Abstract
Objective:
Lipoprotein(a) [Lp(a)] levels vary by race/ethnicity and were recently found to be associated with risk of heart failure (HF). We aimed to determine whether Lp(a)-related risk of HF is similar across different races and whether Lp(a) may further be related to HF with reduced (HFrEF) or preserved ejection fraction (HFpEF).
Approach and Results:
In 6,809 participants of the Multi-Ethnic Study of Atherosclerosis, aged 45–84 years and free of cardiovascular disease (CVD), 308 incident HF events occurred over a median 13-year follow-up. Baseline Lp(a) concentrations were determined by immunoassay. Incident HF was adjudicated, distinguishing HFrEF (EF<45%) from HFpEF (EF ≥45%). Cox regression assessed relations between Lp(a) and HF risk among four races/ethnicities. Lp(a) was examined as a continuous variable (per log unit) and using clinical cutoff values, 30 mg/dL and 50 mg/dL. Lp(a) was related to greater risk of HF in Caucasians alone: per log unit Lp(a): (HR: 1.20; p=0.02); Lp(a)≥30 mg/dL: (HR: 1.69; p=0.01); Lp(a)≥50 mg/dL: (HR: 1.87; p=0.006). No significant relations were found in Black, Hispanic, or Chinese participants, and significant race interactions were observed. Lp(a) was additionally related to greater risk of HFpEF in Caucasian participants: per log unit Lp(a): (HR: 1.48; p=0.001); Lp(a)≥30 mg/dL: (HR: 2.15; p=0.01); Lp(a)≥50 mg/dL: (HR: 2.60; p=0.004). Lp(a)-related risk of HF and HFpEF in Caucasians were independent of aortic valve disease.
Conclusions:
In a multi-ethnic sample, Lp(a)-related risks of HF and HFpEF were only evident in Caucasian participants. If confirmed, these findings have implications in further Lp(a) research and clinical practice.
Keywords: lipids and lipoproteins, heart failure, race and ethnicity
Keywords: epidemiology, risk factors, heart failure, race and ethnicity
INTRODUCTION
Heart failure (HF) represents a major health-economic burden and, with their aging populations, Western nations are poised for a dramatic increase in rates of incident HF (1–3). Considering the deleterious effects of HF on mortality, morbidity, and quality of life (4, 5), the identification and characterization of new HF risk factors is vital. Among these, lipoprotein(a) [Lp(a)] is a genetically-determined low density lipoprotein subclass that may represent one such target.
Lp(a) has been well-characterized for its causal role in coronary heart disease and aortic valve stenosis (6, 7), but was only recently shown to be associated with greater risk of incident HF (8) and HF hospitalizations (9). Potentially complicating this observation, Lp(a) distributions are known to vary widely based on race/ethnicity. Indeed, race-based differences in circulating Lp(a) concentrations are well documented—with Black individuals having, on average, 2–3-fold higher levels than Caucasians (10, 11) and Hispanics (12, 13). Additionally, racial/ethnic differences in Lp(a)-related risk of disease incidence have been suggested in recent studies of coronary heart disease (12) and aortic valve calcification (AVC) (11)—although counter evidence has also been reported (10). It remains unknown whether Lp(a)-related risk of incident HF may differ across race/ethnicity, and no study has examined Lp(a) and risk of HF categorized by ejections fraction.
Overall this study aimed to determine whether Lp(a)-related risk of incident HF is modified by race/ethnicity in Multi-Ethnic Study of Atherosclerosis participants. Analyses were further conducted to determine whether observed relationships were mediated by ischemic-related events. Finally, previous studies (8, 9) have been unable to interrogate HF categorized by reduced ejection fraction (HFrEF) and preserved EF (HFpEF), and these outcomes were examined here.
MATERIALS AND METHODS
The authors declare that all supporting data are available within the article.
Study population
MESA was initiated to investigate the prevalence and progression of subclinical CVD in people initially free of overt clinical CVD, radiation or chemotherapy-treated cancer, other serious major illness, or cognitive impairment in the judgment of the screening interviewer prior to baseline (14). Between 2000 and 2002, 6,814 men and women aged 45–84 years, of Caucasian, Black, Hispanic, or Chinese racial/ethnic backgrounds, and without clinical evidence of overt cardiovascular disease were enrolled from six communities: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN. The institutional review boards at all participating centers approved the study, and all participants signed informed consent. Additional study protocol information is available online at www.mesa-nhlbi.org.
Laboratory measurements
Fasting blood was drawn and EDTA-anticoagulant tubes were processed using a standardized protocol (15). Samples were aliquoted then stored at −70oC until analyte measurements were conducted. Fasting triglyceride, total cholesterol, high density lipoprotein cholesterol (HDL-C) concentrations were measured as described previously (16). Lp(a) mass concentrations served as the exposure variable and were measured at baseline in 6,700 MESA specimens by Health Diagnostics Laboratory (Richmond, Virginia) using a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan) that controls for the heterogeneous sizes of apo(a) (17). Total imprecision was <5%.
Follow-ups for event outcomes
Every 9 to 12 months, telephone interviewers called each participant to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Inpatient and outpatient medical records and information were received in approximately 98% and 95%, respectively. HF events were adjudicated by physicians based on medical records. We used EF reported in the hospital record to define HFrEF < 45% vs. HFpEF ≥ 45% (18). Definite and probable myocardial infarction (MI) cases were identified based on combinations of symptoms, ECG, and cardiac biomarker levels. In most cases, definite or probable MI required either abnormal cardiac biomarkers (2 times upper limits of normal) regardless of pain or ECG findings; evolving Q waves regardless of pain or biomarker findings; or a combination of chest pain, and ST-T evolution or new LBBB, and biomarker levels 1–2 times upper limits of normal.
Aortic Valve Calcification
AVC was identified at baseline by computed tomography. The imaging protocol has been described previously (19). Briefly, computed tomography imaging with either electron beam or multi-detector scanners was used to detect aortic valve calcification. Each participant was scanned twice, and the scans were interpreted at the core laboratory at the Los Angeles Biomedical Research Institute at Harbor-UCLA (University of California Los Angeles) Medical Center by experienced readers who were blinded to the clinical information. Any calcified focus that extended to the aortic root was considered aortic valve calcium.
Statistical analysis
Statistical analysis was conducted using STATA, version 15.0 (StataCorp, College Station, Texas). Baseline characteristics are presented as means (SD) for continuous variables and frequencies (%) for categorical variables. Missing data were excluded when calculating frequencies. Tukey-Kramer HSD was used to test differences between groups. Cox regression determined associations between Lp(a) and incident heart failure across the four races/ethnicities. The proportional hazards assumption was determined using Schoenfeld residuals. To fully interrogate Lp(a) as a clinical CV risk marker, it was treated as both a log-transformed continuous variable and categorized variable using clinical cutoffs. US and European clinics use different Lp(a) clinical cutoff values (30 mg/dL and 50 mg/dL, respectively), so both values were examined here. The regression model was adjusted for age, sex, BMI, field center, education, smoking (pack years), lipid lowering medication, hypertension medication, systolic blood pressure, diastolic blood pressure, baseline glomerular filtration rate, diabetes, HDL-C, total cholesterol, log-transformed triglycerides and AVC at baseline (absent or present). Alcohol intake and baseline income were not related to the exposure or outcome variables and were excluded as covariates. Race/ethnicity was tested as a modifying variable. Unadjusted Nelson–Aalen hazard curves were generated to show differences in cumulative hazards of heart failure between individuals with Lp(a) above and below clinical cutoff values within each race/ethnicity. A secondary analysis assessed whether incident MI or ischemia-related events mediate any observed relationships between Lp(a) and HF. Participants who suffered events were excluded as follows: model 1) incident MI; model 2) incident MI, coronary artery bypass grafting, or percutaneous transluminal coronary angioplasty. Associations between Lp(a) and HFrEF and HFpEF were tested using the above Cox regression model. P-values <0.05 were deemed significant.
RESULTS
In this sample of 6809 adults, there were 308 incident HF cases (3.9 per 1000 person years). Table 1 shows demographic and clinical characteristics of study participants categorized by HF cases and non-cases over the median 13-year follow-up period. Compared to non-cases, individuals who experienced incident heart failure were more likely to be elderly (p<0.001), male (p<0.001), current or former smokers (p=0.03), diabetic (p<0.001), have a higher BMI (p<0.001) and higher systolic and diastolic blood pressure (both p<0.001), taking hypertension medication (p<0.001), have lower levels of total cholesterol (p=0.02) and HDL-C (p=0.005), have suffered an MI over the follow-up period and have AVC at baseline (both p<0.001). Lp(a) levels did not differ between HF cases and non-cases (p=0.29). Lp(a) distributions for each race/ethnicity are presented in Supplemental Figure I.
Table 1.
Demographic and clinical characteristics of 6,809 MESA participants dichotomized by incident HF over the median 13-year follow-up period.
| All | HF cases | Non-cases | p-value | |
|---|---|---|---|---|
| N | 6809 | 308 | 6501 | |
| Age, years (SD) | 62.1 (10.2) | 68.6 (8.8) | 61.8 (10.2) | <0.001 |
| Sex, n (% female) | 3599 (52.9) | 126 (40.9) | 3473 (53.4) | <0.001 |
| Race/ethnicity, n (%) | NS | |||
| Caucasian | 2619 (38.5) | 123 (39.9) | 2496 (38.4) | |
| Chinese | 803 (11.8) | 23 (7.5) | 780 (12.0) | |
| Black | 1892 (27.8) | 96 (31.1) | 1796 (27.6) | |
| Hispanic | 1495 (22.0) | 66 (21.4) | 1429 (22.0) | |
| Education, n (%) | NS | |||
| <High school | 1225 (18.0) | 72 (23.5) | 1153 (17.8) | |
| High school completed/GED | 1236 (18.2) | 58 (18.9) | 1178 (18.2) | |
| Some college | 1933 (28.5) | 82 (26.7) | 1851 (28.6) | |
| Four year degree | 1171 (17.2) | 50 (16.3) | 1121 (17.3) | |
| Professional degree | 1221 (18.0) | 45 (14.7) | 1176 (18.2) | |
| Smoking, n (%) | 0.03 | |||
| Never | 3416 (50.3) | 132 (43.0) | 3284 (50.7) | |
| Former | 2484 (36.6) | 130 (42.4) | 2354 (36.3) | |
| Current | 887 (13.1) | 45 (14.7) | 842 (13.0) | |
| BMI (kg/m2), mean (SD) | 28.3 (5.5) | 29.8 (5.8) | 28.3 (5.5) | <0.001 |
| SBP (mm Hg), mean (SD) | 126.6 (21.5) | 138.5 (23.3) | 126.0 (21.2) | <0.001 |
| DBP (mm Hg), mean (SD) | 71.9 (10.3) | 73.9 (11.7) | 71.8 (10.2) | <0.001 |
| Hypertension medication, n (%) | 2533 (37.2) | 181 (58.8) | 2352 (36.2) | <0.001 |
| Lipid lowering medication, n (%) | 1096 (16.1) | 58 (18.8) | 1038 (16.0) | NS |
| Diabetes, n (%) | 856 (12.6) | 89 (28.9) | 727 (11.8) | <0.001 |
| Total cholesterol (mmol/L), mean (SD) | 5.02 (0.92) | 4.90 (0.91) | 5.03 (0.92) | 0.02 |
| HDL-C (mmol/L) , mean (SD) | 1.32 (0.38) | 1.26 (0.36) | 1.32 (0.39) | 0.005 |
| Triglycerides (mmol/L), mean (SD) | 1.48 (1.00) | 1.59 (1.25) | 1.48 (1.00) | NS |
| Incident myocardial infarction, n (%) | 283 (4.2) | 106 (34.4) | 177 (2.7) | <0.001 |
| Prevalent AVC, n (%) | 913 (13.4) | 94 (30.6) | 819 (12.6) | <0.001 |
| Lp(a) (mg/dL), median (IQR) | 17.3 (33.1) | 17.3 (32.7) | 17.3 (37.45) | NS |
Definitions: HF=heart failure; SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI=body mass index; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; AVC=aortic valve calcification
Cox regression analysis estimated Lp(a)-related risk of HF with adjustments for age, sex, field center, education, BMI, smoking (pack years), systolic blood pressure, diastolic blood pressure, hypertension medication use, baseline glomerular filtration rate, HDL-C, total cholesterol, log triglycerides, lipid lowering medication use, diabetes, and prevalent AVC (Table 3). Lp(a) was found to be related to greater risk of HF over the median 13-year follow-up period in Caucasians alone. Per log unit of Lp(a) was associated with 20% greater risk of HF (p=0.02). Caucasians with Lp(a) levels ≥30mg/dL and ≥50 mg/dL showed 69% (p=0.01) and 87% (p=0.006) greater risks of HF, respectively. Significant race interactions were observed but depended upon the Lp(a) variable: for per log unit of Lp(a), the race-interaction was not significant (p=0.20); categorizing Lp(a) by 30 mg/dL or 50 mg/dL cutoff values revealed differential associations of Lp(a) and HF risk across race/ethnicity that were suggestive of a race interaction (p=0.03 and p=0.08, respectively). Between races/ethnicities, it was found that associations in Caucasians with Lp(a) ≥30mg/dL were different than those in Black (p=0.006) and Hispanic participants (p=0.04). Likewise, associations in Caucasians with Lp(a) ≥50mg/dL were different than those in Black (p=0.02) and Hispanic participants (p=0.05).
Table 3.
Lp(a)-related risk of heart failure in Multi-Ethnic Study of Atherosclerosis participants (N=6,638; 298 HF cases). Cox proportional hazards ratios and 95% confidence intervals are shown (p-values where significant). Lp(a) was modeled as a continuous variable (per log unit) and by clinical cutoff values, 30 and 50 mg/dL. Covariate adjustments were made for age, sex, field center, education, BMI, smoking (pack years), systolic blood pressure, diastolic blood pressure, hypertension medication use, baseline glomerular filtration rate, HDL-C, total cholesterol, log triglycerides, lipid lowering medication use, diabetes, and prevalent AVC. Interactions with race/ethnicity were tested both across race and between individual races/ethnicities.
| Black | Caucasian | Chinese American | Hispanic | Overall race interaction p-value |
|
|---|---|---|---|---|---|
| N | 1795 | 2532 | 786 | 1453 | |
| Cases (%) | 91 (5.1) | 118 (4.7) | 22 (2.8) | 64 (4.4) | |
| per log unit Lp(a) | 1.00 (0.80 – 1.26) |
1.20 (1.03 – 1.40) 0.02 |
0.90 (0.57 – 1.44) | 0.94 (0.77 – 1.13) | 0.33 |
| Lp(a)≥ 30 mg/dL | 0.74 (0.48 – 1.14)* |
1.69 (1.13 – 2.54) 0.01 |
0.84 (0.22 – 3.12) | 0.59 (0.30 – 1.18)* | 0.03 |
| Lp(a)≥ 50 mg/dL | 0.91 (0.57 – 1.44)* |
1.87 (1.19 – 2.93) 0.006 |
1.40 (0.29 – 6.81) | 0.56 (0.24 – 1.31)* | 0.08 |
Definitions: BMI=body mass index; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; AVC=aortic valve calcification
Indicates that the association was significantly different than that in Caucasian participants (p for interactions <0.05)
Unadjusted Nelson–Aalen analysis comparing cumulative hazards of HF for individuals above and below the 50 mg/dL Lp(a) cutoff are presented for each race/ethnicity in Figure 1 (panels A-D). A higher cumulative hazard for incident heart failure was observed in Caucasians with Lp(a)≥50 mg/dL over the study follow-up period. Similar results were found using the 30 mg/dL cutoff value (Supplemental Figure II).
Figure 1.
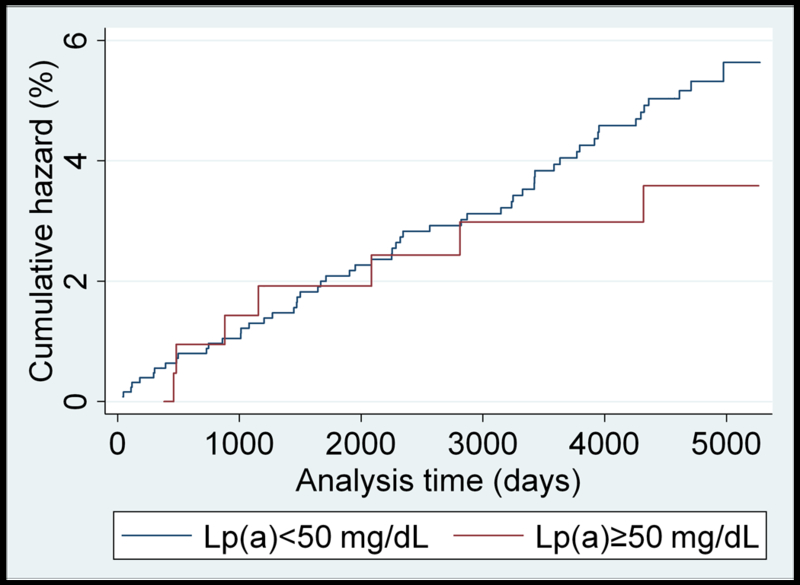
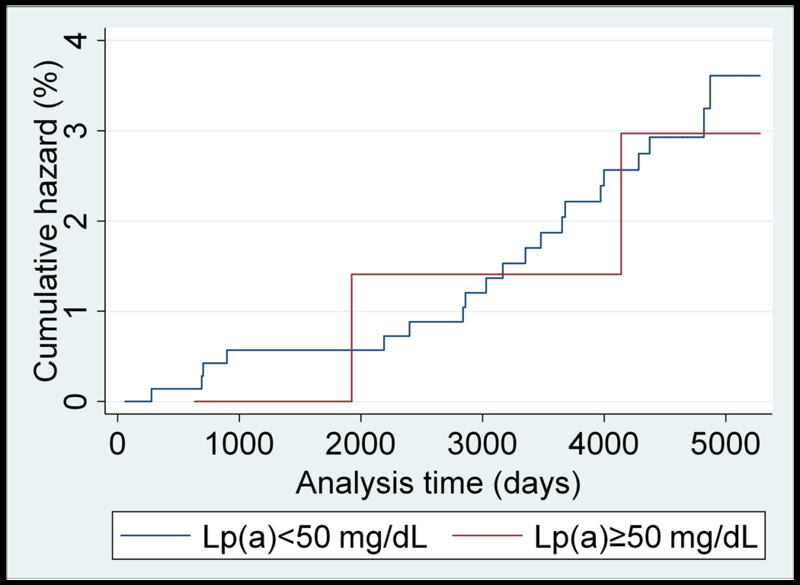
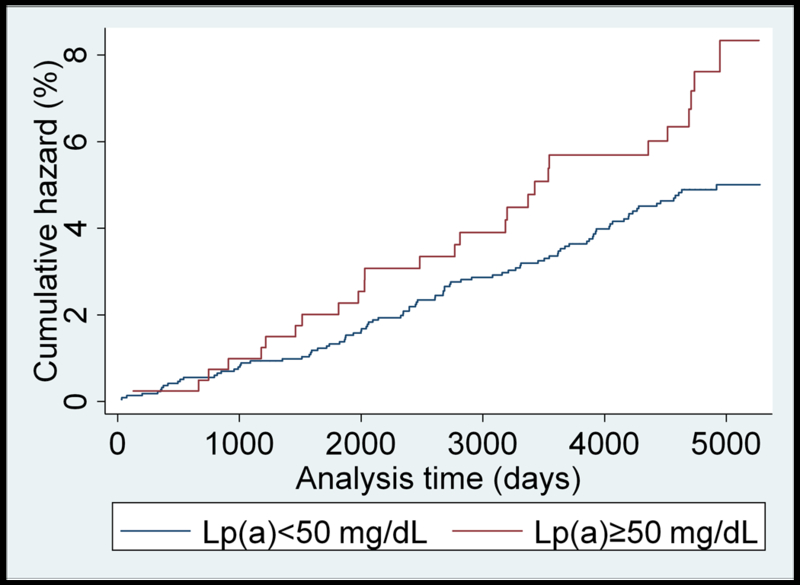
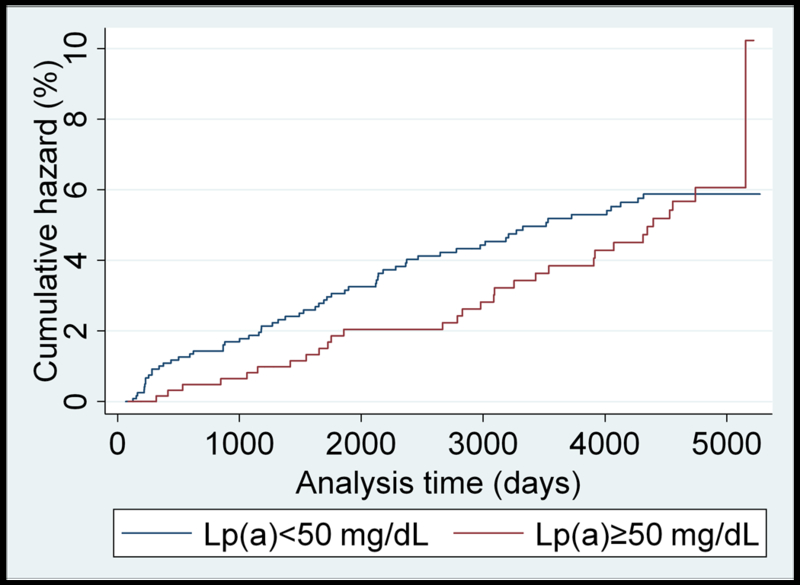
(panels A-D). Unadjusted Nelson-Aalen cumulative hazards curves for incident heart failure in: A) Caucasian; B) Black; C) Chinese-American; and D) Hispanic MESA participants dichotomized by the 50 mg/dL Lp(a) cutoff value.
A secondary analysis evaluated whether Lp(a)-related risk of HF was mediated through ischemia-related events in Caucasian study participants, presented in Table 4. In model 1, individuals who suffered an MI event were excluded from the analysis (n=124). Per log unit of Lp(a) was associated with 37% greater risk of HF (p=0.002). Those with Lp(a) levels ≥30mg/dL and ≥50 mg/dL showed 97% (p=0.006) and 143% (p=0.001) greater risks of HF, respectively. In model 2, in addition to excluding those who suffered an MI, we further excluded participants who underwent coronary artery bypass grafting (n=69) or percutaneous transluminal coronary angioplasty (n=137). Per log unit of Lp(a) was associated with 29% greater risk of HF (p=0.02). Those with Lp(a) levels ≥30mg/dL did not show a significantly greater risk of HF than those below this level (p=0.14), while individuals with Lp(a) levels ≥50 mg/dL showed an approximate two-fold greater risk of HF than those below 50 mg/dL (p=0.03).
Table 4.
Lp(a)-related risk of heart failure in Caucasian participants, excluding individuals who experienced a myocardial infarction or ischemia-related events using a multi-model approach. Lp(a) was modeled as a continuous variable (per log unit) and by clinical cutoff values, 30 and 50 mg/dL. Models were adjusted for age, sex, field center, education, BMI, smoking (pack years), hypertension medication use, lipid lowering medication use, systolic blood pressure, diastolic blood pressure, baseline glomerular filtration rate, HDL-C, total cholesterol, log triglycerides, diabetes, and prevalent AVC.
| Lp(a) variable | HR (95% CI) | p-value |
|---|---|---|
| per log unit Lp(a) | ||
| Model 1 | 1.37 (1.12 – 1.66) | 0.002 |
| Model 2 | 1.29 (1.04 – 1.60) | 0.02 |
| Lp(a)≥ 30 mg/dL | ||
| Model 1 | 1.97 (1.22 – 3.19) | 0.006 |
| Model 2 | 1.53 (0.87 – 2.70) | 0.14 |
| Lp(a)≥ 50 mg/dL | ||
| Model 1 | 2.43 (1.43 – 4.15) | 0.001 |
| Model 2 | 2.04 (1.08 – 3.84) | 0.03 |
Model 1: Excluded individuals with incident myocardial infarction (N=2,414, 82 HF cases)
Model 2: model 1 + excluded individuals who underwent percutaneous transluminal coronary angioplasty or coronary artery bypass grafting (N=2,305, 66 HF cases)
Definitions: BMI=body mass index; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; AVC=aortic valve calcification
In Table 5, HF outcomes were subdivided in to HFrEF (<45%) and HFpEF (≥45%) cases, and Cox regression estimated hazard ratios in Caucasian participants. Per log unit Lp(a) was associated with a 48% greater risk of HFpEF (p=0.002). Those with Lp(a) levels ≥30mg/dL and ≥50 mg/dL showed approximate 2-fold (p=0.02) and 2.5-fold (p=0.004) greater risks of HFpEF than those below these levels, respectively. No significant relations were found between Lp(a) and risk of HFrEF. No significant risk associations were observed between Lp(a) and HFpEF or HFrEF within any of the other racial/ethnic groups (data not shown).
Table 5.
Lp(a)-related risk of incident HFrEF or HFpEF* in Caucasian MESA participants (N=2,516)†. Cox proportional hazards ratios and 95% confidence intervals are shown (p-values where significant). Lp(a) was modeled as a continuous variable (per log unit) and by clinical cutoff values, 30 and 50 mg/dL. Covariate adjustments were made for age, sex, field center, education, BMI, smoking (pack years), systolic blood pressure, diastolic blood pressure, hypertension medication use, lipid lowering medication use, baseline glomerular filtration rate, HDL-C, total cholesterol, log triglycerides, diabetes, and prevalent AVC.
| HFrEF | HFpEF | |
|---|---|---|
| Cases | 47 | 54 |
| per log unit Lp(a) | 1.04 (0.79 – 1.37) |
1.48 (1.17 – 1.89) 0.002 |
| Lp(a)≥ 30 mg/dL | 1.67 (0.91 – 3.06) |
2.09 (1.11 – 3.93) 0.02 |
| Lp(a)≥ 50 mg/dL | 1.60 (0.80 – 3.21) |
2.53 (1.34 – 4.78) 0.004 |
Definitions: HFrEF=heart failure with reduced ejection fraction; HFpEF=heart failure with preserved ejection fraction; BMI=body mass index; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; AVC=aortic valve calcification
HFpEF = ejection fraction≥45%
Individuals with missing covariates or EF data were excluded from the analysis
DISCUSSION
In this multi-ethnic cohort, Lp(a) was found to be a significant risk factor for incident HF in Caucasian individuals alone. Race interactions suggested that Lp(a)-related risk of HF is modified by race/ethnicity. Lp(a)-related risk of HF was found to independent of the known influence of Lp(a) on aortic valve disease and may also be independent of ischemic-related events. Further categorizing HF by EF revealed that higher Lp(a) levels were related to significantly greater risk of HFpEF in Caucasian participants.
To date, two previous cohort studies have examined Lp(a) and HF-related outcomes. Combining two Danish Copenhagen-based cohorts, Kamstrup and Nordestgaard (2016) showed that increasing Lp(a) levels were associated with greater risk of incident HF (p for trend<0.001) (8). Excluding individuals with previous MI or aortic valve stenosis showed that they mediated approximately two-thirds of HF risk, yet the association between Lp(a) and HF remained significant. Similarly, in Atherosclerosis Risk in Communities (ARIC) participants (9), Agarwala et al. (2017) showed that individuals in the top quintile of Lp(a) (23.1–108.2 mg/dL) were at greater risk of HF hospitalization compared to those in the referent quintile (0.02–2.4 mg/dL); however, upon excluding those with prevalent or incident MI, the relationship was rendered non-significant. In addition, and in stark contrast to our findings, ARIC investigators found no evidence of a race interaction with Lp(a) and HF hospitalization (p for race interaction=0.65).
A number of factors may have contributed to the above differential findings. First, the HF outcome variables were distinct—HF hospitalizations were examined in ARIC in contrast to HF incidence in MESA and in the Copenhagen cohorts study. In terms of the exposure variable, Lp(a) was examined using different approaches. The Danish cohort investigators examined Lp(a) based on percentile categories, i.e. (1–33), (34–66), (67–90), (91–99), and (>99). Since MESA did not have the statistical power to mimic this approach, Lp(a) was examined as both a continuous variable and by clinical cutoffs while stratifying by race/ethnicity. In contrast, the ARIC study combined Black and Caucasian individuals and categorized Lp(a) by quintiles. This approach may have biased the results in the ARIC sample considering that there are substantial differences in Lp(a) distributions between Black and Caucasian groups which results in unequal representations of Black and Caucasian individuals across quintiles. Furthermore, we observed no relation between Lp(a) and HF risk in Black MESA participants; therefore, combining these two race groups in ARIC may have weakened Lp(a)-related HF risk in upper quintiles. Ultimately, it may be speculated that the findings in ARIC may have been more consistent with MESA and the Copenhagen cohorts study had the sample been stratified by race and Lp(a) analyzed by a clinical cutoff(s). Supporting this assertion, we applied the statistical approach of the ARIC study to Black and Caucasian MESA participants, and no significant relationship was observed between Lp(a) and incident HF across quintiles (data not shown).
The mechanism through which Lp(a) may influence HF remains unknown, but it was hypothesized that ischemia-related events such as MI would have a mediating influence on Lp(a)-related risk of HF. In a secondary analysis, we used an approach similar to that of previous investigators (8, 9) and excluded individuals who suffered an MI (Table 3, model 1). In a subsequent model (Table 3, model 2), we next excluded those who suffered an MI, underwent coronary artery bypass grafting or percutaneous transluminal coronary angioplasty. With one exception, Lp(a)-related risk of HF was stronger in terms of the magnitudes of associations for Lp(a) exposure variables. While these findings suggest that associations are independent of MI or ischemia-related events in this sample, it must be acknowledged that statistical power was reduced in these analyses. Additional studies are warranted to determine whether Lp(a) is a causal risk factor for HF as found for coronary heart disease and aortic valve stenosis and, if the case, to identify the pathways that are promoting disease development.
Future Research
Despite the thousands of studies conducted on Lp(a) since its identification in 1963 (20, 21), its physiological and pathophysiological mechanisms remain ill-defined, and clinical cutoff values have not been standardized (22). The current finding that Lp(a)-related risk of HF varies across race/ethnicity suggest that the pathogenicity of the Lp(a) particle may be race-dependent or is otherwise heterogeneous across populations. Given the possibility of race-based differences in Lp(a)-related risk, further biomolecular-, genetic-, and cohort-based research is warranted. Specifically, future studies that focus on identifying inter-race differences in the LPA gene [in addition to the well-described KIV type II domain (23)] and in Lp(a) particle components and structure may reveal functions and pathophysiological mechanisms of Lp(a) that remain uncharacterized. The present findings also support the need for standardized, race-dependent clinical cutoffs to discriminate disease risk.
Strengths and Limitations
This study represents the largest evaluation of Lp(a) and incident HF categorized by EF and is the first to show that Lp(a)-related risk of HF is modified by race/ethnicity. The prospective study design and extended follow-up period allowed for the examination of temporality of associations between Lp(a) and HF outcomes. In terms of limitations, the definition of AVC was restricted to the presence of aortic valve calcification at baseline, and we could not evaluate the potential influence of subsequent AVC development on HF incidence. It must also be acknowledged that statistical power may have been limited in analyses of HF categorized by EFs excluding individuals with ischemia-related events; these findings should be interpreted with caution until replicated in other cohorts. MESA is a U.S.-based cohort, and the present results may not be generalizable to other populations. Finally, multiple demographic and clinical adjustments were made within the statistical model, but the possibility of residual confounding cannot be excluded.
Conclusions
In a multi-ethnic cohort, Lp(a) was found to be an independent risk factor for HF and HFpEF in Caucasian individuals alone. Confirmation in other cohorts is warranted, but a race-dependent Lp(a)-related risk of HF has implications in future research and clinical practice.
Supplementary Material
Table 2.
Demographic and clinical characteristics of MESA participants stratified by Lp(a) categories.
| <30 mg/dL | 30 mg/dL <50 mg/dL |
≥50 mg/dL | p-value | |
|---|---|---|---|---|
| N | 4480 | 875 | 1345 | |
| Age, years (SD) | 62.1 (10.3) | 61.9 (10.1) | 62.4 (10.1) | NS |
| Sex, n (% female) | 2273 (50.7) | 470 (53.7) | 796 (59.2) | <0.001 |
| Race/ethnicity, n (%) | <0.001 | |||
| Caucasian | 1936 (43.2) | 231 (26.4) | 412 (31.1) | |
| Chinese | 648 (14.5) | 70 (8.0) | 75 (5.8) | |
| Black | 776 (17.3) | 430 (49.1) | 641 (47.2) | |
| Hispanic | 1120 (25.0) | 144 (16.5) | 217 (15.9) | |
| Education, n (%) | <0.001 | |||
| <High school | 847 (19.0) | 138 (15.9) | 222 (16.5) | |
| High school completed/GED | 809 (18.1) | 152 (17.5) | 260 (19.3) | |
| Some college | 1002 (22.4) | 244 (28.1) | 393 (29.3) | |
| Four year degree | 791 (17.7) | 141 (16.2) | 216 (16.1) | |
| Professional degree | 815 (18.2) | 145 (16.7) | 248 (18.5) | |
| Smoking, n (%) | 0.04 | |||
| Never | 2274 (50.9) | 413 (47.5) | 674 (50.3) | |
| Former | 1653 (37.0) | 324 (37.3) | 472 (35.3) | |
| Current | 543 (12.1) | 132 (15.2) | 193 (14.4) | |
| BMI (kg/m2), mean (SD) | 28.1 (5.4) | 28.7 (5.6) | 28.8 (5.7) | <0.001 |
| SBP (mm Hg), mean (SD) | 125.8 (21.1) | 127.6 (22.2) | 128.2 (22.1) | <0.001 |
| DBP (mm Hg), mean (SD) | 71.6 (10.1) | 72.8 (10.6) | 72.5 (10.4) | <0.001 |
| Hypertension medication, n (%) | 1584 (35.4) | 341 (39.0) | 559 (41.6) | <0.001 |
| Lipid lowering medication, n (%) | 690 (15.4) | 110 (12.6) | 275 (20.5) | <0.001 |
| Diabetes, n (%) | 534 (12.0) | 113 (12.9) | 192 (14.3) | 0.01 |
| Total cholesterol (mmol/L), mean (SD) | 191.1 (34.8) | 195.1 (36.2) | 203.1 (36.5) | <0.001 |
| HDL-C (mmol/L) , mean (SD) | 50.1 (14.5) | 51.5 (14.8) | 53.5 (15.2) | <0.001 |
| Triglycerides (mmol/L), mean (SD) | 138.6 (97.7) | 117.7 (64.8) | 116.8 (66.1) | <0.001 |
| Incident myocardial infarction, n (%) | 176 (3.9) | 28 (3.2) | 66 (4.9) | 0.1 |
| Prevalent AVC, n (%) | 537 (12.0) | 110 (12.6) | 252 (18.7) | <0.001 |
| Heart failure events, n (%) | 200 (4.5) | 33 (3.8) | 67 (5.0) | NS |
Definitions: HF=heart failure; SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI=body mass index; diabetes=treated and untreated cases; HDL-C=high density lipoprotein-cholesterol; AVC=aortic valve calcification
Highlights.
In the Multi-Ethnic Study of Atherosclerosis cohort, Lp(a)-related risk of incident heart failure was only evident in Caucasian study participants
Significant overall race interactions were observed, and Lp(a)-related risk of heart failure was significantly different in Caucasians compared to Black study participants
Categorizing heart by ejection fraction, Lp(a) levels were shown to be related to heart failure with preserved, but not reduced, ejection fraction in Caucasian study participants
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors also thank Denka Seiken (Tokyo, Japan) for providing Lp(a) assay reagents at no cost.
SOURCES OF FUNDING
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
Dr. Bertoni was supported by R01HL127028, NHLBI
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- Lp(a)
Lipoprotein(a)
- HF
Heart Failure
- AVC
Aortic Valve Calcification
- MESA
Multi-Ethnic Study of Atherosclerosis
- HFrEF
Heart Failure with Reduced Ejection Fraction
- HFpEF
Heart Failure with Preserved Ejection Fraction
- ARIC
Atherosclerosis Risk in Communities
Footnotes
DISCLOSURES
None
REFERENCES
- 1 ).Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2 ).Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 3 ).Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 ).Berg J, Lindgren P, Kahan T, Schill O, Persson H, Edner M, Mejhert M. Health-related quality of life and long-term morbidity and mortality in patients hospitalised with systolic heart failure. JRSM Cardiovasc Dis. 2014;3:2048004014548735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 ).Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 ).Arsenault BJ, Boekholdt SM, Dubé MP, Rhéaume E, Wareham NJ, Khaw KT, Sandhu MS, Tardif JC. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet 2014;7:304–10. [DOI] [PubMed] [Google Scholar]
- 7 ).Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 ).Kamstrup PR, Nordestgaard BG. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart Fail. 2016;4:78–87. [DOI] [PubMed] [Google Scholar]
- 9 ).Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, Ndumele C, Shahar E, Heiss G, Boerwinkle E, Konety S, Hoogeveen RC, Ballantyne CM. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis. 2017;262:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 ).Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and Caucasian subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012; 125: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 ).Cao J, Steffen BT, Budoff M, Post WS, Thanassoulis G, Kestenbaum B, McConnell JP, Warnick R, Guan W, Tsai MY. Lipoprotein(a) Levels Are Associated With Subclinical Calcific Aortic Valve Disease in White and Black Individuals: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 ).Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 ).Cao J, Steffen BT, Guan W, Budoff M, Michos ED, Kizer JR, Post WS, Tsai MY. Evaluation of Lipoprotein(a) Electrophoretic and Immunoassay Methods in Discriminating Risk of Calcific Aortic Valve Disease and Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. Clin Chem. 2017;63:1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 ).Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 15 ).Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 ).Tsai MY, Johnson C, Kao WH, Sharrett AR, Arends VL, Kronmal R, Jenny NS, Jacobs DR, Arnett D, O’Leary D, Post W. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Atherosclerosis. 2008;200:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17 ).Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, Couderc R, Dati F, Rifai N, Sakurabayashi I, Tate JR, Steinmetz A. Use of a reference material proposed by the international federation of clinical chemistry and laboratory medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 18 ).Duprez DA, Gross MD, Kizer JR, Ix JH, Hundley WG, Jacobs DR Jr. Predictive Value of Collagen Biomarkers for Heart Failure with and without Preserved Ejection Fraction: the MESA study. J Am Heart Assoc. 2018;7 pii: e007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 ).Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. [DOI] [PubMed] [Google Scholar]
- 20 ).Boffa MB, Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: mechanistic insights from animal models. Clin Biochem. 2004. May;37:333–43. [DOI] [PubMed] [Google Scholar]
- 21 ).Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009. July 22;302:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 ).Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J Am Coll Cardiol. 2018. January 16;71(2):177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ).Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016. July;57:1111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


