Abstract
Background
Smoking remains the single largest preventable cause of death and disease. Smoking-cessation medications provide patients a multitude of benefits and can prevent certain diseases, including some cancers. Because of the limited amount of studies on smoking-cessation medications, we wanted to find general trends about the use of these medications.
Objective
To examine trends in the utilization, pharmacy reimbursement, and prices of smoking-cessation medications and nicotine replacement therapy in the US Medicaid-covered population.
Methods
Using national summary files for outpatient drug utilization and expenditure, we extracted data on smoking-cessation medications from the Centers for Medicare & Medicaid Services in the 25 years from January 1991 through June 2015. We conducted a retrospective drug utilization study to examine the annual (or quarterly) trends of the number of prescriptions, reimbursement expenditures, and the prices of smoking-cessation medications. The study drugs included varenicline (Chantix), bupropion (Zyban), and nicotine. We calculated per-prescription pharmacy reimbursement, which was used as a proxy for drug price, as the total quarterly expenditure for the drug, divided by the total number of prescriptions. All expenditures were inflated to 2015 US dollars using the medical services component of the Consumer Price Index.
Results
The total number of prescriptions for smoking-cessation medications increased rapidly from 46,396 in 1991 to 942,562 in 2014, an increase of more than 1931%. During the same period, the total pharmacy reimbursement for smoking-cessation medications in Medicaid increased by 3562%, from approximately $2.8 million in 1991 to approximately $101 million in 2014. The use of the nonnicotine prescription drugs varenicline and bupropion also increased rapidly, with a high cost expenditure. The price per nonnicotine prescription drug increased over time, ranging from approximately $169 for bupropion to approximately $251 for varenicline in 2015.
Conclusions
The substantial increase in nonnicotine prescription drugs and nicotine replacement therapy between 2007 and 2015 may be attributed to smoking-cessation participants nationwide. Cost-containment policies might have inadvertently prevented Medicaid-covered smokers from obtaining appropriate pharmacotherapy.
Keywords: drug utilization, Medicaid, nicotine replacement therapy, nonnicotine prescription, reimbursement, smoking, smoking-cessation medication
Smoking remains the single largest preventable cause of death and disease, and is responsible for 1 in 5 deaths in the United States each year.1 In 2015, more than 1.1 billion people smoked tobacco worldwide, with a much higher prevalence among men than in women.2 Nearly 171,000 of the approximate 589,400 cancer-related deaths in the United States result from tobacco smoking.3 People who smoke are at an increased risk for various types of cancer.4 Cigarette smoking is also a major risk factor for heart disease, cardiovascular disease, and other serious diseases.5
In addition, smoking imposes a huge economic burden on society. Smoking-related illness in the United States costs more than $300 billion annually, including nearly $170 billion in direct medical care costs for adults and $156 billion in lost productivity.6,7 Smoking-cessation agents can bring a multitude of benefits by lowering a smoker's risk for various cancers, heart disease, and stroke, as well as helping to prevent heart disease and lung cancer in people who are exposed to secondhand smoke.8 A study by Nash and colleagues shows that former smokers had a substantially lower mortality risk after age 70 years compared with current smokers, even among patients who quit tobacco use in their 60s. Therefore, all smokers should be encouraged to stop smoking, regardless of their age.9
KEY POINTS
-
▸
Smoking is the largest preventable cause of disease and death in the United States, but the use of smoking-cessation aids can help smokers successfully quit.
-
▸
This retrospective study examined the quarterly trends of prescribing, utilization, pharmaceutical reimbursement, and smoking-cessation medication cost.
-
▸
The total number of prescriptions for smoking-cessation medications increased more than 1931% from 46,396 in 1991 to 942,562 in 2014.
-
▸
In the same period, the reimbursement for smoking-cessation medications increased 3562%, from approximately $2.8 million in 1991 to approximately $101 million in 2014.
-
▸
The price per nonnicotine prescription increased over time, averaging approximately $169 for Zyban to approximately $251 for Chantix in 2015.
-
▸
The surge in nonnicotine prescription and nicotine replacement therapies from 2007 to 2015 might be attributed to smoking-cessation participants.
-
▸
Cost-containment policies might have unintentionally barred Medicaid-covered smokers from receiving appropriate pharmacotherapy.
The US Food and Drug Administration (FDA) has approved a variety of smoking-cessation aids, including prescription drugs and over-the-counter (OTC) products, such as skin patches, lozenges, and gum. Varenicline (Chantix) and bupropion (Zyban) are smoking-cessation medications that do not contain nicotine. Varenicline is a partial agonist of α4β2 nicotinic acetylcholine receptors that acts in the brain at the sites affected by nicotine. Varenicline provides some nicotinelike effects to lessen the symptoms of withdrawal, and it prevents the effects of nicotine if cigarette users start smoking again after they stop smoking.8 Bupropion is an antidepressant that also helps patients abstain from smoking; however, the precise means by which it accomplishes this is unknown.10
Nicotine replacement agents are one type of smoking-cessation aid. Designed to wean the body off of cigarettes, these nicotine replacements supply smokers with controlled amounts of nicotine, while sparing them from the other chemicals found in tobacco products. Nicotine replacement agents include prescription medicines and OTC products, including nicotine skin patches (eg, Habitrol, Nicoderm), lozenges (eg, Commit), and gum (eg, Nicorette). A prescription-only nicotine replacement medication is Nicotrol, which is available as a nasal spray and as an oral inhaler.8
Some of the most often observed adverse effects associated with the use of varenicline and bupropion include nausea, vomiting, and trouble sleeping.11–15 In addition to warnings about the risks for serious psychiatric problems, the prescribing information for bupropion cites other adverse effects and risks related to this medication, including seizures, hypertension, and allergic reactions.13 In July 2009, the FDA required the product information for varenicline and bupropion to carry new safety information for patients in a boxed warning on their labeling that cites serious neuropsychiatric effects for patients.14,15 In 2016, the FDA determined that the risk for serious neuropsychiatric effects with varenicline and bupropion is lower than previously suspected based on an FDA review of a large clinical trial; thus, the boxed warnings for these 2 drugs have been removed.16 Moreover, this review of the clinical trial results confirmed that varenicline, bupropion, and nicotine replacement medications were all more effective that previously thought in helping people quit smoking.16
To our knowledge, no studies have examined the utilization, expenditures, market-share competition, and patterns of smoking-cessation products among the Medicaid population.17 To fill this gap, the objective of this study was to assess trends in utilization, reimbursement, and the average per-prescription cost of smoking-cessation products and nicotine replacement therapy for the US Medicaid population in the 25 years from 1991 through 2015.
Methods
We conducted a descriptive, retrospective data analysis for the period between the first quarter of 1991 and the second quarter of 2015 using the publicly available National Summary Files from the Medicaid State Drug Utilization Data database maintained by the Centers for Medicare & Medicaid Services (CMS). The database includes Medicaid beneficiaries from 49 states (ie, all US states except Arizona) and the District of Columbia, and is restricted to pharmaceuticals prescribed on an outpatient basis. The CMS data were collected for individual National Drug Code (NDC) drug forms. The National Summary Files were compiled by aggregating data across state databases.
The database was subject to coding errors in 2006 (all 4 quarters) and in quarter 3 of 2007. During those 5 quarters, the number of prescriptions and reimbursements for some individual drugs, including smoking-cessation medications, were incorrectly reported. Therefore, we estimated those values by averaging the previous and subsequent quarters. In this way, we created the reimbursement and utilization estimates to have better face validity. The general results of this study were not affected by this small amount of “data cleaning.”
Each data record in the database included the NDC, drug name (brand or generic), year and quarter of Medicaid expenditure, number of prescriptions, number of units (eg, individual capsules or tablets), and the total pharmacy reimbursement amount, including the costs of the medication and its dispensing fee (ie, administrative fee). The first 5 digits of the NDC number identify the drug manufacturer, and the remaining digits identify the specific drug by strength, dose formulation, and packaging. We searched the database for all smoking-cessation medications by using the brand and generic names (Table 1).
Table 1.
Prescription and OTC Smoking-Cessation Medications Included in the Study
| Smoking-cessation medication typea | Generic name | Brand name | Dosage form | FDA approval date | Extended expiration dateb |
|---|---|---|---|---|---|
| Nonnicotine products | Varenicline | Chantix | Oral | 5/10/2006 | 5/20/2020 |
| Bupropion | Zyban | Oral | 5/14/1997 | Expired | |
| Wellbutrin | Oral | 12/30/1985 | Expired | ||
| Forfivo XL | Extended-release oral | 11/10/2011 | 6/25/2027 | ||
| OTC nicotine replacement | Nicotine | Habitrol | Transdermal patch | 11/27/1991 | Expired |
| Nicoderm | Transdermal patch | 11/7/1991 | 12/15/2019 | ||
| Nicorette | Chewing gum | 1/13/1984 | Expired | ||
| Commit | Oral lozenge | 10/31/2002 | Expired | ||
| Prescription-only nicotine replacement | Nicotrol | Nasal spray | 3/22/1996 | Expired | |
| Prostep | Transdermal patch | 1/28/1992 | Withdrawn |
All data are from www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
All data are from www.accessdata.fda.gov/scripts/cder/ob/index.cfm.
FDA indicates US Food and Drug Administration; OTC, over-the-counter.
For each of the medications in Table 1, the quarterly prescription count and pharmacy reimbursement amounts were calculated by summarizing the data across individual NDCs for individual drugs and then for each class of drugs, respectively. The data for all the generic versions of each drug were aggregated. Market shares for smoking-cessation medications were calculated based on the percentage of the number of prescriptions and the percentage of total Medicaid payments.
Bupropion is a treatment for depression and an aid for quitting smoking, of which Zyban is one brand name. Other brand names for bupropion are Wellbutrin and Forfivo XL, but these are approved for the treatment of major depressive disorder and not for smoking cessation. Thus, we excluded Wellbutrin and Forfivo XL in this study, although some physicians prescribe them off label to patients as smoking-cessation medications.
Quarterly per-prescription pharmacy reimbursements used as a proxy for drug price were computed for all brand-name and generic smoking-cessation medications. Pharmacy reimbursements include the drug ingredient cost and dispensing fee, but they exclude manufacturer rebates (ie, federally mandated rebates and state supplemental rebates have not been subtracted).
The price of medications was calculated as the total pharmacy reimbursements divided by the total number of prescriptions. All expenditures were inflated to 2015 US dollars using the Consumer Price Index medical services component. All data analyses were conducted using Statistical Analysis Software version 9.4 (SAS Institute Inc; Cary, NC). Excel 2013 (Microsoft; Redmond, WA) was used to further develop the data.
Results
The total number of Medicaid-reimbursed prescriptions for smoking-cessation medications increased rapidly from 46,396 in 1991 to 942,562 in 2014, a more than 1931% increase (Table 2). During the same period, the total Medicaid reimbursement for these medications increased by 3562%, from approximately $2.8 million in 1991 to approximately $101.0 million in 2014.
Table 2.
Annual Medicaid Expenditures and Prescriptions for Smoking-Cessation Medications, 1991–2015
| Market share of nonnicotine products and nicotine replacement medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Utilization and expenditure for total | Nonnicotine medication prescriptions, % | Nicotine replacement products prescriptions, % | Nonnicotine medication spending, % | Nicotine replacement products spending, % | Average price claim for smoking cessation | |||
| Prescriptions, N | Expenditure,a $ | Nonnicotine medications,a $ | Nicotine replacement products,a $ | Combined,a $ | |||||
| 1991 | 46,396 | 2,758,822 | 0.0 | 100.0 | 0.0 | 100.0 | 59.46 | 59.46 | |
| 1992 | 212,440 | 22,343,916 | 0.0 | 100.0 | 0.0 | 100.0 | 105.18 | 105.18 | |
| 1993 | 158,292 | 16,216,629 | 0.0 | 100.0 | 0.0 | 100.0 | 102.45 | 102.45 | |
| 1994 | 120,036 | 12,575,937 | 0.0 | 100.0 | 0.0 | 100.0 | 104.77 | 104.77 | |
| 1995 | 101,511 | 10,675,179 | 0.0 | 100.0 | 0.0 | 100.0 | 105.16 | 105.16 | |
| 1996 | 91,148 | 10,130,444 | 0.0 | 100.0 | 0.0 | 100.0 | 111.14 | 111.14 | |
| 1997 | 76,459 | 8,698,608 | 16.8 | 83.2 | 15.2 | 84.8 | 102.63 | 116.02 | 113.77 |
| 1998 | 137,376 | 14,556,134 | 47.6 | 52.4 | 47.3 | 52.7 | 105.48 | 106.39 | 105.96 |
| 1999 | 158,447 | 15,818,461 | 29.7 | 70.3 | 32.8 | 67.2 | 110.28 | 95.42 | 99.83 |
| 2000 | 210,830 | 21,668,737 | 24.7 | 75.3 | 27.5 | 72.5 | 114.45 | 98.94 | 102.78 |
| 2001 | 279,201 | 28,122,815 | 20.3 | 79.7 | 23.2 | 76.8 | 115.16 | 97.06 | 100.73 |
| 2002 | 364,542 | 37,246,437 | 14.5 | 85.5 | 17.2 | 82.8 | 121.03 | 98.97 | 102.17 |
| 2003 | 343,221 | 38,854,841 | 12.6 | 87.4 | 13.3 | 86.7 | 119.05 | 112.36 | 113.21 |
| 2004 | 315,409 | 35,240,744 | 8.3 | 91.7 | 8.8 | 91.2 | 119.66 | 111.02 | 111.73 |
| 2005 | 341,323 | 36,641,967 | 2.7 | 97.3 | 2.2 | 97.8 | 89.31 | 107.85 | 107.35 |
| 2006 | 262,713 | 26,946,527 | 13.7 | 86.3 | 15.0 | 85.0 | 112.27 | 101.03 | 102.57 |
| 2007 | 532,584 | 56,503,817 | 61.6 | 38.4 | 66.2 | 33.8 | 114.08 | 93.28 | 106.09 |
| 2008 | 546,792 | 59,814,467 | 62.5 | 37.5 | 67.9 | 32.1 | 118.87 | 93.61 | 109.39 |
| 2009 | 521,985 | 54,881,076 | 49.5 | 50.5 | 60.9 | 39.1 | 129.32 | 81.42 | 105.14 |
| 2010 | 522,095 | 52,669,511 | 47.3 | 52.7 | 64.2 | 35.8 | 136.80 | 68.61 | 100.88 |
| 2011 | 851,363 | 86,092,415 | 40.6 | 59.4 | 61.4 | 38.6 | 152.92 | 65.70 | 101.12 |
| 2012 | 877,596 | 87,370,367 | 36.5 | 63.5 | 62.3 | 37.7 | 170.03 | 59.10 | 99.56 |
| 2013 | 790,668 | 79,455,151 | 33.7 | 66.3 | 64.8 | 35.2 | 193.44 | 53.31 | 100.49 |
| 2014 | 942,562 | 101,036,840 | 34.4 | 65.6 | 69.5 | 30.5 | 216.61 | 49.78 | 107.19 |
| 2015b | 552,297 | 62,613,536 | 31.3 | 68.7 | 69.3 | 30.7 | 250.95 | 50.70 | 113.37 |
All monetary values were converted to 2015 US dollars using price indices.
Data in 2015 are for quarters 1 and 2 only.
Table 2 also shows utilization and payment market shares for the 2 medications. Nicotine replacement medications had 100% of the Medicaid market share for prescriptions from 1991 to 1996. Their share fell to 37.5% by 2008 and was back to 68.7% by 2015. Since nonnicotine smoking-cessation drugs came into the market in 1997, the expenditure of smoking-cessation medications has continually increased—from 15.2% in 1997 to 69.3% in 2015. Once Chantix became available on the market in 2006, the dollar market share for smoking-cessation drugs suddenly exceeded the dollar market share of nicotine replacement products and remained higher through 2015.
In the first half of 2015, Medicaid programs combined spent more than $62.6 million on smoking-cessation medications overall; 69.3% of that spending was used for nonnicotine smoking-cessation medications, which constituted only 31.3% of the total number of smoking-cessation medications. The average price of nonnicotine medications ($250.95) was almost 5 times the average price of nicotine replacement products ($50.70; Table 2).
The last 3 columns of Table 2 show the annual pharmacy price per prescription for nonnicotine products, nicotine replacement agents, and the 2 medication classes combined. The average pharmacy reimbursement per prescription for nonnicotine products cost the Medicaid program $102.63 in 1997 and rose to $250.95 in 2015. In addition, the average pharmacy reimbursement per prescription for nicotine replacement products rose from $59.46 in 1991 to $116.02 in 1997, and then fell to $50.70 in 2015.
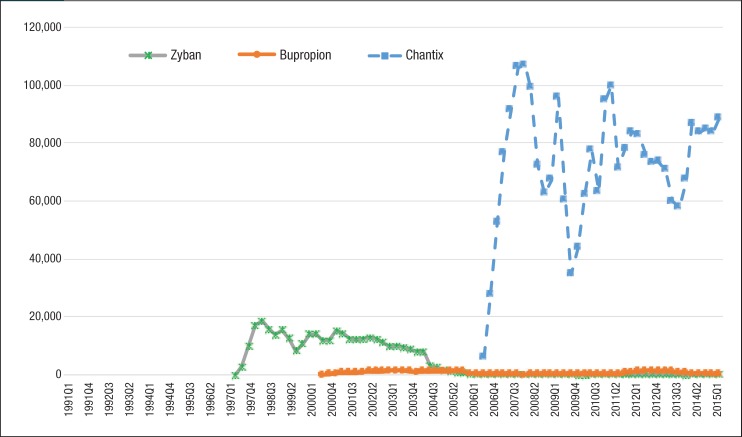
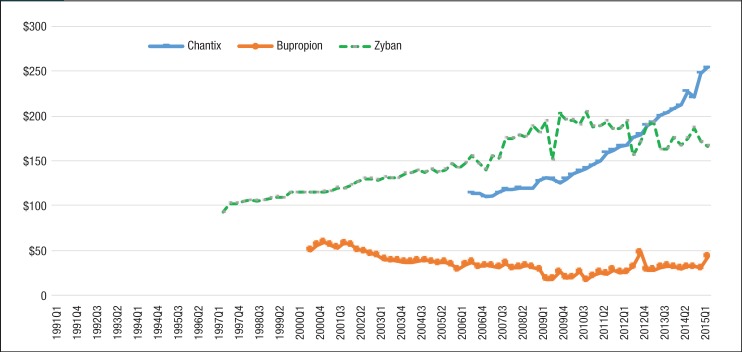
Figure 1 (page 277) and Figure 2 depict the quarterly trends of utilization for nonnicotine products and nicotine replacement medications by generic and brand manufacturers. The most striking trend in Figure 1 is the rising utilization of Chantix among Medicaid beneficiaries. The number of prescriptions for Chantix increased 13,277% from 6215 prescriptions in the third quarter of 2006 to 88,735 in the second quarter of 2015, whereas the number of prescriptions decreased for Zyban and generic bupropion in the same period.
Figure 1. Quarterly Utilization of Nonnicotine Medications for Patients with Medicaid Coverage, 1991 to Mid-2015.
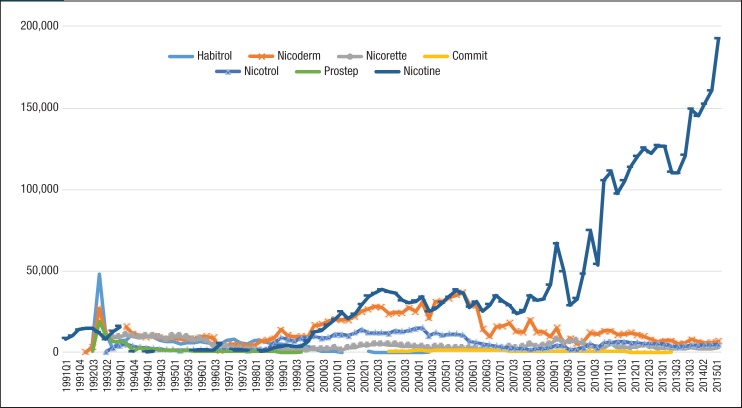
Figure 2. Quarterly Utilization of Nicotine Replacement Products for Patients with Medicaid Coverage, 1991 to Mid-2015.
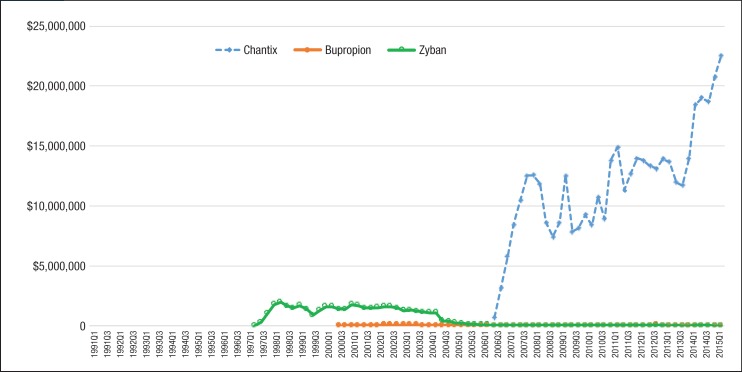
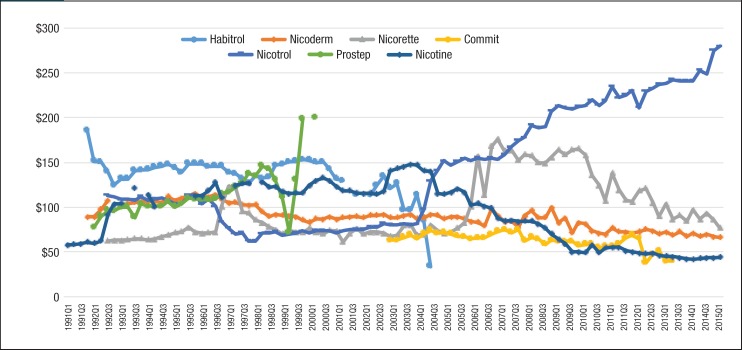
As seen in Figure 2, generic nicotine clearly dominated the nicotine replacement market. The utilization of generic nicotine products skyrocketed during the last 5 years of the study. Figure 3 and Figure 4 show the quarterly trends of Medicaid reimbursement of individual nonnicotine products and nicotine replacement products.
Figure 3. Quarterly Pharmacy Reimbursement for Nonnicotine Medications for Patients with Medicaid Coverage, 1991 to Mid-2015.
NOTE: All reimbursement amounts above were inflated to 2015 US dollars using the medical services component of the Consumer Price Index.
Figure 4. Quarterly Pharmacy Reimbursement for Nicotine Replacement Products for Patients with Medicaid Coverage, 1991 to Mid-2015.
NOTE: All reimbursement amounts were inflated to 2015 US dollars using the medical services component of the Consumer Price Index.
Similar to the utilization trend, the Medicaid spending trend for smoking-cessation medications was dominated by Chantix and Nicotrol. There were 5 brand-name drugs with patents that expired during the study period (ie, Zyban, Nicotrol, Commit, Nicorette, and Habitrol). Because all state Medicaid programs either require or strongly encourage patients to use generic medications, there was an abrupt decrease in the utilization of brand-name medications, such as Zyban, Habitrol, Commit, and Nicotrol. The utilization and reimbursement of Zyban decreased as generic versions of drugs entered the market. (Because Habitrol, Nicotrol, and Commit had low utilization, it was difficult to display in Figure 2 and Figure 4.)
Finally, Prostep was ultimately withdrawn from the market because of severe adverse reactions; therefore, the utilization of Prostep was very limited during the time it was available.
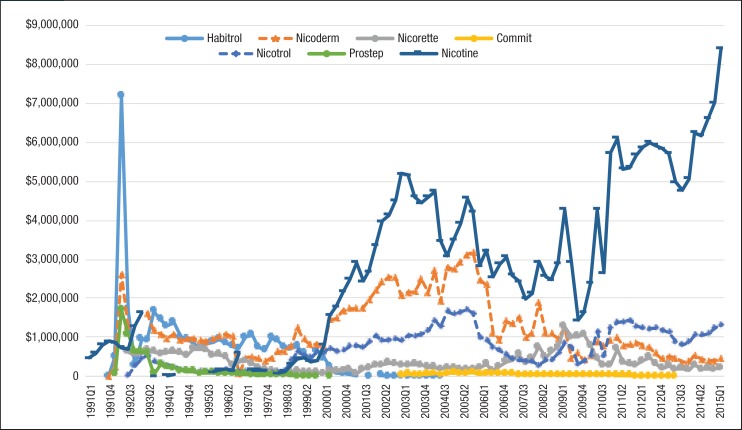
Table 3 shows the estimated drug prices of smoking-cessation medications, which were calculated by dividing Medicaid reimbursement dollars by the number of prescriptions in 2015. Trends in the average reimbursement per prescription for individual drugs are shown in Figure 5 and Figure 6. Each of the smoking-cessation medications has a unique price trend. Although the price of Zyban slowly increased from 1997 to 2015, the price of Chantix climbed abruptly from $113.98 in quarter 3 of 2006 to $254.50 in quarter 2 of 2015.
Table 3.
Average per-Prescription Prices for Smoking-Cessation Medications in 2015
| Smoking-cessation product type | Generic name | Brand name | Average brand-name price, $ | Average generic price, $ |
|---|---|---|---|---|
| Nonnicotine medications | Varenicline | Chantix | 251.31 | NA |
| Bupropion | Zyban | 168.88 | 36.56 | |
| OTC nicotine replacement products | Nicotine | Habitrol | NA | 43.80 |
| Nicoderm | 66.59 | |||
| Nicorette | 81.13 | |||
| Commit | NA | |||
| Prescription-only nicotine replacement products | Nicotrol | 277.45 | ||
| Prostep | NA |
NA indicates not available; OTC, over-the-counter.
Figure 5. Average Reimbursement per Prescription for Nonnicotine Medications for Patients with Medicaid Coverage, 1991 to Mid-2015.
NOTE: All prices were inflated to 2015 US dollars using the medical services component of the Consumer Price Index.
Figure 6. Average Reimbursement per Prescription for Nicotine Replacement Products for Patients with Medicaid Coverage, 1991 to Mid-2015.
NOTE: All prices were inflated to 2015 US dollars using the medical services component of the Consumer Price Index.
Meanwhile, the price of bupropion slowly decreased from 1991 to 2010. Nicotine replacement products had large price changes as well. The price of Nicotrol continued to climb from $113.29 in quarter 3 of 1992 to $279.96 in quarter 2 of 2015. In addition, the price of Habitrol and Commit decreased, because generic manufacturers entered the market after the patent expiration date. As a result of the high price of brand-name nonnicotine medications per prescription, the total spending on nonnicotine agents in Medicaid was much more than nicotine replacement products.
Discussion
During the 25-year study period, a substantial rise in Medicaid expenditures on smoking-cessation medications was observed. The total number of Medicaid-reimbursed prescriptions for smoking-cessation medications increased rapidly from 46,396 in 1991 to 942,562 in 2014, which is a more than 1931% increase. The rise in the number of prescriptions for these medications was much higher than the increase in the number of beneficiaries who enrolled in Medicaid over this same period. The average annual enrollment in Medicaid in 1990 was 22.9 million and increased to 68.9 million in 2015 (after the worst economic recession, between December 2007 and June 2009, in a quarter century), which is a 201% increase in the number of beneficiaries.
The trends seen in this study were similar to the trends seen in Medicaid spending, utilization, and prices for other drug classes studied previously, such as quinolones,18 antiasthma,19 antimigraine triptans,20 antidepressants,21 and antihypertensives.22 According to our analysis, generic nicotine was the most frequently prescribed smoking-cessation medication for Medicaid beneficiaries. Varenicline also was often prescribed as a smoking-cessation medication for Medicaid beneficiaries; however, varenicline was not as popular as generic nicotine, because it was 6 times more expensive than generic nicotine.
Since Chantix came into the market in 2006, the dollar market share for nonnicotine drugs suddenly exceeded the dollar market share of nicotine replacement products and remained higher through 2015. Medicaid programs cannot take advantage of generic versions of these medications before the patent of the FDA-approved medication expires. Until then, Medicaid's expenditures on varenicline are expected to continue to increase for some years. The patent for Chantix will expire in May 2020.23 Generic varenicline was tentatively approved by the FDA in December 2016, and we expect the reimbursement of Chantix to decrease after a generic version enters the market.
We found that once the patent protection expires on a brand-name drug, generic versions of the drug quickly become available. The market competition often leads to substantially lower prices, as was observed for Habitrol in the second quarter of 2004 and for Commit in the second quarter of 2013. By contrast, there was no impact on the expenditure for Medicaid for some brand-name drugs in the market after their patent expiration dates.
For example, Medicaid's expenditures of Zyban fell from $1.3 million to $3700 over the period from the first quarter of 2001 to the second quarter of 2015, but the average price per prescription of these medications rose over this period, because brand-name drug manufacturers raised drug prices to maximize revenue. In addition, some physicians and patients believe that the quality of brand-name drugs is better than that of generic drugs, so they keep using brand-name medications.
Limitations
The present study findings are limited by the available data extracted from the CMS national Medicaid pharmacy file. The OTC nicotine replacement therapy was available since 1996 and was covered by Medicaid since 201421,22; however, state Medicaid programs did not cover the same nicotine replacement therapy products in all states. For example, the nasal spray, the inhaler, and the lozenge forms of nicotine replacement therapy are not all covered in Alaska, Arkansas, District of Columbia, Nebraska, South Dakota, and Texas.22
Although coverage for Zyban is available in each of the 50 states, it is not available in the District of Columbia.22 We were unable to access specific percentage and utilization data on OTC or uncovered nicotine replacement therapy medications in different states. We observed some significant increases of nicotine replacement therapy utilization and expenditures after 2008, which may be a result of Medicaid expansion under the Affordable Care Act in some states23; however, we are unable to measure the specific impact of Medicaid expansion.
In addition, patient-specific information was not available in the national Medicaid database; hence, this study is a descriptive analysis, and other important patient-level information was not adjusted in a multivariate analysis. We cannot determine demographic characteristics and comorbidities, especially respiratory comorbidities, that may be correlated with the use of the different smoking-cessation aids. It was also not possible to determine the prescribed indication for medication use or whether the prescription was filled.
Furthermore, the provider level can influence the prescribing pattern of drugs. For example, certain providers may be more likely to prescribe one drug over another, which is a confounding factor that can result in a study bias. And no information is available about whether the utilization of smoking-cessation products among Medicaid beneficiaries has been appropriate, excessive, or insufficient. Thus, adherence to medication therapy could not be assessed.
Other misclassification biases may be present if the CMS data contained reporting errors. For 2006 (all quarters) and quarter 3 of 2007, some of the expenditure and utilization values had coding errors. To ensure data reliability, a few of the values in this study were imputed. Moreover, these data were from before the start of the Medicaid Drug Rebate Program. Therefore, they may overestimate the actual acquisition cost of US Medicaid programs.
Finally, the results of this study are specific to the Medicaid population, which is heavily comprised of low-income people, especially women and children. Thus, these data do not necessarily represent trends of utilization and expenditure in other populations that are covered by private health insurance or Medicare. Private health insurance companies may have covered more brand-name medications, which requires more investigation in future research. Also, sex and age distributions of Medicaid beneficiaries differ from those of the general US population.
Conclusion
Overall, by analysis of the Medicaid database, Medicaid expenditures and the number of prescriptions for smoking-cessation medications abruptly increased over the study period between 1991 and 2015. Our findings show that the market growth for varenicline far exceeded any other smoking-cessation products over the study years. The substantial increase in nonnicotine prescription and nicotine replacement therapy from 2007 to 2015 may be attributed to smoking-cessation participants nationwide. Cost-containment policies may have inadvertently prevented Medicaid smokers from obtaining the appropriate pharmacotherapy.
Author Disclosure Statement
Ms Yue and Dr Wigle have no conflicts of interest to report. Dr Guo has done research sponsored by Novartis, AstraZeneca, Bristol-Myers Squibb, Janssen Ortho-McNeil, Roche-Genentech, and Eli Lilly, none of which involved smoking-cessation medication.
Contributor Information
Xiaomeng Yue, PhD Student, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center, OH.
Jeff Jianfei Guo, Professor of Pharmacoeconomics & Pharmacoepidemiology, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center, OH.
Patricia R. Wigle, Professor, Pharmacy Practice, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center, OH.
References
- 1. Centers for Disease Control and Prevention. Smoking & tobacco use: tobacco-related mortality. www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm. Accessed July 6, 2018.
- 2. World Health Organization. Global Health Observatory data: prevalence of tobacco smoking. www.who.int/gho/tobacco/use/en/. Accessed July 6, 2018.
- 3. American Cancer Society. Cancer Facts & Figures 2015. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Accessed July 1, 2018.
- 4. US Department of Health & Human Services. How Tobacco Smoke Causes Disease: the Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: 2010. [PubMed]
- 5. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. [DOI] [PubMed] [Google Scholar]
- 6. Xu X, Bishop EE, Kennedy SM, et al. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Department of Health & Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: 2014.
- 8. US Food and Drug Administration. FDA 101: smoking cessation products. 2015. www.fda.gov/forconsumers/consumerupdates/ucm198176.htm. Accessed July 10, 2018.
- 9. Nash SH, Liao LM, Harris TB, Freedman ND. Cigarette smoking and mortality in adults aged 70 years and older: results from the NIH-AARP cohort. Am J Prev Med. 2017;52:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiffman S, Johnston JA, Khayrallah M, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl). 2000;148:33–40. [DOI] [PubMed] [Google Scholar]
- 11. Monárrez-Espino J, Galanti MR, Hansson J, et al. Treatment with bupropion and varenicline for smoking cessation and the risk of acute cardiovascular events and injuries: a Swedish case-crossover study. Nicotine Tob Res. 2018;20:606–613. [DOI] [PubMed] [Google Scholar]
- 12. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zyban (bupropion hydrochloride) sustained-release tablets [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; May 2017. [Google Scholar]
- 14. Lorenz RA, Whitley HP, McCoy EK. Safety of varenicline in patients with mental illness. Prim Psychiatry. 2010;17:60–66. [Google Scholar]
- 15. Chantix (varenicline) tablets [prescribing information]. New York, NY: Pfizer; June 2018. [Google Scholar]
- 16. US Food and Drug Administration. FDA revises description of mental health side effects of the stop smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. www.fda.gov/downloads/Drugs/DrugSafety/UCM532262.pdf. Accessed July 23, 2018.
- 17. Kahende J, Malarcher A, England L, et al. Utilization of smoking cessation medication benefits among Medicaid fee-for-service enrollees 1999–2008. PLoS One. 2017;12:e0170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almalki ZS, Yue X, Xia Y, et al. Utilization, spending, and price trends for quinolones in the US Medicaid programs: 25 years' experience 1991–2015. Pharmacoecon Open. 2017;1:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu SF, Kelton CML, Guo JJ, et al. Utilization, spending, and price trends for short- and long-acting beta-agonists and inhaled corticosteroids in the Medicaid program, 1991–2010. Am Health Drug Benefits. 2011;4(3):140–149. [PMC free article] [PubMed] [Google Scholar]
- 20. Xia Y, Kelton CML, Wigle PR, et al. Twenty years of triptans in the United States Medicaid programs: utilization and reimbursement trends from 1993 to 2013. Cephalalgia. 2016;36:1305–1315. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Kelton CML, Jing Y, et al. Utilization, price, and spending trends for antidepressants in the US Medicaid program. Res Social Adm Pharm. 2008;4:244–257. [DOI] [PubMed] [Google Scholar]
- 22. Bian B, Kelton CML, Guo JJ, Wigle PR. ACE inhibitor and ARB utilization and expenditures in the Medicaid fee-for-service program from 1991 to 2008. J Manag Care Pharm. 2010;16:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US Food and Drug Administration. Orange Book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/patent_info.cfm?Product_No=001&Appl_No=021928&Appl_type=N. Accessed July 23, 2018.
- 24. Amodei N, Lamb RJ. Over–the-counter nicotine replacment therapy: can its impact on smoking cessation be enhanced? Psychol Addict Behav. 2008;22:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henry J. Kaiser Family Foundation. State Health Facts: state Medicaid program coverage of tobacco dependence treatments by type of coverage. Timeframe: 2014. www.kff.org/medicaid/state-indicator/cessation-treatment-under-medicaid/. Accessed February 10, 2018.
- 26. DiGiulio A, Haddix M, Jump Z, et al. State Medicaid expansion tobacco cessation coverage and number of adult smokers enrolled in expansion coverage—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1364–1369. [DOI] [PubMed] [Google Scholar]