Abstract
Background
Family with sequence similarity 129, member B (FAM129B), also called MINERVA, is upregulated and promotes tumor invasion in multiple types of cancer. However, the mechanism and clinicopathological significance of FAM129B remains unclear.
Materials and methods
Online KM-plotter tool and immunohistochemistry were used to predict the prognostic value of FAM129B expression in lung cancer tissues. Western blotting analysis, MTT, colony formation assay and matrigel invasion assay were performed after overexpressing or depleting FAM129B.
Results
In this study, using the online KM-plotter tool, we found FAM129B was correlated with adverse outcome in non-small cell lung cancer (NSCLC) patients (P<0.001). Immunohistochemistry results revealed that FAM129B showed negative or dim expression in normal lung tissues while presented positive cytoplasmic expression in both squamous cell lung carcinoma and lung adenocarcinoma. The positive ratio of FAM129B in clinical NSCLC tissue samples (77/187, 41.2%) was significantly higher than that in normal lung tissue samples (8/68, 11.8%; P<0.001). FAM129B expression associated with advanced TNM staging (P<0.001) and positive regional lymph node metastasis (P<0.001). The results of Kaplan-Meier analysis suggested that the survival time of patients with positive FAM129B expression was significantly shorter than those with negatively FAM129B expression (P<0.001). Proliferation and invasion assay revealed that FAM129B prominently facilitated tumor proliferation and invasion in NSCLC cells. Western blotting results revealed that FAM129B upregulated the expression of MMP2 and Cyclin D1 by enhancing the phosphorylation of FAK at Tyr 397 and Tyr 925. Incorporation of FAK inhibitor in the medium significantly downregulated the phosphorylation of FAK and subsequently attenuated increasing expression of MMP2 and Cyclin D1 induced by FAM129B overexpression.
Conclusion
Our results indicated that FAM129B may be a new prognosis predictor of NSCLC patients and impact tumor invasion and proliferation of NSCLC cells through promoting the activation of FAK signaling.
Keywords: FAM129B, invasion, proliferation, FAK, non-small cell lung cancer
Introduction
Family with sequence similarity 129, member B (FAM129B), also called MINERVA, is upregulated in several human cancers, including breast cancer, renal cancer, colon cancer, lung cancer, endometrial cancer, leukemia and central nervous system tumors.1,2 There is a pleckstrin homology domain near the N-terminus and a proline-rich domain near the C-terminus of FAM129B.3 FAM129B is known to inhibit TNFα-dependent apoptosis and to promote tumor invasion in HeLa cells.3,4 Conrad et al demonstrated that FAM129B facilitated activation of the Wnt/β-catenin signaling pathway, thereby inhibiting apoptosis in melanoma cells.5 However, in the literature, FAM129B served various functions depending on tissue type. In HeLa cells, silencing the FAM129B gene expression did not affect tumor growth,3 whereas in gene-targeted FAM129B-mutant mice, wound healing was significantly delayed. In the same study, overexpression of FAM129B contributed to enhancing cell motility in HaCaT cells.6 Therefore, it is likely that FAM129B may play diverse roles in various types of tissues or tumors.
Previous studies reported that FAM129B functioned as a molecule downstream of EGFR and ERK.3,7 In melanoma cells, knockdown of FAM129B prominently inhibited WNT3A-mediated activation of the Wnt/β-catenin signaling pathway, indicating that FAM129B was an important regulator of Wnt/β-catenin signaling.5 Focal adhesion kinase acts as a key integrator of the growth-factor pathway and the ERK/ MAPK signaling pathway, thereby playing an important role in regulating tumor invasion and migration.8–12 Moreover, FAK protein was also required for the proper regulation of Wnt/ β-catenin signaling.13 Thus, FAK may be a potential downstream regulator of FAM129B, remaining unknown to date.
The purpose of the present study was to investigate the expression and association with clinicopathological features of FAM129B in non-small-cell lung cancer (NSCLC) tissue samples. By transfecting with FAM129B cDNA plasmid or siRNA in NSCLC cell lines, we investigated the effect of FAM129B on cellular invasion, survival and cell movement.
Materials and methods
Patients and clinical specimens
This study was performed with the approval of the local institutional review board of China Medical University. Each of the patients signed informed consent. The tissue samples of 187 patients (111 males and 76 females) were collected from patients who underwent radical surgical excision at the First Affiliated Hospital of China Medical University from 2009 to 2012. No neoadjuvant radiotherapy or chemotherapy was given to these patients prior to surgery. After surgery, all patients received standard chemotherapy according to the NCCN guideline. Sixty-eight of the 187 patients had samples of the corresponding noncancerous tissues. Complete follow-up data were obtained from all 187 lung cancer patients. The survival time was calculated from the day of surgery to the day of death due to recurrence or metastasis or to the end of follow-up. The median age was 60 years old (range 29–83 years). Of the 187 patients, 87 patients were older than 60 years. Histological diagnosis and grading were done according to the fourth edition of WHO classification of tumors of the lung.14 There were 76 squamous cell lung carcinomas and 111 lung adenocarcinomas in our cohort. Of the 187 cases, 71 tumors were well differentiated and 116 were classified as moderately or poorly differentiated. Tumor staging was performed according to the seventh edition of the American Joint Committee on Cancer TNM Staging System for Lung Cancer.15 The tumors included 138 stages I–II and 49 stage III. Lymph node metastases were present in 82 of the 187 patients.
Immunohistochemistry
Using the streptavidin-peroxidase method, immunohistochemistry staining was performed according to the manufacturer’s instructions (Ultrasensitive; MaiXin, Fuzhou, China). The sections were incubated with anti-FAM129B antibodies (mouse anti-human; dilution, 1:200; HPA024312, Sigma-Aldrich, St Louis, MO, USA) at 4°C overnight, followed by biotinylated goat anti-mouse IgG secondary antibodies. The slides were scored by two investigators who were blinded to the clinical data. The scores were obtained by evaluating the staining intensity and percentage of positive cells in representative areas. We used the following strategy to assess the slides: intensity, 0 (no signal), 1 (weak), 2 (moderate) or 3 (high); percentage of cells, 1 (1%–25%), 2 (26%–50%), 3 (51%–75%) or 4 (76%–100%). We multiplied the scores of staining intensity and percentage to obtain a final score (range 0–12). When tumors had scores ≥4, they were defined as positive for FAM129B expression. When tumors had scores <4, they were defined as negative for FAM129B expression.
Online analysis of overall survival in NSCLC patients
The KM Plotter Online Tool (http://www.kmplot.com) was used to evaluate the prognostic value of FAM129B, based on the information on mRNA expression levels from 1,145 NSCLC patients. The details of clinical features of specimens were described in a previous paper.16
Cell culture
The cell lines used in the present study, including NCI-A549 (A549), NCI-H460 (H460) and NCI-H1299 (H1299) were obtained from the Shanghai Cell Bank (Shanghai, China). All the three cell lines were derived from NSCLC. The details of cell characteristics can be found on www.atcc.org. All the three cell lines were cultured in RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Thermo Fisher Scientific), 100 IU/mL penicillin (Sigma-Aldrich) and 100 µg/mL streptomycin (Sigma-Aldrich). The cells were grown in sterile culture dishes and passaged every 2 days after using 0.25% trypsin (Thermo Fisher Scientific).
Western blotting
Total protein was quantified using the Bradford method.17 Intotal, 50 µg of total cellular protein samples was separated by 10% SDS-PAGE and immunoblotted with the following primary antibodies: FAM129B (1:200; HPA024312, Sigma-Aldrich); GAPDH (1:5,000; Sigma-Aldrich); MMP2, MMP9, cyclin A2, cyclin B1, cyclin D1, cyclin E1, p-FAK (Tyr 397), p-FAK (Tyr 576), p-FAK (Tyr 925), FAK (1:1,000; Cell Signaling Technology, Danvers, MA, USA). Immunoreactive bands were visualized using enhanced chemiluminescence (ECL, Pierce, Rockford, IL, USA).
Plasmid transfection and siRNA treatment
The plasmids (pCMV6-ddk-myc and pCMV6-ddk-myc-FAM129B) were purchased from Origene (Rockville, MD, USA). FAM129B-siRNA (sc-92820) and scrambled-siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. Transfection was performed according to the manufacturer’s instructions using the Lipofectamine 3000 reagent (Thermo Fisher Scientific). Cells were transfected with 3 µg of indicated plasmid or 10 µL of indicated siRNA. Forty-eight hours after plasmid/siRNA transfection, cells were used for functional assays and the remaining cells were collected for western blot analysis.
Matrigel invasion assay
Cell invasion assays were performed with 20 µL Matrigel (1:3 dilution; BD Biosciences, San Jose, CA, USA) coated on the upper surface of 24-well Transwell chambers with 8 µm pores (Costar, Cambridge, MA, USA). After incubation for 18 hours, the cells that had passed through the filter were fixed with 4% paraformaldehyde and stained with hematoxylin. Ten randomly selected fields at 400× magnification were counted under a microscope. Each experiment was carried out in triplicate.
Colony formation assay
After the indicated transfection, cells were plated on 6 cm cell culture dishes (1,000 per dish for H460 and A549 cell lines) for 12 days. The cells were then fixed with methyl alcohol for 30 minutes and stained with Giemsa. The numbers of colonies with more than 50 cells were counted. All experiments were carried out in triplicate.
MTT
Cells were seeded on 96-well plates at approximately 3,000 cells per well 24 hours after transfection. After incubation for the indicated number of days, 20 µL of MTT (thiazolyl blue) solution (5 mg/mL) was added to each well and incubated at 37°C for 4 hours. After removing the medium, the resultant MTT formazan was solubilized in 150 µL of DMSO. The absorbance was read at a test wavelength of 490 nm, each carried out in triplicate.
Statistical analysis
SPSS version 22.0 for Windows (SPSS, Chicago, IL, USA) was employed for statistical analyses. The Pearson’s chi-squared test was selected to evaluate possible associations between FAM129B and clinicopathological features. Kaplan–Meier survival analyses were performed for the 187 patient specimens. The log-rank test was carried out to compare the significance. The Mann–Whitney U test was chosen to evaluate the significance of image analysis results of the invasive assay, colony formation assay and MTT test. P<0.05 was considered to indicate statistically significant differences.
Results
FAM129B correlated with tumor development and predicted poor survival of NSCLC patients
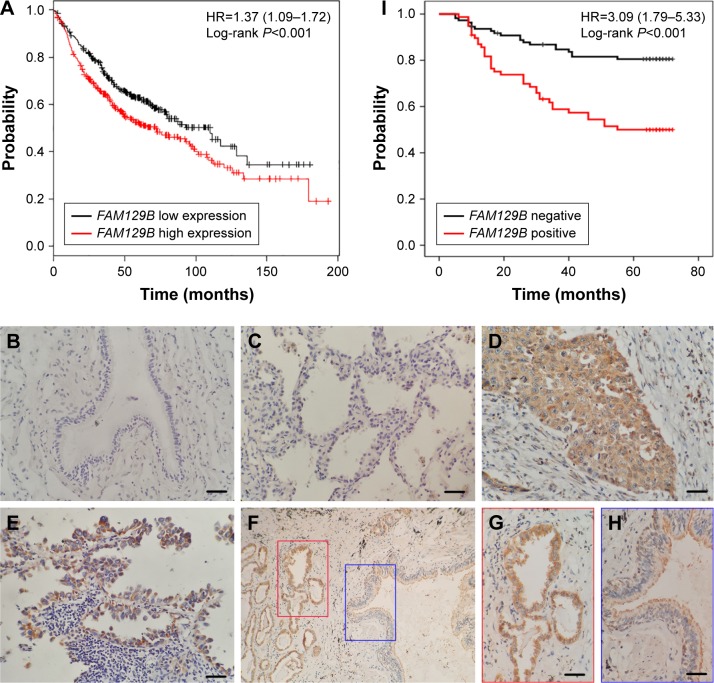
First, we applied the KM-plotter tool to predict the impact of FAM129B gene expression on overall survival. As shown in Figure 1A, we showed that the FAM129B gene was associated with worse outcome in NSCLC patients in terms of overall survival (cut-off value: median; P<0.001). Next, immunohistochemistry staining was performed in 187 cancerous tissue samples and 68 non-cancerous tissue samples. FAM129B was negatively or dimly expressed in the normal bronchial epithelium (Figure 1B) and alveolar epithelium (Figure 1C). However, FAM129B was positively expressed in the cytoplasm of tumor cells in both squamous cell lung carcinoma (Figure 1D) and lung adenocarcinoma (Figure 1E). FAM129B tended to exhibit stronger expression in cancerous tissues than in adjacent non-cancerous tissues (Figure 1F–H). The positive ratio of FAM129B in lung cancer tissues (77/187, 41.2%) was significantly higher than that of adjacent noncancerous lung tissue samples (8/68, 11.8%; P<0.001). FAM129B expression significantly correlated with advanced TNM stage (P<0.001) and positive lymph node metastasis (P<0.001; Table 1). There were no significant correlations between FAM129B expression and other clinicopathological features, including age, gender, tumor size, histological differentiation, histological type and smoking status (P>0.05; Table 1). Kaplan–Meier analysis showed that the overall survival of patients with FAM129B expression was significantly poorer than that of patients without FAM129B expression (P<0.001; Figure 1I).
Figure 1.
FAM129B was associated with worse prognosis of NSCLC patients.
Notes: (A) Results of online software survival prediction (KM plotter). The survival time of patients with higher FAM129B mRNA expression was significantly shorter than that of patients with lower FAM129B mRNA expression. Representative figures of FAM129B expression in normal bronchial (B), alveolar epithelial (C), squamous cell lung carcinoma (D) and lung adenocarcinoma (E). Magnification, 400×, scale bar=50 µm. FAM129B presented higher expression levels in NSCLC tissue samples than in corresponding noncancerous tissues (F, 100×; G and H, inset, 400×, scale bar=50 µm). Kaplan–Meier survival analysis revealed that patients with positive FAM129B expression presented poorer overall survival than did those with negative FAM129B expression (I).
Abbreviation: NSCLC, non-small-cell lung cancer.
Table 1.
The association between FAM129B expression and clini-copathological characteristics
|
FAM129B+ (n=77) |
FAM129B- (n=110) |
P-value | |
|---|---|---|---|
| Age (years) | |||
| <60 | 38 | 62 | 0.344 |
| ≥60 | 39 | 48 | |
| Gender | |||
| Male | 42 | 69 | 0.262 |
| Female | 35 | 41 | |
| Tumor size | |||
| ≤3 cm | 27 | 54 | 0.057 |
| >3 cm | 50 | 56 | |
| TNM staging | |||
| I–II | 46 | 92 | <0.001* |
| IIIA | 31 | 18 | |
| Regional lymph node metastasis | |||
| No | 30 | 75 | <0.001* |
| Yes | 47 | 35 | |
| Histological differentiation | |||
| Well | 24 | 47 | 0.109 |
| Moderate & Poor | 53 | 63 | |
| Histological type | |||
| Squamous cell cancer | 32 | 44 | 0.831 |
| Adenocarcinoma | 45 | 66 | |
| Smoking history | |||
| Never | 27 | 37 | 0.839 |
| Ever | 50 | 73 | |
Note:
P-value <0.05.
Overexpression of exogenous FAM129B promoted tumor invasion and proliferation in NSCLC cells
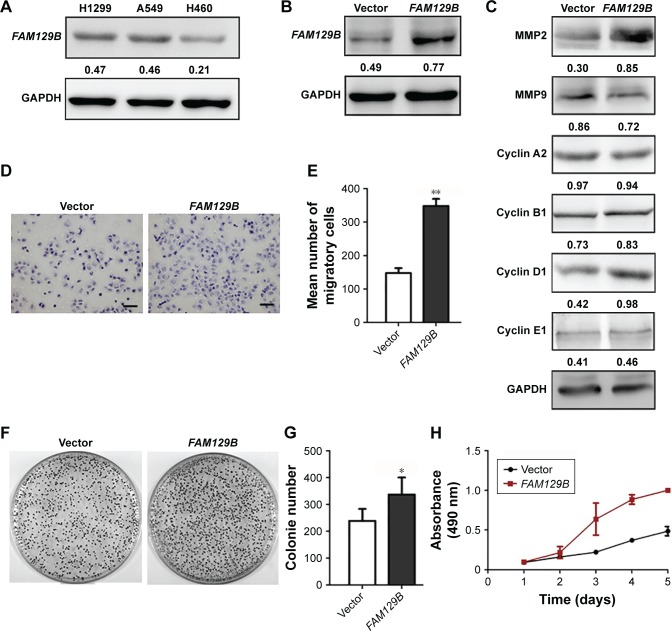
Using western blotting, we evaluated protein levels of FAM129B in 3 NSCLC cell lines (H1299, A549 and H460). FAM129B showed relatively higher protein levels in H1299 and A549 cells than in H460 cells (Figure 2A). Subsequently, we transfected FAM129B cDNA plasmid into H460 cells (Figure 2B) and found that the protein levels of MMP2 and cyclin D1 were significantly elevated following overexpressing FAM129B, whereas other proteins including MMP9, cyclin A2, cyclin B1 and cyclin E1 presented no visible changes (Figure 2C). Subsequent Matrigel invasion assay, colony formation assay and MTT assay revealed that cellular invasion and survival were prominently enhanced by transfecting exogenous FAM129B plasmid (Figure 2D–H).
Figure 2.
Overexpression of exogenous FAM129B upregulated the expression of MMP2 and cyclin D1 and promoted tumor invasion and proliferation of H460 cells.
Notes: (A) Western blotting showed that FAM129B was highly expressed in H1299 cells and A549 cells and weakly expressed in H460 cells. After overexpressing FAM129B in H460 cells (B), MMP2 and cyclin D1 were markedly elevated, whereas other proteins including MMP9, cyclin A2, cyclin B1 and cyclin E1 showed no visible changes (C). Matrigel assay (D and E, 400×, scale bar=50 µm), colony formation assay (F and G) and MTT (H) revealed that tumor invasion and proliferation were enhanced by overexpression of FAM129B. *P<0.05; **P<0.01.
Knockdown of endogenous FAM129B downregulated the expression of MMP2 and cyclin D1, thereby inhibiting tumor invasion and proliferation in NSCLC cells
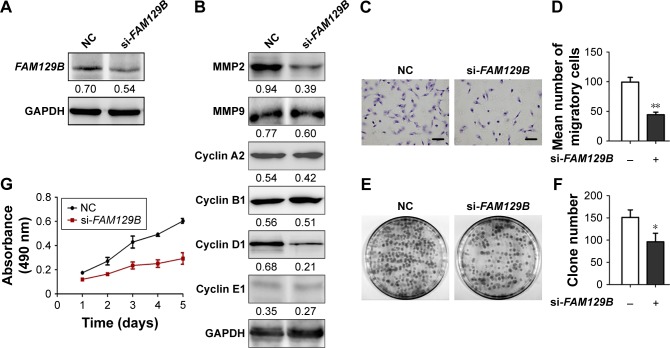
To further elucidate the function of FAM129B, we knocked down endogenous FAM129B by RNAi in A549 cells (Figure 3A). Compared with the control group, FAM129B-specific siRNA strongly decreased protein levels of MMP2 and cyclin D1 (Figure 3B). Correspondingly, depletion of FAM129B significantly inhibited tumor invasion, colony formation and proliferation ability of A549 cells (Figure 3C–G).
Figure 3.
Knockdown of endogenous FAM129B downregulated expression of MMP2 and cyclin D1 and inhibited tumor invasion and proliferation in A549 cells.
Notes: After depleting the expression of FAM129B (A), protein levels of MMP2 and cyclin D1 were prominently downregulated, while other proteins such as MMP9, cyclin A2, cyclin B1 and cyclin E1 showed no significant changes (B). Matrigel assay (C and D, 400×, scale bar=50 µm), colony formation assay (E and F) and MTT (G) revealed that tumor invasion and proliferation were enhanced by overexpression of FAM129B. *P<0.05; **P<0.01.
Abbreviation: NC, negative control.
FAM129B upregulated the expression of MMP2 and cyclin D1 by facilitating the phosphorylation of FAK at Tyr 397 and Tyr 925
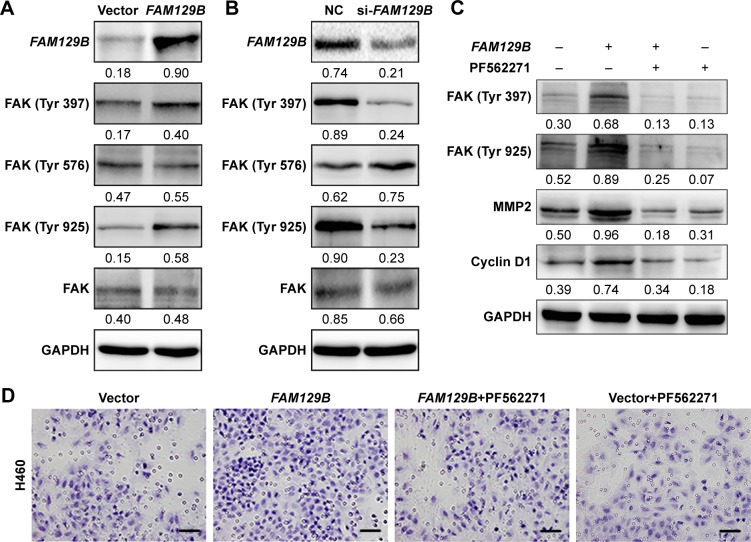
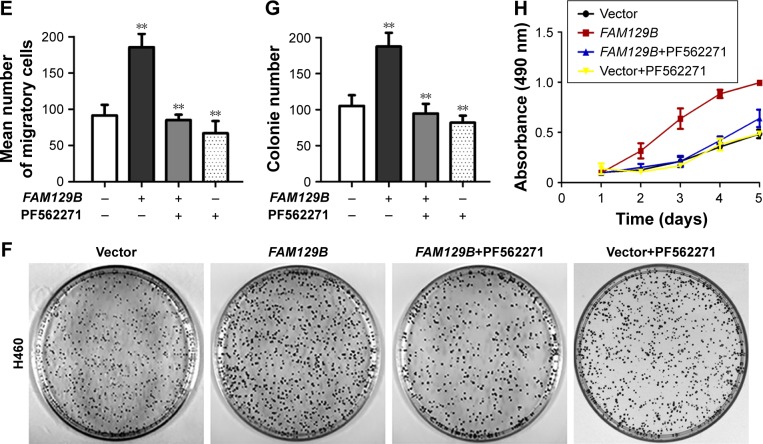
We also investigated the levels of phosphorylated FAK at Tyr 397, Tyr 576 and Tyr 925. Compared with the protein levels of FAK protein, the phosphorylated FAK at Tyr 397 and Tyr 925 were prominently upregulated after overexpression of FAM129B (Figure 4A), while they were downregulated after transfection with FAM129B siRNA (Figure 4B). Subsequently, PF562271, a specific inhibitor of FAK, was added into the medium of H460 cells with or without FAM129B overexpression. Compared with the controls, the elevated expression of FAK (Tyr 397) and FAK (Tyr 925) caused by FAM129B overexpression was significantly inhibited by PF562271 (Figure 4C). Interestingly, the increase of MMP2 and cyclin D1 protein levels caused by FAM129B overexpression was also reversed by FAK inhibitor incorporation (Figure 4C). Similar tendencies were also observed in Transwell assay, colony formation assay and MTT assay (Figure 4D–H).
Figure 4.
FAM129B facilitated tumor invasion and proliferation by promoting the phosphorylation of FAK at Tyr 397 and Tyr 925.
Notes: The phosphorylation of FAK (Tyr 397 and Tyr 925) was increased after overexpression of FAM129B in H460 and was decreased following depleting FAM129B in A549 (A and B). The elevation of MMP2 and cyclin D1 protein levels induced by FAM129B overexpression was reversed by incorporating FAK inhibitor (PF562271; C). The upregulation of tumor invasion (D and E, 400×, scale bar=50 µm), colony formation (F and G) and proliferation (H) caused by FAM129B overexpression were also attenuated by FAK inhibitor incorporation. **P<0.01.
Discussion
Our results showed that FAM129B played an oncogenic role in NSCLC cells, upregulating the protein levels of MMP2 and cyclin D1 through promoting the activation of FAK by inducing its phosphorylation at Tyr 397 and Tyr 925. Furthermore, the expression of FAM129B significantly correlated with advanced TNM stage and lymph node metastasis, and it also predicted poor prognosis of NSCLC patients.
Previous studies demonstrated that FAM129B localized to the cytoplasm during an active ERK1/ERK2 MAPK cascade, while it was localized to cell junctions upon inhibition of the MAPK cascade and upon growth to confluence in HeLa cells and mouse embryonic fibroblasts.3 In our cohort of clinical NSCLC tissue samples, FAM129B was localized in the cytoplasm, and no membranous expression was seen. Moreover, we showed that FAM129B played an oncogenic role in NSCLCs and correlated with tumor development. It is likely that the expression pattern of FAM129B may be tissue specific, therefore requiring more investigation.
In the present study, the expression of FAM129B in both online database (mRNA) and tissue samples (protein) showed significant associations with poor prognosis in NSCLC patients. To our knowledge, there has been no published study addressing the prognostic value of FAM129B. Therefore, FAM129B may be a useful factor for risk stratification and treatment decisions. There is a limitation in the present study: we could not perform an analysis of progression-free survival because of the limited information regarding chemotherapy and/or radiotherapy after surgery. Further studies are required to clarify the prognostic value of FAM129B.
Previous studies showed that FAM129B promoted tumor invasion and cell motility, but not growth.3,5–7 Since MMPs and cyclins played crucial roles in regulating invasion and growth, respectively, we analyzed the expression of multiple MMPs and cyclins after overexpression or depletion of FAM129B.18–21 Our results revealed that FAM129B facilitated both tumor invasion and tumor proliferation through upregu-lation of the expression of MMP2 and cyclin D1 in NSCLC cells. Previous studies were primarily based on experiments in cell lines of melanoma, uterine cervix cancer, glioblastoma and human skin.3,5–7 Despite the fact that one NSCLC cell line was chosen, we postulate that the difference of results between the previous studies and ours was due to different type of cancers or cell lines.
Previous studies focused on the suggestion that FAM129B was activated by ERK or that EGFR signaling induced phosphorylation, thereby promoting downstream proteins such as H-Ras and β-catenin.3,5,7 In our study, we found that FAM129B alone could activate FAK signaling. Previous studies showed that both MMP2 and cyclin D1 could be regulated by FAK signaling, thereby contributing to the effect of FAK signaling on cell migration/invasion or cell cycle progression.22–24 As FAK was also involved in regulating ERK signaling, EGFR signaling and Wnt/β-catenin signaling, our results may shed new light on the downstream signaling of FAM129B; certainly, further studies are needed to further clarify these issues.8–13
In conclusion, our data suggested that FAM129B significantly correlated with advanced TNM staging and positive lymph node metastasis and that it predicted adverse prognosis in NSCLC patients. FAM129B appeared to be an upstream regulator of the FAK signaling pathway, promoting the invasion and proliferation of NSCLC cells by facilitating the phosphorylation of FAK.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81402520 to Ailin Li) and the Research Foundation for the Doctoral Program to Ailin Li (No 20141040).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford S, Dawson E, Forbes S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91(2):355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Evans HG, Evans DR. FAM129B/MINERVA, a novel adherens junction-associated protein, suppresses apoptosis in HeLa cells. J Biol Chem. 2011;286(12):10201–10209. doi: 10.1074/jbc.M110.175273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Old WM, Shabb JB, Houel S, et al. Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma. Mol Cell. 2009;34(1):115–131. doi: 10.1016/j.molcel.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad W, Major MB, Cleary MA, et al. FAM129B is a novel regulator of Wnt/β-catenin signal transduction in melanoma cells. F1000 Res. 2013;2:134. doi: 10.12688/f1000research.2-134.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oishi H, Itoh S, Matsumoto K, et al. Delayed cutaneous wound healing in Fam129b/Minerva-deficient mice. J Biochem. 2012;152(6):549–555. doi: 10.1093/jb/mvs100. [DOI] [PubMed] [Google Scholar]
- 7.Ji H, Lee JH, Wang Y, et al. EGFR phosphorylates FAM129B to promote Ras activation. Proc Natl Acad Sci U S A. 2016;113(3):644–649. doi: 10.1073/pnas.1517112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 9.Long W, Yi P, Amazit L, et al. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell. 2010;37(3):321–332. doi: 10.1016/j.molcel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117(Pt 20):4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 11.Kaneda T, Sonoda Y, Ando K, et al. Mutation of Y925F in focal adhesion kinase (FAK) suppresses melanoma cell proliferation and metastasis. Cancer Lett. 2008;270(2):354–361. doi: 10.1016/j.canlet.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 12.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14(1):92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Fonar Y, Frank D, Fak FD. FAK and WNT signaling: the meeting of two pathways in cancer and development. Anticancer Agents Med Chem. 2011;11(7):600–606. doi: 10.2174/187152011796817673. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Burke A, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8(12):e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 18.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3(4):409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 19.Amălinei C, Căruntu ID, Giuşcă SE, Bălan RA. Matrix metalloprotei-nases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51(2):215–228. [PubMed] [Google Scholar]
- 20.Park MT, Lee SJ. Cell cycle and cancer. J Biochem Mol Biol. 2003;36(1):60–65. doi: 10.5483/bmbrep.2003.36.1.060. [DOI] [PubMed] [Google Scholar]
- 21.Eymin B, Gazzeri S. Role of cell cycle regulators in lung carcinogenesis. Cell Adh Migr. 2010;4(1):114–123. doi: 10.4161/cam.4.1.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Wang W, Ao L, Wang Z, Hao X, Zhang H. Benzo[a]pyrene-7,8-diol-9,10-epoxide suppresses the migration and invasion of human extravillous trophoblast HTR-8/SVneo cells by down-regulating MMP2 through inhibition of FAK/SRC/PI3K/AKT pathway. Toxicology. 2017;386:72–83. doi: 10.1016/j.tox.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Ding Q, Grammer JR, Nelson MA, Guan JL, Stewart JE, Gladson CL. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J Biol Chem. 2005;280(8):6802–6815. doi: 10.1074/jbc.M409180200. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12(12):4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]