Abstract
Recent evidence suggests that specialized proresolving lipid mediators (SPMs) generated from docosahexaenoic acid (DHA) can modulate the vascular injury response. However, cellular sources for these autacoids within the vessel wall remain unclear. Here, we investigated whether isolated vascular cells and tissues can produce SPMs and assessed expression and subcellular localization of the key SPM biosynthetic enzyme 5-lipoxygenase (LOX) in vascular cells. Intact human arteries incubated with DHA ex vivo produced 17-hydroxy DHA (17-HDHA) and D-series resolvins, as assessed by liquid chromatography-tandem mass spectrometry. Addition of 17-HDHA to human arteries similarly increased resolvin production. Primary cultures of human saphenous vein endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) converted 17-HDHA to SPMs, including resolvin D1 (RvD1) and other D-series resolvins and protectins. This was accompanied by a rapid translocation of 5-LOX from nucleus to cytoplasm in both ECs and VSMCs, potentially facilitating SPM biosynthesis. Conditioned medium from cells exposed to 17-HDHA inhibited monocyte adhesion to TNF-α–stimulated EC monolayers. These downstream effects were partially reversed by antibodies against the RvD1 receptors ALX/FPR2 and GPR32. These results suggest that autocrine and/or paracrine signaling via locally generated SPMs in the vasculature may represent a novel homeostatic mechanism of relevance to vascular health and disease.—Chatterjee, A., Komshian, S., Sansbury, B. E., Wu, B., Mottola, G., Chen, M., Spite, M., Conte, M. S. Biosynthesis of proresolving lipid mediators by vascular cells and tissues.
Keywords: resolution of inflammation, resolvins, omega-3 fatty acids
Interventions such as endarterectomy, balloon angioplasty, and bypass grafting are used to treat patients with advanced coronary and peripheral artery occlusive diseases. The vascular injury associated with these procedures initiates an inflammatory response that results in intimal thickening (i.e., neointimal hyperplasia) and development of restenosis. Inflammation plays a critical role in this process, and it is hypothesized that chronic inflammation promotes endothelial cell (EC) and vascular smooth muscle cell (VSMC) dysfunction central to the restenotic injury response (1, 2). Although the processes that initiate vascular inflammation in the context of acute vessel injury are well understood, less is known about endogenous mediators that counter-regulate these responses. During acute inflammation, the inflammatory response is self-limited and actively resolves (3). Resolution is mediated in part by endogenous specialized proresolving lipid mediators (SPMs), such as the resolvins, that were originally discovered by unbiased lipidomics analysis of self-resolving inflammatory exudates (4–7). These SPMs are produced from ω-3 polyunsaturated fatty acid (PUFA) precursors, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as the ω-6 PUFA arachidonic acid (3, 4, 6, 7).
Resolvins and related SPMs have demonstrated potent bioactivity in several models of inflammation by regulating leukocyte trafficking and effector functions (e.g., macrophage efferocytosis), and they also enhance host defense (3). Recent studies suggest that resolvins may have important vasculo-protective properties in settings of vascular injury (8–11). Indeed, DHA-derived (D-series) resolvins attenuate VSMC migration and proliferation and reduce neointimal hyperplasia in animal models. Moreover, previous studies have shown that SPMs counter-regulate leukocyte-EC interactions, diminish expression of EC adhesion receptors, and blunt the production of proinflammatory chemokines by ECs [reviewed by Sansbury and Spite (12)]. Nonetheless, the relative contributions of resident vascular cells to the local biosynthesis of SPMs in these and other contexts are incompletely understood.
D-series resolvins D1–6 (RvD1–6), maresins, and protectins are produced from DHA via a series of enzymatic reactions involving lipoxygenase (LOX), including 5-LOX, 12-LOX, and 15-LOX, as well as hydrolases. For instance, 15-LOX facilitates the conversion of DHA to a 17S-hydroperoxide intermediate that is the precursor to D-series resolvins and protectins (4). Whereas protectins can be produced directly from this intermediate, production of D-series resolvins requires the sequential action of 15-LOX and 5-LOX (4). Alternatively, the R epimer of 17-hydroxy(H)DHA can be formed by cyclooxygenase 2 (COX-2) in the presence of aspirin and can give rise to the aspirin-triggered resolvins (6). Recently, statin-mediated S-nitrosylation of COX-2 was shown to contribute to the biosynthesis of novel 13-series resolvins generated from docosapentaenoic acid (13). Dietary supplements containing ω-3 PUFA lessen the severity of inflammatory diseases in mice and may have cardioprotective effects in humans (14–16). Dietary supplementation with DHA and/or EPA increases levels of SPMs in plasma, and SPMs have been measured from a wide array of human body fluids and tissues using targeted metabololipidomics approaches (17–20). SPMs are produced from immune cells, such as neutrophils and monocytes/macrophages, as well as during leukocyte-EC interactions via transcellular biosynthesis (3, 12). Furthermore, recent studies suggest that in macrophages, 5-LOX localization (i.e., nucleus vs. cytoplasm) is a critical determinant of whether this enzyme participates in biosynthesis of proinflammatory mediators [e.g., leukotrienes (LTs)] or SPMs (21). Whether similar mechanisms are operative in vascular cells is unclear.
In the present study, we investigated the biosynthesis of DHA-derived SPMs in primary cultured vascular cells and freshly harvested blood vessels from humans and examined the expression and localization of 5-LOX, a key enzyme involved in this pathway. Furthermore, we examined the potential autocrine/paracrine effects of SPMs locally generated by vascular ECs on inflammatory responses.
MATERIALS AND METHODS
Reagents and cells
Primary cultures of human greater saphenous vein ECs and VSMCs were prepared from human saphenous veins discarded at the time of bypass operation in a University of California–San Francisco Institutional Review Board–approved protocol as described previously (11). VSMCs were maintained in low-glucose DMEM (HyClone, Logan, UT, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and penicillin/streptomycin/amphotericin B (Lonza, Basel, Switzerland) and used between passages 2 and 5. ECs (passage 2–5) were maintained in Media 199 with Earle’s balanced salt solution (Hyclone) supplemented with 10% fetal bovine serum, penicillin/streptomycin/amphotericin B (Lonza 1760), ECGS (BD Biosciences, San Jose, CA, USA), and heparin (17.5 U/ml) (Sigma-Aldrich, St. Louis, MO, USA). DHA and 17R/S-HDHA were obtained from Cayman Chemicals (Ann Arbor, MI, USA) and were supplied in ethanol. Diluted solutions were prepared in aqueous medium with ethanol vehicle (<0.35% v/v). TNF-α was purchased from Sigma-Aldrich. Lamin B1 and glyceraldehyde 3-phosphate dehydrogenase antibodies were purchased from Cell Signaling (Danvers, MA, USA).
SPM production by human arteries
Segments of arterial branches from the aortic arch (carotid, subclavian, and brachiocephalic) were collected by California Transplant and Donor Network West, transported in ice-cold PBS within 3 h of surgical resection, and then cleaned in a sterile environment. Arterial segments (∼1 cm length) were incubated in sterile centrifuge tubes in a cell culture incubator with DHA or 17-HDHA (both at 1 μM) in serum-free medium, and SPMs were measured (tissue + medium) after 6 h by liquid chromatography-tandem mass spectrometry (LC-MS/MS) or ELISA.
Identification and quantification of SPMs by LC-MS/MS and ELISA
Medium (phenol red free and serum free) collected from ECs, VSMCs, or arterial tissues after treatment without or with DHA or 17-HDHA (1 µM) were combined with 2 volumes of ice-cold methanol and stored at −80°C. Arterial tissues were finely minced in ice-cold methanol prior to extraction. Internal deuterium-labeled standards, including d5-RvD2, d5-LXA4, d4-LTB4, and d8-5-HETE, were then added to assess extraction recovery. Solid-phase extraction and LC-MS/MS analysis was carried out essentially as described in Colas et al. (17). Briefly, lipid mediators were extracted by C18 column chromatography, and methyl formate fractions were taken to dryness under a stream of N2 gas prior to suspension in methanol:water (50:50). Samples were analyzed using a high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) coupled to a QTrap5500 mass spectrometer (Sciex, Framingham, MA, USA). The instrument was operated in negative ionization mode, and lipid mediators were identified and quantified using multiple reaction monitoring transitions, information-dependent acquisition, and enhanced product ion scanning (17) after normalization to extraction recovery based on internal deuterium-labeled standards and calibration curves for external authentic standards for each mediator. Upon comprehensive SPM profiling and positive identification of RvD1 by LC-MS/MS, in some experiments, RvD1 was quantified by ELISA. For this, immunoreactive RvD1 was measured in cell culture supernatants using a commercially available ELISA kit as per the manufacturer’s instructions (Cayman Chemicals). The sensitivity for this assay is >15 pg/ml.
Western blotting
VSMCs and ECs were lysed in CellLytic M lysis buffer (Sigma-Aldrich). After three pulses of 2–3 watt sonication on ice, whole cell homogenates were centrifuged at 18,000 g for 20 min. The supernatants were evaluated for protein concentration and then heated at 100°C in Laemlli buffer for 7 min. Lysates (25 μg) were then run on NuPage 10% Bis-Tris gels (Thermo Fisher Scientific) and transferred to PVDF membranes that were probed with anti–5-LOX antibody (Novus Biologicals, Littleton, CO, USA) and anti–β-actin (1:4000) (Sigma-Aldrich) using a QDot 625 Western blotting kit (Thermo Fisher Scientific). Nuclear and cytoplasmic extracts were prepared by the following method. Endothelial cells were pelleted at 280 g for 5 min and washed once with ice-cold PBS, followed by lysing with 50 mM Tris-HCl with 2 mM EDTA containing protease inhibitors and digitonin (final concentration: 50 μg/ml). Cell viability was assessed by Trypan blue exclusion, and the time of incubation with digitonin was optimized to induce plasma membrane lysis for >95% cells. The lysates were centrifuged at 1000 g for 15 min, and the supernatants were collected as the cytoplasmic extracts. The pellets were washed several times with 50 mm Tris-HCl with 2 mM EDTA and were incubated on a rocker for 1 h in the same lysis buffer with 3% TritonX-100 for nuclear membrane lysis. The lysates were then centrifuged at 18,000 g for 20 min, and nuclear extracts were collected. Lamin B1 and glyceraldehyde 3-phosphate dehydrogenase served as markers of nuclear and cytoplasmic fractions, respectively.
Immunofluorescence
Immunofluorescence staining was performed on 8-well chamber slides (Millipore, Billerica, MA, USA). After treatment, cells were briefly rinsed in PBS and fixed with 4% paraformaldehyde for 20 min at 37°C, followed by permeabilization in ice-cold acetone (10 min at −20°C) and 1% Triton-X100 (20 min at room temperature). Cells were then incubated in a humidified chamber at 4°C overnight with anti–5-LOX antibody, followed by goat anti-rabbit IgG-conjugated Alexa Fluor 488 (Thermo Fisher Scientific). Fluorescence images were taken with an Olympus BX51 microscope (Olympus America, Center Valley, PA, USA) with an EXFO X-cite 120 system (Exfo Photonic Solutions, Mississauga, ON, Canada), an Olympus DP70 digital microscope camera, and DP-Controller software (Olympus America). Ten images in total (at ×20 magnification) were taken at random from all 4 quadrants of the wells, and immunofluorescence values were represented as average relative fluorescence units per well. 5-LOX localization was quantified through comparison of relative mean cytoplasmic to nuclear intensity with ImageJ software. A 5-LOX blocking peptide was used (Novus Biologicals) before incubation of cells with anti–5-LOX antibody to confirm specificity of the 5-LOX signal.
Monocyte adhesion assay
ECs were incubated with 17-HDHA (1 μM) in serum-free medium for 24 h, and conditioned medium was collected. Confluent ECs grown in 24-well plates were exposed to control (ctrl) or 17-HDHA–conditioned medium followed by TNF-α (10 ng/ml) without or with the anti-RvD1 receptor antibodies anti-GPR32 (Genetex, Irvine, CA, USA) and anti-ALX/FPR2 (Abcam, Cambridge, United Kingdom). After 18 h, ECs were washed with PBS and incubated with U937 monocytes labeled with Calcein-AM (Thermo Fisher Scientific), and adhesion was measured using a spectrophotometer as previously described (10).
Statistical analysis
Data are shown as means ± sem. Direct comparisons were made using unpaired Student's t tests, and multiple group comparisons were made using 2-way ANOVA followed by Bonferroni’s post hoc tests where appropriate. In all cases, P ≤ 0.05 was considered significant.
RESULTS
Biosynthesis of SPMs by isolated human arteries
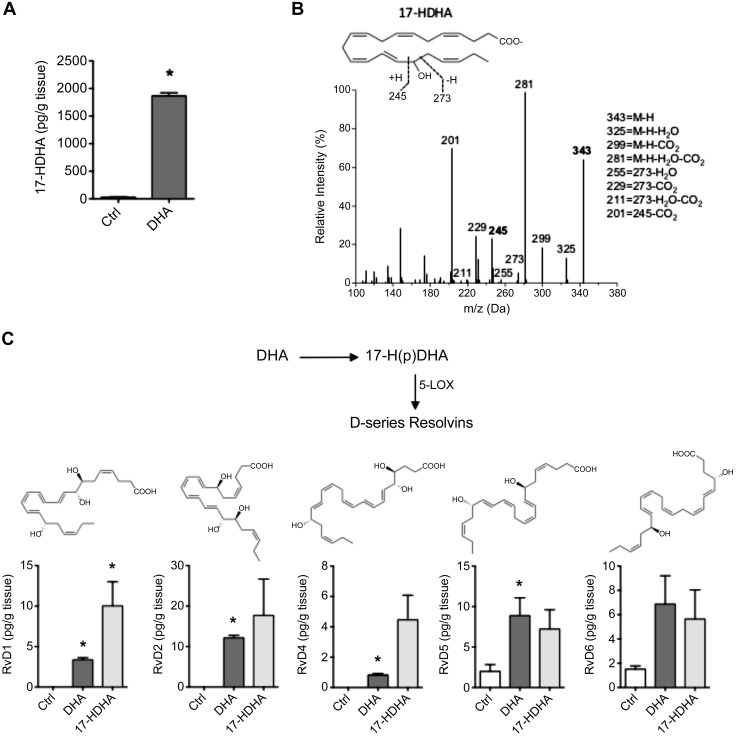
To evaluate the biosynthetic capacity for SPMs by vascular cells, we first asked whether human arteries could produce 17-HDHA from its PUFA precursor because this product is a common biosynthetic pathway marker for D-series resolvins and protectins. For this, human aortic branch segments of 1 cm length were incubated with DHA in serum-free medium for 6 h at 37°C. Under baseline conditions, in the absence of additional DHA, aortic segments produced detectable amounts of 17-HDHA. The addition of DHA resulted in an increase in production of 17-HDHA (Fig. 1A). A representative MS/MS spectra of 17-HDHA, with diagnostic ion assignments, is shown in Fig. 1B. Targeted multiple reaction monitoring profiling by LC-MS/MS also indicated that DHA was further transformed by these aortic segments into D-series resolvins, including RvD1, RvD2, RvD4, RvD5, and RvD6 (Fig. 1C), whereas RvD3 was not identified. We then bypassed the initial step of this biosynthetic pathway and incubated cells directly with 17R/S-HDHA, which similarly led to an increase in D-series resolvin production above baseline values. We also identified the 17R epimer of RvD1, which increased during incubation with the racemic 17-HDHA (ctrl: 1.4 ± 0.7; DHA: 1.2 ± 0.4; 17-HDHA: 7.4 ± 3.3 pg/g). We did not identify PD1 under these conditions, although its double dioxygenation isomer, 10S, 17S-diHDHA, increased in the presence of DHA and 17-HDHA (ctrl: 3.5 ± 0.7; DHA: 20.8 ± 7.4; 17-HDHA: 13.9 ± 6.2 pg/g). These results collectively indicate that intact human arteries are capable of producing resolvins and protectins.
Figure 1.
LC-MS/MS analysis of SPMs generated in human arteries. A) Levels of 17-HDHA (medium + arterial tissue) after 6-h incubation with DHA. B) Representative MS/MS spectra of 17-HDHA from A, with diagnostic fragmentation ions shown as inset. C) Levels of D-series resolvins from human arterial tissue incubated with DHA or 17-HDHA for 6 h. Structures shown as inset. *P ≤ 0.05 vs. ctrl (means ± sem; n = 3 per group).
Biosynthesis of SPMs by cultured human VSMCs and ECs
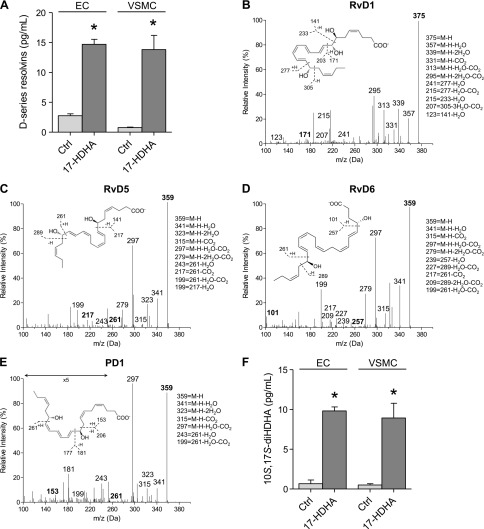
To elucidate the relative contribution of ECs vs. VSMCs in the biosynthesis of resolvins observed in intact human arteries, we next assessed their production by isolated vascular cells. For this, human saphenous vein ECs or VSMCs were incubated with 17-HDHA for 6 h, and the mediators produced were assessed by LC-MS/MS as described above. In these experiments, we identified basal levels of SPMs in medium from each cell type; the total levels of D-series resolvins (RvD1–6) increased significantly in both ECs and VSMCs incubated with 17-HDHA (Fig. 2A). Of these, individual increases were observed for RvD1 (ctrl: 0.13 ± 0.05; 17-HDHA: 0.2 ± 0.07 pg/ml), RvD5 (ctrl: 0.8 ± 0.5; 17-HDHA: 4.3 ± 0.2 pg/ml), and RvD6 (ctrl: 0.3 ± 0.01; 17-HDHA: 8.3 ± 0.6 pg/ml) in ECs. In VSMCs, individual increases in RvD1 (ctrl: 0.04 ± 0.006; 17-HDHA: 2.0 ± 0.6 pg/ml), RvD2 (ctrl: 0.07 ± 0.1; 17-HDHA: 0.4 ± 0.2 pg/ml), RvD5 (ctrl: 0.3 ± 0.1; 17-HDHA: 4.4 ± 0.8 pg/ml), and RvD6 (ctrl: 0.03 ± 0.003; 17-HDHA: 4.5 ± 0.7 pg/ml) were observed, whereas RvD4 was identified in ECs but not in VSMCs. Representative MS/MS spectra used for identification of individual resolvins are shown in Fig. 2B–D, with diagnostic fragmentation ions shown as insets. We note that 17R-RvD3 was present in ECs in the absence of 17-HDHA and did not increase further with the addition of exogenous 17-HDHA (ctrl: 3.8 ± 0.3; 17-HDHA: 2.6 ± 0.6 pg/ml). In addition to D-series resolvins, we identified members of the protectin family, including PD1 (Fig. 2E) and its double dioxygenation isomer, 10S, 17S-diHDHA (Fig. 2F). As observed with human arteries, PD1 was not substantially increased by incubation with 17-HDHA (data not shown), whereas 10S, 17S-diHDHA significantly increased in the presence of 17-HDHA. This likely reflects the fact that PD1 is produced from enzymatic hydrolysis of an epoxide formed from 17-hydroperoxyDHA, whereas 10S, 17S-diHDHA is produced by lipoxygenation of 17-HDHA via 5-LOX (22).
Figure 2.
Production of SPM by ECs and VSMCs. A) Levels of D-series resolvins in medium from ECs (RvD1–6) and VSMCs (RvD1–3, RvD5, and RvD6) after incubation with 17-HDHA for 24 h. (B–D) Representative MS/MS spectra of RvD1, RvD5, and RvD6 (with proposed stereochemistry) generated in VSMCs in the presence of 17-HDHA. E) Representative MS/MS spectra of PD1 in VSMCs at baseline. F) Levels of 10S, 17S-diHDHA from ECs and VSMCs incubated with 17-HDHA. *P ≤ 0.05 vs. ctrl of same cell type. Data are means ± sem (n = 3 per group).
Time- and concentration-dependent production of RvD1 in VSMCs and ECs
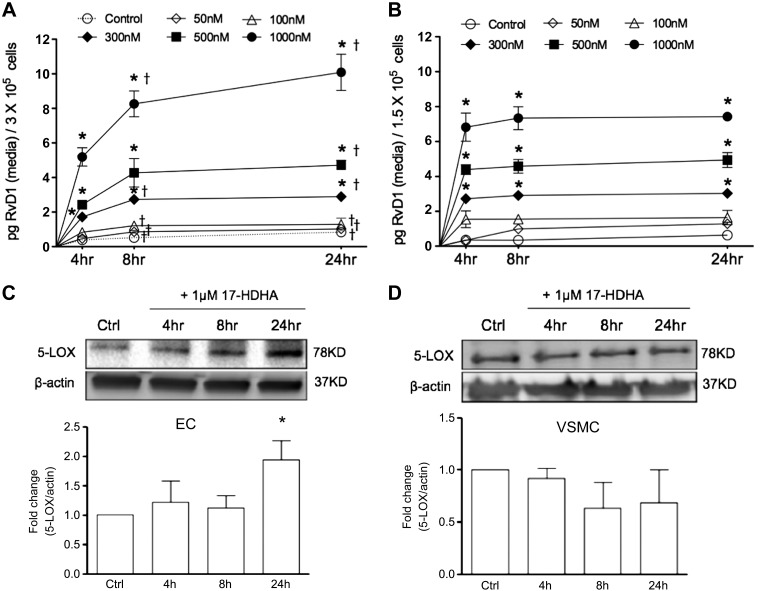
After comprehensive analysis of SPM production in the presence of 17-HDHA, we focused our attention on RvD1 in order to determine the time and concentration dependence of its biosynthesis. The production of RvD1 in ECs and VSMCs was highly dependent upon the amount of 17-HDHA incubated with the cells, with significant increases in RvD1 observed with as little as 300 nM 17-HDHA added to the cells (Fig. 3A, B). The production of RvD1 showed a clear time-dependent increase in both ECs and VSMCs between baseline and 8 h, whereas its production largely plateaued by 24 h.
Figure 3.
RvD1 biosynthesis and 5-LOX expression in ECs and VSMCs. A, B) Time- and concentration-dependent formation of RvD1 in ECs (A) or VSMCs (B) incubated with 17-HDHA as indicated. Results are shown as net RvD1 produced (pg/ml medium recovered; n = 3). *P ≤ 0.05 compared with untreated ctrl at respective time points. †P ≤ 0.05 compared with 4-h treatment (2-way ANOVA with Bonferroni's post hoc test). C, D) Time-dependent expression of 5-LOX in ECs (C) or VSMCs (D) treated with 17-HDHA (1 µM) as assessed by Western blot analysis. Data are means ± sem (n = 3). *P ≤ 0.05 compared with ctrl (unpaired Student’s t test).
Expression of 5-LOX ECs and VSMCs
Because the production of RvD1, as well as other SPMs found to be increased in cells incubated with 17-HDHA (Fig. 2), is dependent upon 5-LOX, we next sought to evaluate the abundance and regulation of this enzyme in each cell type. We found that both human ECs and VSMCs expressed 5-LOX in the basal cultured state (Fig. 3C, D). After incubation with 17-HDHA, 5-LOX expression in ECs increased by nearly 2-fold (94%) at 24 h (Fig. 3C), whereas no significant increase in 5-LOX expression was observed in VSMCs (Fig. 3D).
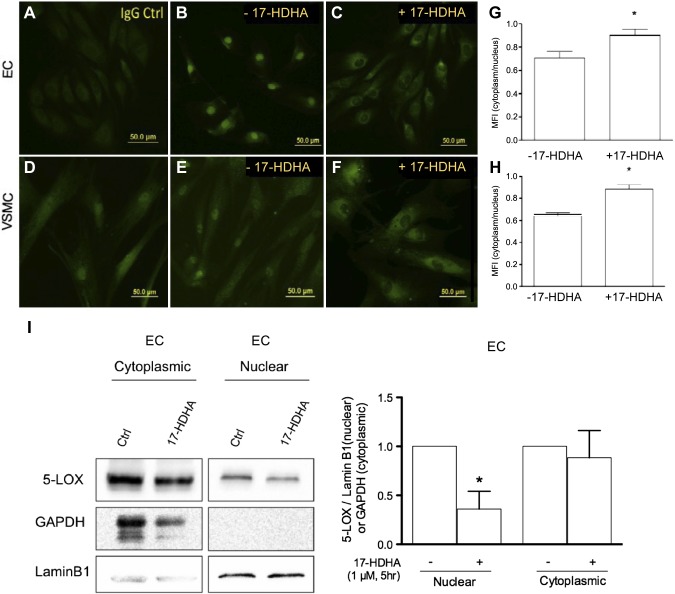
Recent evidence indicates that 5-LOX substrate preference can be altered by its subcellular location, where in macrophages, cytoplasmic 5-LOX is associated with SPM biosynthesis, and nuclear 5-LOX is associated with proinflammatory LT production (21). The translocation of 5-LOX from the nucleus to the cytoplasm is mediated in part by RvD1, which acts on its specific receptor to modulate 5-LOX phosphorylation (21). Thus, we next examined the subcellular distribution of 5-LOX in vascular cells and in the presence of 17-HDHA at a time point at which RvD1 is generated (i.e., 5 h). In both ECs and VSMCs, incubation with 17-HDHA caused a significant, rapid shift in 5-LOX localization from the nucleus to the cytoplasm (Fig. 4A–F). Quantification of the immunofluorescent images revealed an approximately 28% (ECs) and 37% (VSMCs) increase in relative 5-LOX expression in cytoplasm compared with the nucleus in cells incubated with 17-HDHA (Fig. 4G, H). We further confirmed 5-LOX localization in ECs by probing 5-LOX expression in the nuclear and cytoplasmic fractions by Western blotting. We found no significant differences in 5-LOX expression in cytoplasmic extracts of 17-HDHA–treated and untreated cells, but nuclear 5-LOX was significantly reduced in cells incubated with 17-HDHA (Fig. 4I). Collectively, these results suggest that, consistent with the proposed biosynthesis of SPMs in immune cells, vascular cell–dependent production of SPMs is also associated with 5-LOX subcellular redistribution.
Figure 4.
Effect of 17-HDHA on subcellular distribution of 5-LOX. A–F) Representative immunofluorescence images of 5-LOX distribution in ECs (A–C) and VSMCs (D–F) with images of IgG ctrl (A, D), untreated ctrl (B, E), and 17-HDHA–treated cells (1 µM for 5 h; C, F). G, H) Quantitative analysis of 5-LOX distribution (ratio of cytoplasmic to nuclear fluorescence) in ECs (G) and VSMCs (H) (n ≥ 3). *P ≤ 0.05 compared with ctrl (unpaired Student’s t test). I) Western blot of 5-LOX in nuclear and cytosolic extracts from ECs exposed to 17-HDHA (1 µM) for 5 h. *P ≤ 0.05 compared with untreated ctrl. Data are means ± sem.
Conditioned medium from ECs incubated with 17-HDHA attenuates cytokine-stimulated leukocyte-EC interactions
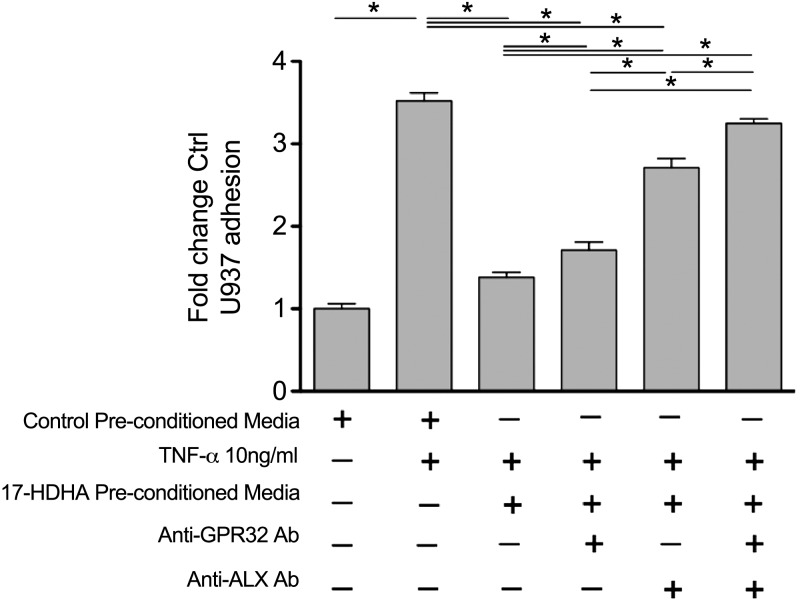
We hypothesized that SPMs locally produced by vascular cells could have important autocrine/paracrine effects on inflammatory responses in the vessel wall. Treatment of ECs with conditioned medium collected from ECs incubated with 17-HDHA (1 µM, 24 h) resulted in marked attenuation of TNF-α–stimulated monocyte adhesion. TNF-α stimulation of ECs treated with unconditioned medium resulted in a 3.5-fold (sem ± 0.09) increase in monocyte adhesion from baseline, whereas pretreatment of ECs with conditioned medium from ECs incubated with 17-HDHA resulted in near complete ablation of this response (P < 0.05) (Fig. 5). Because RvD1 has previously been shown to regulate leukocyte-EC interactions via its specific receptor (23) and because our results demonstrate that RvD1 is produced in cells incubated with 17-HDHA, we next questioned whether RvD1 production plays a causal role in the aforementioned effects observed with conditioned medium from ECs exposed to 17-HDHA. To this end, we incubated ECs with conditioned medium from ECs exposed to 17-HDHA in the presence of antibodies (anti-GPR32 and anti-ALX/FPR2) against the human RvD1 receptors (24). Incubation with each RvD1 receptor antibody significantly reversed the effects of conditioned medium from ECs incubated with 17-HDHA. Interestingly, dual blockade of both RvD1 receptors was additive, resulting in a near-complete abrogation of the counter regulation of leukocyte adhesion observed with cells treated with conditioned medium alone. The concentration of RvD1 in the conditioned medium equates to 0.15 nM, which is within its bioactive range (24). We note that RvD5, an additional major product in ECs, is also a ligand for GPR32 (25). Collectively, these results suggest that SPMs produced by ECs from 17-HDHA mediate, in part, the effects of 17-HDHA on monocyte adhesion. These results also demonstrate that SPMs play a direct autocrine and/or paracrine role in ECs to regulate vascular inflammation.
Figure 5.
Preconditioned medium from 17-HDHA–treated ECs attenuates EC inflammatory activation. Monocyte adhesion to ECs incubated without or with TNF-α and in the absence or presence of conditioned medium prepared from ECs incubated with 17-HDHA. Data are means ± sem (n ≥ 3 per group). In some groups, anti-GPR32 and anti-ALX/FPR2 antibodies (Ab) were added to the incubation in the presence of 17-HDHA–preconditioned medium. *P ≤ 0.05 (unpaired Student’s t test for indicated comparisons).
DISCUSSION
Resolvins are SPMs generated during the resolution phase of inflammation from ω-3 fatty acids, such as DHA. It is well established that SPMs can be produced by immune cells, including neutrophils and macrophages, as well as by transcellular biosynthesis involving interactions between neutrophils/macrophages and epithelial/endothelial cells during acute inflammation (3, 6, 7). In this study, we found that cells and tissues of vascular origin in humans have the capacity to produce SPMs in the absence of leukocytes. We also demonstrate that biologic activity of these products released from vascular cells, suggesting that locally derived SPMs may be involved in vascular homeostasis in an autocrine and/or paracrine fashion.
Previous studies have documented that endothelial and epithelial cells can convert endogenous PUFA into precursors to SPMs. For instance, aspirin-acetylation of COX-2 in endothelial cells produces 15R-hydroxyeicosatetraenoic acid and 18-hydroxyEPA, which can be converted to lipoxins or E-series resolvins, respectively, by leukocytes expressing 5-LOX (5, 26). These SPMs then counter-regulate leukocyte-EC interactions, providing a key negative feedback system to regulate excessive leukocyte recruitment during acute inflammation. Here, we show that human arteries are capable of converting DHA to both 17-HDHA and downstream resolvins and that isolated human ECs express 5-LOX to facilitate a biosynthetic step normally carried out in leukocytes. Moreover, our results suggest that, in addition to ECs, VSMCs may be a previously underappreciated contributor to SPM biosynthesis in the vasculature.
Fish oil dietary supplements that contain DHA have been shown to dampen chronic inflammation in humans, with varying levels of clinical success in cardiovascular disease, inflammatory bowel disease, and asthma (27–32). In human studies of fish oil supplementation, the concentration of DHA in blood can reach roughly 100–500 μM (33, 34). Interestingly, 17-HDHA has also been observed in the plasma after fish oil supplementation (20). The levels of these precursors generally exceed that of SPMs in the plasma, likely because SPMs are potent autacoids. It should be noted that most of the physiologic DHA is esterified in phospholipid and triglyceride pools, although unesterified DHA carried by serum proteins can also be delivered to inflammatory exudates for rapid conversion to SPMs (35). The results presented here show that both DHA and 17-HDHA can be converted to SPMs by the vasculature, indicating that local conversion of these substrates at the blood-vascular interface could potentially contribute to vascular health in individuals taking fish oil supplements. We have observed a similar biosynthetic capability in isolated rabbit aortae (data not shown) and in injured rabbit aortae in vivo (11), confirming that this vascular pathway is active in nonhuman species as well.
5-LOX is primarily expressed in neutrophils, monocytes/macrophages, dendritic cells, mast cells, B-lymphocytes, and some epithelial cells (e.g., oral, colon, lung, etc.), whereas relatively low levels of expression have been observed in ECs (36) and VSMCs (37). Notably, under certain conditions, such as infection with cytomegalovirus, 5-LOX can be induced in VSMCs (38), whereas allergic airway inflammation and pulmonary hypertension have been shown to increase 5-LOX in pulmonary ECs from humans (39, 40). Thus, the expression of 5-LOX in primary human vascular cells observed in our study is surprising and could potentially be due to the vascular bed or patient characteristics (e.g., extent of vascular disease, other comorbidities). Nonetheless, we found that 17-HDHA exposure led to increased 5-LOX levels in ECs above baseline expression levels. Transcriptional regulation of 5-LOX has been linked to the transcription factors Sp1, Egr-1, and p53 (41, 42). The mechanisms through which 17-HDHA causes these changes in 5-LOX expression in vascular cells require further investigation.
Upon stimulation, 5-LOX translocates to the perinuclear region where, in close proximity with FLAP (5-LOX activating protein), cPLA2 liberates arachidonic acid from membrane phospholipids, which is then transferred via FLAP to 5-LOX to initiate biosynthesis of proinflammatory LTB4 and LTC4 (43). The products of lipoxygenases include both proinflammatory and proresolving lipid mediators, and a “class-switch” toward SPMs is an important step in resolution (44, 45). Multiple lines of evidence suggest a proinflammatory role of 5-LOX, and, as a result, 5-LOX inhibitors have been developed to block its activity in chronic inflammation (46). However, the role of 5-LOX is context and substrate dependent. Here, we show that 5-LOX translocation from nucleus to cytoplasm is associated with SPM biosynthesis in vascular cells. These results are consistent with a recent study reporting that RvD1 induced translocation of nuclear 5-LOX to the cytoplasmic compartment in human macrophages, which was associated with reduced production of proinflammatory LTB4 and increased production of LXA4, another proresolving lipid mediator (21). This promotes a positive feedback loop to shift 5-LOX activity from generating proinflammatory mediators to proresolving mediators. Our results in ECs and VSMCs support this concept by defining a subcellular location-specific biosynthetic association of 5-LOX and SPM biosynthesis in vascular cells. It is possible that 17-HDHA–induced cytoplasmic translocation of 5-LOX in ECs and VSMCs was mediated by SPMs because RvD1 was generated at a time point at which 5-LOX redistribution occurred. A recent study has shown a key role of 17-HDHA in obesity-induced inflammation in mice (47), whereas in humans 17-HDHA reduces expression of inflammatory cytokines in adipocytes (47), enhances IgM/IgG production (48), and inhibits IgE production in activated B cells (49). There are limited studies on the direct effects of 17-HDHA on leukocytes or other cells (50). In our in vitro experiments with ECs and VSMCs, we show that 17-HDHA–conditioned medium attenuated cytokine (TNF-α)-driven EC activation. We also show that RvD1 and/or RvD5 plays a major role in mediating these effects because the adhesion of monocytes to TNF-stimulated ECs was largely inhibited using antibodies to the receptors ALX/FPR2 and GPR32. Thus, it is possible that effects attributed to 17-HDHA could be due to rapid conversion to SPMs. Indeed, 17-HDHA was converted to RvD1 within 4 h in both ECs and VSMCs, and RvD1 production plateaued thereafter, potentially because of substrate consumption (e.g., higher amounts of 17-HDHA increased RvD1 production). Future studies are required to fully interrogate the kinetics of these biosynthetic relationships.
Resolution of inflammation is now appreciated as an actively orchestrated biologic process involving SPMs (3) that exert potent anti-inflammatory and proresolving actions (e.g., macrophage efferocytosis and tissue repair). Protective actions of SPMs have been demonstrated in numerous models of inflammation, including atherosclerosis, diabetes, peritonitis, colitis, and airway inflammation (3, 21, 51, 52). We have recently shown that DHA-derived SPMs, such as RvD1, RvD2, and maresin 1, attenuate intimal hyperplasia in models of acute vascular injury (9–11) and have described a novel strategy for local RvD1 delivery to the injured vessel using a biodegradable polymer wrap (53, 54). Acute vascular injury resulting from vascular interventions performed for occlusive cardiovascular disease (e.g., angioplasty, stents, bypass grafts, etc.) often results in the development of neointimal hyperplasia and restenosis injury. Thus, the availability of proresolving lipid mediators in the vasculature is critical for the resolution of acute inflammation that promotes downstream neointimal hyperplasia. Our findings demonstrate a potentially important homeostatic mechanism in the vasculature, wherein locally generated SPMs of vascular origin can promote resolution of the inflammatory response. Further studies are needed to understand the mechanisms governing vascular lipoxygenase specificity and SPM production in a physiologic context and whether these observations may be leveraged in a preventive or therapeutic fashion.
ACKNOWLEDGMENTS
This work was supported by American Heart Association Grant 13SDG16940069 (to A.C.), and by U.S. National Institutes of Health (NIH) Heart, Lung, and Blood Institute Grants HL119508 (to M.S.C.) and HL106173, and NIH National Institute of General Medical Sciences Grant GM095467 (to M.S.). A.C. and S.K. are cofirst authors. The authors declare no conflicts of interest.
Glossary
- 17-HDHA
17-hydroxy docosahexaenoic acid
- COX-2
cyclooxygenase 2
- ctrl
control
- DHA
docosahexaenoic acid
- EC
endothelial cell
- EPA
eicosapentaenoic acid
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygenase
- LT
leukotriene
- PUFA
polyunsaturated fatty acid
- RvD1–6
resolvin D1–6
- SPM
specialized proresolving lipid mediators
- VSMC
vascular smooth muscle cell
AUTHOR CONTRIBUTIONS
M.S. Conte designed research, analyzed the data, and wrote the manuscript; A. Chatterjee designed and performed the research, and wrote the manuscript; S. Komshian performed the research and analyzed the data; B. Wu performed the research; and M. Spite and B. E. Sansbury designed and performed the research, analyzed the data, and contributed to the writing of the manuscript.
REFERENCES
- 1.Weintraub W. S. (2007) The pathophysiology and burden of restenosis. Am. J. Cardiol. 100(5A), 3K–9K [DOI] [PubMed] [Google Scholar]
- 2.Tanaskovic S., Isenovic E. R., and Radak D. (2011) Inflammation as a marker for the prediction of internal carotid artery restenosis following eversion endarterectomy: evidence from clinical studies. Angiology 62, 535–542 [DOI] [PubMed] [Google Scholar]
- 3.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S., Gronert K., Devchand P. R., Moussignac R. L., and Serhan C. N. (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 278, 14677–14687 [DOI] [PubMed] [Google Scholar]
- 5.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., and Gronert K. (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., and Moussignac R. L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., and Serhan C. N. (2005) Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 8.Pope N. H., Salmon M., Davis J. P., Chatterjee A., Su G., Conte M. S., Ailawadi G., and Upchurch G. R., Jr.. (2016) D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 30, 4192–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akagi D., Chen M., Toy R., Chatterjee A., and Conte M. S. (2015) Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 29, 2504–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A., Sharma A., Chen M., Toy R., Mottola G., and Conte M. S. (2014) The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One 9, e113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara T., Runge S., Chatterjee A., Chen M., Mottola G., Fitzgerald J. M., Serhan C. N., and Conte M. S. (2013) D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 27, 2220–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansbury B. E., and Spite M. (2016) Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 119, 113–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalli J., Chiang N., and Serhan C. N. (2015) Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raederstorff D., Pantze M., Bachmann H., and Moser U. (1996) Anti-inflammatory properties of docosahexaenoic and eicosapentaenoic acids in phorbol-ester-induced mouse ear inflammation. Int. Arch. Allergy Immunol. 111, 284–290 [DOI] [PubMed] [Google Scholar]
- 15.Tiesset H., Pierre M., Desseyn J. L., Guéry B., Beermann C., Galabert C., Gottrand F., and Husson M. O. (2009) Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro- and antiinflammatory responses in mice with Pseudomonas aeruginosa lung infection. J. Nutr. 139, 82–89 [DOI] [PubMed] [Google Scholar]
- 16.Calder P. C. (2015) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851, 469–484 [DOI] [PubMed] [Google Scholar]
- 17.Colas R. A., Shinohara M., Dalli J., Chiang N., and Serhan C. N. (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Hjorth E., Vedin I., Eriksdotter M., Freund-Levi Y., Wahlund L. O., Cederholm T., Palmblad J., and Schultzberg M. (2015) Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. J. Lipid Res. 56, 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prüss H., Rosche B., Sullivan A. B., Brommer B., Wengert O., Gronert K., and Schwab J. M. (2013) Proresolution lipid mediators in multiple sclerosis: differential, disease severity-dependent synthesis. A clinical pilot trial. PLoS One 8, e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mas E., Croft K. D., Zahra P., Barden A., and Mori T. A. (2012) Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58, 1476–1484 [DOI] [PubMed] [Google Scholar]
- 21.Fredman G., Ozcan L., Spolitu S., Hellmann J., Spite M., Backs J., and Tabas I. (2014) Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl. Acad. Sci. USA 111, 14530–14535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C. N., Gotlinger K., Hong S., Lu Y., Siegelman J., Baer T., Yang R., Colgan S. P., and Petasis N. A. (2006) Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 176, 1848–1859 [DOI] [PubMed] [Google Scholar]
- 23.Norling L. V., Dalli J., Flower R. J., Serhan C. N., and Perretti M. (2012) Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol. 32, 1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C. H., Yang R., Petasis N. A., and Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., and Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clària J., and Serhan C. N. (1995) Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA 92, 9475–9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ley M., de Vos R., Hommes D. W., and Stokkers P. (2007) Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. (4):CD005986. [DOI] [PubMed] [Google Scholar]
- 28.Turner D., Steinhart A. H., and Griffiths A. M. (2007) Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. (3):CD006443. [DOI] [PubMed] [Google Scholar]
- 29.Turner D., Zlotkin S. H., Shah P. S., and Griffiths A. M. (2009) Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. (1):CD006320. [DOI] [PubMed] [Google Scholar]
- 30.Yang H., Xun P., and He K. (2013) Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis. PLoS One 8, e80048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisgaard H., Stokholm J., Chawes B. L., Vissing N. H., Bjarnadóttir E., Schoos A. M., Wolsk H. M., Pedersen T. M., Vinding R. K., Thorsteinsdóttir S., Følsgaard N. V., Fink N. R., Thorsen J., Pedersen A. G., Waage J., Rasmussen M. A., Stark K. D., Olsen S. F., and Bønnelykke K. (2016) Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 375, 2530–2539 [DOI] [PubMed] [Google Scholar]
- 32.Massaro M., Scoditti E., Carluccio M. A., and De Caterina R. (2008) Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot. Essent. Fatty Acids 79, 109–115 [DOI] [PubMed] [Google Scholar]
- 33.Geppert J., Kraft V., Demmelmair H., and Koletzko B. (2005) Docosahexaenoic acid supplementation in vegetarians effectively increases omega-3 index: a randomized trial. Lipids 40, 807–814 [DOI] [PubMed] [Google Scholar]
- 34.Yurko-Mauro K., Kralovec J., Bailey-Hall E., Smeberg V., Stark J. G., and Salem N., Jr. (2015) Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., and Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y. Y., Walker J. L., Huang A., Keaney J. F., Clish C. B., Serhan C. N., and Loscalzo J. (2002) Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem. J. 361, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipollone F., Mezzetti A., Fazia M. L., Cuccurullo C., Iezzi A., Ucchino S., Spigonardo F., Bucci M., Cuccurullo F., Prescott S. M., and Stafforini D. M. (2005) Association between 5-lipoxygenase expression and plaque instability in humans. Arterioscler. Thromb. Vasc. Biol. 25, 1665–1670 [DOI] [PubMed] [Google Scholar]
- 38.Qiu H., Strååt K., Rahbar A., Wan M., Söderberg-Nauclér C., and Haeggström J. Z. (2008) Human CMV infection induces 5-lipoxygenase expression and leukotriene B4 production in vascular smooth muscle cells. J. Exp. Med. 205, 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu S. J., Tang L. O., Watney E., Chi E. Y., and Henderson W. R., Jr. (2000) In situ amplification of 5-lipoxygenase and 5-lipoxygenase-activating protein in allergic airway inflammation and inhibition by leukotriene blockade. J. Immunol. 165, 4640–4648 [DOI] [PubMed] [Google Scholar]
- 40.Wright L., Tuder R. M., Wang J., Cool C. D., Lepley R. A., and Voelkel N. F. (1998) 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 157, 219–229 [DOI] [PubMed] [Google Scholar]
- 41.Gilbert B., Ahmad K., Roos J., Lehmann C., Chiba T., Ulrich-Rückert S., Smeenk L., van Heeringen S., Maier T. J., Groner B., and Steinhilber D. (2015) 5-Lipoxygenase is a direct p53 target gene in humans. Biochim. Biophys. Acta 1849, 1003–1016 [DOI] [PubMed] [Google Scholar]
- 42.Silverman E. S., Du J., De Sanctis G. T., Rådmark O., Samuelsson B., Drazen J. M., and Collins T. (1998) Egr-1 and Sp1 interact functionally with the 5-lipoxygenase promoter and its naturally occurring mutants. Am. J. Respir. Cell Mol. Biol. 19, 316–323 [DOI] [PubMed] [Google Scholar]
- 43.Rådmark O., Werz O., Steinhilber D., and Samuelsson B. (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 1851, 331–339 [DOI] [PubMed] [Google Scholar]
- 44.Buckley C. D., Gilroy D. W., Serhan C. N., Stockinger B., and Tak P. P. (2013) The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 [DOI] [PubMed] [Google Scholar]
- 45.Levy B. D., Clish C. B., Schmidt B., Gronert K., and Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 46.Osher E., Weisinger G., Limor R., Tordjman K., and Stern N. (2006) The 5 lipoxygenase system in the vasculature: emerging role in health and disease. Mol. Cell. Endocrinol. 252, 201–206 [DOI] [PubMed] [Google Scholar]
- 47.Neuhofer A., Zeyda M., Mascher D., Itariu B. K., Murano I., Leitner L., Hochbrugger E. E., Fraisl P., Cinti S., Serhan C. N., and Stulnig T. M. (2013) Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 62, 1945–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramon S., Gao F., Serhan C. N., and Phipps R. P. (2012) Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189, 1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim N., Ramon S., Thatcher T. H., Woeller C. F., Sime P. J., and Phipps R. P. (2016) Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur. J. Immunol. 46, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu C. Y., Gomolka B., Dierkes C., Huang N. R., Schroeder M., Purschke M., Manstein D., Dangi B., and Weylandt K. H. (2012) Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm. Res. 61, 967–976 [DOI] [PubMed] [Google Scholar]
- 51.Spite M., Clària J., and Serhan C. N. (2014) Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19, 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., Connolly E. S., Solomon R., Jones D. M., Heyer E. J., Spite M., and Tabas I. (2016) An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu B., Mottola G., Chatterjee A., Lance K. D., Chen M., Siguenza I. O., Desai T. A., and Conte M. S. (2017) Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J. Vasc. Surg. 65, 207–217.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lance K. D., Chatterjee A., Wu B., Mottola G., Nuhn H., Lee P. P., Sansbury B. E., Spite M., Desai T. A., and Conte M. S. (2017) Unidirectional and sustained delivery of the proresolving lipid mediator resolvin D1 from a biodegradable thin film device. J. Biomed. Mater. Res. A 105, 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]