Abstract
Background:
Atrial fibrillation (AF) is the most common sustained arrhythmia; it affects 1%–2% of the general population [1]. Many studies demonstrated an association between atrial fibrosis and AF [2]. There is increasing evidence that even in patients with lone AF; the AF is an arrhythmic manifestation of a structural atrial disease which has been described as fibrotic atrial cardiomyopathy [3]. It is unknown whether the presence of atrial fibrosis has any impact on post pulmonary vein antrum isolation outcome.
Purpose of the study:
This study aims to determine the incidence of atrial fibrosis in patients with non-valvular AF and its impact on recurrence after pulmonary vein antrum isolation.
Patients and Methods:
This study included twenty eight consecutive patients referred for first-time pulmonary vein antrum isolation for the treatment of symptomatic recurrent non-valvular AF not responding to medical treatment, Isolation of the pulmonary veins antra was performed using three dimensional electroanatomical mapping, detailed voltage map was done in the right and left atrium, before ablation and Low-voltage zones were identified. Follow up of the patients was done for 6 months after the procedure to detect recurrence of AF.
Results:
Left atrium fibrosis was present in 6 (21.4%) cases, right atrium fibrosis was present only in 1 (3.6%) case and recurrence of atrial fibrillation after 6 months occurred in 12 (42.9%) cases. AF burden was significantly higher in the recurrence group [50.33 ±19.7 (48) (hour/month)] as compared to no recurrence group [29.5 ± 6.99 (32) (hour/month)] with P-value 0.002 and the incidence of left atrium fibrosis was significantly higher in the recurrence group as compared to no recurrence group with P-value 0.024. The only significant predictors of recurrence were the presence of left atrium fibrosis (OR 10.71, 95% CI 1.05 to 109.78; P=0.046) and AF burden (OR 1.14, 95% CI 1.02 to 1.27; P=0.023). The only significant predictor of the presence of left atrium fibrosis was AF burden (OR 1.06, 95% CI 1.01 to 1.13; P=0.031)
Conclusions
The presence of the atrial fibrosis in the left atrium is an independent predictor of atrial fibrillation recurrence after pulmonary vein antrum isolation after 6 months without left atium substrate modification.
Keywords: AF, Atrial Fibrosis, Electrophysiology, Radiofrequency Ablation
Introduction
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia; it affects 1%–2% of the general population. Many studies demonstrated an association between atrial fibrosis and AF. There is increasing evidence that even in patients with lone AF; the AF is an arrhythmic manifestation of a structural atrial disease which has been described as fibrotic atrial cardiomyopathy. It is unknown whether the presence of atrial fibrosis has any impact on post pulmonary vein antrum isolation outcome.
Purpose of the study
This study aims to determine the incidence of atrial fibrosis in patients with non-valvular AF and its impact on recurrence after pulmonary vein antrum isolation.
Patients and methods:
This study enrolled 30 patients referred to the cardiology department at Ain Shams University hospitals for first-time pulmonary vein antrum isolation for the treatment of symptomatic paroxysmal non-valvular AF. Of these patients catheter ablation was postponed in 2 patients because of the development of cardiac tamponade during transseptal puncture, thus the 28 patients were included in the study.
All patients included were free of hypertension, diabetes mellitus, coronary artery disease, and cardiomyopathy thus they were defined as having lone AF.
Inclusion criteria included patients with symptomatic paroxysmal atrial fibrillation who were younger than sixty years and AF was documented by 12 lead ECG or Holter monitoring.
Exclusion criteria included patients with history of previous pulmonary vein isolation, patients with history of previous cardiac ablation procedures, patients with history of previous cardiac surgery, patients with persistent or permanent AF and patients with valvular heart disease.
After giving informed written consent and approval of the ethical committee, the selected patients were subjected to the thorough history taking, physical examination electrocardiogram, echocardiogram and transesophageal echocardiography. Oral anticoagulation was discontinued about 3 days before the procedure itself with bridging with enoxaparin 1mg/kg/12h.
Appropriate preparation including at least 6 hours of fasting before the procedure was necessary. All procedures were done under general anesthesia. After proper local anesthesia, two left femoral venous punctures were done for introduction of a quadripolar 6 Fr catheter at His region (landmark for trans-septal puncture) and a decapolar 6Fr steerable catheter in CS. Two right femoral venous punctures were done followed by exchange wiring with 2 long sheaths (Biosense Webster-PREFACE®) 8Fr caliber, which were introduced via 2 separate trans-septal punctures into LA cavity using the Brockenbrough technique. A circular decapolar mapping catheter (Lasso® catheter, Biosense Webster, Inc.; adjustable 15 to 25 mm circumference; 1–2 mm interelectrode spacing) was introduced to the LA via one long sheath and the ablation catheter (EZ Steer® ThermoCool® Nav catheter Biosense Webster, Inc.; tip electrode 3.5 mm, interelectrode spacing 2-5-2 mm) through the other one. 10,000 IU of heparin were given immediately after successful trans-septal punctures followed by continuous infusion of 1000 I.U. per hour, activated clotting time (ACT) measurement was performed every 30 - 40 minutes to maintain it in the range of 300–400 seconds. The Lasso catheter was positioned in every PV ostium to map PVP. Three dimensional Electro-anatomical mapping was done using CARTO® Platform (CARTO-3; Biosense Webster). The CARTO mapping system utilizes a low-level magnetic field delivered from 3 separate coils in a located pad beneath the patient. The magnetic field strength from each coil is detected by a location sensor embedded proximal to the tip of the specialized mapping catheter. Thus, by integrating each coil's field strength and converting this measurement into a distance, the location sensor (and therefore, catheter tip location) can be triangulated into space. Electro-anatomical voltage mapping: Complete maps of both atria were constructed with recording sites of anatomic relevance and areas of low endocardial voltage. The Atrial shell was created using point by point acquisition. Criteria for an adequate LA shell were ≥100 points that were homogenously distributed to create the entire each atrium with a fill threshold of ≤15 [4].
Right and left atrial voltage maps were constructed from the contact bipolar electrograms obtained from the ablation catheter during sinus rhythm. When low voltage zones (LVZ) were apparently identified, we looped the catheters and carefully recorded the voltages to prevent an insufficient wall contact force. For patients in AF at the beginning of the procedure, external direct current cardioversion was performed to restore sinus rhythm and a 15 min interval was taken before mapping. Low-voltage zones were defined as areas with bipolar peak-to-peak voltage amplitudes of < 0.5 mV. The presence of LVZs was defined as that covering >5% of LA body surface area. This cut-off point is equivalent to the minimum grade of LA fibrosis as reported by Mahnkopf et al using DE-MRI evaluation of the LA [5]. Pulmonary vein antrum isolation was performed; RF ablation was jointly performed by a continuous lesion all around the ipsilateral superior and inferior PVs using an open-irrigation catheter creating Wide area of circumferential ablation (WACA). RF ablation was performed with a temperature limit of 40°C, power limit of 25– 30W, and infusion rate of 13–20 mL/min with repositioning of the catheter tip every 20 s, but the power was reduced to 20–25W at the LA posterior wall near the oesophagus. The endpoint of the PVAI was entrance and exit block from the PV. All patients remained under in-hospital supervision for 24 hours after the procedure. Oral anticoagulation with Warfarin was initiated in all patients after 4-6 hours of the procedure and also concomitant enoxaparin 1mg/kg/12h until target international normalized ratio (INR) of 2-3 is reached. All patients received the oral anticoagulation for 3 months after the procedure. Anticoagulation strategy after 3 months varied, for patients with CHA2DS2-VASc score of ≥1 long-term warfarin treatment with target INR of 2-3 was continued [6]. A postablation blanking period was observed for 3 months and all patients were kept on the same AAD regimen they were prescribed before the ablation procedure throughout this period. Early recurrences within 3 months were treated with direct current cardioversion, AADs or both to restore sinus rhythm. AADs were discontinued at the end of the blanking period. Follow up for recurrence was done for a duration of 6 months after the procedure by a cardiologist who was blinded to the electro-physiologic procedure results to detect success rate including: history taking as regards recurrence of the symptoms, Surface ECG was taken and repeated after 1 day, 7 days, 1 month, 3 months and 6 months after the procedure or earlier if they developed symptoms consistent with recurrent AF, holter monitoring at 6months or earlier if they developed symptoms consistent with recurrent AF, cases were always asked to record their ECG when symptomatic. Recurrence was defined as any symptomatic or asymptomatic atrial tachyarrhythmia sustained for longer than 30 seconds documented with ECG or Holter monitoring without AAD treatment following the 3 month blanking period [7].
Statistical analysis: Results were analyzed by statistical package for social sciences (SPSS) Data were expressed as mean ± SD and percentage.
Results
The study population included 28 patients referred for first-time pulmonary vein antrum isolation for the treatment of symptomatic paroxysmal AF. Mean age was 42.43 ± 10.42 years; 23 patients were males (82.1%); mean BMI was 29.83 ± 5.13 Kg/m2; mean AF duration was 6.13 ± 6.47 (median 3.5) years; mean AF burden was 38.43 ± 17.19 (median 34) hours/month; AF burden (prior to the procedure) per month (which was defined as the total number of hours of symptomatic AF episodes per month in the last 3 months). 4 (14.3%) patients responded to medical treatment (they desired drug-free lifestyle) ; mean left atrium antero-posterior diameter was 39.75 ± 5.77 mm; mean ejection fraction 63.36 ± 4.68 %; mean lateral mitral annulus E’ was 10.46 ± 1.91 cm/s; as regard diastolic dysfunction 18 (64.3%) patients had no diastolic dysfunction, 9 (32.1%) patients had diastolic dysfunction grade 1 and 1 patient (3.6%) had diastolic dysfunction grade 3. Mean left atrium fibrosis as a percentage of whole left atrium was 10.23 ± 5.51 % [Table 1].
Table 1. Baseline characteristics of the study population .
| Baseline characteristics | Study sample n = 28 | |
|---|---|---|
| Age (years) | 42.43 ± 10.42 | |
| Male gender (number, %) | 23 (82.1) | |
| BMI (Kg/m2) | 29.83 ± 5.13 | |
| AF duration (years) | 6.13 ± 6.47 (median 3.5) | |
| AF burden prior to the procedure (hours/month) | 38.43 ± 17.19 (median 34) | |
| Response to medical treatment (number, %) | 4 (14.3) | |
| LA anteroposterior diameter (mm) | 39.75 ± 5.77 | |
| EF (%) | 63.36 ± 4.68 | |
| Lateral mitral Annulus E’ (cm/s) | 10.46 ± 1.91 | |
| Diastolic Dysfunction grade | 0 | 19 (67.9) |
| 1 | 9(32.1) |
As regards the procedural details 22 (78.6%) of patients underwent PVI only while 6 (21.4%) underwent PVI plus another ablation lesions (2 cases included ablation inside the coronary sinus, 2 cases included ablation of cavo-tricuspid isthmus for counterclockwise typical atrial flutter, 1 case included ablation of right free wall accessory pathway, and 1 case included ablation of Mahaim pathway), Left atrium fibrosis was present in 6 (21.4%) cases, right atrium fibrosis was present only in 1 (3.6%) case and recurrence of atrial fibrillation after 6 months occurred in 12 (42.9%) cases. While there were no major complications during ablation regarding those 28 patients, minimal inguinal hematoma was observed in 1 (3.6%) patient that was relieved conservatively. AF ablation, 'major complication’ was defined as a complication which resulted in permanent injury or death, prolonged or required hospitalization’ or required intervention for treatment. Two patients were found in AF rhythm the morning of the procedure and they were cardioverted electrically just before 3D mapping. In Comparison between patients with and without AF recurrence: There were no significant differences between the 2 groups regarding age, gender BMI, AF duration (which is the time since the AF diagnosis was made), response to medical treatment, ejection fraction, left atrium size, lateral mitral annulus E’ wave, diastolic dysfunction grade, ablation strategy and the presence of right atrium fibrosis [Table 2].
Table 2. Comparison between patients with and without recurrence .
| Variable | Recurrence | No Recurrence | P-value | |
|---|---|---|---|---|
| n=12 | n=16 | |||
| Male gender (number) | 9 | 14 | 0.624 | |
| Response to medical treatment (number) | 3 | 1 | 0.285 | |
| Diastolic Dysfunction grade (number) | 0 | 8 | 10 | |
| 1 | 3 | 6 | 0.431 | |
| 3 | 1 | 0 | ||
| Strategy (number) | PVI | 8 | 14 | |
| PVI and other ablation lesions | 4 | 2 | 0.354 | |
| LA fibrosis (number) | 5 | 1 | 0.024 | |
| RA fibrosis (number) | 1 | 0 | 0.429 |
However, AF burden was significantly higher in the recurrence group [50.33 ±19.7 (48) (hour/month)] as compared to no recurrence group [29.5 ± 6.99 (32) (hour/month)] with P-value 0.002 and the incidence of left atrium fibrosis was significantly higher in the recurrence group as compared to no recurrence group with P-value 0.024.[Figure 1]
Figure 1. Recurrence of AF after ablation was significantly higher in the presence of left atrium fibrosis with P-value 0.024.
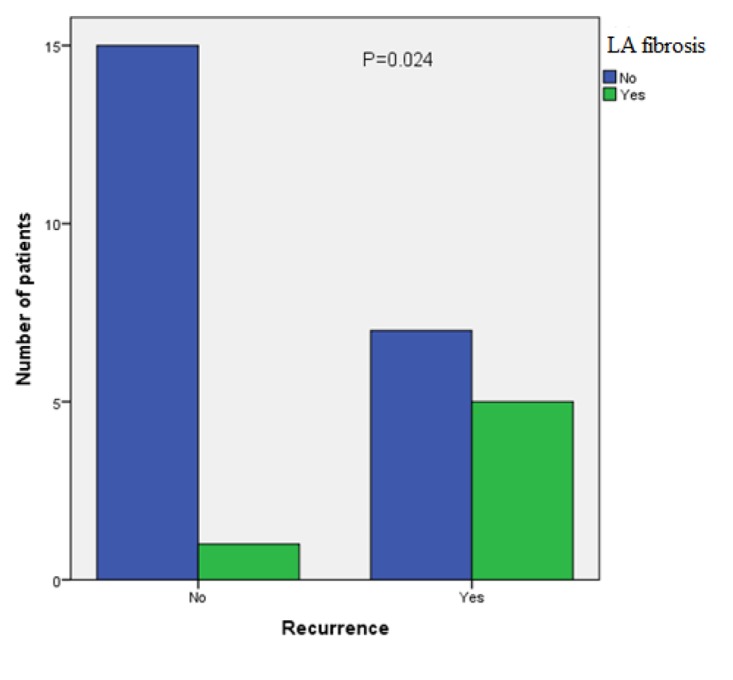
Univariate logistic regression analysis was used to identify variables predicting AF recurrence after 6 months. Analyzed variables included presence of left atrium fibrosis, AF duration, AF burden, left atrium size, age, gender, BMI, ejection fraction, lateral mitral annulus E’ wave, procedure strategy and the response to medical treatment. The only significant predictors of recurrence were the presence of left atrium fibrosis (OR 10.71, 95% CI 1.05 to 109.78; P=0.046) and AF burden (OR 1.14, 95% CI 1.02 to 1.27; P=0.023). Univariate logistic regression analysis was used to identify variables predicting the presence of left atrium fibrosis. Analyzed variables included age, gender, BMI, response to medical treatment, AF duration, AF burden, left atrium size, ejection fraction, lateral mitral annulus E’ wave. The only significant predictor of the presence of left atrium fibrosis was AF burden (OR 1.06, 95% CI 1.01 to 1.13; P=0.031). No multivariate analysis was done.
Discussion
Our study included 28 patients referred for first-time pulmonary vein antrum isolation for the treatment of symptomatic paroxysmal lone AF, there were no significant differences regarding the Baseline Clinical characteristics of the whole study sample. Recurrence of atrial fibrillation after 6 months occurred in 12 (42.9%). Left atrium fibrosis was present in 6 (21.4%) cases; right atrium fibrosis was present only in 1 (3.6%) case. AF burden was significantly higher in the recurrence group [50.33 ±19.7 (48) (hour/month)] as compared to no recurrence group [29.5 ± 6.99 (32) (hour/month)] with P-value 0.002. The incidence of left atrium fibrosis was significantly higher in the recurrence group as compared to no recurrence group with P-value 0.024.Univariate analyses showed that the only significant predictors of recurrence were the presence of left atrium fibrosis (OR 10.71, 95% CI 1.05 to 109.78; P=0.046) and AF burden (OR 1.14, 95% CI 1.02 to 1.27; P=0.023) and The only significant predictor of the presence of LA fibrosis was AF burden (OR 1.06, 95% CI 1.01 to 1.13; P=0.031). The idea that LA fibrosis was associated with recurrence after AF ablation can be explained by: (1) a LVZ may aggravate an interatrial conduction delay, resulting in the formation of circuits for reentry and thus promote AF perpetuation and (2) a LVZ may act as for the occurrence of non-PV ectopic sites. In 2015 Canpolat et al study showed that LA fibrosis was present in65.9% patients with lone paroxysmal AF with a median enhancement of 5% of the LA surface area. After cyoablation 78.1% of patients remained free of AF recurrence. Plasma TGF-β1 level (P = 0.001) was found to be the predictor of the extent of LA fibrosis. Multivariate Cox regression analysis pointed out that the extent of LA fibrosis (HR: 1.127, P = 0.007) and early AF recurrence (HR: 1.442, P = 0.011) were the independent predictors of AF recurrence in late follow-up. They concluded that higher levels of TGF-β1 are associated with more extensive LA fibrosis and extent of LA fibrosis predict recurrences in patients undergoing cryoablation for lone AF [8]. In 2014 Yamaguchi et al demonstrated LVZs in 32% of AF patients. During 24±7 months of follow-up 63% with LVZs and 19% without had AF recurrences off antiarrhythmic drugs (log-rank P < 0.001). A multivariate logistic regression analysis revealed that LVZ and ATP-induced reconnection were significant predictors of recurrence [9].
Conclusion
The presence of the left atrial fibrosis is an independent predictor of atrial fibrillation recurrence after pulmonary vein antrum isolation after 6 months without LA substrate modification.
References
- 1.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Oakes Robert S, Badger Troy J, Kholmovski Eugene G, Akoum Nazem, Burgon Nathan S, Fish Eric N, Blauer Joshua J E, Rao Swati N, DiBella Edward V R, Segerson Nathan M, Daccarett Marcos, Windfelder Jessiciah, McGann Christopher J, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009 Apr 07;119 (13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kottkamp Hans. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur. Heart J. 2013 Sep;34 (35):2731–8. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 4.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto Koji, Tsuchiya Takeshi, Narita Sumito, Yamaguchi Takanori, Nagamoto Yasutsugu, Ando Shin-ichi, Hayashida Kiyoshi, Tanioka Yoshito, Takahashi Naohiko. Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation. Europace. 2009 Dec;11 (12):1597–605. doi: 10.1093/europace/eup352. [DOI] [PubMed] [Google Scholar]
- 6.Joundi Raed A, Cipriano Lauren E, Sposato Luciano A, Saposnik Gustavo. Ischemic Stroke Risk in Patients With Atrial Fibrillation and CHA2DS2-VASc Score of 1: Systematic Review and Meta-Analysis. Stroke. 2016 May;47 (5):1364–7. doi: 10.1161/STROKEAHA.115.012609. [DOI] [PubMed] [Google Scholar]
- 7.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Canpolat Uğur, Oto Ali, Hazirolan Tuncay, Sunman Hamza, Yorgun Hikmet, Şahiner Levent, Kaya Ergün Bariş, Aytemir Kudret. A prospective DE-MRI study evaluating the role of TGF-β1 in left atrial fibrosis and implications for outcomes of cryoballoon-based catheter ablation: new insights into primary fibrotic atriocardiomyopathy. J. Cardiovasc. Electrophysiol. 2015 Mar;26 (3):251–9. doi: 10.1111/jce.12578. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Takanori, Tsuchiya Takeshi, Nagamoto Yasutsugu, Miyamoto Koji, Murotani Kenta, Okishige Kaoru, Takahashi Naohiko. Long-term results of pulmonary vein antrum isolation in patients with atrial fibrillation: an analysis in regards to substrates and pulmonary vein reconnections. Europace. 2014 Apr;16 (4):511–20. doi: 10.1093/europace/eut265. [DOI] [PubMed] [Google Scholar]


