Abstract
We examined the impact of sex on high fat diet induced renal alterations in Dahl salt sensitive and Sprague Dawley rats. In Dahl rats, high fat diet (60% kcal from fat for 24–26 weeks starting at weaning) significantly and equally increased blood pressure in males and females when compared to rats fed a control diet (10% kcal from fat). Male Dahl rats on high fat diet exhibited progressive renal histological injury, and moderately increased renal macrophage infiltration at 10 and 24 weeks of feeding when compared to males on control diet. Female Dahl rats had lower grade renal injury and less macrophage infiltration (except at 17 weeks) than males regardless of diet. Male Dahl rats on both diets showed progressively increasing numbers of renal T-cells, a pattern not observed in females. High fat diet per se did not significantly affect renal T-cell number. Male Dahl rats had lower renal T-reg cell ratio than females at 24 weeks. Renal macrophage and T-cell infiltrations were highly correlated to final MAP levels in males but not in females. Sprague Dawley rats fed high fat diet were normotensive without significant renal injury/inflammation after 24 weeks of feeding. In summary, high fat diet feeding fails to increase arterial blood pressure in Sprague Dawley rats, but strongly promotes hypertension in both male and female Dahl salt sensitive rats. Only Dahl males, however, exhibited blood pressure-associated renal inflammation and injury. Maintenance of T-reg ratio may protect against hypertension associated renal injury/inflammation but not high fat diet-induced hypertension.
Keywords: Sex differences, Dahl salt sensitive rats, High fat diet, Hypertension, Renal injury/inflammation
Introduction:
High fat diet (HFD) consumption causes obesity, dyslipidemia, insulin resistance, diabetes, chronic low grade inflammation, hypertension and renal dysfunction. 1, 2 HFD impairs endothelial function,3 increases arterial stiffness,4 and sympathetic outflow,5 activates the renin-angiotensin system, and increases renal water and salt retention.6 These alterations can promote and sustain hypertension. Sex differences in the prevalence and progression of obesity and cardiovascular and kidney diseases are well established.7, 8 Females are often protected from these disorders before menopause.9, 10 Obesity, male sex and hypertension are risk factors for the developing chronic kidney disease. 7
The mechanisms responsible for sex differences in blood pressure regulation and hypertension, and renal injury, have been investigated in several animal models of hypertension, including spontaneously hypertensive rats (SHR),11–13 Dahl salt sensitive (SS) hypertensive rats with high salt intake (4% NaCl),14, 15 angiotensin II-induced hypertension,16 aged Sprague-Dawley (SD) rats treated with an angiotensin II type 1 receptor antagonist during the nephrogenic period,17 and high salt diet fed mice.18 These studies support the hypothesis that sex differences in blood pressure, the renin-angiotensin system, and in renal inflammatory responses, contribute to sex differences in hypertension associated renal injury.8, 19, 20 Renal protection in hypertensive females has been attributed to the relatively lower blood pressure in females and to lower T-cell infiltration,16 or higher infiltration of regulatory T-cells (T-reg) compared to males.11, 12 However, the lower prevalence and risk of hypertension-induced end-organ damage in females is not be completely explained by sex differences in blood pressure regulation, since hypertensive males developed far greater end-organ damage than females even when blood pressures were comparable.21 Interestingly, female sex hormones protect against hypertension in Dahl SS rats but not in SHR. 22 Conflicting results were also reported, where no sex differences in the development of hypertension and renal injury were found in Dahl SS rats on high salt diet, however, female Dahl SS rats were protected from renal injury at “normal” salt diet (0.4%).15 It is unclear whether the kidneys in hypertensive females are protected in other animal models of hypertension. Obesity and HFD increase the incidence of salt sensitive hypertension and chronic kidney diseases,7 a globally fast-growing demographic in men and women. It is therefore important to determine if there are sex differences in HFD induced hypertension and renal injury using salt sensitive animal models. Dahl SS rats are to mimic salt sensitive hypertension in humans. Interestingly, HFD fed male Dahl SS rats develop hypertension even when ingesting a “normal salt” rodent diet, 0.3% NaCl.23–25 However, there is no information about HFD associated hypertension and renal inflammation/injury in female Dahl SS rats. Therefore, we tested the hypothesis that HFD fed female Dahl SS rats are protected against hypertension and renal inflammation/injury and sought to identify the underlying mechanisms.
Materials and Methods:
All raw data that support the findings of this study are available from the corresponding author on reasonable request.
Animals and diets:
We used HFD fed Dahl SS rats, a reliable rat model of HFD-induced hypertension,23–25 which allowed us to investigate hypertension-related renal injury/inflammation. As not all overweight or obese humans become hypertensive, we used Sprague Dawley (SD) rats to mimic this population apparently resistant to adiposity-associated hypertension.
Male and female Dahl SS and SD rats were purchased from Charles River Laboratory at 3 weeks of age and were randomly fed a normal salt (0.3% NaCl) control fat diet (CD, D12450J, kcal from saturated fat 10% + carbohydrate 70% + protein 20%) or a high fat diet (HFD, D12492, kcal from saturated fat 60% + carbohydrate 20% + protein 20%) (Research Diets, Inc.) ad libitum for 10, 17 or 24 weeks for Dahl SS rats, and 24 weeks for SD rats. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996) and approved by the Michigan State University Institutional Animal Care and Use Committee.
Measurement of arterial blood pressure and evaluation of neurogenic pressor activity, blood sampling and tissue collection, metabolic and biochemical measures, histological assessment of renal injury, histological assessment of renal inflammatory responses and detection of renal cytokines and MCP-1 expression by qRT-PCR are presented in On-line Only Supplemental Materials and Data.
Statistics:
Data are reported as mean ± SEM. Changes in body weight and MAP following 24 weeks of feeding in different groups were compared using a mixed design two-way ANOVA followed by Student-Newman-Keuls test. MAP and cell counts in kidney sections at 10, 17 and 24 weeks were compared using one-way ANOVA followed by post-hoc testing with Bonferroni test. Unpaired t-tests were used for comparing two groups, such as HFD vs CD rats or males vs females on the plasma metabolic measures, renal injury scores, cell counting and mRNA expression at 17 and 24 weeks. Data were analyzed using GraphPad Prism 6.0 software. A P-value of <0.05 was considered statistically significant.
Results:
Body weight (BW) and metabolic measures for HFD Dahl SS rats:
After 24 weeks, BW and most metabolic measures from HFD fed males and females were comparable with CD fed rats (Fig. 1A, Table S1). HFD fed male and female rats showed some abdominal adipose tissue accumulation and greater BW adjusted left ventricular mass than CD fed rats, and the changes were similar in HFD fed males and females. HFD moderately increased plasma leptin levels in males and females, but did not change plasma levels of glucose, insulin, cholesterol, triglycerides, BUN, creatinine, or the ratio of BUN/creatinine in males or females (Table S1). HFD males had significantly lower plasma albumin levels than CD fed males and HFD fed females (Table S1). Plasma albumin levels were similar in all females.
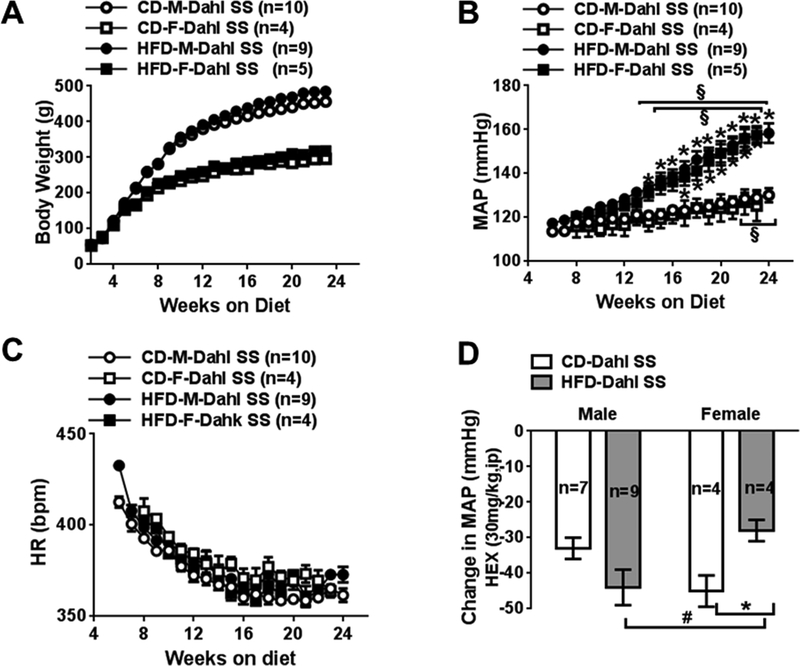
Fig 1:
Comparison of body weight (A) and weekly 24-h mean arterial blood pressure (B), and heart rate (C) measured following 24 weeks of control and HFD with normal salt in Dahl salt sensitive (SS) male and female rats. D, maximal changes of MAP after injection of hexamethonium (HEX) in Dahl SS rats at 24 weeks. CD, control fed; HFD, high fat fed; MAP, mean arterial blood pressure; HR, heart rate, M, male; F, female. Data are presented as mean ± SE. *P<0.05, HFD vs CD; #, P<0.05 M vs F; § P<0.05, vs MAP at their 10 weeks.
Blood pressure and heart rate in HFD fed Dahl SS rats:
HFD progressively increased mean arterial pressure (MAP) in male and female Dahl SS rats (Fig 1B, Table S2). At 10 weeks, 24-h MAPs in HFD rats did not differ from CD rats. At 17 weeks, MAP in all HFD rats was significantly higher than in the CD rats. At 24 weeks, MAP in all HFD rats was remarkably higher than CD rats. After 24 weeks, MAP in CD males was also slightly higher than their MAP at week 10, but not in CD females. The overall progression of HFD-induced hypertension was similar in male and female rats during 24 weeks (Fig. 1B, Table S2). HFD did not affect heart rate in all Dahl SS rats (Fig 1C, Table S2).
Neurogenic depressor responses in HFD fed Dahl SS rats:
At 24 weeks, sympathetic support of blood pressure was evaluated following treatment with ganglion blocker hexamethonium (a nicotinic ACh receptor antagonist, 30mg/kg, ip). Maximal changes in MAP were within 30 minutes after injection. Hexamethonium caused a slightly larger depressor response in HFD male Dahl SS rats than CD males. Hexamethonium caused smaller depressor responses in HFD females than CD females and HFD males (Fig 1D).
Renal histological changes to HFD in Dahl SS rats:
At 10 weeks (Fig 2B, S1, S3B), all male rats showed similar, low grade renal histological injury, identified as hyaline casts, interstitial fibrosis (peritubular), glomerular sclerosis, tubular atrophy, arterial hypertrophy and perivascular fibrosis; these changes were not seen in females.
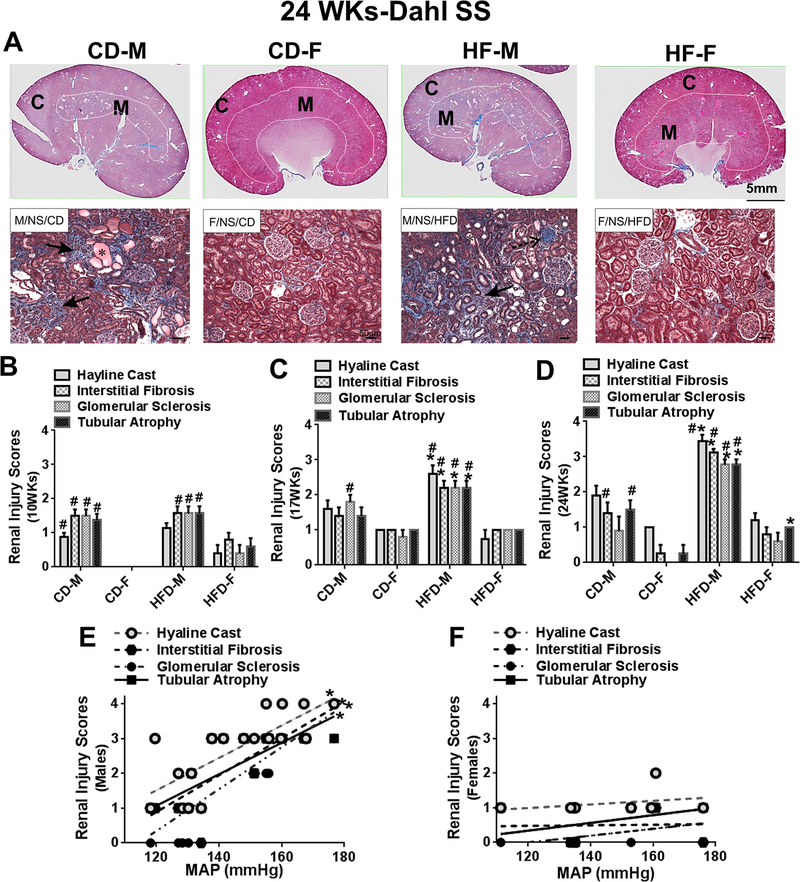
Fig 2:
A, Representative light photomicrographs taken from Masson’s Trichrome-stained whole renal sections and higher magnified cortical areas from male and female Dahl SS rats at 24 weeks (24WKs). Comparisons of semi-quantified renal injury scores in CD and HFD Dahl SS male and female rats at 10 (B), 17 (C), and 24 (D) weeks. Indicating the occurrence of histological changes in kidney sections: *hyaline cast; ←interstitial fibrosis (peritubular); stippled arrow, glomerular sclerosis. C, cortex; M, medulla. Data are mean ± SE. *P<0.05, HFD vs CD; #P<0.05 M vs F.
At 17 weeks (Fig 2C, S2), HFD male rats displayed greater renal histological injury than CD male rats and HFD females. CD males also had more glomerular sclerosis than CD female rats. Females had lower grade renal histological injury scores than males. HFD did not cause more arterial hypertrophy and perivascular fibrosis in males (Fig S3B). HFD females had lower arterial hypertrophy and perivascular fibrosis than HFD males (Fig S3B).
At 24 weeks (Fig 2A, 2D), when MAPs in HFD males and females were significantly higher (~35 mmHg) than in CD rats, HFD caused more severe renal histological injury in males, but these changes were not detected in kidneys from HFD fed females. CD male rats also showed greater interstitial fibrosis and tubular atrophy than did CD females. HFD female rats showed slight tubular atrophy compared to CD fed female rats, but overall renal injury scores were lower in all females compared to males. When MAP from individual HFD and CD fed rats was plotted against the renal injury scores, the renal injury scores were highly correlated with MAP levels in males (Fig 2E), but not in females (Fig 2F). HFD and CD males had significant arterial hypertrophy and perivascular fibrosis, these changes were minimal in CD and HFD females (Fig S3B).
Renal inflammatory responses to HFD fed in Dahl SS rats:
Macrophage infiltration:
At 10 weeks (Fig 3B, S3A), cortical and medullary macrophage infiltration (CD68+) was greater in males than females. All females had low numbers of cortical and medullary macrophages. HFD males had moderately higher cortical macrophage numbers than did CD males.
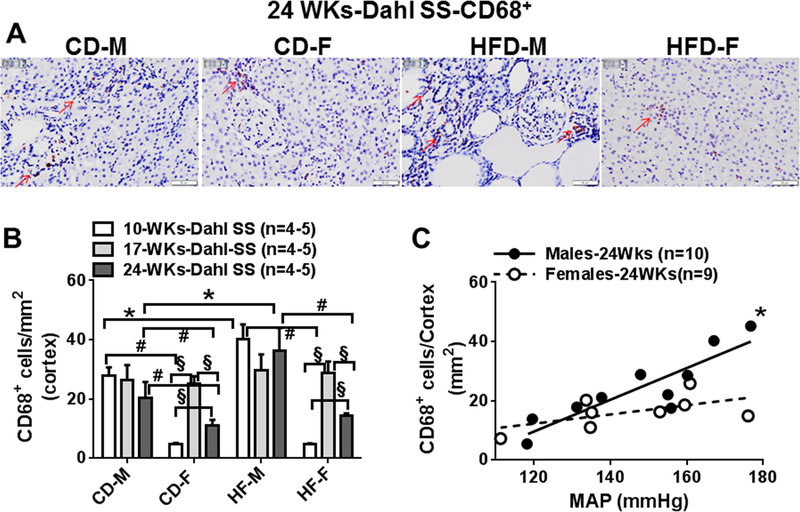
Fig 3:
A, Representative images (400x) of renal CD68+ positive cells in renal cortex taken from CD and HFD Dahl SS males and females at 24 weeks. B, Comparisons of cortical macrophages in CD and HFD males and females at 10 (10WKs), 17 (17WKs) and 24 weeks (24WKs). C Correlation between blood pressure and cortical CD68+ infiltrations in male and female Dahl SS rats at 24 weeks. Red arrow indicates CD68+ positive cells. Data are mean ± SE. *P<0.05, HFD vs CD; #P<0.05 M vs F, §; P<0.05, 10WKs vs 17WKs or 24WKs.
At 17 weeks (Fig 3B, S3A), cortical and medullary macrophages were similar to 10 weeks in all male rats. Female rats had increased (versus 10 weeks) cortical and medullary macrophages regardless of diet. Macrophage cell counts were similar in male and female rats.
At 24 weeks (Fig 3A, 3B, S3A), cortical and medullary macrophages were not different in male rats compared with 10 and 17 week rats. 24 weeks female rats had significantly fewer cortical and medullary macrophages compared to 17 week female rats, but macrophage numbers at this time point were greater than those detected in 10 week CD and HFD female rats. Cortical and medullary macrophage counts were greater in male compared to female rats.
HFD increased cortical and medullary macrophages in male rats at 10 and 24 weeks, but not at 17 weeks (Fig 3B, S3A). HFD did not affect cortical and medullar macrophage numbers in female rats. When cortical and medullary macrophage infiltration at 24 weeks were plotted against blood pressure levels, infiltration levels were highly correlated to MAP in male, but not female rats (Fig 3C, S3B).
T-cell infiltration
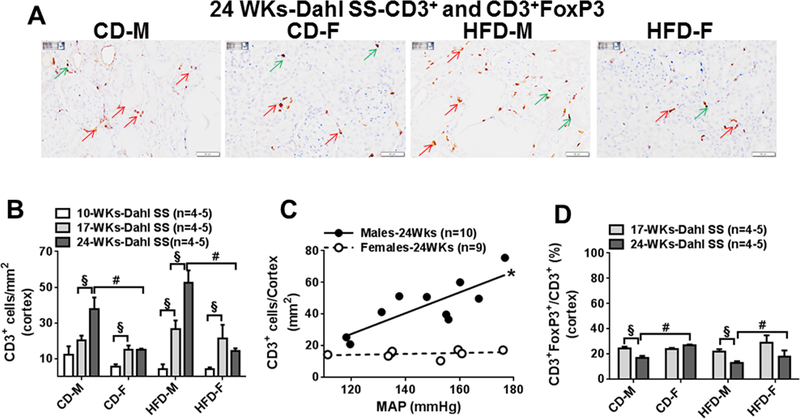
At 10 weeks (Fig 4B, S4A), all male and female rats had lower numbers of cortical and medullary T-cells (CD3+). At 17 weeks (Fig 4B, S4A), T-cell numbers increased further in male and female rats and by 24 weeks male (Fig 4A, 4B, S4A), but not female, rats exhibited a further increase in cortical and medullary T-cells. Following 24 weeks of feeding, HFD did not affect the cortical and medullar T-cell infiltrations significantly in males or females (Fig 4A, 4B, S4A). When cortical and medullar T-cell infiltration at 24 weeks was plotted against blood pressure, cortical T-cell numbers, but not medullary, were highly correlated with MAP in male rats (Fig 4C, S4B). Renal T-cell infiltration and blood pressure were not correlated in female rats (Fig 4C, S4B).
Fig 4:
A, Representative images (400x) of renal CD3+ and CD3+FoxP3+ positive cells in cortex from CD and HFD males and females at 24 weeks. B, Comparisons of cortical CD3+ cells in CD and HFD males and females at 10 (10WKs), 17 (17WKs) and 24 weeks (24WKs). C, Correlation between blood pressure and cortical CD3+ infiltrations in male and female Dahl SS rats at 24 weeks. D, Comparisons of the ratio of T-reg cells (CD3+FoxP3+/CD3+ cells) in cortex area in CD and HFD Dahl SS male and female rats at 17 (17WKs) and 24 weeks (24WKs). Red arrow indicates CD3+ positive cells; green arrow indicates CD3+FoxP3 positive cells. Data are mean ± SE. #P<0.05 M vs F, §; P<0.05, 10WKs vs 17WKs or 24WKs
T-reg cell infiltration, cytokine and MCP-1 responses
To examine a possible role of T-reg cell infiltration in protecting against HFD-induced kidney injury, the ratios of T-reg cells (CD3+FoxP3+ vs. total CD3+) were compared in cortical and medullary areas at 17 and 24 weeks, when T-cell numbers and renal injury were significant (Fig 4A, 4D, S4C). At 17 weeks, ~20% of CD3+ cells in the cortex and medulla were also FoxP3+ positive, and the ratio of T-reg cells was similar in all male and female rats. At 24 weeks compared with 17 weeks, the ratio was unchanged in female rats, but it was significantly lower in the cortex of CD and HFD males and lower in the medulla from HFD male rats when compared with female rats. Following 24 weeks of feeding, HFD did not affect the ratio of cortical and medullary T-reg cells significantly in male or female rats.
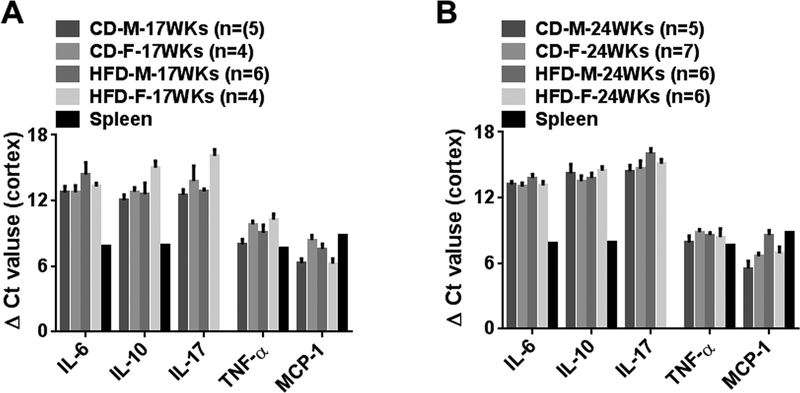
mRNA expression for IL-6, IL-17, IL-10, TNF-α and MCP-1 were determined in renal cortex from 17 and 24 week CD and HFD male and female rats (Fig 5A, 5B). Gene expressions for these inflammatory mediators were similar in cortex and medulla. mRNA expression in rat spleen was used as a positive control. ΔCt values for cortical IL-6, IL-17, IL-10, TNF-α and MCP-1 were similar in male and female rats at 17 and 24 weeks and were unaffected by diet (Fig 5).
Fig 5:
A and B, Comparisons of cortical mRNA expression of cytokine and MCP-1 in CD and HF males and females at 17 (17WKs) and 24 weeks (24WKs). Data are mean ± SE. #P<0.05 M vs F, §; P<0.05, 17WKs vs 24WKs
BW, blood pressure, renal histological changes and inflammatory responses to HFD in SD rats:
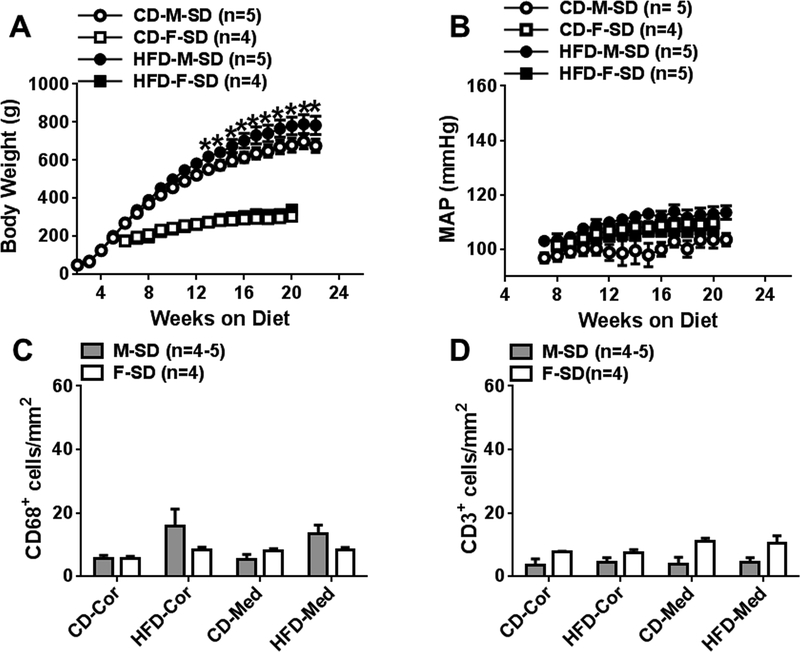
At 24 weeks, HFD fed SD males gained more weight than CD fed male rats, while BW in HFD and CD fed female rats were similar (Fig 6A). Weekly 24-h MAPs in HFD fed male and female rats did not change significantly throughout the study time course (Fig 6B). HFD male and female rats did not show significant renal histological injury (Fig S5). Cortical and medullary macrophage and T-cell numbers were low in all male and female rats (Fig. 6C, 6D). HFD promoted visceral adipocyte accumulation and hyperleptinemia without hyperglycemia and hyperinsulinemia in male rats. HFD female rats had higher leptin levels than CD female rats, but the levels were much lower than HFD male SD rats (Table S3).
Fig 6:
Comparison of body weight (A) and weekly 24-h mean arterial blood pressure (B) measured following 24 weeks of CD and HF feeding with normal salt in Sprague-Dawley (SD) male and female rats. C and D, comparisons of cortical and medullar CD68+ and CD3+ cells in CD and HFD males and females at 24 weeks. Data are mean ± SE.
Discussion
Our goal was to identify the impact of sex on the relationships between diet induced adiposity, blood pressure and histologically defined renal injury in an animal model of salt-sensitive hypertension. A key focus was on the relative time courses of renal injury versus hypertension as a guide to separate cause and effect between these variables. In addition, we examined the role of inflammation and immune cells in renal injury.
HF/normal salt diet promotes comparable hypertension in Dahl SS male and female rats
High salt diets (>0.3% NaCl) accelerate hypertension and renal inflammation/injury in Dahl SS rats, these rats are often used as an animal model of salt- sensitive hypertension and renal injury. Early studies reported sex differences in blood pressure regulation and renal function in HFD Dahl SS rats,14, 15, 26 similar to other animal models of hypertension.16–18, 21 Dahl SS females on high salt diet commonly have lower blood pressure and better renal function than hypertensive male rats.14, 27 In our study, HFD Dahl SS males developed hypertension similar to what has been previously reported by some,23–25 but not all,28 investigators. We report here for the first time that female Dahl SS rats given a normal salt HFD were also hypertensive, and that both the time course and magnitude of hypertension development are identical to males. Murphy et al also reported similar hypertension development in 4% NaCl fed male and female Dahl SS rats.15 This observation is surprising in light of the abundant evidence that in both “natural” and “induced” models of hypertension in animals, blood pressure is higher in males than females.27 One exception is renal wrap hypertension in rats, in which blood pressure is comparable in males and females.21 It should be noted that blood pressure in females has not been measured or reported in many of the newer genetic models of hypertension.
HFD caused minimal metabolic disturbance in male or female Dahl rats after 24–26 weeks of feeding, therefore, metabolic disorders likely play a minimal role in hypertension and renal injury in these rats. Although HFD caused slightly increased leptin levels in both sexes, our previous and current studies do not support that obesity and higher plasma leptin levels are necessarily associated with hypertension and renal injury. HFD SD rats and C57BL mice are obese with hyperleptinemia but they are normotensive without significant renal inflammation/injury.29 Other studies on HFD male Dahl SS rats also showed that metabolic changes were minimal.23 Furthermore, both sexes exhibit only moderately higher body weights and visceral fat accumulation when on HFD versus CD. Thus, our model resembles the clinical condition labeled “normal weight obesity” (NWO)30 comprised of individuals (the majority female) with increased visceral fat, but body weights in the normal range. Such individuals are clearly at increased risk for hypertension and cardiovascular morbidity and mortality.30,31 Patients with non-diabetic chronic kidney disease and NWO recently were shown to have a worse prognosis than non-obese individuals.31
HFD Dahl SS female rats are resistant to hypertension associated renal injury
Hypertension is the second leading cause of end stage renal disease after diabetes.32 Sex differences in hypertension associated renal injury and dysfunction are observed in clinical and experimental animal studies.7, 8 The data generally indicate that men are at higher risk for hypertension associated renal dysfunction/injury than women.8, 33, 34 Animal studies also show that hypertensive males develop more severe and rapid renal dysfunction/injury than hypertensive females.8, 14, 18 It is important to note, however, that the apparent resilience of females against hypertensive renal injury/dysfunction may depend on the specific index utilized to assess renal function or integrity.8
We used established histological measures to evaluate renal injury because these appear early in the course of hypertension and can precede the onset of microalbuminuria, another commonly used index of hypertensive renal damage.35 We found that renal injury was consistently lower in HFD Dahl SS female versus male rats, even though blood pressure was similar in the two groups. Male HFD Dahl SS rats exhibited greater hyaline cast (tubular proteinosis), peritubular interstitial fibrosis, glomerular sclerosis, and tubular atrophy compared to female HFD Dahl SS rats. Renal injury occurred in male rats at 10 weeks on the HFD, before hypertension developed. After 24 weeks of HFD, the severity of renal injury was positively correlated to blood pressure in male, but not female rats. All rats had normal plasma BUN, creatinine and BUN/creatinine ratio, even after 24 weeks on the HFD. These measures may be insufficiently sensitive to mark the severity of renal injury in our studies. However, the severe renal injury in males was indirectly supported by the lower plasma albumin levels. We found that all Dahl SS female rats had lower plasma albumin levels than those reported in other strain of rats (normally >3.3 g/dl,36 although the Dahl SS female rats did not show significant histological renal injury up to 17 weeks on the HFD. Compared to female rats, HFD caused further reduction in plasma albumin levels in male rats at 24 weeks. Lower plasma albumin levels may indicate greater proteinuria, since Dahl SS rats develop hypertension, proteinuria and tubular atrophy with aging,37 as shown in our studies. However, we did not measure renal function comprehensively and histological injury may not correlate with functional changes. Overall male Dahl SS rats had thicker renal arterial walls and greater perivascular fibrosis compared to females; however, HFD did not exacerbate these changes, at least in larger intrarenal arteries. The role of vascular remodeling in renal injury needs to be studied further.
The mechanisms responsible for the apparent resistance of Dahl SS female rats to renal injury secondary to adiposity-associated hypertension need further study. Previous work in other models indicate that renal protection in females is mediated by estrogen,10, 19, 22 lower blood pressure than males,11, 14, 28 sex differences in renin-angiotensin responses16, 18, 20 and sex differences in renal inflammation. 11–13, 16, 19 We chose to investigate possible sex differences in renal inflammation and immune cell infiltration.
Sex differences in renal inflammatory responses in Dahl SS rats
The contributions of inflammation in development of hypertension and associated renal injury have been established in humans and in animal models of hypertension.8 Hypertension is associated with an increase in renal macrophage and T-cell infiltration, and immunosuppression attenuates hypertension and renal injury.38 Male and female Dahl SS rats develop hypertension and renal inflammation/injury with aging.37, 39 High salt intake or feeding a HFD with normal salt accelerates hypertension development and proteinuria, renal T-cell and macrophage infiltration and renal injury in Dahl SS male rats.23, 40–42 Dahl SS rats are particularly useful for investigating hypertensive kidney injury because Dahl SS rats have a genetically determined, heightened propensity for renal inflammation and injury43 relative to other rat strains. We confirmed this by comparing renal macrophage and T-cell infiltration in Dahl SS rats to that in male SD rats (on CD or HFD). We found renal macrophage and T-cell infiltration were higher in Dahl SS male and female rats compared to male SD rats, regardless of diet. Since SD rats on HFD gained weight and adiposity, but showed minimal changes in blood pressure even after 24 weeks, it seems likely that, in rodents anyway, the main adverse effects of adiposity on renal injury are related to increased blood pressure, rather than adiposity per se.
Regarding sex differences, high salt associated increases in blood pressure and renal injury were less severe in Dahl SS female rats compared to male rats when BP in females was lower than males.44 Interestingly, other studies also reported that high salt intake caused similar increases in blood pressure and renal injury in male and female Dahl SS rats.15 However, there is no information about hypertension associated renal inflammation in HFD/normal salt fed Dahl SS female rats. Therefore, we examined the progression of renal macrophage and T-cell infiltrations in Dahl SS male and female rats at 10 weeks of HFD (pre-hypertensive), 17 weeks of HFD (moderate increase in blood pressure), and 24 weeks of HFD (hypertensive). We expected to see progressively increased renal macrophage and T-cell infiltration during the time course of hypertension development in HFD male and female rats. But differences between HFD and CD Dahl SS rats in renal macrophage and T-cell infiltration were not significant in males or females at any time point.
Nevertheless, at 24 weeks of feeding, renal macrophage and T-cell infiltrations was highly correlated to blood pressure in male, but not female, Dahl SS rats. Differences in renal injury and inflammation between CD versus HFD male rats may be diminished by spontaneously developing hypertension (increased ~12 mmHg at 24 weeks) and renal inflammation in CD Dahl SS males. This may mean that HFD induces renal injury and inflammation through at least two mechanisms, only one of which is hypertension. Males seem more prone to hypertension-associated renal injury, while injury in females may primarily reflect other effects of HFD.
Female Dahl SS rats showed comparable renal macrophage infiltration to male rats at 17 weeks on the HFD, whereas macrophage infiltration was less than in male rats at 10 and 24 weeks. Dahl SS female rats also had much less renal T-cell infiltration than males at 24 weeks. Therefore, our studies support the previously proposed idea that renal protection in Dahl SS female rats is likely due to less pressure-associated renal macrophage and T-cell infiltration than male rats.45 Why Dahl SS female rats show less pressure-dependent renal inflammation compared to male rats is unknown.
T-reg cells maintain immune system homeostasis by suppressing activation of conventional effector T-cells.46 Sullivan et al showed that lower blood pressure and proteinuria in female compared to male SHR is due to increased renal T-reg cells.8, 11, 12 We speculated that there would be higher renal T-reg cells in Dahl SS female rats too, however, we found that at 17 weeks renal T-reg/total T-cell ratio was comparable in HFD and CD fed male and female rats. Furthermore, HFD and CD female rats maintained this ratio, and total T-cell numbers, after 24 weeks on the HFD. Male rats had more total T-cells, with lower percentage of T-reg cells, compared to female rats. Our studies suggest that maintained T-reg cell number along with lower total T-cells in hypertensive Dahl SS female rats may play a key role in sex differences in hypertension associated renal injury. Our study is strongly supported by a recent study on pulmonary hypertension. Maintenance of T-reg cells appeared more important in protecting against pulmonary hypertension in female than male rats 47, since female rats with T-reg cell deficiency developed more severe pulmonary hypertension than male rats.
T-reg cells release IL-10, which protects against renal injury caused by the pro-inflammatory cytokines IL-6, IL-17, TNF-α.46 We analyzed renal mRNA expression of these cytokines and MCP-1 (macrophage activation) but did not find any differences among the various groups of rats at 17 and 24 weeks of HFD and CD feeding. This could indicate that increased release of cytokines has no role in the renal injury associated with HFD. It is also possible that mRNA expression and immune cell counts in renal sections may not represent the cytokine synthesis and release in whole kidney. We did not measure cytokine protein levels in the kidney, as it has been reported the levels in rat kidney are below the detection threshold of commercially available ELISA assays.12 We noted that renal macrophage infiltration was similar in males and females at 17 weeks of feeding, so it is crucial to know if there are different populations of M1 and M2 macrophages in males and females. More studies on immune cell profiles using flow cytometry analysis are needed, e.g. determination of renal M1 and M2 macrophage populations and detailed profiles of T-cell infiltration.11, 12
Increased sympathetic activation in renal T-cell infiltration?
Increased sympathetic activity could contribute to HFD or obesity associated hypertension5 and T-cell infiltration, and the extent and timing of increases in sympathetic activation are sex dependent.48,49 HFD increased sympathetic support of blood pressure in male, but not female, mice, although the mice remained normotensive.49 We measured the sympathetic support of blood pressure by injection of hexamethonium. Our results were consistent with the previous report: hexamethonium caused a larger depressor response in HFD male Dahl SS rats than CD males, but hexamethonium caused smaller depressor responses in HFD females than CD females and HFD males.49 We have no explanation for why HFD females showed reduced sympathetic support of blood pressure compared to CD females. Therefore, increased systemic sympathetic activity may be more responsible for hypertension and increased renal T-cell infiltration in HFD males than in female Dahl SS rats. Alternatively, since renal injury/inflammation may be a key stimulus for sympathoexcitation, it is possible that the greater T-cell infiltration in males versus females could account for the larger sympathetic support of blood pressure in HFD males.
Limitations and future studies:
Sex hormones affect both hypertension development and associated target organ damage in other experimental models. WE think that it is highly likely that sex hormones levels, and receptor function in autonomic, vascular, renal and immune systems, contribute to sex differences in hypertension associated renal inflammation/injury. One limitation of the work here is that we did not investigate those possibilities in our model; we will attempt to provide insight in future investigations. Another limitation of this study is that we used only high fat diet to drive adipose tissue accumulation. An increase in caloric intake through other means could dramatically change experimental outcomes, so the effects of other types of high calorie diets on hypertension and renal injury/inflammation in Dahl SS rats need to be investigated.
Conclusions:
Our studies show that feeding HFD from weaning promotes moderate adiposity and hypertension equally in adult Dahl SS male and female rats with minimal associated metabolic disorders, and exacerbates renal injury in male, but not female rats. Multiple mechanisms appear to be responsible for HFD feeding induced renal inflammation/injury in Dahl SS males, only one of which is hypertension. Dahl SS female rats may be protected against hypertension-associated renal injury via a blood pressure-independent moderation of renal inflammation. Maintained renal T-reg cell ratios may also contribute to the renal protection in Dahl SS female rats.
Perspective:
Hypertension is one of the leading causes of chronic kidney diseases. There are sex differences in hypertension development and the hypertension associated kidney diseases. Females usually are protected against hypertension associated renal injury via relatively lower blood pressure than males. In our studies, renal protection in hypertensive female Dahl SS rats is mediated by lower renal inflammatory responses and maintained T-reg cell ratios, not lower blood pressure. Our studies suggested that drugs that have potential properties to maintain the differentiation of T-reg from T cells would be novel treatments for the prevention or treatment of hypertension associated renal injury in males.
Clinical Implications:
Hypertension is the second leading cause of end stage renal disease.32 Our experimental model of HFD associated hypertension in a susceptible rat strain resembles clinical observations of sex differences in hypertension associated renal injury and dysfunction,7, 8 specifically findings that males are at higher risk for hypertension associated renal dysfunction/injury than females.8, 33, 34 Our studies suggest that because the severity of renal injury/dysfunction does not depend exclusively on blood pressure levels, moderation of renal immune responses may be an effective way to protect against hypertension associated renal diseases, especially in males.
Supplementary Material
Novelty and Significance:
1). What Is New:
High fat diet associated hypertension can occur with minimal changes in body weight and metabolic measures in the salt-sensitive Dahl SS rat.
There are sex differences in the occurrence of renal injury/inflammation in Dahl SS rats on HFD that are independent of blood pressure.
2). What Is Relevant:
Males and females had similar blood pressure responses to high fat feeding,
Renal injury/inflammation is highly correlated to the blood pressure levels in male but not in female salt sensitive rats.
Compared to males, females had lower grade renal injury with lower renal macrophage and T-cell infiltration and maintained T-reg cells.
3). Summary:
Maintenance of T-reg cell ratio may be a more important protection against hypertension associated renal injury than lower blood pressure.
Acknowledgment:
We would like to thank the staff at the histology laboratory at Michigan State University for tissue processing, histological and immune cell staining.
Funding support:
This study is supported by NHLBI 2P01HL070687 for G.D.F, J.J.G. and H.X.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement
None
References:
- 1.Brands MW, Hall JE. Insulin resistance, hyperinsulinemia, and obesity-associated hypertension. Journal of the American Society of Nephrology : JASN. 1992;3:1064–1077. [DOI] [PubMed] [Google Scholar]
- 2.Odermatt A The western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. American journal of physiology. Renal physiology 2011;301:F919–931. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 3.Lambert EA, Phillips S, Belski R, Tursunalieva A, Eikelis N, Sari CI, Dixon JB, Straznicky N, Grima M, Head GA, Schlaich M, Lambert GW. Endothelial function in healthy young individuals is associated with dietary consumption of saturated fat. Frontiers in physiology. 2017;8:876. doi: 10.3389/fphys.2017.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. American journal of physiology. Regulatory, integrative and comparative physiology. 2018;314:R387–R398. doi: 10.1152/ajpregu.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim K, Jackson KL, Sata Y, Head GA. Factors responsible for obesity-related hypertension. Current hypertension reports. 2017;19:53. doi: 10.1007/s11906-017-0750-1. [DOI] [PubMed] [Google Scholar]
- 6.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. Journal of the American Society of Nephrology : JASN. 2001;12:1211–1217. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan JC, Gillis EE. Sex and gender differences in hypertensive kidney injury. American journal of physiology. Renal physiology. 2017;313:F1009–F1017. doi: 10.1152/ajprenal.00206.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy E, Lagranha C, Deschamps A, Kohr M, Nguyen T, Wong R, Sun J, Steenbergen C. Mechanism of cardioprotection: What can we learn from females? Pediatric cardiology. 2011;32:354–359. doi: 10.1007/s00246-010-9877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silbiger SR. Raging hormones: Gender and renal disease. Kidney international. 2011;79:382–384. doi: 10.1038/ki.2010.474. [DOI] [PubMed] [Google Scholar]
- 11.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory t lymphocyte infiltration than males. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;303:R359–367. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory t cells in response to elevations in blood pressure. Hypertension. 2014;64:557–564. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipton AJ, Musall JB, Crislip GR, Sullivan JC. Greater transforming growth factor-beta in adult female shr is dependent on blood pressure, but does not account for sex differences in renal t-regulatory cells. American journal of physiology. Renal physiology. 2017;313:F847–F853. doi: 10.1152/ajprenal.00175.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr. Chromosome substitution reveals the genetic basis of dahl salt-sensitive hypertension and renal disease. American journal of physiology. Renal physiology 2008;295:F837–842. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ. Renoprotective effects of anti-tgf-beta antibody and antihypertensive therapies in dahl s rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;303:R57–69. doi: 10.1152/ajpregu.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in t-lymphocyte tissue infiltration and development of angiotensin ii hypertension. Hypertension. 2014;64:384–390.doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saez F, Reverte V, Paliege A, Moreno JM, Llinas MT, Bachmann S, Salazar FJ. Sex-dependent hypertension and renal changes in aged rats with altered renal development. American journal of physiology. Renal physiology. 2014;307:F461–470. doi: 10.1152/ajprenal.00198.2014. [DOI] [PubMed] [Google Scholar]
- 18.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. American journal of physiology. Renal physiology. 2014;307:F901–907. doi: 10.1152/ajprenal.00288.2014. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R1573–1579. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin ii-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H, Pesce C, Zheng W, Kim J, Zhang Y, Menini S, Haywood JR, Sandberg K. Sex differences in renal injury and nitric oxide production in renal wrap hypertension. American journal of physiology. Heart and circulatory physiology. 2005;288:H43–47. [DOI] [PubMed] [Google Scholar]
- 22.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. American journal of physiology. Regulatory, integrative and comparative physiology. 2014;307:R149–157. doi.org/10.1152/ajpregu.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spradley FT, De Miguel C, Hobbs J, Pollock DM, Pollock JS. Mycophenolate mofetil prevents high-fat diet-induced hypertension and renal glomerular injury in dahl ss rats. Physiological reports. 2013;1:e00137. doi: 10.1002/phy2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in dahl salt-sensitive rats. Clin Exp Hypertens. 2009;31:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension. 2012;60:404–410. doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayorh MA, Socci RR, Eatman D, Wang M, Thierry-Palmer M. The role of gender in salt-induced hypertension. Clin Exp Hypertens. 2001;23:241–255. [DOI] [PubMed] [Google Scholar]
- 27.Sandberg K, Ji H. Sex differences in primary hypertension. Biology of sex differences. 2012;3:7. doi: 10.1186/2042-6410-3-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clinical and experimental pharmacology & physiology. 2005;32:825–831. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Garver H, Fernandes R, Phelps JT, Harkema JJ, Galligan JJ, Fink GD. Bk channel beta1-subunit deficiency exacerbates vascular fibrosis and remodelling but does not promote hypertension in high-fat fed obesity in mice. Journal of hypertension. 2015;33:1611–1623. doi: 10.1097/HJH.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco LP, Morais CC, Cominetti C. Normal-weight obesity syndrome: Diagnosis, prevalence, and clinical implications. Nutrition reviews. 2016;74:558–570. doi: 10.1093/nutrit/nuw019. [DOI] [PubMed] [Google Scholar]
- 31.Lin TY, Lim PS, Hung SC. Normal-weight obesity and clinical outcomes in nondiabetic chronic kidney disease patients: A cohort study. The American journal of clinical nutrition. 2018;107:664–672. doi: 10.1093/ajcn/nqy006. [DOI] [PubMed] [Google Scholar]
- 32.Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. Journal of human hypertension. 2014;28:74–79. doi: 10.1038/jhh.2013.55. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Xie D, Xu X, Qin X, Tang G, Wang B, Wang Y, Hou F, Wang X. Blood pressure and renal function decline: A 7-year prospective cohort study in middle-aged rural chinese men and women. Journal of hypertension. 2015;33:136–143. doi: 10.1097/HJH.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 34.Muiesan ML, Ambrosioni E, Costa FV, Leonetti G, Pessina AC, Salvetti M, Trimarco B, Volpe M, Pontremoli R, Deferrari G, Rosei EA. Sex differences in hypertension-related renal and cardiovascular diseases in italy: The i-demand study. Journal of hypertension. 2012;30:2378–2386. doi: 10.1097/HJH.0b013e328359b6a9. [DOI] [PubMed] [Google Scholar]
- 35.Ofstad J, Iversen BM. Glomerular and tubular damage in normotensive and hypertensive rats. American journal of physiology. Renal physiology. 2005;288:F665–672. [DOI] [PubMed] [Google Scholar]
- 36.Rose R, Klemcke HG. Relationship between plasma albumin concentration and plasma volume in 5 inbred rat strains. Journal of the American Association for Laboratory Animal Science : JAALAS. 2015;54:459–464. [PMC free article] [PubMed] [Google Scholar]
- 37.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging dahl salt sensitive rat. Journal of the American Society of Nephrology : JASN. 2004;15:1546–1556. [DOI] [PubMed] [Google Scholar]
- 38.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in dahl salt-sensitive rats. American journal of physiology. Renal physiology. 2016;311:F555–561. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female dahl salt-sensitive rats. Hypertension. 2004;44:405–409. [DOI] [PubMed] [Google Scholar]
- 40.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. American journal of physiology. Renal physiology. 2014;307:F499–508. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating t lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. American journal of physiology. Renal physiology. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in dahl salt-sensitive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrett MR, Joe B, Yerga-Woolwine S. Genetic linkage of urinary albumin excretion in dahl salt-sensitive rats: Influence of dietary salt and confirmation using congenic strains. Physiological genomics. 2006;25:39–49. [DOI] [PubMed] [Google Scholar]
- 44.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489. [DOI] [PubMed] [Google Scholar]
- 45.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal t-cell infiltration in the dahl salt-sensitive rat. Hypertension. 2017;70:543–551. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vignali DA, Collison LW, Workman CJ. How regulatory t cells work. Nature reviews. Immunology. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamosiuniene R, Manouvakhova O, Mesange P et al. Dominant role for regulatory T Cells in protecting females against pulmonary hypertension. Circ Res. 2018;8:122(12):1689–1702. doi: 10.1161/CIRCRESAHA.117.312058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. 13. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50(5):862–868. [DOI] [PubMed] [Google Scholar]
- 49.Bruder-Nascimento T, Ekeledo OJ, Anderson R, Le HB, Belin de Chantemèle EJ. Long Term High Fat Diet Treatment: An Appropriate Approach to Study the Sex-Specificity of the Autonomic and Cardiovascular Responses to Obesity in Mice. Front Physiol. 2017;8:32. doi: 10.3389/fphys.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.