Abstract
Retinopathy of prematurity (ROP) is the leading cause of childhood blindness, but current anti-VEGF therapy is concerned with delayed retinal vasculature, eye, and brain development of preterm infants. The clinical observation of reduced ROP severity in premature infants after caffeine treatment for apnea suggests that caffeine may protect against ROP. Here, we demonstrate that caffeine did not interfere with normal retinal vascularization development but selectively protected against oxygen-induced retinopathy (OIR) in mice. Moreover, caffeine attenuated not only hypoxia-induced pathologic angiogenesis, but also hyperoxia-induced vaso-obliteration, which suggests a novel protection window by caffeine. At the hyperoxic phase, caffeine reduced oxygen-induced neural apoptosis by adenosine A2A receptor (A2AR)–dependent mechanism, as revealed by combined caffeine and A2AR-knockout treatment. At the hypoxic phase, caffeine reduced microglial activation and enhanced tip cell formation by A2AR-dependent and -independent mechanisms, as combined caffeine and A2AR knockout produced additive and nearly full protection against OIR. Together with clinical use of caffeine in neonates, our demonstration of the selective protection against OIR, effective therapeutic window, adenosine receptor mechanisms, and neuroglial involvement provide the direct evidence of the novel effects of caffeine therapy in the prevention and treatment of ROP.—Zhang, S., Zhou, R., Li, B., Li, H., Wang, Y., Gu, X., Tang, L., Wang, C., Zhong, D., Ge, Y., Huo, Y., Lin, J., Liu, X.-L., Chen, J.-F. Caffeine preferentially protects against oxygen-induced retinopathy.
Keywords: retinopathy of prematurity, adenosine A2A receptor, vaso-obliteration, neovascularization
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness in many parts of the world (1–3). ROP is caused by oxygen-induced damage to the developing retinal vasculature, which leads to hyperoxia-induced vaso-obliteration and subsequent hypoxia-induced pathologic neovascularization (4, 5) driven by the VEGF signaling pathway in the retina (5, 6). Conventional therapies for ROP are limited to laser treatment to ablate the avascular retina to prevent retinal detachment as a result of ROP (7), but the efficacy of ablative laser therapy is limited and is associated with destruction to the retina that causes clinically significant loss of the visual field. Current therapeutic developments focus on anti-VEGF therapy (e.g., intravitreal injection of anti–VEGF-A antibody bevacizumab) that has demonstrated some effect in a randomized controlled trial (8), but the long-term effect of intravitreal bevacizumab remains unclear, with reported persistent avascular retina (9) and recurrent intravitreal neovascularization (10). VEGF acts as an angiogenic and neurotrophic factor for normal retinal neural and vascular development (9, 11, 12). Retinal vasculature undergoes critical postnatal developmental changes, from forming superficial capillaries to sprouting vertically in the retina to establishing the intraretinal deep vascular plexus (13, 14), which makes it particular sensitive and vulnerable to environmental factors, such as oxygen and vascular and neuronal factors. There is serious concern about the unintended effects of anti-VEGF agents on delayed retinal vasculature, eye growth, and brain development of preterm infants (15–17). Thus, identifying effective pharmacologic strategies for targeting ROP without affecting normal retinal vascularization is critically needed.
Caffeine, a ubiquitous trimethylxanthine, has been routinely used in the neonatal intensive care unit for apnea of prematurity (AOP) since 2006 (18). Of interest, the 2-yr follow-up observation of the initial large, randomized caffeine trial for AOP reported that the severity of ROP is reduced in the caffeine treatment group compared with the control group (19). Considering the safety profile of caffeine (20) and the ability of caffeine to control angiogenic factors, hypoxia-inducible factor-1α and VEGF (21, 22), angiogenesis (22, 23) and apoptosis of endothelium cells (24), and other vascular actions (25), this clinical observation raises an exciting possibility that caffeine may protect against pathologic neovascularization in ROP; however, this therapeutic strategy against ROP has not been directly tested. A recent study demonstrated that ocular ketorolac and systemic caffeine individually or synergistically reduced retinal neovascularization in rat oxygen-induced retinopathy (OIR) (26). Several critical issues, such as the selectivity of caffeine effects on normal retinal vascularization development from pathologic angiogenesis, effective therapeutic window, and the actual molecular pharmacologic targets have not been addressed experimentally.
Here, we demonstrate that caffeine does not affect normal retinal vascularization during early postnatal development, but selectively modulates hyperoxia-induced vaso-obliteration and hypoxia-induced pathologic angiogenesis in OIR. We defined the effective therapeutic windows of caffeine during postnatal development in controlling OIR. Furthermore, by coupling caffeine treatment with the adenosine A2A receptor (A2AR) and adenosine A1 receptor (A1R)-knockout (KO) mice, we identified that caffeine acted at A2AR, but not A1R, as well as non-A2AR targets to exert its protective effect against OIR. We uncovered the neuronal (hyperoxic phase) and microglial (hypoxic phase) changes that were associated the caffeine’s protection against OIR. Our demonstration of the efficacy, selectivity, and therapeutic windows and identification of the receptor and neuroglial mechanisms whereby caffeine protects against OIR—together with widely accepted safety profile of caffeine (20)—provide proof-of-principle preclinical evidence to translate this novel caffeine therapy for the prevention and treatment of ROP.
MATERIALS AND METHODS
Animals: A1R- and A2AR-KO mice
A2AR-KO mice were developed in our laboratory as previously described (27–29). Congenic A2AR-KO mice in a C57BL/6 background were generated by backcrossing A2AR-KO in mixed 129-Steel × C57BL/6 background to C57BL/6 mice for >10 generations. Heterozygous A1R-KO (A1R+/−) or A2AR-KO (A2AR+/−) mouse cross-breeding was used to generate homozygous A1R-KO (A1R−/−) and homozygous A2AR-KO (A2AR−/−) mice and their corresponding wild-type (WT) littermates (A1R+/+ and A2AR+/+), all from the same breeding pairs. A1R−/− and A1R+/+ or A2AR−/−and A2AR+/+ mice with matched sexes at age 12 or 17 d were used for this study. Mouse genotypes were determined by PCR analysis of genomic DNA that was isolated from mouse tails by using the 3 primer sets targeted to neocassettes and the adjacent A1R or A2AR gene as previously described (27–29).
Mouse model of OIR
The OIR model was described previously by Smith and colleagues (13). In brief, 7-d-old C57BL/6J mice or A1R- and A2AR-KO mice and their corresponding WT littermates were kept with foster/nursing mothers and exposed to 75% oxygen for 5 d [from postnatal day (P)7 to -12] to induce vaso-obliteration. On P12, mice were returned to room air (21% oxygen) to induce retinal neovascularization, which was maximal at P17. Age-matched mice kept in room air throughout postnatal development (P0–17) served as room-air controls. Neonatal mice that weighed <6 g were excluded from the study. Foster mothers were rotated between the pups who were exposed to hyperoxia and the mice in room air environments every 8 h.
Caffeine treatments
Caffeine solution (Sigma-Aldrich, St. Louis, MO, USA) was freshly prepared at concentrations of 0.1, 0.3, and 1 g/L in drinking water. Pups were exposed to caffeine via nursing mothers who were given caffeine in drinking water. The foster mother and newborn pups were kept together in cages until P17. Caffeine treatment was commenced on the day mice were exposed to caffeine in drinking water at different postnatal developmental stages (P0, -7, or -12) and for different periods (P0–17, P7–12, P12–17). OIR mice that were exposed to water only served as vehicle treatment.
To ensure that the effective concentration of caffeine in the blood of pups was reached after feeding foster mothers with caffeine in drinking water (1 g/L) in OIR mice from P0 to P12, we collected blood from pups with anesthesia (chloral hydrate, 4%, 0.1 ml/10 g). Blood was centrifuged at 6000 rpm for 10 min to isolate plasma. Plasma was then mixed with acetonitrile and centrifuged at 13,000 rpm for 10 min. Supernatants were used for ultra-high-performance liquid chromatography–mass spectrometry analysis.
To examine the effect of direct injection of caffeine into pups, we intraperitoneally injected caffeine (10 mg/kg first dose at P7, then 2.5 mg/kg for the remainder of the treatment, daily, P8–16) into neonates. The control group was treated with saline of the same injection volume of caffeine. We analyzed the avascular area of these mice at P17.
Fluorescence immunostaining in whole-mount retinas
Fluorescence staining of whole-mounted retinas was performed as previously described (30). Mice were euthanized on P3, -6, -12, and -17, and eyes were enucleated and fixed with 4% paraformaldehyde for 1 h. Corneas were removed with scissors along the limbus, and intact retinas were dissected. Retinas were blocked and permeabilized in PBS that contained 0.5% Triton X-100 overnight at 4°C. Retinas were incubated with 10 µg/ml isolectin B4 (Thermo Fisher Scientific, Waltham, MA, USA) overnight at 4°C. Retinas were then incubated with anti–glial fibrillary acidic protein mouse mAb (1:500; Sigma-Aldrich) for 12 h at 4°C, followed by incubation with fluorescence-conjugated second Ab (1:500; Thermo Fisher Scientific) for 2 h, and then whole mounted. Retinas were washed with PBS and mounted on microscope slides in mounting medium. Retinas were examined by Laser Scanning Microscope (Zeiss 510; Carl Zeiss, Jena, Germany). Areas of vaso-obliteration and vitreoretinal neovascular tufts were quantified by using Adobe Photoshop CS 5 software (Adobe, San Jose, CA, USA). Eight nonoverlapping and randomly selected microscopic fields per retina were imaged by confocal scanning laser microscopy (LSM 710; Carl Zeiss) to assess the formation of endothelial tip cells, and 4 nonoverlapping and randomly selected microscopic fields in the avascular area per retina were imaged by confocal scanning laser microscopy to assess astrocyte function.
Fluorescence immunostaining in retinal section
At P17 of OIR, mouse eyes were dissected and embedded in optimum cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). Three cryostat sections with optic nerve head per retina were blocked and permeabilized, then incubated with anti–Iba-1 rabbit mAb (1:100; Wako, Chuo-ku, Osaka, Japan) overnight at 4°C. Fluorescence-conjugated second Abs (1:500; Thermo Fisher Scientific) were applied to detect positive signals. For anti–proliferating cell nuclear antigen (PCNA) staining, mouse eyes were dissected and embedded in paraffin at P17 of OIR. After being deparaffinized and heated in 10 mM sodium citrate for antigen repairing, 3 retinal paraffin sections were blocked and permeabilized, then incubated with PCNA rabbit polyclonal Ab (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Fluorescence-conjugated second Abs (1:500; Thermo Fisher Scientific) were applied to detect positive signals.
Assessment of developmental retinal angiogenesis
To assess normal postnatal development of retinal angiogenesis, C57BL/6J pups that were exposed to caffeine in drinking water or treated with KW6002 were euthanized and eyes were harvested on P17. Whole-mount retinas were stained with isolectin B4. Eight nonoverlapping and randomly selected microscopic fields per retina and whole-mounted retina were assessed for morphology and distribution of retinal vessels at P17. Vessels in the 3 layers—superficial, intermediate, and deep—were skeletonized (31), and total vessel length in each microscopic field was calculated by using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). Vessel density was quantified as the ratio of the total vessel length to microscopic field.
Neovascular quantification
Quantification of neovascularization was performed according to the procedure described by Smith and colleagues (13). Eyes from OIR mice were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin. The extent of neovascularization was evaluated by counting the number of intravitreal vascular nuclei, which were defined as the nuclei of cells that extended beyond the inner limiting membrane of the retina into the vitreous. In this study, eyes of 6–10 mice from each group were examined and analyzed. For each eye, 20 retinal sections, excluding the optic nerve, were evaluated. All intravitreal vascular nuclei were counted under ×400 magnification with hematoxylin and eosin–stained retinal sections by an investigator who was blinded to the specific treatment group.
TUNEL assay
Retinal cell apoptosis was evaluated on P12. TUNEL staining was performed with the Roche In Situ Cell Death Detection kit (Roche Diagnostics, Basel, Switzerland) according to manufacturer instructions. Cryostat sections (12 μm thick) with optic nerve head were permeabilized, and antigen retrieval was performed by 0.1% sodium citrate buffer solution with 0.5% Triton X-100 for 5 min. After washing 3 times, sections were incubated in TUNEL reaction solutions for 1 h in 37°C, then washed and stained for another 5 min by using DAPI (1:2000; Beyotime Biotechnology, Shanghai, China). Fluorescence images were taken by using a fluorescence microscope (Zeiss 510; Carl Zeiss). TUNEL-positive cells were counted from 3 sections that crossed the optic nerve per retina.
RNA isolation and quantitative PCR
Eye tissues were homogenized in TRIzol reagent (Thermo Fisher Scientific), and total RNA was extracted according to manufacturer instructions. The concentration and purity of total RNA were then determined by using NanoDrop ND 1000 (Thermo Fisher Scientific). The first cDNA chain was synthesized from 0.5 μg of extracted total RNA using PrimeScript RT Master Mix (Perfect Real Time; Takara, Tokyo, Japan) according to manufacturer protocol. Quantitative PCR was performed with SYBR-Green premix Extaq (Takara) and detected by a Real-Time PCR System (CFX96; Bio-Rad, Hercules, CA, USA). β-Actin was used as an internal control gene. The sequences of quantitative PCR primers were as follows: A2AR: forward, 5′-CCGAATTCCACTCCGGTACA-3′; reverse, 5′-CAGTTGTTCCA GCCCAGCAT-3′; A1R: forward, 5′-ATCCCTCTCCGGTACAAGACAGT-3′; reverse, 5′-ACTCAGGTTGTTCCAGCCAAAC-3′; VEGF: forward, 5′-GAAAGGGTCAAAAACGAAAGC-3′; reverse, 5′-CGCTCTGAACAAGGCTCAC-3′; TNF-α:forward, 5′-CGGGGTGATCGGTCCCCAA AG-3′; reverse, 5′-GGAGGGCGTTGGCGCGCTGG-3′; TGF-β1: forward, 5′-TGGAGCAACATGTGGAACTC-3′; reverse, 5′-CGTCAAAAGACAGCCACTCA-3′; β-actin: forward, 5′-GAGATTACTGCCCTGGCTCCTA-3′; reverse, 5′-TCATCGTACTCCTGCTTGCTGAT-3′. A standard 2-step PCR amplification was performed, with a melting step at 95°C for 15 s and annealing and elongation at 60°C for 30 s, for 40 cycles. After PCR amplification, a first derivative melting-curve analysis was conducted to confirm the specificity of the PCR. Ct values were converted to relative quantification by using the 2−∆∆Ct method.
Statistics
Data are presented as means ± se. The difference between 2 experimental groups (caffeine vs. water) was analyzed by independent Student’s t test. The comparison among multiple (>2) groups of one experimental factor (caffeine dose response curve and therapeutic window) was analyzed by 1-way ANOVA followed by Bonferroni post hoc test. The effect of 2 independent experiment factors (caffeine treatment and genetic KO) and their interactions was analyzed by 2-way ANOVA followed by Bonferroni post hoc test. Statistical analysis was performed by using SPSS 13.0 (SPSS, Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Caffeine had no effect on postnatal retinal vasculature development
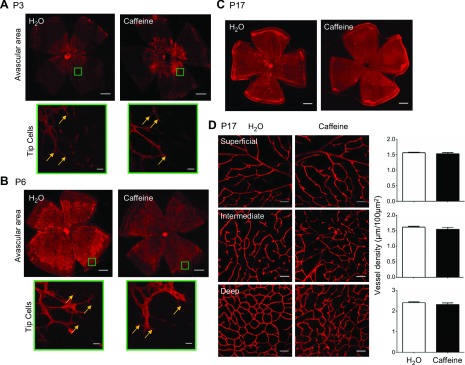
As a critical therapeutic concern is whether caffeine affects postnatal retinal vasculature development, we first analyzed the development of the retinal vascular networks of C57BL/6 mice after caffeine treatment by isolectin B4 analysis in the whole-mount retinas that were scanned with laser scanning microscopy. After exposure to room air from birth, mice displayed normal development of the retinal vascularization. Both the superficial and deep vascular layers from the optic disc to the periphery were well developed by P17. Of importance, the peripheral avascular areas at P3, -6, and -17 were comparable between mice that were treated with water and those with caffeine (Fig. 1A–C). Caffeine concentrations in pups at P12 after feeding the mother caffeine in drinking water (1 g/L) from P0 to -12 were 359 ± 96 µg/L (i.e., ∼1.8 μM), and 0 μM (n = 5/group) after feeding the mother with water, which was consistent with the literature and the above effective concentration for blocking adenosine receptors and other caffeine pharmacologic targets (32, 33).Thus, the superficial retinal vascular layer grew with similar rates and indistinguishable patterns of vessel distribution for mice that were treated with water or caffeine. We also found that caffeine treatment did not affect the development of endothelial tip cells, as morphology and the number of tip cells were indistinguishable between caffeine- or water-treated mouse pups (Fig. 1A, B). We further analyzed development and morphologies of 3 vessel layers under confocal scanning laser microscopy on P17 after treatment with caffeine or water. Arterioles in the superficial layer had numerous dichotomous branches, whereas vessels in the deep layer formed anastomotic grids, with vessels in the intermediate layer displaying short capillary segments. Caffeine treatment did not affect retinal vasculature in the superficial, intermediate, or deep vascular plexus (Fig. 1D). Quantitative analysis confirmed that retinal blood vessel densities were indistinguishable between mice treated with water or caffeine (Fig. 1D). Together, these analyses unambiguously demonstrated that caffeine treatment throughout postnatal development stage (P0–17) did not affect normal development of morphology, distribution, and density of retinal vascularization for mice raised in room air.
Figure 1.
Caffeine treatment does not affect normal postnatal retinal vasculature development. A, B) Mouse whole-mount retinas from caffeine- and water-treated mice were harvested and immune-stained with isolectin B4. Scale bars, 500 μm. Selected areas of the retina were magnified (green rectangle; scale bar, 20 μm) to show representative images of retinal tip cells (yellow arrow) at P3 (A) and P6 (B) after exposure to room air. The morphology of retinal vasculatures and the numbers of tips cells were indistinguishable between caffeine- or water-treated groups. C) Retinal vasculature morphologies were indistinguishable between caffeine- or water-treated pups at P17 in room air. Scale bars, 500 μm. D) Retinal vasculatures of the superficial, intermediate, and deep vascular layers were examined at P17 by isolectin B4 staining of whole-mount retinas. The distributions of 3 retinal vascular layers were displayed in distinct confocal planes. Vessel density was quantified as the ratio of the total vessel length to microscopic field. Vessel densities of the superficial, intermediate, and deep plexuses were indistinguishable between caffeine- and water-treated pups. P > 0.05, Student’s t test (n = 9/group). Scale bars, 50 μm.
Caffeine reduced retinal vaso-obliteration and pathologic angiogenesis in OIR
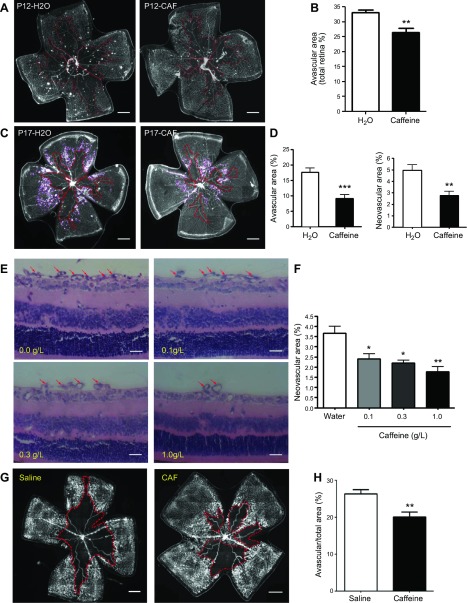
We next evaluated the effect of caffeine treatment for 12 d (P0–12) or 17 d (P0–17) on vaso-obliteration and neovascularization in OIR mice by analyzing the avascular area and vitreoretinal neovascular area with isolectin B4 staining in whole-mounted retinas. Caffeine treatment (1.0 g/L in drinking water) for 12 d (P0–12) significantly decreased retinal avascular area compared with that in water-treated OIR mice (Fig. 2A, B). Furthermore, caffeine treatment for 17 d (P0–17) decreased the retinal avascular area by 51.5% and the retinal neovascular area by 44.3% (Fig. 2C, D). Quantitation of intravitreal vascular nuclei numbers in cross-sections demonstrated that caffeine treatment for 17 d markedly reduced pathologic retinal angiogenesis, with reduced intravitreal vascular nuclei in retinas of OIR mice compared with water-treated mice (42.9 ± 3.2 vs. 87.0 ± 4.5). Moreover, at the concentration range of 0.1–1.0 g/L in drinking water, caffeine treatment reduced retinal vaso-proliferation in a dose-dependent manner. A dose-response study of caffeine showed that caffeine treatment reduced the retinal neovascular area by 51.5% at a dose of 1.0 g/L caffeine and by 40.0% at 0.3 g/L caffeine (Fig. 2E, F).
Figure 2.
Caffeine treatment reduces retinal vaso-obliteration at P12 and pathologic angiogenesis at P17 in OIR. Starting from the first day after delivery, pups were exposed to caffeine (Caf) via feeding nursing mothers with 0.1–1.0 g/L caffeine in drinking water. A, C, E) Whole-mount retinas (A, C) or retinal cross-section (E) from the water-OIR and caffeine-OIR groups were harvested on P12 (A) or -17 (C, E) and examined by immunostaining with isolectin B4, or histologic analysis of intravitreoretinal neovascular nuclei was performed. Representative images show avascular areas (indicated by red dotted line) at P12 (A) and -17 (C) and vitreoretinal neovascular tufts (indicated by purple line) in retinal whole-mounts at P17 (C). Scale bars, 500 μm. B, D) Areas of vaso-obliteration (B, D) and vitreoretinal neovascular tufts (D) were quantified and analyzed. **P < 0.01, ***P < 0.001; Student’s t test (n = 10/group). E, F) Representative images (E) show neovascular nuclei (red arrow) after treatment with caffeine at doses of 0–1.0 g/L in drinking water. Vitreoretinal neovascular tuft areas in whole-mount retinas were quantified and analyzed (F). Caffeine treatment dose-dependently reduced pathologic neovascularization. Scale bars, 20 μm. *P < 0.05, **P < 0.01; 1-way ANOVA, Bonferroni post hoc test (n = 10/group). G, H) Representative images (G) show areas of vaso-obliteration (indicated by red dotted line) after direct intraperitoneal injection of caffeine (10 mg/kg at P7 and 2.5 mg/kg P8–-16 daily) into pups. Quantitative analysis (H) shows that direct injection of the caffeine treatment reduced the avascular area compared with saline-treated pups. Scale bars, 500 μm. **P < 0.01; Student’s t test (n = 7–8/group).
We further validated the protective effect of caffeine by direct injection of caffeine into pups to exclude the possibility that caffeine intake by the mother may affect the secretion or other composition of milk to influence pups’ OIR response. We demonstrated that direct injection of caffeine into pups at P7–16 also attenuated the avascular area at P17 by 23.8% (Fig. 2G, H; n = 7–8/group). Direct caffeine treatment also reduced the retinal neovascular area (data not shown), which was in agreement with our standard caffeine treatment paradigm of feeding foster mothers with caffeine in drinking water (Fig. 2C, D). Together, these findings demonstrate that caffeine treatment selectively and dose-dependently reduced retinal vaso-obliteration and pathologic angiogenesis in OIR.
The effective therapeutic window of caffeine: the hyperoxic and hypoxic phases
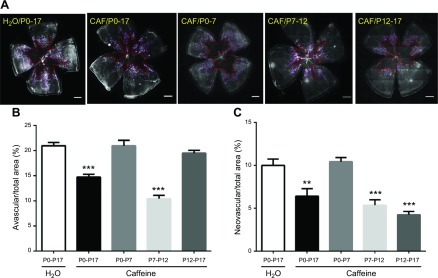
As retinal vascularization and caffeine action are remarkably different at different postnatal developmental stages (P0–17), and as the OIR model of ROP pathogenesis is characterized by 2 phases [the hyperoxic phase (P7–12) with vaso-obliteration, and the hypoxic phase (P12–17) with pathologic angiogenesis (30, 34)], we sought to define the specific developmental stage and disease phase whereby caffeine exerted its protection against and prevention of OIR. We treated mice with caffeine (1 g/L) in 4 different treatment paradigms (i.e., at 4 different postnatal developmental periods: P0–7, P7–12, P12–17, and P0–17, with P0 being the day for pup delivery). Consistent with the results shown in Fig. 2C, D, caffeine treatment from P0–17 decreased avascular areas and neovascular tufts toward the vitreous of OIR mice (Fig. 3). Of interest, analysis of the avascular area revealed that caffeine treatment at P7–P12, but not P12–P17, was effective in reducing vaso-obliteration (Fig. 3A, B); however, caffeine treatment at P7–12 (5.4 ± 0.6%) and P12–17 (4.2 ± 0.4%) was effective in reducing neovascularization tufts toward the vitreous comparing with OIR water-treated control mice (10.0 ± 0.8%; Fig. 3A, C). In addition, pretreatment with caffeine from P0–7, before exposure to 75% oxygen, was not sufficient to confer a preventive effect on development of OIR. Together, these results identified the effective therapeutic windows of caffeine: treatment with caffeine at P7–12 (i.e., the hyperoxic phase) was effective in reducing vaso-obliteration, whereas treatment with caffeine at P7–12 and P12–17 was effective in reducing neovascularization in OIR.
Figure 3.
Effective therapeutic windows for caffeine to confer protection against OIR. A) Pups were treated with water or caffeine (1 g/L) in different therapeutic windows (P0–17, P0–7, P7–12, and P12–17), and retinal vasculatures were analyzed by whole-mount isolectin B4 staining at P17 of OIR. Avascular areas are shown by red dotted line. Neovascularization tufts are indicated by purple line. Scale bar, 500 μm. B) Avascular area (%) was quantified as a percentage of the whole retinal surface (n = 7–9/group). C) Neovascularization tuft area (%) was quantified as a percentage of whole retinal area. **P < 0.01, ***P < 0.001; 1-way ANOVA, Bonferroni post hoc test (n = 7–9/group).
Caffeine’s protection was associated with neural, microglial, and endothelial mechanisms
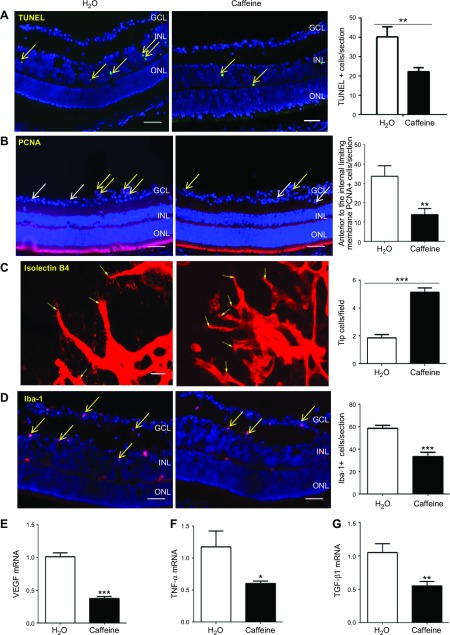
As the first step to uncover the cellular basis for caffeine-mediated protection against OIR, we performed TUNEL analyses of cellular apoptosis in retinas at P12 of OIR mice, as vaso-obliteration in the hyperoxic phase is mainly a result of neuronal and endothelial apoptosis by a combination of direct toxicity of reactive oxygen species to neurons and a down-regulation of hypoxia-inducible factor-1α–dependent VEGF expression to endothelial cells (35). Consistent with our recent study (36) and other studies (37–39), TUNEL-positive signals were mainly detected within the inner nuclear layer—mainly neurons, such as amacrine cells and bipolar cells—of the avascular area, with scattered TUNEL-positive cells detected in the ganglion cell layer and outer nuclear layer in 2 groups (Fig. 4A). Quantitative analysis showed that caffeine treatment (P0–12) decreased TUNEL-positive cells in retinas compared with WT water-treated OIR mice (Fig. 4A).
Figure 4.
In OIR, the effect of caffeine treatment on neural apoptosis at P12, and cellular proliferation, tip cell numbers, and microglial activation and mRNA levels for VEGF, TNF-α, and TGF-β1 in retinas at P17. A) Apoptotic cells in retinas were analyzed by TUNEL (green) staining and individual cells were visualized by DAPI (blue) staining at P12 of OIR. Retinal TUNEL-positive cells (yellow arrow) were quantified and analyzed. Caffeine treatment (1 g/L) reduced cellular apoptosis in the inner nuclear layer (INL) cells of retinas. GLC, ganglion cell layer; ONL, outer nuclear layer. Scale bars, 50 μm. Data are presented as means ± sem. **P < 0.01; Student’s t test (n = 8–9/group). B) Cell proliferation in retinas was assessed by immunohistochemistry of PCNA at P17 of OIR. PCNA-positive cells inside retinas (white arrow) and exceeding the membrane (yellow arrow) were quantified. Caffeine treatment reduced total PCNA-positive cells in retinas and, in particular, PCNA-positive cells that exceeded the internal limiting membrane but had no effect on intraretinal PCNA-positive cells. Scale bars, 50 μm. **P < 0.01; Student’s t test (n = 7/group). C) Endothelial tip cells in retinas were detected by isolectin B4 staining at P17 of OIR. Representative retinal endothelial tip cells are indicated by yellow arrows. Quantification of the number of endothelial tip cells revealed that caffeine treatment increased tip cell numbers in OIR. Scale bars, 20 μm. ***P < 0.001; Student’s t test (n = 8/group). D) Microglial activation in retinas was assessed by immunohistochemistry of Iba-1 at P17 of OIR. Expression of Iba-1 (yellow arrow) was detected mainly in the GCL and INL of retinas. Quantification of Iba-1–positive cells reveal that caffeine treatment attenuated microglial activation in retina of OIR mice. Scale bar, 50 μm. ***P < 0.001; Student’s t test (n = 8/group). E–G) mRNA levels for VEGF (E), TNF-α (F), and TGF-β1 (G) in retinas after caffeine treatment (1 g/L in drinking water, P0–P17) were determined by quantitative PCR at P17. Caffeine treatment reduced mRNA levels for VEGF, TNF-α, and TGF-β1 compared with the water-treated mice. *P < 0.05, **P < 0.01 (n = 7–8).
In the hypoxic phase, revascularization and pathologic angiogenesis could be induced by proliferation of endothelial cells within retinas, or into vitreous, respectively. We first analyzed the expression of PCNA, a marker for cellular proliferation, at P17 of OIR (Fig. 4B). Caffeine treatment decreased the numbers of PCNA-positive cells anterior to the internal limiting membrane, but did not affect intraretinal PCNA-positive cells. We next evaluated the formation of endothelial tip cells at the junction between vascularized and avascular areas at P17 of OIR, as endothelial tip cells play an important role during angiogenesis by extending typical filopodia in retinal sprout tips along VEGF gradient derived from astrocytes. Quantitative PCR analysis demonstrated that chronic caffeine treatment from P0 to P17 significantly reduced VEGF mRNA levels at P17 (Fig. 4E). This finding, together with our previous finding that VEGF mRNA was reduced in A2AR-KO mice compared with WT littermates in the OIR model (40), suggests that the effect of caffeine on pathologic angiogenesis in OIR, in part, is mediated by reduced VEGF expression in retina. As seen in Fig. 4C, quantitative analysis revealed that the numbers of endothelial tip cells in retinas were significantly increased by caffeine treatment compared with the WT water-treated group (Fig. 4C). Lastly, we examined whether caffeine treatment modulates retinal vascularization in OIR by affecting microglial activation, as A2AR expression is up-regulated in retinal microglial cells in the OIR model (41, 42) and because activation of microglial cells promotes endothelial apoptosis and growth of immature retinal vasculatures, which contributes to the development of OIR (43, 44). Analysis of the Iba-1–positive signal indicated that microglial activation at P17 was observed in the ganglion cell, inner plexiform layer, and inner nuclear layers of retinas of the water and caffeine groups. Of importance, activated microglial cells (Iba-1–positive) were significantly attenuated by caffeine treatment (Fig. 4D). Attenuation of microglial activation by caffeine may lead to an improved vascularization environment as indicated by enhanced tip cell formation in retinas. Quantitative PCR analyses demonstrated that caffeine treatment from P0 to 17 decreased TNF-α and TGF-β1 expression (Fig. 4F, G). The reduced proinflammatory cytokine, TNF-α mRNA, is consistent with the notion that caffeine protects against OIR by modulating inflammation. The apparent reduction of anti-inflammatory cytokine, TGF-β1 mRNA, is likely a result of that, whereas inflammatory response of OIR mice has largely subsided at P17 and this stage is characterized by hypoxia-driven proliferative angiogenesis in retinas.
Together, these finding suggest that caffeine may protect against OIR, in part, by modulating hyperoxia-induced neuronal apoptosis at P12 and by reduced proliferation of intravitreous endothelial cells and microglial activation with enhanced tip cell formation in retinas of OIR mice at P17.
Caffeine’s protection against OIR is independent of A1Rs
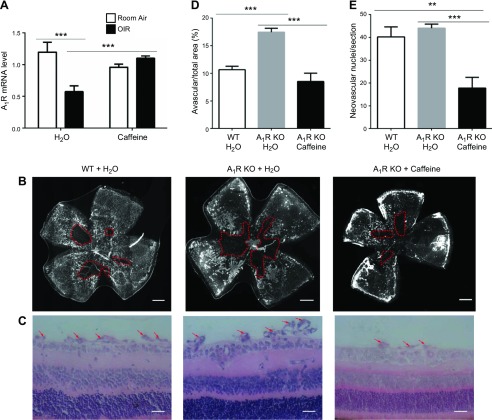
As caffeine at the concentration used in this study has similar affinity for both A1R and A2AR (20), we explored the possible involvement of A1R in the effect of caffeine by administering caffeine to WT and A1R-KO mice. Quantitative PCR analysis indicated that the A1R mRNA level was markedly decreased in OIR mice compared with the control group under room air. Caffeine treatment from P0 to P17 did not affect the expression of A1R mRNA in control mice (room air), but reversed the reduction of A1R mRNA in OIR mice when comparing the OIR caffeine-treated group with the OIR water-treated group (P < 0.001; Fig. 5A). Changes in retinal A1R mRNA after OIR and caffeine treatment indicated altered A1R signaling in OIR and possible caffeine effects; however, further analysis indicated that, consistent with our recent report (36), A1R KO indeed exacerbated retinal vascular damage, as evident by increased avascular areas compared with WT littermates in OIR models. Of importance, when caffeine was administered to A1R-KO mice, we still observed the protective effect of caffeine, which reversed the increased avascular area of A1R-KO mice to that observed in WT littermates that received no caffeine treatment (Fig. 5B–E). Thus, A1R activation confers a protective effect against OIR and caffeine-mediated protection was independent of A1Rs.
Figure 5.
Caffeine’s protection against OIR is independent of A1R activation. A) Mouse retinas from room air/OIR and water/caffeine (1 g/L) groups were harvested on P17 and analyzed for A1R mRNA levels in retinas by quantitative PCR. Caffeine reversed the down-regulation of retinal A1R mRNA expression in OIR. **P < 0.01, ***P < 0.001; 2-way ANOVA, Bonferroni post hoc test (n = 6/group). B) Retinal vasculature was visualized by whole-mount isolectin B4 staining at P17 of OIR. Representative images show avascular area (red dotted line) from WT and A1R-KO mice (with or without caffeine treatment). Scale bar, 500 μm. C) Retinal pathologic angiogenesis (red arrow) from WT and A1R-KO mice (with or without caffeine treatment) were detected by using hematoxylin and eosin staining at P17. Scale bar, 20 μm. D) Avascular areas (%) from WT and A1R-KO mice (with or without caffeine treatment) in 3 groups were quantified as percentages of the whole retinal surface. Whereas A1R KO increased avascular areas, caffeine treatment still reduced avascular areas in A1R-KO mice. ***P < 0.001; 1-way ANOVA, Bonferroni post hoc test (n = 8/group). E) The number of vascular nuclei on the vitreal side of the inner limiting membrane in 3 groups was quantified and analyzed. Caffeine treatment reduced intravitreal vascular nuclei in A1R-KO mice. **P < 0.01, ***P < 0.001; 1-way ANOVA, Bonferroni post hoc test (n = 6–8/group).
Caffeine protects against OIR by A2AR-dependent and -independent mechanisms
To evaluate whether A2AR mediates the effect of caffeine in OIR, we examined A2AR mRNA expression in eyes of mice that were exposed to OIR and after caffeine treatment (1 g/L) for 17 d (P0–17). Caffeine treatment for 17 d increased retinal A2AR mRNA in the eyes of control (room air) mice. There was a further up-regulation of A2AR mRNA with caffeine treatment in OIR mice (Fig. 6A). Up-regulation of retinal A2AR mRNA levels after caffeine treatment suggests the possible alteration of A2AR signaling in caffeine’s modulation of OIR.
Figure 6.
Caffeine protects against OIR by A2AR-dependent and -independent mechanisms. A) Room air or OIR mouse retinas [with or without caffeine treatment (1 g/L)] were harvested on P17 for quantitative PCR analysis of the A2AR mRNA expression in retinas. Chronic caffeine (Caf) treatment increased A2AR mRNA expression in retinas of normal and OIR mice. **P < 0.01, ***P < 0.001; 2-way ANOVA, Bonferroni post hoc test (n = 6–11/ mice). B) Retinal vasculature was visualized by whole-mount isolectin B4 staining at P12 of OIR. Avascular area is shown by red dotted line. Scale bar, 500 μm. C) Avascular area (%) was quantified as a percentage of the whole retinal surface. *P < 0.05; ***P < 0.001, 2-way ANOVA, Bonferroni post hoc test (n = 16–22/group). D) Retinal vasculature was visualized by whole-mount isolectin B4 staining at P17 of OIR. Avascular area is shown by red dotted line. Neovascularization tufts are indicated by purple line. Scale bar, 500 μm. E) Avascular area (%) was quantified as a percentage of the whole retinal surface. ***P < 0.001, 2-way ANOVA, Bonferroni post hoc test (n = 14–16/group). F) The neovascularization tuft area (%) was quantified as a percentage of whole retinal area. Caffeine treatment (1 g/L) or A2AR KO decreased avascular areas, and combined caffeine and A2AR-KO treatment produced a synergistic effect and completely reversed OIR pathology. ***P < 0.001; 2-way ANOVA, Bonferroni post hoc test (n = 12–16/group).
To establish the role of A2AR in mediating caffeine’s protection against OIR, we assessed the effects of caffeine, A2AR KO, and caffeine–A2AR KO combined on vaso-obliteration in the hyperoxia phase of OIR at P12. Compared with water-treated mice, caffeine treatment and A2AR KO decreased avascular areas by 18.2 and 29.2%, respectively, compared with water/vehicle control (Fig. 6B, C). However, the combined caffeine–A2AR-KO treatment did not produce a further reduction in vaso-obliteration, as the avascular areas were indistinguishable between the A2AR-KO–caffeine and A2AR-KO–water groups.
We further examined the effects of caffeine, A2AR KO, and their combination on OIR in the hypoxic phase of OIR at P17. Both caffeine treatment and A2AR KO reduced avascular areas compared with the WT water-treated control (Fig. 6D). Quantitative analysis revealed that caffeine treatment or A2AR KO decreased avascular areas by 47.5 and 61.6%, respectively, compared with the WT water-treated control (Fig. 6E). Remarkably, combined caffeine and A2AR-KO treatment produced a synergistic effect and completely reversed OIR pathology, with the avascular area being nearly 0 (Fig. 6E). This full protection was largely attributed to the attenuation of pathologic angiogenesis, as neovascularization tufts toward the vitreous were attenuated by caffeine and by A2AR KO, but more prominently by their combined treatment in a synergistic manner (Fig. 6F). The finding that caffeine exerted additional protection on top of A2AR-KO strongly suggests that both the A2AR-dependent and -independent mechanisms contribute to the full protection provided by the combined caffeine and A2AR inactivation strategy.
DISCUSSION
The present study demonstrates that caffeine—by feeding the foster mother with caffeine in drinking water or by direct intraperitoneal injection of caffeine into pups—protects against hyperoxia-induced vaso-obliteration and hypoxia-induced pathologic angiogenesis in mouse OIR, which is consistent with the protective effect on neovascularization by the systemic administration of caffeine in a rat intermittent (50–12% oxygen) hypoxia model of ROP (26). This study identified 4 critical features of caffeine’s protection against OIR: 1) caffeine selectively attenuated OIR pathology, but did not affect normal retinal vascular development, conferring therapeutic advantage of caffeine by targeting ROP without an unwanted effect on normal postnatal retinal vascularization; 2) caffeine treatment was effective in protecting OIR not only at the hypoxic phase (P12–17), but also at the hyperoxic phase (P7–12), which suggests a novel therapeutic window; 3) caffeine protects against OIR by an A2AR-mediated mechanism (the hyperoxic phase) and by A2AR-dependent and -independent mechanisms (the hypoxic phase) with no involvement of A1Rs (of note, the combined treatment of caffeine and A2AR KO achieves nearly full protection against OIR); 4) caffeine’s protection is associated with reduced neuronal cell death in the inner nuclear layer in the hypoxic phase and with reduced microglial activation with enhanced endothelial tip cell formation in the hypoxic phase. Together with the noted safety profile (20) and routine use of caffeine in neonate care (18), these findings collectively provide the required preclinical studies to translate this novel caffeine therapy into the prevention and treatment of ROP and other proliferative retinopathies.
Caffeine protects against OIR at the hypoxic and hyperoxic phases
During postnatal development, retinal vasculature undergoes critical developmental changes, from forming the superficial capillaries at P7, to sprouting vertically in retinas to form the deep vascular layer at P8, to completing the intermediate vascular plexus in mice at P15 (13, 14). These developing retinal vasculatures may be particularly sensitive and vulnerable to hypoxia and pharmacologic manipulation. Furthermore, the 2-stage pathology of ROP is modeled in OIR by the hyperoxic phase at P7–12, and by the hypoxic phase at P12–17. In addition, caffeine treatment at different postnatal stages produces different steady-state levels of caffeine in humans and rodents. For example, the half-life of caffeine in humans decreases dramatically from 8 to 23 h for the full-term newborn infant (45–47) to 14 h at postnatal age 3–5 mo, and to 2.6 h in 5- to 6-mo-old infants (48, 49). Depending on the developmental stage, caffeine has been shown to exert opposite effects on synaptic plasticity (50) and white matter injury (51) and, thus, may have distinct effects on retinal vasculature development. Thus, it is critical to define the specific postnatal developmental stages and 2 phases of ROP that are sensitive to caffeine treatment.
Of importance, our data show that caffeine treatment at P7–12 (i.e., the hyperoxic phase) was effective in reducing vaso-obliteration, whereas treatment with caffeine at P7–12 and P12–17 was effective in reducing neovascularization in OIR. Despite clear vaso-obliteration at the retina center, there is no hypoxia in retinas in the hyperoxic phase, as shown by in vivo detection with nitroimidazole EF5 (52). In fact, ecto-5′-nucleotidase (CD73) and, presumably, adenosine expression levels are low during the hyperoxic phase (53). Our finding is nonetheless surprising, however, as adenosine levels are expected to be low and the pharmacologic effect of caffeine (as an adenosine receptor blocker) is less effective at the hyperoxic phase. Yet, the hyperoxic phase at P7–12 is most critical to confer protection against OIR by caffeine. This intriguing finding may be attributed to the particular sensitivity of developing retinal vasculature at P7–12 to caffeine treatment and to significantly higher concentrations of caffeine at P7–12 than at P12–17 as result of different caffeine metabolisms during postnatal development. This finding indicates that hyperoxic damage to developing retinal vasculatures is the primary and critical effect during OIR pathogenesis, despite the fact that pathologic angiogenesis is most evident at the hypoxic phase of ROP (5, 34). We argue that caffeine treatment should be primarily targeted at the hyperoxic phase, that is, when premature infants receive oxygen treatment.
Since the 2006 publication of the results of caffeine therapy for AOP, caffeine has been routinely used in neonate care with the noted safety. Of note, plasma caffeine and metabolite levels in dams that we obtained in our study are 0.3 mg/L, which is consistent with earlier studies (32, 33) and comparable with regular human consumption (1.5 mg/L) (20), with reported umbilical cord plasma in the newborns of mothers who consumed coffee (0.5–2 mg/L) (54), and with the serum from breast-fed infants and caffeine-exposed mothers (0.25–3.2 mg/L) (48, 55)—all being, in fact, 10- to 20-fold lower than caffeine levels achieved by caffeine treatment for AOP infants (∼15 mg/L) (45, 46, 48, 49). Currently, caffeine treatment for AOP is usually initiated within the first 10 d of life in the preterm infant (56). Our findings indicate that the treatment of caffeine at the hypoxic phase and, in particular, the hyperoxic phase (i.e., at the early stage of ROP) suggest that this caffeine treatment regime for AOP might be adapted and tested in clinical trials for the prophylaxis and treatment of ROP.
Caffeine protects against OIR by A2AR-dependent and -independent mechanisms
Caffeine has a complex pharmacology, with multiple molecular targets. Caffeine at levels of 0.1 to 1.0 g/L in drinking water fed dams in this study can give plasma caffeine and metabolite levels in dams at the concentration of 0.2 to 1 μM (mg/L) (32, 33), which is comparable with concentrations obtained from the umbilical cord plasma of newborns whose mothers consumed coffee [0.5–2 μM (mg/L)] (54) and in the serum of breast-fed infants of caffeine-exposed mothers (48, 55). Among multiple molecular targets of caffeine, adenosine receptor blockade by caffeine occurs at concentrations (2–50 μM) that are 20- to 100-fold less than that required for phosphodiesterase or GABAA receptor inhibition and calcium release (>200 μM) (20, 57).
Involvement of adenosine receptors (A2AR and A1R) in the caffeine-mediated control of OIR is also consistent with our finding that down-regulation of A1R and up-regulation of A2AR in OIR (36, 40) are reversed by chronic caffeine treatment (Figs. 5A and 6A). Of importance, our analysis using combined caffeine treatment and A2AR-KO model reveals that the protection against vaso-obliteration and neuronal apoptosis by caffeine is mediated by A2AR in the hyperoxic phase, as combined treatment of caffeine and A2AR KO produced similar and partial protection at P12 to caffeine or A2AR KO alone. At P17, administering caffeine to A2AR-KO mice produces additive/synergistic protection against OIR, which demonstrates the A2AR-dependent and -independent effects of caffeine. The nature of the A2AR-independent effect of caffeine during the hypoxic phase is not clear. A1R blockade did not contribute to caffeine’s protection against OIR, as A1R KO exacerbated OIR damage, and administering caffeine to A1R-KO mice still produced protection. However, A2BR may contribute to OIR pathology, as A2BR antagonists can inhibit oxygen-induced retinal neovascularization in vivo (58, 59). Because retinal membrane preparations exhibit Ca2+-independent [3H]ryanodine binding and inhibition by caffeine (60, 61), caffeine may also act at specific isoforms of ryanodine receptor to control calcium release, which contributes to the protective effect of caffeine in OIR.
Caffeine may protect against OIR by endothelial and neuro-glial mechanisms
Caffeine modulation of retinal vascularization during development and OIR likely involves close interaction among endothelial cells, neurons, and glial cells in retinas. In retinas, A1Rs and A2ARs are largely expressed in ganglion cells, inner plexiform layers, inner and outer nuclear layers, and the choriocapllaris and endothelium (53, 62, 63). In retinal endothelial cells, A2AR activation increases production of VEGF and GLUT-1 (64, 65), which indicates a proangiogenic effect of A2AR. In the hypoxic phase of ROP, increased endothelial sprouting and proliferation likely play major roles in control of pathologic angiogenesis (66–68) as well as developmental blood vessel formation and physiologic revascularization (69). This is consistent with the experimental finding that genetic inactivation of A2AR or caffeine treatment reduces endothelial cell proliferation and VEGF mRNA expression in the OIR model (40). Caffeine may reduce avascular areas and promote revascularization, likely by enhancing the functions of endothelial tip cells, as caffeine reverses OIR reduction of endothelial tip cells in retinas. Caffeine may directly act on A2AR or other molecular targets in endothelial cells to control vaso-obliteration and vaso-proliferation. Future studies using endothelial A2AR-KO mice are needed to determine the exact mechanism by which endothelial A2ARs remain in control of ROP.
Alternatively, caffeine may exert its effect indirectly by acting on molecular targets (e.g., A2AR and non-A2AR) at neurons (e.g., stimulating platelet-derived growth factor) or microglial (e.g., releasing glutamate and TNF-α) to affect endothelial cell function in retinas. We found that, in parallel with reduced avascular area, caffeine attenuates TUNEL-positive cells in the inner nuclear layer (neuronal cells) of retinas, which suggests that caffeine likely protects against hyperoxia-induced damage to developing retinal vessels by modulating neuronal apoptosis. In keeping with this notion, caffeine exposure during the first 7 d of life has been shown to affect neonate sensitivity to neuronal insults, including ischemic brain injury (32), white matter injury (70), and seizure susceptibility (33).
At the hypoxic phase, we found that caffeine modulation of retinal vascularization in OIR at P17 is associated with attenuated microglia activation at P17 of OIR. This finding is consistent with the critical contribution of microglial cells to OIR development, as depletion of microglial cells during vascular development resulted in suppression of retinal neovascularization in OIR (43), whereas activated macrophages induce endothelial cell apoptosis (44). This finding is also in agreement with our previous studies that indicated that inactivation of A2ARs in microglial cells attenuates microglial activation and brain injury (71–73). As endothelial cells are the final common pathway of retinal vascularization under physiologic and pathologic conditions, indirect modulation of endothelial proliferation by neuro-glial actions of A2ARs may explain the selective control of pathologic angiogenesis without affecting normal retinal vasculature development.
Therapeutic advantages and clinical implications of caffeine treatment in ROP
Our demonstration of the therapeutic efficacy and selectivity, effective therapeutic window, and cellular and receptor mechanisms of caffeine’s protection against OIR suggest potential caffeine-based therapeutic strategies with 2 distinct advantages over the current anti-VEGF treatment in ROP. First, caffeine treatment preferentially controls retinal pathologic vaso-obliteration and neovascularization in OIR, but does not affect postnatal retinal vascularization (with normal morphology, density, and distribution of retinal vessels). This critical finding is entirely consistent with our earlier study that reported that genetic inactivation of A2ARs (40) or A1Rs (36) selectively controlled pathologic OIR without affecting normal retinal development, and with selective effects of A1R and A2AR antagonists on ischemic retinal injury without modification of normal retinal function (36, 40). Second, to the best of our knowledge, this is the first study to demonstrate that combined caffeine and A2AR inactivation can produce nearly full protection against OIR. The mechanism that underlies this selective and robust caffeine effect on OIR may be related to local increase of adenosine–adenosine receptor signaling in response to stress, hypoxia, and inflammation, as we postulated (74). This is produced by rapid (minutes) local increase of adenosine and delayed (∼24 h) up-regulation of adenosine receptors and enzymes that are responsible for generating and maintaining adenosine concentration, as demonstrated in OIR (41, 42) and other pathologic conditions (74–76). Aberrant adenosine signaling thus represents a local “find-me” signal and serves as a unique purinergic chemotaxis for a local resolution to pathologic conditions (74). Thus, the preferential protection of caffeine against OIR without affecting normal vascular development confers a critical advantage over anti-VEGF Ab strategy: VEGF activity is necessary not only for pathologic angiogenesis, but also for normal retinal vascularization during development (77). As a result, anti-VEGF Ab effect is limited by delayed vasculature and neural development of preterm infants (9, 11, 12). Further dissection of A2AR signaling interactions with other molecular pathways, such as VEGF, that lead to distinct physiologic development and pathologic angiogenesis may achieve the maximal protective effect of caffeine while maintaining minimal influence on normal retinal vascularization.
ACKNOWLEDGMENTS
This study was sponsored by the Start-up Fund from Wenzhou Medical University (Grants 89211010 and 89212012 to J.-F.C.), the National Natural Science Foundation of China (Grants 81630040 to J.-F.C., 81600753 to S.Z., and 81100672 to R.Z.), the Zhejiang Provincial Special Funds (Grant 604161241 to J.-F.C.), Key Laboratory of Vision Science, Ministry of Health, China (Grant 601041241 to J.-F.C.), the National Key Basic Research Program of China (Grant 2012CB910402 to J.-F.C.), the Central Government Special Fund for Local Universities’ Development (Grant 474091314 to J.-F.C.), and Zhejiang Provincial Natural Science Foundation (Grant LY12H12007 to X.-L.L.). This study was also funded by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK095862 to Y.H.), NIH National Heart, Lung, and Blood Institute (Grants HL095556 and HL108922 to Y.H.), and Boston University School of Medicine Special Research Fund DTD 4-30-14 (to J.-F.C.). The authors thank Dr. Zailong Chi (Wenzhou Medical University) for critical reading of the manuscript, and Drs. Zhe Wang and Congcong Wen (both from Wenzhou Medical University) for assistance in ultra-high-performance liquid chromatography–mass spectrometry analysis.
Glossary
- A1R
adenosine A1 receptor
- A2AR
adenosine A2A receptor
- AOP
apnea of prematurity
- KO
knockout
- OIR
oxygen-induced retinopathy
- PCNA
proliferating cell nuclear antigen
- ROP
retinopathy of prematurity
- WT
wild type
AUTHOR CONTRIBUTIONS
S. Zhang, R. Zhou, X.-L. Liu, and J.-F. Chen designed the experiment, analyzed the data, and wrote the paper; S. Zhang, R. Zhou, B. Li, H. Li, Y. Wang, X. Gu, L. Tang, C. Wang, D. Zhong, and Y. Ge performed the experiments and collected and analyzed the data; and Y. Huo and J. Lin analyzed the data and wrote the paper.
REFERENCES
- 1.Chen Y., Li X. X., Yin H., Gilbert C., Liang J. H., Jiang Y. R., and Zhao M. W.; Beijing ROP Survey Group . (2008) Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br. J. Ophthalmol. 92, 326–330 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert C. (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 84, 77–82 [DOI] [PubMed] [Google Scholar]
- 3.Dhaliwal C., Wright E., Graham C., McIntosh N., and Fleck B. W. (2009) Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. Br. J. Ophthalmol. 93, 355–359 [DOI] [PubMed] [Google Scholar]
- 4.Fleck B. W., and McIntosh N. (2008) Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum. Dev. 84, 83–88 [DOI] [PubMed] [Google Scholar]
- 5.Cavallaro G., Filippi L., Bagnoli P., La Marca G., Cristofori G., Raffaeli G., Padrini L., Araimo G., Fumagalli M., Groppo M., Dal Monte M., Osnaghi S., Fiorini P., and Mosca F. (2014) The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 92, 2–20 [DOI] [PubMed] [Google Scholar]
- 6.Penn J. S., Madan A., Caldwell R. B., Bartoli M., Caldwell R. W., and Hartnett M. E. (2008) Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 27, 331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark D., and Mandal K. (2008) Treatment of retinopathy of prematurity. Early Hum. Dev. 84, 95–99 [DOI] [PubMed] [Google Scholar]
- 8.Mintz-Hittner H. A., Kennedy K. A., and Chuang A. Z.; BEAT-ROP Cooperative Group . (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 364, 603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga C. C., Mitton K. P., Dailey W., Massoll C., Roumayah K., Guzman E., Tarabishy N., Cheng M., and Drenser K. A. (2014) Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 55, 1884–1892 [DOI] [PubMed] [Google Scholar]
- 10.Hu J., Blair M. P., Shapiro M. J., Lichtenstein S. J., Galasso J. M., and Kapur R. (2012) Reactivation of retinopathy of prematurity after bevacizumab injection. Arch. Ophthalmol. 130, 1000–1006 [DOI] [PubMed] [Google Scholar]
- 11.Robinson G. S., Ju M., Shih S. C., Xu X., McMahon G., Caldwell R. B., and Smith L. E. (2001) Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 15, 1215–1217 [DOI] [PubMed] [Google Scholar]
- 12.McCloskey M., Wang H., Jiang Y., Smith G. W., Strange J., and Hartnett M. E. (2013) Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 54, 2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D’Amato R., Sullivan R., and D’Amore P. A. (1994) Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 35, 101–111 [PubMed] [Google Scholar]
- 14.Stahl A., Connor K. M., Sapieha P., Chen J., Dennison R. J., Krah N. M., Seaward M. R., Willett K. L., Aderman C. M., Guerin K. I., Hua J., Löfqvist C., Hellström A., and Smith L. E. (2010) The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 51, 2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijima K., Ng Y. S., Zhong L., Bradley J., Schubert W., Jo N., Akita J., Samuelsson S. J., Robinson G. S., Adamis A. P., and Shima D. T. (2007) Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 171, 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Geniez M., Maharaj A. S., Walshe T. E., Tucker B. A., Sekiyama E., Kurihara T., Darland D. C., Young M. J., and D’Amore P. A. (2008) Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One 3, e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin J., Luu T. M., Superstein R., Ospina L. H., Lefebvre F., Simard M. N., Shah V., Shah P. S., and Kelly E. N.; Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network Investigators . (2016) Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 137, e20153218. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Hady H., Nasef N., Shabaan A. E., and Nour I. (2015) Caffeine therapy in preterm infants. World J. Clin. Pediatr. 4, 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt B., Roberts R. S., Davis P., Doyle L. W., Barrington K. J., Ohlsson A., Solimano A., and Tin W.; Caffeine for Apnea of Prematurity Trial Group . (2007) Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 357, 1893–1902 [DOI] [PubMed] [Google Scholar]
- 20.Fredholm B. B., Bättig K., Holmén J., Nehlig A., and Zvartau E. E. (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133 [PubMed] [Google Scholar]
- 21.Merighi S., Benini A., Mirandola P., Gessi S., Varani K., Simioni C., Leung E., Maclennan S., Baraldi P. G., and Borea P. A. (2007) Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol. Pharmacol. 72, 395–406 [DOI] [PubMed] [Google Scholar]
- 22.Ryzhov S., McCaleb J. L., Goldstein A. E., Biaggioni I., and Feoktistov I. (2007) Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J. Pharmacol. Exp. Ther. 320, 565–572 [DOI] [PubMed] [Google Scholar]
- 23.Hsu S. J., Lee F. Y., Wang S. S., Hsin I. F., Lin T. Y., Huang H. C., Chang C. C., Chuang C. L., Ho H. L., Lin H. C., and Lee S. D. (2015) Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 61, 1672–1684 [DOI] [PubMed] [Google Scholar]
- 24.Li H., Jin S. Y., Son H. J., Seo J. H., and Jeong G. B. (2013) Caffeine-induced endothelial cell death and the inhibition of angiogenesis. Anat. Cell Biol. 46, 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echeverri D., Montes F. R., Cabrera M., Galán A., and Prieto A. (2010) Caffeine’s vascular mechanisms of action. Int. J. Vasc. Med. 2010, 834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aranda J. V., Cai C. L., Ahmad T., Bronshtein V., Sadeh J., Valencia G. B., Lazzaro D. R., and Beharry K. D. (2016) Pharmacologic synergism of ocular ketorolac and systemic caffeine citrate in rat oxygen-induced retinopathy. Pediatr. Res. 80, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J. F., Huang Z., Ma J., Zhu J., Moratalla R., Standaert D., Moskowitz M. A., Fink J. S., and Schwarzschild M. A. (1999) A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 19, 9192–9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day Y. J., Huang L., McDuffie M. J., Rosin D. L., Ye H., Chen J. F., Schwarzschild M. A., Fink J. S., Linden J., and Okusa M. D. (2003) Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 112, 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Q. Y., Wei C., Yu L., Coelho J. E., Shen H. Y., Kalda A., Linden J., and Chen J. F. (2006) Adenosine A2A receptors in bone marrow-derived cells but not in forebrain neurons are important contributors to 3-nitropropionic acid-induced striatal damage as revealed by cell-type-selective inactivation. J. Neurosci. 26, 11371–11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor K. M., Krah N. M., Dennison R. J., Aderman C. M., Chen J., Guerin K. I., Sapieha P., Stahl A., Willett K. L., and Smith L. E. (2009) Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornfield T. E., and Newman E. A. (2014) Regulation of blood flow in the retinal trilaminar vascular network. J. Neurosci. 34, 11504–11513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bona E., Adén U., Fredholm B. B., and Hagberg H. (1995) The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr. Res. 38, 312–318 [DOI] [PubMed] [Google Scholar]
- 33.Georgiev V., Johansson B., and Fredholm B. B. (1993) Long-term caffeine treatment leads to a decreased susceptibility to NMDA-induced clonic seizures in mice without changes in adenosine A1 receptor number. Brain Res. 612, 271–277 [DOI] [PubMed] [Google Scholar]
- 34.Hartnett M. E., and Penn J. S. (2012) Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 367, 2515–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alon T., Hemo I., Itin A., Pe’er J., Stone J., and Keshet E. (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1, 1024–1028 [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Li H., Li B., Zhong D., Gu X., Tang L., Wang Y., Wang C., Zhou R., Li Y., He Y., Chen M., Huo Y., Liu X. L., and Chen J. F. (2015) Adenosine A1 receptors selectively modulate oxygen-induced retinopathy at the hyperoxic and hypoxic phases by distinct cellular mechanisms. Invest. Ophthalmol. Vis. Sci. 56, 8108–8119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan L. J., Takeda K., and Fong G. H. (2011) Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am. J. Pathol. 178, 1881–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludewig P., Flachsbarth K., Wegscheid C., Tiegs G., Richard G., Wagener C., Bartsch U., and Horst A. K. (2014) CEACAM1 confers resistance toward oxygen-induced vessel damage in a mouse model of retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 55, 7950–7960 [DOI] [PubMed] [Google Scholar]
- 39.Narayanan S. P., Xu Z., Putluri N., Sreekumar A., Lemtalsi T., Caldwell R. W., and Caldwell R. B. (2014) Arginase 2 deficiency reduces hyperoxia-mediated retinal neurodegeneration through the regulation of polyamine metabolism. Cell Death Dis. 5, e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X. L., Zhou R., Pan Q. Q., Jia X. L., Gao W. N., Wu J., Lin J., and Chen J. F. (2010) Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest. Ophthalmol. Vis. Sci. 51, 6625–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsherbiny N. M., Naime M., Ahmad S., Elsherbini A. M., Mohammad S., Fulzele S., El-Remessy A. B., Al-Gayyar M. M., Eissa L. A., El-Shishtawy M. M., Han G., White R., Haroldo T. F., and Liou G. I. (2013) Potential roles of adenosine deaminase-2 in diabetic retinopathy. Biochem. Biophys. Res. Commun. 436, 355–361 [DOI] [PubMed] [Google Scholar]
- 42.Lutty G. A., and McLeod D. S. (2003) Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog. Retin. Eye Res. 22, 95–111 [DOI] [PubMed] [Google Scholar]
- 43.Ishida S., Yamashiro K., Usui T., Kaji Y., Ogura Y., Hida T., Honda Y., Oguchi Y., and Adamis A. P. (2003) Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat. Med. 9, 781–788 [DOI] [PubMed] [Google Scholar]
- 44.Lobov I. B., Rao S., Carroll T. J., Vallance J. E., Ito M., Ondr J. K., Kurup S., Glass D. A., Patel M. S., Shu W., Morrisey E. E., McMahon A. P., Karsenty G., and Lang R. A. (2005) WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aranda J. V., Cook C. E., Gorman W., Collinge J. M., Loughnan P. M., Outerbridge E. W., Aldridge A., and Neims A. H. (1979) Pharmacokinetic profile of caffeine in the premature newborn infant with apnea. J. Pediatr. 94, 663–668 [DOI] [PubMed] [Google Scholar]
- 46.Aranda J. V., Gorman W., Bergsteinsson H., and Gunn T. (1977) Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J. Pediatr. 90, 467–472 [DOI] [PubMed] [Google Scholar]
- 47.Le Guennec J. C., and Billon B. (1987) Delay in caffeine elimination in breast-fed infants. Pediatrics 79, 264–268 [PubMed] [Google Scholar]
- 48.Parsons W. D., and Neims A. H. (1981) Prolonged half-life of caffeine in healthy tem newborn infants. J. Pediatr. 98, 640–641 [DOI] [PubMed] [Google Scholar]
- 49.Pearlman S. A., Duran C., Wood M. A., Maisels M. J., and Berlin C. M., Jr. (1989) Caffeine pharmacokinetics in preterm infants older than 2 weeks. Dev. Pharmacol. Ther. 12, 65–69 [PubMed] [Google Scholar]
- 50.Cabral-Miranda F., Serfaty C. A., and Campello-Costa P. (2011) A time-dependent effect of caffeine upon lesion-induced plasticity. Neurosci. Res. 71, 99–102 [DOI] [PubMed] [Google Scholar]
- 51.Rivkees S. A., and Wendler C. C. (2011) Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr. Res. 69, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott A., and Fruttiger M. (2010) Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond.) 24, 416–421 [DOI] [PubMed] [Google Scholar]
- 53.Taomoto M., McLeod D. S., Merges C., and Lutty G. A. (2000) Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 41, 230–243 [PubMed] [Google Scholar]
- 54.Hentges C. R., Guedes R. R., Silveira R. C., and Procianoy R. S. (2010) Serum levels of caffeine in umbilical cord and apnea of prematurity. J. Pediatr. (Rio J.) 86, 137–142 [DOI] [PubMed] [Google Scholar]
- 55.Ryu J. E. (1985) Caffeine in human milk and in serum of breast-fed infants. Dev. Pharmacol. Ther. 8, 329–337 [DOI] [PubMed] [Google Scholar]
- 56.Schoen K., Yu T., Stockmann C., Spigarelli M. G., and Sherwin C. M. (2014) Use of methylxanthine therapies for the treatment and prevention of apnea of prematurity. Paediatr. Drugs 16, 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daly J. W. (2007) Caffeine analogs: biomedical impact. Cell. Mol. Life Sci. 64, 2153–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afzal A., Shaw L. C., Caballero S., Spoerri P. E., Lewin A. S., Zeng D., Belardinelli L., and Grant M. B. (2003) Reduction in preretinal neovascularization by ribozymes that cleave the A2B adenosine receptor mRNA. Circ. Res. 93, 500–506 [DOI] [PubMed] [Google Scholar]
- 59.Mino R. P., Spoerri P. E., Caballero S., Player D., Belardinelli L., Biaggioni I., and Grant M. B. (2001) Adenosine receptor antagonists and retinal neovascularization in vivo. Invest. Ophthalmol. Vis. Sci. 42, 3320–3324 [PubMed] [Google Scholar]
- 60.Shoshan-Barmatz V., Zakar M., Shmuelivich F., Nahon E., and Vardi N. (2007) Retina expresses a novel variant of the ryanodine receptor. Eur. J. Neurosci. 26, 3113–3125 [DOI] [PubMed] [Google Scholar]
- 61.Shoshan-Barmatz V., Orr I., Martin C., and Vardi N. (2005) Novel ryanodine-binding properties in mammalian retina. Int. J. Biochem. Cell Biol. 37, 1681–1695 [DOI] [PubMed] [Google Scholar]
- 62.Braas K. M., Zarbin M. A., and Snyder S. H. (1987) Endogenous adenosine and adenosine receptors localized to ganglion cells of the retina. Proc. Natl. Acad. Sci. USA 84, 3906–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kvanta A., Seregard S., Sejersen S., Kull B., and Fredholm B. B. (1997) Localization of adenosine receptor messenger RNAs in the rat eye. Exp. Eye Res. 65, 595–602 [DOI] [PubMed] [Google Scholar]
- 64.Takagi H., King G. L., Robinson G. S., Ferrara N., and Aiello L. P. (1996) Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci. 37, 2165–2176 [PubMed] [Google Scholar]
- 65.Takagi H., King G. L., and Aiello L. P. (1998) Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 47, 1480–1488 [DOI] [PubMed] [Google Scholar]
- 66.Xu Z., Gong J., Maiti D., Vong L., Wu L., Schwarz J. J., and Duh E. J. (2012) MEF2C ablation in endothelial cells reduces retinal vessel loss and suppresses pathologic retinal neovascularization in oxygen-induced retinopathy. Am. J. Pathol. 180, 2548–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horowitz A., and Simons M. (2008) Branching morphogenesis. Circ. Res. 103, 784–795 [DOI] [PubMed] [Google Scholar]
- 68.Carmeliet P., and Tessier-Lavigne M. (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 [DOI] [PubMed] [Google Scholar]
- 69.Surmeier D. J., and Graybiel A. M. (2012) A feud that wasn’t: acetylcholine evokes dopamine release in the striatum. Neuron 75, 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Back S. A., Craig A., Luo N. L., Ren J., Akundi R. S., Ribeiro I., and Rivkees S. A. (2006) Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 60, 696–705 [DOI] [PubMed] [Google Scholar]
- 71.Yu L., Shen H. Y., Coelho J. E., Araújo I. M., Huang Q. Y., Day Y. J., Rebola N., Canas P. M., Rapp E. K., Ferrara J., Taylor D., Müller C. E., Linden J., Cunha R. A., and Chen J. F. (2008) Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann. Neurol. 63, 338–346 [DOI] [PubMed] [Google Scholar]
- 72.Saura J., Angulo E., Ejarque A., Casadó V., Tusell J. M., Moratalla R., Chen J. F., Schwarzschild M. A., Lluis C., Franco R., and Serratosa J. (2005) Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J. Neurochem. 95, 919–929 [DOI] [PubMed] [Google Scholar]
- 73.Dai S. S., Zhou Y. G., Li W., An J. H., Li P., Yang N., Chen X. Y., Xiong R. P., Liu P., Zhao Y., Shen H. Y., Zhu P. F., and Chen J. F. (2010) Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J. Neurosci. 30, 5802–5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J. F., Eltzschig H. K., and Fredholm B. B. (2013) Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 12, 265–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frick J. S., MacManus C. F., Scully M., Glover L. E., Eltzschig H. K., and Colgan S. P. (2009) Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol. 182, 4957–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schingnitz U., Hartmann K., Macmanus C. F., Eckle T., Zug S., Colgan S. P., and Eltzschig H. K. (2010) Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 184, 5271–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LeBlanc K. H., Maidment N. T., and Ostlund S. B. (2013) Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One 8, e61355. [DOI] [PMC free article] [PubMed] [Google Scholar]