Abstract
Background
Targeted disease-modifying antirheumatic drug (DMARD) options for rheumatoid arthritis (RA) include tumor necrosis factor (TNF) inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab) or alternative mechanisms of action (MOAs), such as a T-cell co-stimulation modulator (abatacept), Janus kinase inhibitor (tofacitinib), or interleukin-6 inhibitor (tocilizumab).
Objective
To examine treatment persistence and healthcare costs in patients with RA who changed therapy by cycling therapy (ie, switching within the same drug class), or switching between, the TNF inhibitors and alternative MOA medication classes.
Methods
We analyzed medical and pharmacy claims for commercially insured patients who cycled or switched between targeted DMARD agents between January 1, 2010, and September 30, 2014 (ie, the index date), to determine treatment patterns (ie, treatment switching, discontinuation, restarting after a gap ≥60 days, or persistence) and costs (plan- and patient-paid) for 1 year postindex. The cost per persistent patient was the total healthcare cost divided by the number of treatment-persistent patients.
Results
The analysis included 6203 patients who cycled between TNF inhibitors, 2640 patients who switched from TNF inhibitors to alternative MOA agents, 699 patients who cycled between alternative MOA agents, and 687 patients who switched from alternative MOA agents to TNF inhibitors. The 1-year treatment persistence rates (with P values vs TNF inhibitor cyclers) were 45.2% for TNF inhibitor cyclers, 50.3% for TNF inhibitor-alternative MOA switchers (P <.001), 51.4% for alternative MOA agent cyclers (P = .002), and 46.1% for alternative MOA-TNF inhibitor switchers (P = .63). Compared with TNF inhibitor cyclers, the cost per persistent patient was lower for TNF inhibitor-alternative MOA switchers (–$16,853 RA-related; –$19,280 targeted DMARDs), alternative MOA agent cyclers (–$21,662 RA-related; –$25,153 targeted DMARDs), and alternative MOA-TNF inhibitor cyclers (–$7206 RA-related; –$7919 targeted DMARDs).
Conclusion
Among patients with RA, patients who switched from a TNF inhibitor to an alternative MOA agent and those who cycled between alternative MOA agents had significantly higher treatment persistence rates and a substantially lower cost per persistent patient than those who cycled between TNF inhibitors. These findings support the evaluation of switching medication classes for patients with RA when a targeted therapy fails.
Keywords: alternative mechanisms of action, cost, persistence, rheumatoid arthritis, switching treatment, synthetic DMARD, treatment cycling, treatment persistence, tumor necrosis factor inhibitors
Treatment guidelines for rheumatoid arthritis (RA) recommend initiating therapy with a conventional synthetic disease-modifying antirheumatic drug (DMARD), such as methotrexate, leflunomide, sulfasalazine, or hydroxychloroquine.1,2 If response to the synthetic DMARD is inadequate and disease activity remains moderate or high, then targeted DMARD therapy can be added. Several targeted DMARDs are available for the treatment of patients with RA, including tumor necrosis factor (TNF) inhibitors, such as adalimumab, certolizumab, etanercept, golimumab, or infliximab, or medications with alternative mechanisms of action (MOAs), such as a T-cell co-stimulation modulator (abatacept), a Janus kinase inhibitor (tofacitinib), or interleukin-6 inhibitors (tocilizumab and sarilumab).1,2
Older treatment guidelines for RA generally recommended the use of a TNF inhibitor before an agent with an alternative MOA.3–7 Recently, the RA treatment guidelines were updated to reflect emerging clinical evidence.1,2 When synthetic DMARD monotherapy fails, the guidelines now place an alternative MOA agent or a TNF inhibitor at the same level for initial targeted therapy; when TNF inhibitor therapy fails, the recommendations are less clear and mention the use of any targeted DMARD.1,2
Real-world studies consistently report that patients with RA are more likely to cycle TNF inhibitors (ie, switch within the same class) than to switch from a TNF inhibitor to an alternative MOA agent in clinical practice.8–19 Because TNF inhibitors were introduced to clinical practice before drugs with alternative MOAs, physicians may be more comfortable with cycling between TNF inhibitors instead of using newer agents with alternative MOAs. In addition, health plan formulary restrictions that require patients to cycle through several TNF inhibitors before they can switch to an agent with an alternative MOA may have contributed to higher rates of TNF inhibitor cycling in clinical practice.
Several studies have reported that patients who switch from a TNF inhibitor to an alternative MOA agent have greater treatment effectiveness16–25 and better treatment persistence14–17 than patients who cycle from one TNF inhibitor to another TNF inhibitor. The objective of this study was to examine treatment persistence, healthcare (ie, medical and pharmacy) costs per patient, and healthcare costs per persistent patient after cycling therapy within, or switching between, TNF inhibitor and alternative MOA drug classes.
Methods
This retrospective analysis is based on inpatient medical claims, outpatient medical claims, and pharmacy claims from the Truven Health MarketScan Commercial Database. The database includes claims for approximately 35 million employees and their dependents in fee-for-service and managed care health plans. No identifiable protected health information was extracted or accessed during the study, pursuant to the US Health Insurance Portability and Accountability Act (HIPAA). The study did not involve collection, use, or transmittal of individually identifiable data; this compliance with HIPAA regulation meant that patient consent and Institutional Review Board approval to conduct this study were not necessary.
KEY POINTS
-
▸
The choice of targeted DMARD options for RA is guided by treatment persistence and cost.
-
▸
In this study, medical and pharmacy claims data were used to evaluate treatment persistence and costs in patients who changed treatment within the drug class or switched from one class to another.
-
▸
Switching from a TNF inhibitor to an alternative MOA agent or cycling within alternative MOA agents resulted in greater odds of treatment persistence than cycling within TNF inhibitors.
-
▸
The 1-year persistence rates were lowest for TNF inhibitor cyclers and highest for those cycling among agents with alternative MOAs.
-
▸
The cost per persistent patient was lower for those switching from a TNF inhibitor to an alternative MOA agent or among agents with alternative MOAs than those cycling within TNF inhibitors.
-
▸
These findings support switching to a medication with an alternative MOA instead of a TNF inhibitor when the initial treatment with a targeted DMARD fails.
Study Population
The analysis included only patients who changed targeted DMARD therapy. Patients were included in the analysis if they had at least 1 claim for a targeted DMARD, including a TNF inhibitor (ie, etanercept, adalimumab, infliximab, golimumab, or certolizumab) or an agent with an alternative MOA (ie, abatacept, tocilizumab, or tofacitinib) between January 1, 2010, and September 30, 2014, and at least 1 claim for a targeted DMARD that was different from the index therapy in the previous 12 months. The first time in the study period that a patient cycled to another targeted DMARD in the same class or switched to a targeted DMARD in the other class, was designated as the index event.
Patients who switched to anakinra on the index date were not included in the study, because the total patient sample was too small. Patients who switched to rituximab on the index date were not included, because treatment persistence could not be determined accurately from the claims data as a result of the drug's unusual dosing schedule (every 6 months in 2 infusions separated by 1 week). Any targeted DMARD that was approved by the US Food and Drug Administration after September 2014 was not included.
Patients were required to have an insurance claim for only 1 targeted DMARD on the index date, but they could receive 1 or more synthetic DMARDs (ie, hydroxychloroquine, leflunomide, methotrexate, or sulfasalazine) concurrently with the index therapy. Participating patients were required to have continuous health plan enrollment and pharmacy benefits for ≥12 months before the index date (ie, baseline period) and for ≥12 months after the index date (ie, postindex period). The postindex follow-up for each patient varied in length, ending on September 30, 2015, or at disenrollment from the health plan, if earlier.
Eligible patients had to be aged ≥18 years at index and have ≥1 nondiagnostic medical claims with a diagnosis code for RA (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD-9-CM] code 714.0x) in the primary or secondary position in the baseline period or 30 days postindex.
Patients were excluded from the study if they had a claim in any position in the baseline period or after the index date with a diagnosis code for any autoimmune conditions for which targeted DMARDs are used, including ankylosing spondylitis (ICD-9-CM code 720.0); Crohn's disease (ICD-9-CM code 555.x); juvenile idiopathic arthritis (ICD-9-CM code 714.3); plaque psoriasis (ICD-9-CM code 696.1x); psoriatic arthritis (ICD-9-CM code 696.0); ulcerative colitis (ICD-9-CM code 556.x); chronic lymphocytic leukemia (ICD-9-CM code 204.1x); or non-Hodgkin lymphoma (ICD-9-CM codes 200.xx and 202.xx).
Outcomes
The demographic characteristics (ie, age, sex, geographic region, urban/rural residence, insurance plan type, and index year) were determined for the index date. Clinical characteristics (ie, Charlson Comorbidity Index, DMARDs used, total healthcare expenditures, RA-related expenditures, and healthcare utilization) were determined from claims data in the baseline period.
Possible treatment patterns during the 1-year postindex period were treatment discontinuation, switching, restarting, or persistence. Treatment discontinuation was defined as a gap in therapy of ≥60 days for index therapy until the end of the postindex period. Switching treatment was defined as a claim for another targeted DMARD postindex, other than the index therapy.
Restarting treatment was defined as a treatment gap of ≥60 days for the index therapy, followed by another claim for the index therapy as the next targeted DMARD after the treatment gap. Treatment persistence was defined as no treatment discontinuation, switching, or restarting in the postindex period. A nonpersistent patient could have more than 1 nonpersistence event (ie, discontinuation, switching, or restarting) reported postindex. The duration of treatment persistence for each patient was calculated from the index date until the first treatment gap or switch in therapy (for nonpersistent patients) or until the end of the postindex period (for persistent patients).
The total healthcare costs included plan-paid and patient-paid amounts for all medical claims (ie, inpatient, emergency department, and outpatient) and pharmacy claims in the postindex period, with no adjustment for rebates. The RA-related costs for medical claims included inpatient medical claims with a diagnosis of RA in the primary position and nondiagnostic medical claims with RA in any position on an outpatient claim.
The RA-related costs for pharmacy claims included targeted DMARDs and synthetic DMARDs. The targeted DMARD costs included pharmacy claims for the index therapy, as well as for the postindex targeted DMARDs that patients used after switching to another therapy. The cost estimates were inflated to 2015 dollars using the medical care component of the Consumer Price Index and a biologic price modifier from price evolution data.
Statistical Analysis
The study variables were evaluated in 1 of 4 cohorts, which were based on each patient's preindex and index therapies and included TNF inhibitor cyclers, TNF inhibitor-alternative MOA switchers, alternative MOA cyclers, and alternative MOA-TNF inhibitor switchers. The TNF inhibitors included in the study were adalimumab, certolizumab, etanercept, golimumab, or infliximab. The alternative MOA agents were abatacept, anakinra, rituximab, tocilizumab, or tofacitinib.
Multivariable analyses were conducted to examine the predictors of persistence in the 1-year postindex period. The covariates included treatment cohort, demographic characteristics at index, clinical characteristics at baseline, and treatment adherence at baseline as measured by the proportion of days covered for all of the baseline therapies. The total RA-related costs and targeted DMARD costs in the 1-year postindex period were analyzed descriptively, and P values were determined between the cohorts with TNF inhibitor cyclers as the reference cohort; P <.05 was considered statistically significant. Within each cohort, the costs in the 1-year postindex period were divided by the total number of patients who were persistent for 1 year to calculate the cost per persistent patient during 1 year.
Results
Of 334,172 patients with at least 1 targeted DMARD claim, 44,150 patients cycled or switched therapy during the study period, including 10,229 patients who satisfied all the study eligibility criteria and were included in the analysis (Table 1).
Table 1.
Study Sample Attrition
| Parameter | Patients, N (%) |
|---|---|
| ≥1 claims for a targeted DMARDa 1/1/2010-9/30/2014 | 334,172 |
| ≥1 pharmacy or medical claims for a different targeted DMARDa during baseline period | 44,150 |
| 1 targeted DMARDa on index date | 43,928 |
| ≥12 months of continuous enrollment and pharmacy benefits before index date (baseline) | 33,220 |
| ≥12 months continuous enrollment and pharmacy benefits after index date (postindex) | 24,070 |
| Aged ≥18 years at index | 23,364 |
| ≥1 nondiagnostic medical claims with an ICD-9-CM diagnosis code (714.0x) for RAb | 13,050 |
| No claims for other autoimmune conditions for which biologics are usedc | 10,231 |
| Alive at end of 1 year postindex | 10,229 |
| Switching cohorts of interest (baseline to index) | |
| TNF inhibitor cyclers | 6203 (60.6) |
| TNF inhibitor-alternative MOA switchers | 2640 (25.8) |
| Alternative MOA cyclers | 699 (6.8) |
| Alternative MOA-TNF inhibitor switchers | 687 (6.7) |
Abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab, tocilizumab, or tofacitinib.
RA diagnosis in the primary or secondary position during baseline or 30 days postindex.
No inpatient or outpatient nondiagnostic claims in any diagnosis code position during baseline or any follow-up with an ICD-9-CM code for any other autoimmune condition for which biologics are used.
DMARD indicates disease-modifying antirheumatic drug; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; MOA, mechanism of action; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
The study cohorts included 6203 (60.6%) patients who cycled from a TNF inhibitor at baseline to another TNF inhibitor at the index date (TNF inhibitor cyclers), 2640 (25.8%) patients who switched from a TNF inhibitor at baseline to an alternative MOA agent at the index date (TNF inhibitor-alternative MOA switchers), 699 (6.8%) patients who switched from an alternative MOA medication at baseline to another alternative MOA agent at the index date (alternative MOA cyclers), and 687 (6.7%) patients who switched from an alternative MOA drug at baseline to a TNF inhibitor at the index date (alternative MOA-TNF inhibitor switchers).
The mean patient age was 50.3 years, and 82.3% of patients were female. Significant differences between the cohorts were observed for several demographic characteristics at the index date and in clinical characteristics in the baseline period (Table 2). For example, TNF inhibitor cyclers had fewer outpatient visits, lower healthcare costs, and fewer comorbid conditions at baseline than the other cohorts.
Table 2.
Patient Demographics (at Index Date) and Clinical Characteristics (at Baseline)
| Parameter | All patients (N = 10,229) | TNF inhibitor cyclers (N = 6203) | TNF inhibitor-alternative MOA switchers (N = 2640) | P valuea | Alternative MOA cyclers (N = 699) | P valuea | Alternative MOA-TNF inhibitor switchers (N = 687) | P valuea |
|---|---|---|---|---|---|---|---|---|
| Age, yrs, mean ± SD | 50.3 ± 9.4 | 49.7 ± 9.6 | 51.0 ± 9.2 | <.001 | 52.0 ± 8.5 | <.001 | 51.0 ± 8.5 | <.001 |
| Sex, N (%) | ||||||||
| Male | 1808 (17.7) | 1167 (18.8) | 428 (16.2) | .004 | 112 (16.0) | .072 | 101 (14.7) | .008 |
| Female | 8421 (82.3) | 5036 (81.2) | 2212 (83.8) | 587 (84.0) | 586 (85.3) | |||
| Geographic region, N (%) | ||||||||
| Northeast | 1592 (15.6) | 983 (15.8) | 391 (14.8) | .016 | 110 (15.7) | .670 | 108 (15.7) | .170 |
| North Central | 2129 (20.8) | 1274 (20.5) | 569 (21.6) | 150 (21.5) | 136 (19.8) | |||
| South | 4488 (43.9) | 2657 (42.8) | 1197 (45.3) | 310 (44.3) | 324 (47.2) | |||
| West | 1932 (18.9) | 1238 (20.0) | 457 (17.3) | 123 (17.6) | 114 (16.6) | |||
| Unknown | 88 (0.9) | 51 (0.8) | 26 (1.0) | 6 (0.9) | 5 (0.7) | |||
| Residence, N (%) | ||||||||
| Urban | 8515 (83.2) | 5152 (83.1) | 2205 (83.5) | .410 | 586 (83.8) | .810 | 572 (83.3) | .990 |
| Rural | 1630 (15.9) | 1004 (16.2) | 409 (15.5) | 107 (15.3) | 110 (16.0) | |||
| Unknown | 84 (0.8) | 47 (0.8) | 26 (1.0) | 6 (0.9) | 5 (0.7) | |||
| Insurance plan type, N (%) | ||||||||
| Traditional | 379 (3.7) | 201 (3.2) | 118 (4.5) | <.001 | 33 (4.7) | .033 | 27 (3.9) | .370 |
| EPO/PPO | 6369 (62.3) | 3829 (61.7) | 1698 (64.3) | 411 (58.8) | 431 (62.7) | |||
| HMO | 1272 (12.4) | 831 (13.4) | 273 (10.3) | 76 (10.9) | 92 (13.4) | |||
| POS | 867 (8.5) | 532 (8.6) | 202 (7.7) | 74 (10.6) | 59 (8.6) | |||
| POS with capitation | 44 (0.4) | 26 (0.4) | 13 (0.5) | 3 (0.4) | 2 (0.3) | |||
| Other | 872 (8.5) | 528 (8.5) | 238 (9.0) | 64 (9.2) | 42 (6.1) | |||
| Unknown | 426 (4.2) | 256 (4.1) | 98 (3.7) | 38 (5.4) | 34 (4.9) | |||
| Index year, N (%) | ||||||||
| 2010 | 2206 (21.6) | 1492 (24.1) | 413 (15.6) | <.001 | 65 (9.3) | <.001 | 236 (34.4) | <.001 |
| 2011 | 2404 (23.5) | 1511 (24.4) | 526 (19.9) | 220 (31.5) | 147 (21.4) | |||
| 2012 | 1991 (19.5) | 1195 (19.3) | 575 (21.8) | 120 (17.2) | 101 (14.7) | |||
| 2013 | 2080 (20.3) | 1159 (18.7) | 654 (24.8) | 166 (23.7) | 101 (14.7) | |||
| 2014 | 1548 (15.1) | 846 (13.6) | 472 (17.9) | 128 (18.3) | 102 (14.8) | |||
| Length of follow-up, days, mean ± SD | 927.0 ± 439.3 | 943.9 ± 445.3 | 889.1 ± 417.3 | <.001 | 892.7 ± 427.2 | .004 | 954.5 ± 468.2 | .560 |
| Charlson Comorbidity Index score, mean ± SD | 1.4 ± 0.9 | 1.4 ± 0.8 | 1.5 ± 1.0 | <.001 | 1.6 ± 1.0 | <.001 | 1.6 ± 1.0 | <.001 |
| Targeted DMARDs, N (%) | ||||||||
| Abatacept IV | 661 (6.5) | 0 (0.0) | 0 (0.0) | 277 (39.6) | <.001 | 384 (55.9) | <.001 | |
| Abatacept SC | 130 (1.3) | 0 (0.0) | 0 (0.0) | 72 (10.3) | <.001 | 58 (8.4) | <.001 | |
| Adalimumab | 2819 (27.6) | 2051 (33.1) | 768 (29.1) | <.001 | 0 (0.0) | <.001 | 0 (0.0) | <.001 |
| Anakinra | 28 (0.3) | 0 (0.0) | 0 (0.0) | 14 (2.0) | <.001 | 14 (2.0) | <.001 | |
| Certolizumab | 286 (2.8) | 155 (2.5) | 131 (5.0) | <.001 | 0 (0.0) | <.001 | 0 (0.0) | <.001 |
| Etanercept | 4069 (39.8) | 3168 (51.1) | 901 (34.1) | <.001 | 0 (0.0) | <.001 | 0 (0.0) | <.001 |
| Golimumab IV | 1 (0.0) | 0 (0.0) | 1 (0.0) | .30 | 0 (0.0) | 0 (0.0) | ||
| Golimumab SC | 449 (4.4) | 303 (4.9) | 146 (5.5) | .21 | 0 (0.0) | <.001 | 0 (0.0) | <.001 |
| Infliximab | 1220 (11.9) | 526 (8.5) | 693 (26.3) | <.001 | 0 (0.0) | <.001 | 1 (0.1) | <.001 |
| Rituximab | 432 (4.2) | 0 (0.0) | 0 (0.0) | 274 (39.2) | <.001 | 158 (23.0) | <.001 | |
| Tocilizumab IV | 89 (0.9) | 0 (0.0) | 0 (0.0) | 46 (6.6) | <.001 | 43 (6.3) | <.001 | |
| Tocilizumab SC | 7 (0.1) | 0 (0.0) | 0 (0.0) | 4 (0.6) | <.001 | 3 (0.4) | .001 | |
| Tofacitinib | 38 (0.4) | 0 (0.0) | 0 (0.0) | 12 (1.7) | <.001 | 26 (3.8) | <.001 | |
| Targeted DMARD PDC, mean ± SD | 0.53 ± 0.27 | 0.53 ± 0.27 | 0.55 ± 0.27 | .011 | 0.47 ± 0.28 | <.001 | 0.50 ± 0.28 | .006 |
| No. of targeted DMARDs, mean ± SD | 1.03 ± 0.18 | 1.02 ± 0.13 | 1.02 ± 0.16 | .008 | 1.09 ± 0.29 | <.001 | 1.12 ± 0.35 | <.001 |
| Baseline csDMARDs, N (%) | ||||||||
| Hydroxychloroquine | 2367 (23.1) | 1478 (23.8) | 552 (20.9) | .003 | 172 (24.6) | .65 | 165 (24.0) | .91 |
| Leflunomide | 1736 (17.0) | 1004 (16.2) | 472 (17.9) | .051 | 148 (21.2) | <.001 | 112 (16.3) | .94 |
| Methotrexate | 5531 (54.1) | 3554 (57.3) | 1334 (50.5) | <.001 | 306 (43.8) | <.001 | 337 (49.1) | <.001 |
| Sulfasalazine | 806 (7.9) | 488 (7.9) | 201 (7.6) | .68 | 52 (7.4) | .69 | 65 (9.5) | .14 |
| Baseline total healthcare cost, $, mean ± SD | 41,195 ± 28,481 | 39,359 ± 25,939 | 44,673 ± 30,520 | <.001 | 44,739 ± 38,501 | <.001 | 40,802 ± 28,807 | .17 |
| Baseline RA-related cost, $, mean ± SD | 29,703 ± 18,395 | 29,059 ± 17,292 | 32,207 ± 21,045 | <.001 | 28,868 ± 16,682 | .78 | 26,737 ± 17,812 | <.001 |
| Outpatient office visits, mean ± SD | 5.5 ± 3.3 | 5.3 ± 3.1 | 5.8 ± 3.4 | <.001 | 6.4 ± 4.1 | <.001 | 6.4 ± 3.7 | <.001 |
P value compared with TNF inhibitor cyclers.
csDMARDs indicates conventional synthetic disease-modifying antirheumatic drugs; DMARD, disease-modifying antirheumatic drug; EPO, exclusive provider organization; HMO, health maintenance organization; IV, intravenous; MOA, mechanism of action; PDC, proportion of days covered; POS, point of service; PPO, preferred provider organization; RA, rheumatoid arthritis; SC, subcutaneous; SD, standard deviation; TNF, tumor necrosis factor.
Postindex Treatment Patterns
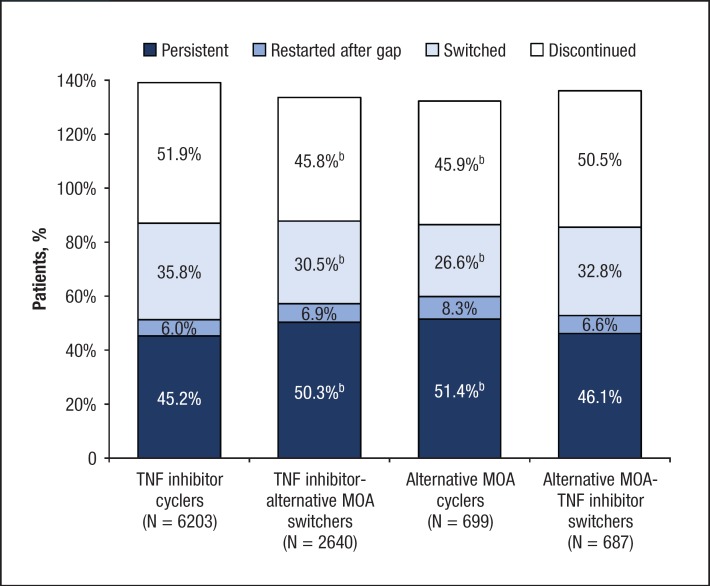
For 1 year after a change in therapy (ie, postindex), 47.0% of patients were persistent with the index therapy, 33.6% switched to another therapy, 6.4% had a treatment gap of 60 days and then restarted the index therapy, and 49.8% discontinued the index therapy without switching or restarting. The 1-year treatment persistence rates by cohort (with P values vs TNF inhibitor cyclers) were 45.2% for TNF inhibitor cyclers, 50.3% for TNF inhibitor-alternative MOA switchers (P <.001), 51.4% for alternative MOA cyclers (P = .002), and 46.1% for alternative MOA-TNF inhibitor switchers (P = .63).
In the 1-year postindex period, TNF inhibitor-alternative MOA switchers and alternative MOA cyclers also were significantly less likely than TNF inhibitor cyclers to switch to another therapy and significantly less likely to discontinue the index therapy (Figure 1).
Figure 1. Treatment Patterns During the 1-Year Postindex Perioda.
aEach column totals >100%, because nonpersistent patients could have >1 treatment pattern.
bP <.05 compared with TNF inhibitor cyclers cohort.
MOA indicates mechanism of action; TNF, tumor necrosis factor.
A multivariable analysis of predictors for treatment persistence included treatment cohort, as well as baseline variables for baseline patient characteristics (Table 3). In this multivariable analysis, compared with TNF inhibitor cyclers, TNF inhibitor-alternative MOA switchers had 28.0% greater odds of treatment persistence (odds ratio [OR], 1.280; 95% confidence interval [CI], 1.165–1.406; P <.001), and alternative MOA cyclers had 43.5% greater odds of treatment persistence (OR, 1.435; 95% CI, 1.220–1.689; P <.001). Alternative MOA-TNF inhibitor switchers did not have significantly different odds of treatment persistence compared with TNF inhibitor cyclers (OR, 1.108; 95% CI, 0.941–1.304; P = .219).
Table 3.
Multivariable Analysis of Predictors for Treatment Persistence
| Variable | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Cohort (ref: TNF inhibitor cyclers) | ||
| TNF inhibitor-alternative MOA switchers | 1.280 (1.165–1.406) | <.001 |
| Alternative MOA cyclers | 1.435 (1.220–1.689) | <.001 |
| Alternative MOA-TNF inhibitor switchers | 1.108 (0.941–1.304) | .219 |
| Age-group, yrs (ref: 55–64 yrs) | ||
| 18–34 | 0.880 (0.749–1.033) | .118 |
| 35–44 | 0.832 (0.741–0.934) | .002 |
| 45–54 | 0.897 (0.819–0.984) | .021 |
| Male (ref: female) | 1.090 (0.982–1.209) | .105 |
| Plan type (ref: EPO/PPO) | ||
| Comprehensive/indemnity | 1.003 (0.809–1.242) | .982 |
| HMO | 0.886 (0.782–1.004) | .058 |
| POS | 1.027 (0.889–1.187) | .717 |
| POS with capitation | 0.957 (0.524–1.747) | .886 |
| Other | 1.075 (0.931–1.242) | .323 |
| Missing/unknown | 0.969 (0.788–1.192) | .766 |
| Index year (ref: 2011) | ||
| 2010 | 1.007 (0.894–1.134) | .912 |
| 2012 | 0.936 (0.829–1.056) | .283 |
| 2013 | 0.758 (0.672–0.855) | <.001 |
| 2014 | 0.747 (0.655–0.852) | <.001 |
| Region (ref: South) | ||
| Northeast | 1.095 (0.972–1.233) | .137 |
| North Central | 1.201 (1.080–1.336) | .001 |
| West | 1.162 (1.041–1.297) | .008 |
| Unknown | 1.030 (0.669–1.584) | .894 |
| Rural (ref: urban) | 0.998 (0.896–1.112) | .973 |
| Charlson Comorbidity Index | 0.942 (0.898–0.988) | .014 |
| Any hydroxychloroquine in baseline | 1.002 (0.912–1.101) | .965 |
| Any leflunomide in baseline | 0.900 (0.808–1.002) | .054 |
| Any methotrexate in baseline | 1.145 (1.055–1.241) | .001 |
| Any sulfasalazine in baseline | 1.176 (1.015–1.362) | .031 |
| >1 targeted DMARDs preindexa | 0.863 (0.674–1.105) | .244 |
| Baseline total healthcare cost | 0.999 (0.997–1.001) | .146 |
| Baseline RA-related cost | 1.002 (0.998–1.006) | .348 |
| Baseline number of RA-related office visits | 0.997 (0.985–1.009) | .648 |
| Baseline PDC for all therapies | 1.951 (1.581–2.409) | <.001 |
Using a variable preindex period; other baseline measures used 12-month baseline preindex.
DMARDs indicates disease-modifying antirheumatic drugs; EPO, exclusive provider organization; HMO, health maintenance organization; MOA, mechanism of action; PDC, proportion of days covered; POS, point of service; PPO, preferred provider organization; RA, rheumatoid arthritis; ref, reference; TNF, tumor necrosis factor.
Other statistically significant predictors of better treatment persistence in the model were North Central region or West region (compared with South), methotrexate or sulfasalazine use at baseline, and treatment adherence for all targeted DMARDs at baseline (calculated as the proportion of days covered; Table 3). The significant predictors of worse treatment persistence were ages 35 to 44 years or 45 to 54 years (compared with ages 55 to 64 years), index dates in 2013 or 2014 (compared with 2011), and a higher Charlson Comorbidity Index score at baseline.
Postindex Healthcare Costs
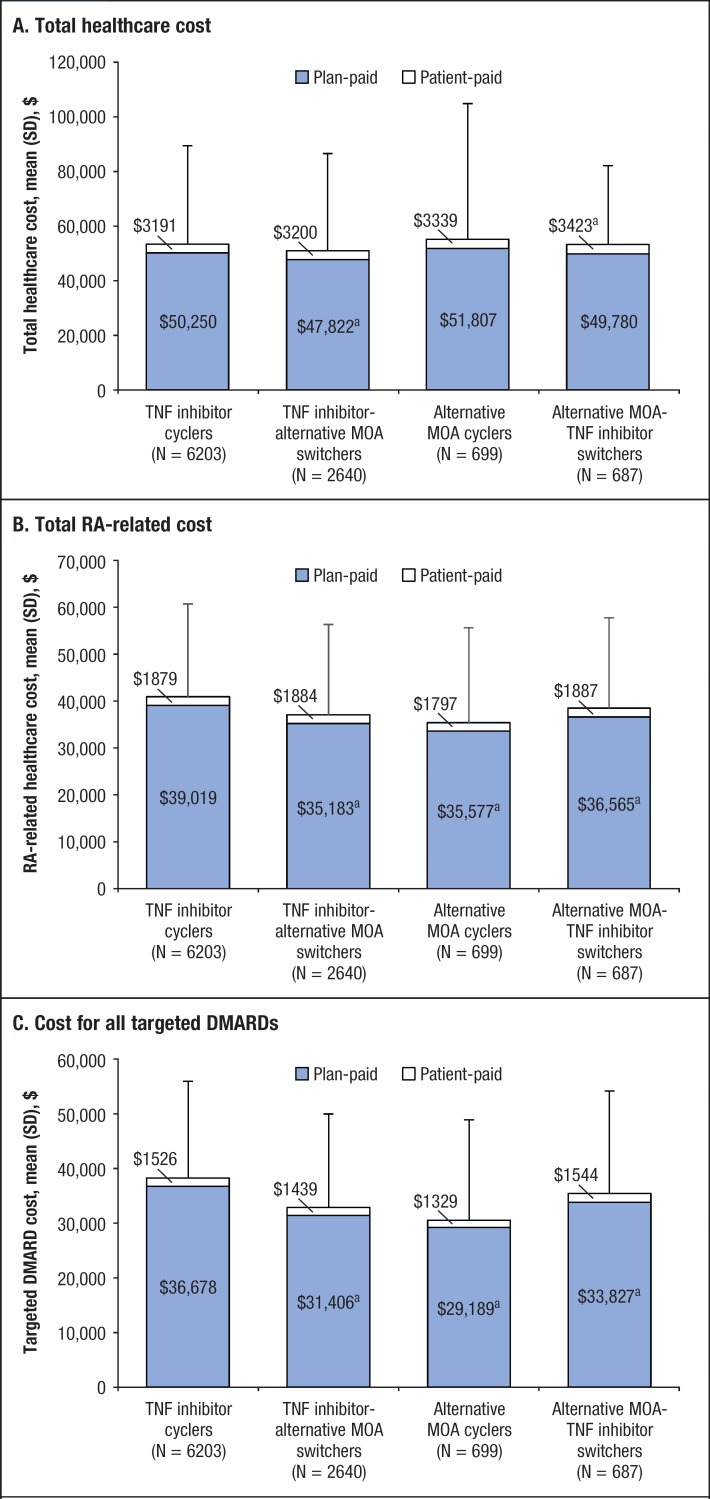
In the 12 months postindex, the plan-paid total healthcare costs were significantly higher for TNF inhibitor cyclers than for TNF inhibitor-alternative MOA switchers (mean, $50,250 vs $47,822; P = .003). The patient-paid total healthcare costs were significantly lower for TNF inhibitor cyclers than for alternative MOA-TNF inhibitor switchers (mean, $3191 vs $3423; P = .047). Other comparisons between TNF inhibitor cyclers and the other cohorts for total healthcare costs (plan-paid or patient-paid) were not significantly different (Figure 2A).
Figure 2. 1-Year Plan-Paid and Patient-Paid Healthcare Costs After a Change in Therapy.
aP <.05 compared with TNF inhibitor cyclers.
DMARD indicates disease-modifying antirheumatic drug; MOA, mechanism of action; RA, rheumatoid arthritis; SD, standard deviation; TNF, tumor necrosis factor.
The plan-paid RA-related healthcare costs for TNF inhibitor cyclers (mean, $39,019) were significantly higher than those for TNF inhibitor-alternative MOA switchers (mean, $35,183; P <.001), alternative MOA cyclers (mean, $33,577; P <.001), and alternative MOA-TNF inhibitor switchers (mean, $36,565; P = .002). The patient-paid RA-related healthcare costs were not significantly different between TNF inhibitor cyclers and the other cohorts (Figure 2B).
The plan-paid targeted DMARD costs, including those for any TNF inhibitor or alternative MOA used in the 12 months postindex, for TNF inhibitor cyclers (mean, $36,678) were significantly higher than those for TNF inhibitor-alternative MOA switchers (mean, $31,406; P <.001), alternative MOA cyclers (mean, $29,189; P <.001), and alternative MOA-TNF inhibitor switchers (mean, $33,827; P <.001). The patient-paid targeted DMARD costs were not significantly different between TNF inhibitor cyclers and the other cohorts (Figure 2C).
When plan-paid and patient-paid amounts were summed (Table 4), the total RA-related costs and targeted DMARD costs were significantly higher for TNF inhibitor cyclers compared with the other cohorts. Compared with TNF inhibitor cyclers, the total RA-related costs per persistent patient were $16,853 lower among TNF inhibitor-alternative MOA switchers (P <.001), $21,662 lower among alternative MOA cyclers (P <.001), and $7206 lower among alternative MOA-TNF inhibitor switchers (P = .002); targeted DMARD costs per persistent patient were $19,280 (P <.001), $25,153 (P <.001), and $7919 (P <.001) lower, respectively (Table 4).
Table 4.
Cost Per 1-Year Treatment-Persistent Patient After a Change in Therapy
| Parameter | TNF inhibitor cyclers (N = 6203) | TNF inhibitor-alternative MOA switchers (N = 2640) | Alternative MOA cyclers (N = 699) | Alternative MOA-TNF inhibitor switchers (N = 687) |
|---|---|---|---|---|
| Treatment-persistent patients, N (%) | 2802 (45.2) | 1328 (50.3) P <.001a | 359 (51.4) P = .002a | 317 (46.1) P = .63a |
| Total RA-related cost | ||||
| Mean (SD), $ | 40,898 (19,789) | 37,066 (19,239) P <.001a | 35,375 (20,237) P <.001a | 38,452 (19,294) P = .002a |
| Cost per persistent patient, $ | 90,539 | 73,686 | 68,877 | 83,333 |
| Difference from TNF inhibitor to TNF inhibitor, $ | –16,853 | –21,662 | –7206 | |
| All targeted DMARDs cost | ||||
| Mean (SD), $ | 38,204 (17,718) | 32,845 (17,100) P <.001a | 30,518 (18,354) P <.001a | 35,371 (18,800) P <.001a |
| Cost per persistent patient, $ | 84,574 | 65,294 | 59,421 | 76,655 |
| Difference from TNF inhibitor to TNF inhibitor, $ | –19,280 | –25,153 | –7919 |
P value versus TNF inhibitor to TNF inhibitor.
DMARDs indicates disease-modifying antirheumatic drugs; MOA, mechanism of action; RA, rheumatoid arthritis; SD, standard deviation; TNF, tumor necrosis factor.
Discussion
This study examined the treatment patterns and healthcare costs from a large, nationwide database of medical and pharmacy claims for more than 10,000 patients with RA who cycled therapy within, or switched between, TNF inhibitors and targeted DMARDs with an alternative MOA. Other alternative MOA agents, such as sarilumab, became available after 2014 and thus were not included in the study. More than 85% of patients with a change in therapy on the index date switched from a TNF inhibitor at baseline, cycling to another TNF inhibitor (60.6%) or switching to an alternative MOA agent (25.8%) at the index date.
These findings were consistent with previous reports that TNF inhibitor cycling historically predominated over alternative MOA medication switching in patients with RA.8–19 Treatment recommendations that were released before or during the study period (from 2010–2014)3–6 supported this approach, because they recommend a TNF inhibitor over an alternative MOA agent as the initial targeted DMARD therapy in patients with RA. Notably, all the TNF inhibitors in this study were approved before the study period, but some of the alternative MOA medications became available during the study period (ie, tocilizumab in January 2010 and tofacitinib in November 2012).
Updated treatment guidelines for RA place alternative MOA drugs and TNF inhibitors at the same level for initial therapy.1,2 This study also included patients who received an alternative MOA medication at baseline, but only 1 of 8 patients cycled from an alternative MOA agent to another alternative MOA agent (6.7%) or switched from an alternative MOA agent to a TNF inhibitor (6.8%) on the index date.
It is possible that the use of TNF inhibitors will continue to predominate as first-line targeted DMARD therapy in clinical practice, as a result of a combination of clinical preference, a longer history of experience with the TNF inhibitor class, and formulary and payer restrictions. As newer real-world data become available, it remains to be seen whether physicians will change their clinical practice and payers will change their formularies to align with the recent updates to treatment guidelines.
Regardless of the reasons for the observed treatment choices, this study shows that TNF inhibitor-alternative MOA switching was associated with significantly better treatment persistence and significantly lower healthcare costs than TNF inhibitor cycling. Alternative MOA cycling was associated with significantly better treatment persistence and significantly lower RA-related healthcare costs and targeted DMARD costs than TNF inhibitor cycling.
The 1-year treatment persistence rates were more than 50% in each cohort that switched to an alternative MOA agent at index, and less than 50% in cohorts that switched to a TNF inhibitor at index. Compared with TNF inhibitor cyclers, patients who switched from a TNF inhibitor to an alternative MOA therapy had 28.0% greater odds of treatment persistence, and patients who cycled between 2 alternative MOA medications had 43.5% greater odds of treatment persistence in multivariable analyses. Switching from an alternative MOA agent to a TNF inhibitor was not associated with significantly better treatment persistence than TNF inhibitor cycling.
The claims database did not include treatment outcomes, but previous studies reported that treatment persistence is associated with improvements in RA disease activity and disability.26–28 Treatment persistence with targeted DMARDs is also a key component of a validated algorithm that can be used to estimate treatment effectiveness in RA from claims data.16,29–31 Other studies in patients with various conditions have shown that greater patient satisfaction with therapy is linked to better treatment compliance and persistence.32
An important consideration is whether the total healthcare cost, including the drug and nondrug costs, associated with treatment persistence is higher or lower than that for nonpersistence. In this study, the RA-related healthcare costs for TNF inhibitor cyclers were significantly higher than the costs for TNF inhibitor-alternative MOA switchers and for alternative MOA cyclers. The cost of a targeted DMARD therapy should be higher in a persistent patient than in a nonpersistent patient, simply because of the increased number of insurance claims for a persistent patient.
However, this study also accounted for the cost of postindex therapies among patients whose nonpersistence with the index therapy resulted from switching again to another therapy after the index date. Using this approach, the cost of a targeted DMARD use was significantly higher for TNF inhibitor cyclers than for TNF inhibitor-alternative MOA switchers and alternative MOA cyclers.
These results demonstrate the importance of including postindex treatments in cost calculations for nonpersistent patients. Our analysis also examined plan-paid and patient-paid costs separately, showing that increased plan-paid costs were responsible for significant increases in targeted DMARD- and RA-related healthcare costs, with no significant impact on the out-of-pocket costs for patients.
Combining treatment persistence rates with healthcare costs showed that compared with TNF inhibitor cyclers the RA-related cost per persistent patient was substantially lower among TNF inhibitor-alternative MOA switchers and alternative MOA cyclers. The RA-related cost per persistent patient also was lower for alternative MOA-TNF inhibitor switchers versus TNF inhibitor cyclers, but the observed difference was smaller than in the other 2 cohorts. In each cohort, the cost of targeted DMARD use per patient was the primary driver of the observed differences.
Many studies have examined the cost and cost-effectiveness of targeted DMARD therapy in RA; a full review of those studies was beyond the scope of this study. A few previous studies that examined the cost of treatment persistence with targeted DMARD therapy in patients with RA showed that nondrug costs were significantly lower among patients who were persistent with targeted therapy than for nonpersistent patients.33–35 Switching targeted DMARD therapy is associated with higher treatment costs than not switching.9,35
Other studies have compared costs associated with TNF inhibitor cycling or TNF inhibitor-alternative MOA switching and have reported inconsistent results; 2 analyses reported that TNF inhibitor cyclers had higher treatment costs36 or higher RA-related costs,17 and 2 other analyses reported that higher TNF inhibitor-alternative MOA switchers had higher RA-related costs14 or total healthcare costs.9
In our study, the drug costs and RA-related healthcare costs significantly favored TNF inhibitor-alternative MOA switching or alternative MOA cycling over TNF inhibitor cycling, and the total healthcare costs significantly favored TNF inhibitor-alternative MOA switching over TNF inhibitor cycling. Even if the cost for TNF inhibitor-alternative MOA switchers had been 10% higher than for TNF inhibitor cyclers in this study, the cost per persistent patient still would have favored TNF inhibitor-alternative MOA switchers because of the significantly higher treatment persistence rate in this group.
This retrospective, claims database analysis could not control for the baseline differences among patients that may have influenced physicians to switch a patient to a TNF inhibitor versus an alternative MOA agent. Multivariable analyses were used to adjust for these differences, and significant differences favoring TNF inhibitor-alternative MOA switchers and alternative MOA cyclers versus the TNF inhibitor cyclers were maintained in multivariable analyses. Other significant predictors of better treatment persistence were geographic region, methotrexate or sulfasalazine use at baseline, and treatment adherence before the patient switched to the index drug.
Significant predictors of worse treatment persistence were younger patient age, year of index date (later vs earlier), and greater comorbidity at baseline. A prospective analysis could control for these characteristics to provide greater confidence that observed differences in treatment persistence among treatment cohorts resulted from treatment choices rather than other patient characteristics. Notably, higher postindex costs after TNF inhibitor cycling in the current analysis were not attributable to the selection of patients for this treatment strategy who required costlier treatment, because TNF inhibitor cyclers had significantly lower baseline healthcare costs, fewer comorbid conditions at baseline, and fewer outpatient visits at baseline compared with other cohorts.
Limitations
Claims databases do not contain reasons for changes in treatment. Thus, it was not possible in this study to determine if patients stopped treatment for efficacy, safety, formulary requirements, or other reasons: when they switched from baseline treatment to index treatment; or when they were nonpersistent on index treatment during follow-up. Some patients who were nonpersistent might have achieved remission and stopped taking their targeted DMARD or might have increased the dosing interval after remission. In these patients, nonpersistence would reflect a desirable outcome rather than an undesirable outcome.
The study was designed to compare patients who cycled therapy within, or switched between, 1 of the 2 broad categories of TNF inhibitors or alternative MOAs. Individual therapies within each class may differ in their efficacy and safety profiles, particularly for alternative MOAs, which included a T-cell co-stimulation modulator, a Janus kinase inhibitor, and an interleukin-6 inhibitor during the study period. Different MOAs for therapies within the alternative MOA drug class may explain why treatment persistence was significantly better among alternative MOA cyclers than TNF inhibitor cyclers in this analysis; switching among alternative MOA agents represents switching more than cycling. Individual therapies within each drug class may be associated with different costs per persistent patient, but this study was neither designed nor large enough for meaningful comparisons across individual agents.
In addition, rituximab was not included in the alternative MOA cohorts for this analysis because treatment persistence could not be determined accurately as a result of variable dosing schedules for rituximab. Previous studies consistently reported that switching to rituximab was more effective than TNF inhibitor cycling.18–24 If rituximab had been included in the TNF inhibitor-alternative MOA switching and alternative MOA cycling cohorts in this study, it probably would not have changed the major findings with respect to treatment persistence.
The costs were based on plan-paid and patient-paid amounts in adjudicated claims, which did not include rebates. Copay assistance was not reflected in the patient-paid amounts. Thus, the actual patient burden may be significantly less than that reported in a claims data set.
Finally, an analysis of more recent claims data that includes all medications that were available during the study period might provide different results regarding treatment patterns and costs.
Conclusions
In this study of patients with RA who changed targeted DMARD therapy, switching to an alternative MOA agent (from a TNF inhibitor or another alternative MOA therapy) was associated with significantly higher treatment persistence rates and significantly lower costs for total healthcare, RA-related healthcare, and targeted DMARDs than with TNF inhibitor cycling. As a result, patients with RA who switched to an alternative MOA agent (from another alternative MOA drug or a TNF inhibitor) had a substantially lower cost per persistent patient than patients who cycled between TNF inhibitors. These findings support drug formulary designs that allow patients to switch to an alternative MOA therapy instead of a TNF inhibitor when initial treatment with a targeted DMARD fails.
Acknowledgments
Jonathan Latham, of PharmaScribe, LLC, assisted with the preparation of the manuscript, and Prime Global, a medical communications group, provided editorial assistance.
Funding Source
This study was funded by Sanofi and Regeneron Pharmaceuticals.
Author Disclosure Statement
Dr Bonafede is an employee of Truven Health Analytics; Ms McMorrow is a consultant to Truven Health Analytics; Dr Proudfoot was an employee of and is a shareholder of Sanofi, and is currently an employee of ViiV/GSK; Ms Shinde was an employee of Sanofi; Dr Kuznik is an employee of Regeneron Pharmaceuticals; Ms Chen is an employee of and has stock options in Regeneron.
Contributor Information
Machaon M. K. Bonafede, Senior Director of Outcomes Research, Truven Health Analytics, an IBM Company, Cambridge, MA.
Donna McMorrow, Lead Analyst, Truven Health Analytics, an IBM Company.
Clare Proudfoot, Evidence Lead, Sarilumab, Sanofi, Bridgewater, NJ.
Shraddha Shinde, Research Analyst, Health Economics and Outcomes Research, Sanofi.
Andreas Kuznik, Senior Director, Health Economics and Outcomes Research, Regeneron Pharmaceuticals, Tarrytown, NY.
Chieh-I Chen, Director, Health Economics and Outcomes Research, Regeneron Pharmaceuticals.
References
- 1. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. [DOI] [PubMed] [Google Scholar]
- 3. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. [DOI] [PubMed] [Google Scholar]
- 4. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. Erratum in: Ann Rheum Dis. 2011;70:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39:1559–1582. [DOI] [PubMed] [Google Scholar]
- 8. Bonafede M, Fox KM, Watson C, et al. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29:664–674. [DOI] [PubMed] [Google Scholar]
- 9. Harnett J, Wiederkehr D, Gerber R, et al. Real-world evaluation of TNF-inhibitor utilization in rheumatoid arthritis. J Med Econ. 2016;19:91–102. [DOI] [PubMed] [Google Scholar]
- 10. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718–724. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds A, Koenig AS, Bananis E, Singh A. When is switching warranted among biologic therapies in rheumatoid arthritis? Expert Rev Pharmacoecon Outcomes Res. 2012;12:319–333. [DOI] [PubMed] [Google Scholar]
- 12. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford). 2014;53:1664–1668. Erratum in: Rheumatology (Oxford). 2015;54:1337. [DOI] [PubMed] [Google Scholar]
- 13. Bergman MJ, Elkin EP, Ogale S, et al. Response to biologic disease-modifying anti-rheumatic drugs after discontinuation of anti-tumor necrosis factor alpha agents for rheumatoid arthritis. Rheumatol Ther. 2014;1:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baser O, Ganguli A, Roy S, et al. Impact of switching from an initial tumor necrosis factor inhibitor on health care resource utilization and costs among patients with rheumatoid arthritis. Clin Ther. 2015;37:1454–1465. [DOI] [PubMed] [Google Scholar]
- 15. Rotar Z, Hočevar A, Rebolj Kodre A, et al; for the Slovenian Rheumatologists. Retention of the second-line biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis failing one tumor necrosis factor alpha inhibitor: data from the BioRx.si registry. Clin Rheumatol. 2015;34:1787–1793. [DOI] [PubMed] [Google Scholar]
- 16. Bonafede MM, Curtis JR, McMorrow D, et al. Treatment effectiveness and treatment patterns among rheumatoid arthritis patients after switching from a tumor necrosis factor inhibitor to another medication. Clinicoecon Outcomes Res. 2016;8:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chastek B, Becker LK, Chen CI, et al. Outcomes of tumor necrosis factor inhibitor cycling versus switching to a disease-modifying anti-rheumatic drug with a new mechanism of action among patients with rheumatoid arthritis. J Med Econ. 2017;20:464–473. [DOI] [PubMed] [Google Scholar]
- 18. Finckh A, Ciurea A, Brulhart L, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010;69:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatzidionysiou K, van Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190–195. [DOI] [PubMed] [Google Scholar]
- 20. Soliman MM, Hyrich KL, Lunt M, et al; for the British Society for Rheumatology Biologics Register. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res (Hoboken). 2012;64:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrold LR, Reed GW, Magner R, et al. Comparative effectiveness and safety of rituximab versus subsequent anti-tumor necrosis factor therapy in patients with rheumatoid arthritis with prior exposure to anti-tumor necrosis factor therapies in the United States Corrona registry. Arthritis Res Ther. 2015;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kekow J, Mueller-Ladner U, Schulze-Koops H. Rituximab is more effective than second anti-TNF therapy in rheumatoid arthritis patients and previous TNFα blocker failure. Biologics. 2012;6:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez-Reino JJ, Maneiro JR, Ruiz J, et al; for the MIRAR Study Group. Comparative effectiveness of switching to alternative tumour necrosis factor (TNF) antagonists versus switching to rituximab in patients with rheumatoid arthritis who failed previous TNF antagonists: the MIRAR Study. Ann Rheum Dis. 2012;71:1861–1864. [DOI] [PubMed] [Google Scholar]
- 24. Malottki K, Barton P, Tsourapas A, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gottenberg JE, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316:1172–1180. [DOI] [PubMed] [Google Scholar]
- 26. Contreras-Yáñez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: how persistence with therapy matters. Arthritis Res Ther. 2015;17:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pascual-Ramos V, Contreras-Yáñez I, Villa AR, et al. Medication persistence over 2 years of follow-up in a cohort of early rheumatoid arthritis patients: associated factors and relationship with disease activity and with disability. Arthritis Res Ther. 2009;11:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther. 2015;17:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curtis JR, Chastek B, Becker L, et al. Further evaluation of a claims-based algorithm to determine the effectiveness of biologics for rheumatoid arthritis using commercial claims data. Arthritis Res Ther. 2013;15:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonafede M, Johnson BH, Princic N, et al. Cost per patient-year in response using a claims-based algorithm for the 2 years following biologic initiation in patients with rheumatoid arthritis. J Med Econ. 2015;18:376–389. [DOI] [PubMed] [Google Scholar]
- 32. Barbosa CD, Balp MM, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang B, Rahman M, Waters HC, Callegari P. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther. 2008;30:1375–1384. [DOI] [PubMed] [Google Scholar]
- 34. Dalén J, Svedbom A, Black CM, et al. Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int. 2016;36:987–995. [DOI] [PubMed] [Google Scholar]
- 35. Degli Esposti L, Favalli EG, Sangiorgi D, et al. Persistence, switch rates, drug consumption and costs of biological treatment of rheumatoid arthritis: an observational study in Italy. Clinicoecon Outcomes Res. 2017;9:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manders SH, Kievit W, Adang E, et al. Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial. Arthritis Res Ther. 2015;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]