57), however sPE/HELLP+Orencia rats had significantly decreased CD4+ T compared to NP (p=0.02) and sPE/HELLP rats (p=0.03, Figure 5A). sPE/HELLP rats had a significant increase in splenic CD4+ T cells compared to NP rats (p=0.0006) which was significantly decreased in sPE/HELLP+Orencia rats (p=0.0004; Figure 5A). In the kidney sPE/HELLP rats had significantly more CD4+ T cells compared to NP rats (p=0.0003) which was reduced in sPE/HELLP+Orencia rats (p=0.0001; Figure 5A). There were no statistically significant differences in CD4+ T cells in the liver between the groups (p=0.31, Figure 5A).
Figure 5. T-cells are suppressed in post-partum sPE/HELLP+Orencia rats.
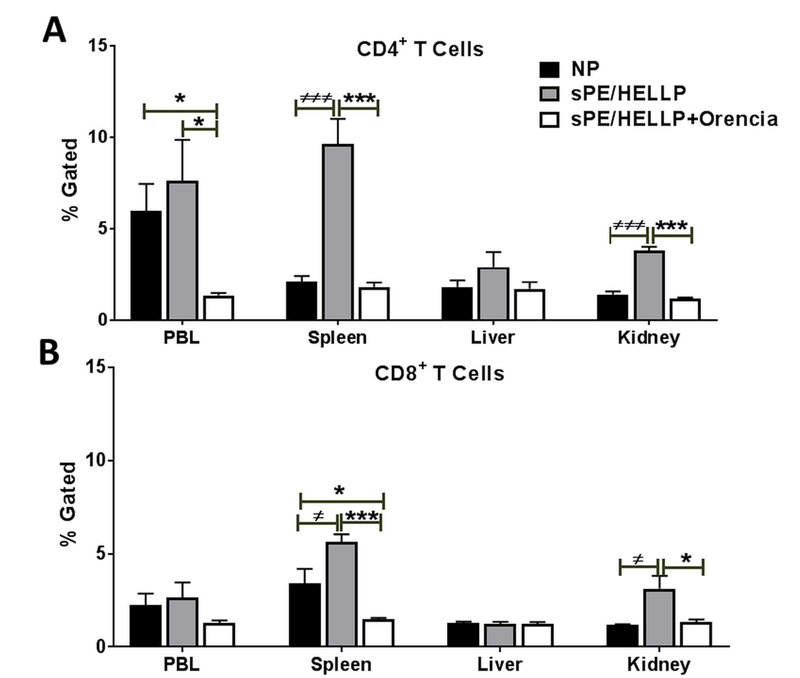
CD4+ T cells are significantly decreased in sPE/HELLP+Orencia rats compared to untreated sPE/HELLP rats and/or NP rats (A) as are CD8+ T cells (B). ≠, ≠≠≠ denotes p<0.05, p<0.0005 between NP and sPE/HELLP; * , *** denotes p<0.05, p<0.0005 between indicated groups; n=5/group.
Circulating CD8+ T cells were not significantly different between the groups of rats (p=0.26), however, splenic CD8+ T cells were significantly increased in sPE/HELLP compared to NP rats (p=0.04, Figure 5B). sPE/HELLP+Orencia rats had significantly decreased CD8+ T cells in the compared to NP (p=0.04) and sPE/HELLP rats (p=0.0001, Figure 5B). sPE/HELLP rats had a significant increase in kidney CD8+ T cells compared to NP rats (p=0.04) which was reduced in sPE/HELLP+Orencia rats (p=0.05; Figure 5B).There were no statistically significant differences in CD8+ T cells in the liver between the three groups (p=0.95, Figure 5B).
BBB damage persists into the post-partum period
Similar to what occurs during pregnancy there were no statistically significant changes in BBB permeability in the frontal cortex of post-partum sPE/HELLP rats compared to NP rats regardless of Orencia administration (p=0.537; Figure 6). BBB permeability was significantly increased in the posterior cortex of sPE/HELLP rats compared to NP rats (p=0.03; Figure 6) but not sPE/HELLP+Orencia rats (p=0.91). In the brainstem/cerebellum region, sPE/HELLP rats had significantly more BBB permeability compared to NP rats (p=0.03; Figure 6) which was significantly reduced with Orencia treatment (p=0.008).
Figure 6. Post-partum sPE/HELLP rats have increased BBB permeability.
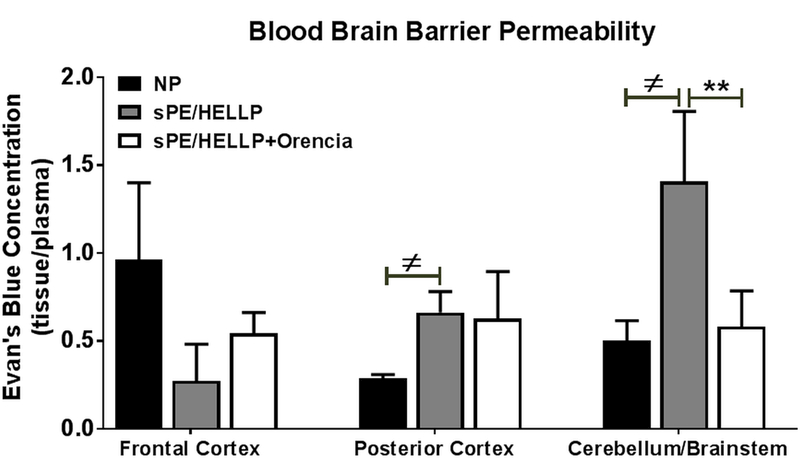
Post-partum sPE/HELLP rats had significantly more Evan’s blue leakage in the posterior cortex and in the brainstem/cerebellum regions of the brain compared to normal pregnant (NP) rats. sPE/HELLP+Orencia rats significantly decreased EB leakage into the cerebellum/brainstem during the post-partum period compared to untreated sPE/HELLP rats. ≠ denotes p<0.05 between NP and sPE/HELLP; ** denotes p<0.005 between sPE/HELLP and sPE/HELLP+Orencia rats; n=5/group.
There were no statistically significant differences in permeability in the liver (p=0.37), spleen (p=0.99) or kidney (p=0.18) when measured by one-way ANOVA (please see S1 http://hyper.ahajournals.org).
DISCUSSION
It is well documented that women with a history of high-risk pregnancies have an increased risk of developing cardiovascular and cerebrovascular disease later in life27, 28. More recently an increased risk in cognitive and mental disorders has been identified29–32. In the current study we modeled sPE/HELLP syndrome by chronically infusing sFlt-1 and sEng into pregnant rats beginning on GD12. The imbalance in sFlt-1 and sEng is similar to what is seen in women with sPE/HELLP syndrome33 and induces sPE/HELLP syndrome in pregnant rats22, 23, 34. We have previously reported that infusion of sFlt-1 and sEng into pregnant rats results in hypertension, increased CD4+ and CD8+ T cells, had no significant effect on B cell populations, increased inflammatory cytokines and BBB disruption by GD19 of pregnancy22. Furthermore, infusion of Orencia to HELLP rats attenuated all of the above factors. In the current study we sought to determine if infusion of sFlt-1 and sEng during pregnancy resulted in chronic hypertension, inflammation and BBB disruption in post-partum rats once the source of sFlt-1 and sEng had been removed. We also wanted to determine if a single infusion of Orencia during mid-pregnancy had any long-lasting effects into the post-partum period and finally whether this experimental model of sPE/HELLP was associated with changes in anhedonia and anxiety in the post-partum period. In the current study, post-partum rats with a history of sPE/HELLP had evidence of hyperactivity and increased anxiety compared to NP rats. Importantly, these changes in behavior were associated with hypertension, increased splenic and renal T cells and increased BBB permeability to Evan’s blue dye. However, there were no changes in sFlt-1, sEng or TNFα levels between post-partum sPE/HELLP and NP rats, suggesting that the behavioral changes and persistent hypertension may not be a results of current systemic inflammatory processes.
Several studies have identified positive associations between PE and HELLP during pregnancy and the development of post-partum depression, anxiety and/or PTSD35–38. In the current study we demonstrated that rats with a history of sPE/HELLP had increased anxiety relative to rats with a normal pregnancy and sPE/HELLP rats infused with Orencia. These results are confirmed by clinical studies which have reported that women with PE and/or HELLP have increases in PTSD5, 39. As to be expected, PTSD after pregnancy has been associated with traumatic birth events and infant morbidity and mortality40. To compensate for pup morbidity/mortality problems, all pups were removed from dams within 24hrs of delivery. There was not a significant decrease in anhedonia in rats due to sPE/HELLP when measured via the two-bottle sucrose test, however sPE/HELLP rats treated with Orencia during pregnancy did have a greater preference for sucrose compared to untreated sPE/HELLP rats. Motor activity, assessed by total distance traveled and movement time was positively associated with increased anxiety behavior in sPE/HELLP rats. As with the anxiety-like behaviors, Orencia infusion during pregnancy to sPE/HELLP rats prevented the alterations in behavior.
While we only used marble burying, elevated plus maze and NSF to assess changes in anxiety in the current study, futures studies examining behaviors specific to PTSD and studies assessing maternal-pup interaction will need to be conducted. We also cannot overlook the importance of other biological factors such as the hypothalamic-pituitary-adrenal axis which plays an important role in contributing to anxiety and depression41. Interestingly, diurnal salivary cortisol excretion was not found to be increased in women with either PE or HELLP compared to normal pregnant women when tested during pregnancy42, indicating that changes in maternal stress hormones may not be detectable until late pregnancy of after delivery.
There was not a significant change in circulating sEng, sFlt-1 or TNFα between NP and sPE/HELLP post-partum rats and IL-17 was not detectable via ELISA in the circulation. These findings are consistent with those reported by others who also did not find evidence of a continued immune response in the post-partum period after PE when assessed via ELISA43, 44. Rats treated with Orencia had little to no detection of TNFα and sFlt-1 compared to NP and sPE/HELLP rats whereas sEng levels were not affected by Orencia treatment suggesting that perhaps sEng is not regulated by T cells by maybe by a different immune pathway. When we examined the levels of CD4 and CD8 T cells they were similar to our ELISA results in that there were not any significant changes in circulating T cells between post-partum NP and sPE/HELLP However, both the spleen and the kidney had significantly increased levels of T cells relative to NP rats.
We have previously reported that Orencia infused during pregnancy attenuates hypertension, the increase in T Cells, systemic inflammation and BBB permeability in pregnant HELLP rats22. We were surprised to see that this single infusion of Orencia during pregnancy not only affected the behavioral changes in post-partum rats with sPE/HELLP but also significantly decreased T cells in select organs compared to sPE/HELLP rats. The lack of residual anti-angiogenic imbalance in this model was unexpected but is similar to what is seen in the more mild model of placental ischemia induced via a reduction in utero placental perfusion43. The studies examining the chronic effects of Orencia are few, but both experimental and clinical studies have found that Orencia treatment prevents chronic disease progression and immune cell suppression when it was administered at multiple time points45–47. During pregnancy Orencia infusion into NP rats was not found to have a statistically significant effect on hypertension, inflammation or BBB permeability so we did not include this group of rats for post-partum examination22, 48. However, future studies will need to examine the effects of T cell suppression during pregnancy in NP rats to determine if there are any behavioral or immune changes due to Orencia alone.
sPE/HELLP rats had a significant increase in BBB permeability in the posterior cortex and in the brainstem/cerebellum compared to NP rats which was not protected by Orencia in the case of the posterior cortex. We have also previously reported that during pregnancy rats with HELLP did not have a significant increase in BBB permeability in the frontal cortex22, which is similar to what is currently reported. This is consistent with a recent study by Clayton et al reported a reduction in the tight junction occludin expression in the posterior cerebrum of post-partum placental ischemic rats, indicative of BBB damage49. Damage to the posterior region of the brain in post-partum rats is also supported by clinical studies indicating brain damage in the subcortical organs of women with a history of PE and HELLP31, 50. In general the posterior regions of the brain have been reported to have increased white matter damage in women in a history of PE31, 50. We did not assess white matter damage in the current study but will plan to examine not only white matter but also cognitive function.
It is possible that the chronic increase blood pressure that we see in post-partum sPE/HELLP rats could also contribute to long-lasting changes in BBB permeability. Even though some women with sPE/HELLP during pregnancy have a resolution in hypertension prior to hospital discharge, between 41–57% of women still experience hypertension in the post-partum period even while taking anti-hypertensives51, 52. It is also important to note that in the clinical setting women are often treated with anti-hypertensives and magnesium sulfate to decrease hypertension and offer neuroprotection53. This was not the case in untreated rats with sPE/HELLP which may be one reason for the continued hypertension. Our current results differ from previous post-partum studies of animal models of placental ischemia which do not have significant increases in MAP relative to post-partum NP rats43, 49, 54. It is important to note that in these previous studies placental ischemia during pregnancy was induced through either a mechanical reduction of uteroplacental blood flow or via lipopolysaccharide administration, which does not create sPE or HELLP in rats34, 55, 56. This difference in the severity of disease pathology might be one explanation for the difference in hypertension during the post-partum period despite the similarities in post-partum inflammation.
Hypertension and inflammation during pregnancy, such as that seen in sPE/HELLP, is associated with increased BBB permeability, impairments in cerebral blood flow autoregulation, cerebral edema and neuroinflammation57–61. While the exact mechanism(s) responsible for the CNS complications associated with sPE/HELLP are not known it is believed that the cerebral circulation is involved. It is well established in both human and animal studies that circulating factors, in subjects with PE or HELLP contribute to BBB disruption and neuroinflammation22, 57, 60, 62. Therefore one could postulate that the increase in BBB permeability or decrease in BBB function could contribute to changes in the neural environment during pregnancy or the post-partum period leading to behavioral changes63, 64. Though we did not directly test this theory in the current study we do believe that the post-partum behavioral changes that are present in the current model are due, at least in part, to neuroinflammatory mechanisms associated with the increase in BBB disruption.
PERSPECTIVES
Cerebral complications associated with sPE/HELLP contribute significantly to maternal morbidity and mortality both during pregnancy and in postpartum periods. Hypertension and BBB disruption during pregnancy, mediated partly by systemic inflammation and neuroinflammation, may play an important role in the underlying pathophysiology of the varying CNS complications and ensuing post-partum behavioral modifications associated with sPE/HELLP. In the current study, for the first time to our knowledge, a relationship between hypertension and increased BBB permeability during pregnancy with hypertension and negative behavioral outcomes in the post-partum period in an animal model has been demonstrated. Inactivation of T cells may serve as a potential therapy for the treatment of sPE/HELLP, which may lead to improvement in overall maternal and fetal morbidity and mortality. In order to further understand the underlying mechanism of CNS complications associated with sPE/HELLP, the role of specific T helper cells and evaluation of neuroinflammation needs to be further studied.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Anxiety, anhedonia, inflammation, chronic hypertension and BBB permeability were examined in rats infused with sFlt-1 and sEndoglin during pregnancy.
A single infusion of Orencia during pregnancy prevented anxiety, inflammation, chronic hypertension and BBB permeability in post-partum rats with sPE/HELLP.
What is relevant?
sPE/HELLP in pregnant rats is associated with post-partum anxiety
Immune suppression during pregnancy may offer prevent the onset of post-partum anxiety
Summary
The importance of immune system activation during pregnancy on both the vasculature and central nervous system has long-lasting consequences.
Acknowledgments
Funding. This work was supported by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from NIH/NIGMS P20GM103476 and NIH/NIGMS P30GM103328–01A1 to KW.
Footnotes
Disclosures. None.
REFERENCES
- 1.Magee L, Pels A, Helewa M, Rey E, Von Dadelszen P, Canadian Hypertensive Disorders of Pregnancy (HDP) working g. The hypertensive disorders of pregnancy (29.3). Best Pract Res Clin Obstet Gynaecol 2015;29:643–657. [DOI] [PubMed] [Google Scholar]
- 2.Mogos M, Salemi J, Spooner K, McFarin B, Salihu H. Hypertensive disorders of pregnancy and postpartum readmission in the United States: national surveillance of the revolving door. J Hypertension 2018;36:608–618. [DOI] [PubMed] [Google Scholar]
- 3.Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;166:117–123. [DOI] [PubMed] [Google Scholar]
- 4.Delahaije D, Dirksen C, Peeters L, Smits L. Anxiety and depression following preeclampsia or HELLP syndrome. A systematic review. Acta Obstetricia Gynecologica Scandinavica 2013;92:746–761. [DOI] [PubMed] [Google Scholar]
- 5.Van Pampus M, Wolf H, Weijmar Schultz W, Neeleman J, Aarnoudse J. Posttraumatic stress disorder following preeclampsia and HELLP syndrome. J Psychosom Obstet Gynaecol 2004;25:183–187. [DOI] [PubMed] [Google Scholar]
- 6.Fields J, Garovic V, Mielke M, Kantarci K, Jayachandran M, White W, Butts A, Graff-Radford J, Lahr B, Bailey K, Miller V. Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol 2017;217:74.e71–74.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hara M, McCabe J. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 2013;9:379–407. [DOI] [PubMed] [Google Scholar]
- 8.Gavin N, Gaynes B, Lohr K, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005;106:1071–1083. [DOI] [PubMed] [Google Scholar]
- 9.Miller R, Pallant J, Negri L. Anxiety and stress in the postpartum: Is there more to postnatal distress than depression? BMC Psychiatry 2006;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross L, McLean L. Anxiety disorders during pregnancy and the postpartum period: A systematic review. J Clin Psychiatry 2006;67:1285–1298. [DOI] [PubMed] [Google Scholar]
- 11.Hoedjes M, Berks D, vogel I, Franx A, Bangma M, Darlington A, Visser W, Duvekot J, Habbema J, Steegers E, Raat H. Postpartum depression after mild and severe preeclampsia. J Womens Health 2011;20:1535–1542. [DOI] [PubMed] [Google Scholar]
- 12.Osborne L, Monk C. Perinatal depression - the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 2013;38:1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Gelston C, Mitchell B. Recent advances in Immunity and Hypertension. Am J Hypertension 2017;30:643–652. [DOI] [PubMed] [Google Scholar]
- 14.Harrison D. The Immune System in Hypertension. Trans Am Clin Climatol Assoc 2014;125:130–138. [PMC free article] [PubMed] [Google Scholar]
- 15.Harmon A, Cornelius D, Amaral L, Faulkner J, Cunningham M Jr, Wallace K, LaMarca B. The role of inflammation in the pathophysiology of preeclampsia. Clinical Science 2016;130:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triggianese P, Perricone C, Chimenti M, De Carolis C, Perricone R. Innate Immune System at the Maternal-Fetal Interface: Mechanisms of Disease and Targets of Therapy in Pregnancy Syndromes. Am J Reprod Immunol 2016;76:245–257. [DOI] [PubMed] [Google Scholar]
- 17.Shariq A, Brietzke E, Rosenblat J, Barendra V, Pan Z, McIntyre R. Targeting cytokines in reduction of depressive symptoms: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2018;83:86–91. [DOI] [PubMed] [Google Scholar]
- 18.Harris R, Zhou J, Youngblood B, Smagin G, Ryan D. Failure to change exploration or saccharin prefernece in rats exposed to chronic mild stress. Physiol Behav 1997;63:91–100. [DOI] [PubMed] [Google Scholar]
- 19.Njung’e K, Handley S. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav 1991;38:63–67. [DOI] [PubMed] [Google Scholar]
- 20.Pellow S, Chopin P, File S, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985;14:149–167. [DOI] [PubMed] [Google Scholar]
- 21.Stedenfeld K, Clinton S, Kerman I, Akil H, Watson S, Sved A. Novelty-Seeking Behavior Predicts Vulnerability in a Rodent Model of Depression. Physiol Behav 2011;103:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bean C, Spencer S, Bowles T, Kyle P, Williams J, Gibbens J, Wallace K. Inhibition of T cell-activation attenuates hypertension, TNF-alpha, IL-17 and blood-brain barrier permeability in pregnant rats with angiogenic imbalance. Am J Reprod Immunol 2016;76:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace K, Morris R, Kyle P, Cornelius D, Darby M, Scott J, Moseley J, Chatman K, Lamarca B. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertension in Pregnancy 2014;33:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris R, Spencer S, Kyle P, Williams J, Harris A, Owens M, Wallace K. Hypertension in an animal model of HELLP syndrome is associated with activation of endothelin-1. Reproductive Science 2016;130:409–419. [DOI] [PubMed] [Google Scholar]
- 25.Wallace K, Cornelius D, Scott J, Heath J, Moseley J, Chatman K, LaMarca B. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension 2014;64:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace K, Richards S, Dhillion P, Weimer A, Edholm E, Bengten E, Wilson M, Martin JJ, Lamarca B. CD4+ T Helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 2011;57:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald S, Malinowski A, Zhou Q, Yusuf S, Devereaux P. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008;156:918–930. [DOI] [PubMed] [Google Scholar]
- 28.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 29.Amaral L, Cunningham M Jr, Cornelius D, Lamarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag 2015;11:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral L, Wallace K, Owens M, Lamarca B. Pathophysiology and Current Clinical Management of Preeclampsia. Curr Hypertens Rep 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postma I, Bouma A, De Groot J, Aukes A, Aarnoudse J, Zeeman G. Cerebral white matter lesions, subjective cognitive failures, and objective neurocognitive functioning: A follow-up study in women after hypertensive disorders of pregnancy. J Clin Exp Neuropsychol 2016;38:585–598. [DOI] [PubMed] [Google Scholar]
- 32.Muela H, Costa-Hong V, Yassuda M, Moraes N, Memoria C, Machado M, Macedo T, Shu E, Massaro A, Nitrini R, Mansur A, Bortolotto L. Hypertension severity is associated with impaired cognitive performance. J Am Heart Associ 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaarschmidt W, Rana S, Stephan H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med 2013;41:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature Medicine 2006;12:642–649. [DOI] [PubMed] [Google Scholar]
- 35.Blom E, Jansen PV, FC, Hofman A, Raat H, Jaddoe V, Coolman M, Steegers E, Tiemeier H. Perinatal complications increase the risk of postpartum depression. The Generation R Study. British Journal of Obstetrics and Gynaecology 2010;117:1390–1398. [DOI] [PubMed] [Google Scholar]
- 36.Habli M, Eftekhari N, Wiebracht E, Bombrys A, Khabbaz M, How H, Sibai B. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol 2009;201:385e381–385. [DOI] [PubMed] [Google Scholar]
- 37.Mommersteeg P, Drost J, Ottervanger J, Maas A. Long-term follow-up of psychosocial distress after early onset preeclampsia: the Preeclampsia Risk Evaluation in FEMales cohort study. J Psychosom Obstet Gynaecol 2016;37:101–109. [DOI] [PubMed] [Google Scholar]
- 38.Abedian Z, Soltani N, Mokhber N, Esmaily H. Depression and anxiety in pregnancy and postpartum in women with mild and severe preeclampsia. Iran J Nurs Midwifery Res 2015;20:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelhard I, van Rij M, Boullart I, Ekhart T, Spaanderman M, van den Hout M, Peeters L. Posttraumatic stress disorder after pre-eclampsia: an exploratory study. General Hospital Psychiatry 2002;24:260–264. [DOI] [PubMed] [Google Scholar]
- 40.DeMier R, Hynan M, Harris H, Manniello R. Perinatal stressors as predictors of symptoms of posttraumatic stress in mothers of infants at high risk. J Perinatol 1996;16:276–280. [PubMed] [Google Scholar]
- 41.Yehuda R Post-traumatic stress disorder. New England Journal of Medicine 2002;346:108–114. [DOI] [PubMed] [Google Scholar]
- 42.Sikkema J, Robles de Medina P, Schaad R, Mulder E, Bruinse H, Buitelaar J, Visser G, Franx A. Salivary cortisol levels and anxiety are not increased in women destined to develop preeclampsia. Journal of Psychosomatic Research 2001;50:45–49. [DOI] [PubMed] [Google Scholar]
- 43.Paauw N, Joles J, Spradley F, Bakrania B, Zsengeller Z, Franx A, Verhaar M, Granger J, Lely A. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 2017;312:R664–R670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy M, Tayade C, Smith G. Evidence of inflammation and predisposition toward metabolic syndrome after pre-eclampsia. Pregnancy Hypertens 2015;5:354–358. [DOI] [PubMed] [Google Scholar]
- 45.Via C, Rus V, Nguyen P, Linsley P, Gause W. Differential Effect of CTLA4Ig on Murine Graft-Versus-host Disease (GVHD) Development. Journal of Immunology 1996;157:4258–4267. [PubMed] [Google Scholar]
- 46.Nahas M, Soiffer R, Kim H, et al. Phase 1 clinical trial evaluating abatacept in patients with steroid-refractory chronic graft-versus-host disease. Blood 2018;131:2836–2845. [DOI] [PubMed] [Google Scholar]
- 47.Mease P, Gottlieb A, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman D. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Annals of the Rheumatic Diseases 2016;76:1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novotny S, Wallace K, Herse F, Moseley J, Darby M, Heath J, Gill J, Wallukat G, Martin J Jr., Dechend R, LaMarca B. CD4+ T cells play a critical role in mediating hypertension in response to placental ischemia. Journal of Hypertension 2013;2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clayton A, Shao Q, Paauw N, Giambrone A, Granger J, Warrington J. Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy. Brain Behavior Immun 2018;70:376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siepmann T, Boardman H, Bilderbeck A, Griffanti L, Kenworthy Y, Zwager C, McKean D, Francis J, Neubauer S, Yu G, Lewandowski A, Sverrisdottir Y, Leeson P. Long-term cerebral white and gray matter changes after preeclampsia. Neurology 2017;88:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ditisheim A, Wuerzner G, Ponte B, Vial Y, Irion O, Burnier M, Boulvain M, Pechere-Bertschi A. Prevalance of Hypertensive Phenotypes After Preeclampsia. Hypertension 2018;71:103–109. [DOI] [PubMed] [Google Scholar]
- 52.Benschop L, Duvekot J, Versmissen J, van Broekhoven V, Steegers E, Roeters van Lennep J. Blood Pressure Profile 1 Year after Severe Preeclampsia. Hypertension 2018;71:491–498. [DOI] [PubMed] [Google Scholar]
- 53.Folk D Hypertensive Disorders of Pregnancy: Overview and Current Recommendations. J Midwifery Womens Health 2018;63:289–300. [DOI] [PubMed] [Google Scholar]
- 54.Brennan L, Morton J, Quon A, Davidge S. Postpartum Vascular Dysfunction in the Reduced Uteroplacental Perfusion Model of Preeclampsia. PLos One 2016;11:e0162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isler C, Bennett W, Rinewalt A, Cockrell K, Martin J Jr., Morrison J, Granger J. Evaluation of a rat model of preeclampsia for HELLP syndrome characteristics. J Soc Gynecol Investig 2003;10:151–153. [DOI] [PubMed] [Google Scholar]
- 56.Ushida T, Macdonald-Goodfellow S, Quadri A, Tse M, Winn L, Pang S, Adams M, Kotani T, Kikkawa F, Graham C. Persistence of risk factors associated with maternal cardiovascular disease following aberrant inflammation in rat pregnancy. Biology of Reproduction 2017;97:143–152. [DOI] [PubMed] [Google Scholar]
- 57.Amburgey O, Chapman A, May V, Bernstein I, Cipolla M. Plasma from preeclamptic women increases blood-brain barrier permeability. Hypertension 2010;56:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cipolla M, Pusic A, Grinberg Y, Chapman A, Poynter M, Kraig R. Pregnant serum induces neuroinflammation and seizure activity via TNFalpha. Experimental Neurology 2012;234:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson A, Tremble S, Chan S, Moseley J, Lamarca B, Nagle K, Cipolla M. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLos One 2014;9:e113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallace K, Tremble S, Owens M, Morris R, Cipolla M. Plasma from patients with HELLP Syndrome Increases Blood-brain barrier permeability. Reproductive Science 2015;22:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warrington J, Fan F, Murphy S, Roman R, Drummond H, Granger J, Ryan M. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol Rep 2014;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson A, Hammers E, Sakkaki S, Tremble S, Holmes G, Cipolla M. Inhibition of blood-brain barrier efflux transporters promotes seizures in pregnant rats: Role of circulating factors. Brain, Behavior, and Immunity 2017;67:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoshnam S, Farbood Y, Fathi Moghaddam H, Sarkaki A, Badavi M, Khorsandi L. Vanillic acid attenuates cerebral hyperemia, blood-brain barrier disruption and anxiety-like behaviors in rats following transient bilateral common carotid occlusion and reperfusion. Metab Brain Dis 2018;33:785–793. [DOI] [PubMed] [Google Scholar]
- 64.Yang F, Huang S, Cheng I. Behavioral alterations following blood-brain barrier disruption stimulated by focused ultrasound. Oncotarget 2016;7:27916–27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


