Abstract
Background
Diabetes is associated with substantial clinical and economic burdens on patients and on the US healthcare system. Treatment options for patients with type 1 or type 2 diabetes have increased significantly, from only 3 drug classes in 1995 to more than 12 distinct classes today. Although several of the newer treatments are reported to have improved efficacy and safety profiles, they are often substantially more costly than older medications. Consequently, as drug options increase, the cost of diabetes management continues to grow.
Objectives
To estimate the annual real-world costs of type 1 and 2 diabetes, as well as diabetes prevalence, treatment patterns, care quality, and resource utilization during 8 years.
Methods
In this cross-sectional study, we examined 8 annual cohorts of patients with type 1 or type 2 diabetes, on a biennial basis, using claims data from the HealthCore Integrated Research Database between 2006 and 2014. Patients were matched with controls by age, sex, residency, and health plan type. We assessed the prevalence of diabetes, treatment patterns, care quality measures, and all-cause and diabetes-related healthcare costs using 2 methods. Method 1 calculated the annual costs as the difference in all-cause costs between patients with diabetes and matched controls. Method 2 calculated the costs for healthcare encounters based on specific codes for a diabetes diagnosis or for antidiabetes medications.
Results
Between 346,486 and 410,234 patients with type 2 diabetes and between 21,176 and 26,228 patients with type 1 diabetes were included in each study year cohort. Between 2007 and 2014, the prevalence of type 2 diabetes increased from 4.9% to 6.3%. The costs associated with using Method 1 were almost double the cost estimates in Method 2 during most of the study period. For patients with type 1 diabetes, the associated costs were twice greater with Method 1 than with Method 2. Projections to the entire US population in 2014 indicated a total of 19.3 million individuals with diabetes and associated direct costs of $314.8 billion that year.
Conclusion
Cost estimates can guide the prioritization of healthcare expenditures. The results of this study showed that costs attributable to diabetes differed by approximately 2-fold, depending on the estimation method. The management of the escalating expenses for diabetes management in the United States requires judicious selection of the methods for estimating costs.
Keywords: cost-estimation method, cost projection, diabetes, disease prevalence, healthcare utilization, prevalence, type 1 diabetes, type 2 diabetes
KEY POINTS
-
▸
The cost of diabetes continues to increase, reflecting growing disease prevalence and cost of treatment.
-
▸
This study was based on real-world claims data for patients with type 1 or type 2 diabetes in the United States, using 2 cost-estimation methods.
-
▸
Method 1 estimated the difference in all-cause costs between patients and matched controls.
-
▸
The largest cost drivers in Method 1 were hospitalizations, outpatient services, and medications.
-
▸
Method 2 costs were restricted to healthcare encounters based on diagnostic and pharmacy codes.
-
▸
Costs in Method 2 were mostly driven by increased medications and outpatient services.
-
▸
Cost estimates based on Method 1 were almost double those from Method 2 during most years.
Diabetes mellitus imposes a substantial clinical and economic burden on the US healthcare system.1 National estimates reported in 2017 indicate that 9.4% of the US population, or 30.3 million Americans, have diabetes; however, only 23.1 million have actually been diagnosed with the disease, which means that 7.2 million people (or 23.8% of those with diabetes) were undiagnosed.2
In 2017, the annual expenditure on diabetes care in the United States was approximately $327 billion, consisting of $237 billion in direct medical costs and $90 billion in indirect costs.1–4 The mean annual cost for individuals diagnosed with diabetes was approximately $16,750, with approximately $9600 attributable to direct diabetes costs.4 The average medical expenditure for individuals diagnosed with diabetes was estimated to be 2.3 times greater than for people without diabetes.2–4 A recent study by Rowley and colleagues estimated that between 2015 and 2030, the prevalence of diabetes would increase by 54%, and the annual total costs of the disease would rise by 53% to more than $622 billion.5
Diabetes-related cost drivers include the high-cost management of common comorbidities, which can affect patients' daily functioning and quality of life, and may increase mortality risk.6 The majority of adults with diabetes have a comorbid condition,7 and approximately 40% of them have at least 3 comorbidities.6,8,9 Comorbidities profoundly affect diabetes care, particularly self-care (eg, the self-injection of medications, self-monitoring of blood glucose, and lifestyle management, such as diet and exercise), which is essential for successful treatment outcomes.6 Patients may also have a range of microvascular and macrovascular complications.10
Glucose-lowering drug classes increased from 3 (ie, insulin, metformin, and sulfonylureas) in 1995 to more than 12 today, as well as appropriate combination therapies, and many of the newer agents have potential clinical benefits, such as a reduced risk for hypoglycemia or a potential for weight loss.11 In addition, newer insulin formulations with improved pharmacokinetic-pharmacodynamic profiles, such as rapid-acting insulin analogs, lower blood glucose and have more favorable side-effect profiles than nonanalog insulins.12,13
The prices of newer drugs may substantially add to the cost of diabetes treatments.1,14–16 An accurate estimation of diabetes-related costs is essential to the management of often-limited financial resources to guide policy decisions and optimize limited healthcare resources.
To our knowledge, only 1 study, by Tunceli and colleagues, evaluated and compared the annual healthcare costs of patients with type 1 or type 2 diabetes using the cost-estimation approaches of diabetes-attributable cost and the all-cause cost for matched cohorts of patients with and without diabetes.17 That study, which used administrative claims data in a commercially insured population for 1 year (ie, 2006), showed that a diabetes-attributable cost-estimation method substantially underestimated costs relative to the all-cause case-control approach for patients with type 1 or 2 diabetes.17
The primary objective of our study was to estimate the annual costs of managing type 1 and type 2 diabetes using the 2 different methodologies described in the study by Tunceli and colleagues.17 An important goal of our study was to add a substantial temporal component to the method, by analyzing costs during an extended period (ie, 8 years) to gain insights into diabetes-related costs in a commercially insured population over time. We also applied a projection methodology18 to calculate nationally representative estimates of the prevalence and cost of care associated with diabetes,19 and examined the attainment of healthcare quality metrics based on Healthcare Effectiveness Data and Information Set (HEDIS) criteria20 over time.
Methods
In this retrospective, cross-sectional study, we selected and independently analyzed 8 annual cohorts on a biennial basis between January 1, 2006, and December 31, 2014, using administrative claims data from the HealthCore Integrated Research Database. This database is a nationally representative managed care repository of longitudinal administrative claims information for commercial private health plan members and Medicare Advantage enrollees from 14 regional Anthem health plans. The database incorporates a range of health plan types, including preferred provider organizations and health maintenance organizations.
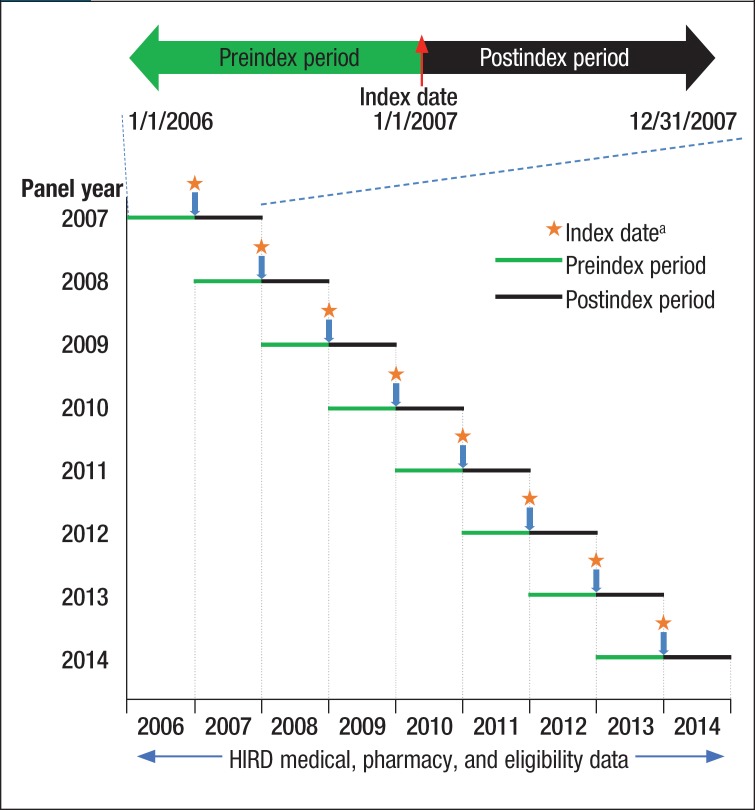
Data from the US Census American Community Survey were used to perform national diabetes cost projections.19 The first day of the second calendar year (ie, January 1, 2007) was defined as the index date, and the 12-month periods before and after the index date were designated as the preindex and postindex periods, respectively (Figure 1).
Figure 1. Study Design.
aIndex date is the first day in the year of the analysis. For the 2007 panel, the index date will be 1/1/2007 for patients and for controls.
HIRD indicates HealthCore Integrated Research Database.
Inclusion in each biennial cohort required continuous health plan enrollment with medical and pharmacy benefits during the 2-year period encompassing the 12 months before and 12 months after the index date. Each cohort was based on 2 consecutive calendar years between 2007 and 2014. For example, in the 2006–2007 panel, the presence of diabetes was confirmed in the first (ie, preindex) calendar year, 2006, and healthcare utilization and treatment patterns were identified in the subsequent (ie, postindex) year, 2007. For the control cohorts, the absence of diabetes was confirmed for the pre- and postindex years.
We used a limited data set that was defined by the Health Insurance Portability and Accountability Act Privacy Rule, because our observational study did not require direct patient identifiers, and patient privacy was safeguarded throughout.
Patients with diabetes were identified during the preindex period based on ≥2 medical claims for diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 250.xx) or 1 pharmacy claim for an antidiabetes medication (Generic Product Identifier [GPI] code 27x, excluding 2730x, namely medications for hypoglycemia).
Patients who were included in the type 1 diabetes cohort had to have ≥2 medical claims for type 1 diabetes (ICD-9-CM code 250.x1 and 250.x3); ≥1 pharmacy claim for insulin (GPI code 2710) or ≥1 medical claims for an insulin pump (Current Procedural Terminology code E0784, J1817, ICD-9-CM code V53.91); and not have a claim for a noninsulin antidiabetes medication, except metformin, during the preindex period.
Patients who were not identified as having type 1 diabetes were classified as having type 2 diabetes if they had ≥2 medical claims for type 2 diabetes (ICD-9-CM code 250.x0 and 250.x2), or ≥1 medical claims for type 2 diabetes (ICD-9-CM code 250.x0 and 250.x2) and ≥1 pharmacy claims for an antidiabetes medication (GPI code 27x) during the preindex period.
Patients with diabetes who did not satisfy the diagnosis criteria for type 1 or type 2 diabetes were excluded from the analysis, as were patients with gestational diabetes (ICD-9-CM code 648.8x). The excluded patients were reconsidered for inclusion in the subsequent year. The control cohorts consisted of individuals without a diagnosis of diabetes who had no claim for antidiabetes medications during the entire study period. The controls had ≥1 medical or pharmacy claims, which verified their active health plan enrollment, and their medical or pharmacy costs were unrelated to diabetes. No age restriction was applied.
Patients with type 1 or type 2 diabetes were directly matched to controls in a 1:1 ratio according to age, sex, health plan type, and state of residence, using the exact attribute-matching technique. Matching was performed for each data panel independently, and for type 1 and type 2 diabetes separately.
A weighting projection framework18 was used to project the national prevalence and the total cost of care for diabetes. The estimates were first weighted by age, sex, and geographic region of residence by comparing the study population with the US population projected in the US Census American Community Survey.19 The weighted estimates were projected to the national population level by multiplying the inverse proportion of patients included in our study by the US population.
We examined the all-cause and diabetes-attributable healthcare resource utilization and costs during the postindex period for each data panel. The utilization categories were hospitalizations, emergency department visits, outpatient encounters, skilled nursing facility visits, and pharmacy prescriptions. The medical utilization categories were selected to represent the full spectrum of available services provided and represented all medical services received by patients under their health plan benefits. Total costs included plan-paid and patient-paid amounts. All costs were adjusted to 2014 US dollars based on the Consumer Price Index of the US Bureau of Labor Statistics.21
We estimated the diabetes-attributable costs using 2 different methodologies. Method 1 compared the difference in all-cause costs between patients with diabetes and matched controls, and the difference was considered to be the diabetes-associated costs. Method 2 estimated the diabetes-attributable costs by directly counting the claims data associated with a diagnosis of diabetes or the use of an antidiabetes medication.
The healthcare quality for patients with diabetes was assessed if claims for at least 1 HEDIS measure were available during the postindex period of each data panel; we reported the quality of care as the proportion of patients receiving at least 1 hemoglobin (Hb) A1c, low-density lipoprotein (LDL) cholesterol, or nephropathy test, or a diabetic retinal examination.
Statistical Analysis
Descriptive statistics, including means for continuous data and frequencies for categorical data, were calculated. The values were descriptively compared for the patients with diabetes and the matched controls without diabetes, as well as for cost-estimation methods 1 and 2. All analyses were performed using SAS version 9.4 statistical software (SAS Institute, Inc; Cary, NC).
Because of the descriptive nature of the study objectives and the large sample size, which overinflates statistical significance, no statistical testing was performed.
Results
As shown in Table 1, more than 7.05 million eligible members were identified in the initial 2007 cohort. The total sample size increased to 7.45 million in 2009, and then gradually decreased to 5.89 million in 2014, because of a decrease in members with complete data who were eligible for this study.
Table 1.
Prevalence and Demographics of Patients with Diabetes in the United States, 2007–2014
| Demographics | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|
| Total patient cohort, N, in millions | 7.05M | 7.44M | 7.45M | 7.27M | 7.11M | 6.63M | 6.35M | 5.89M |
| Prevalence of diabetes,a % | 5.2 | 5.6 | 5.7 | 5.9 | 6.1 | 6.5 | 6.6 | 6.7 |
| Type 1, % | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Type 2, % | 4.9 | 5.2 | 5.4 | 5.5 | 5.8 | 6.1 | 6.3 | 6.3 |
| Type 1 diabetes | ||||||||
| Patients, N | 23,073 | 24,683 | 26,228 | 26,164 | 25,876 | 24,986 | 23,881 | 21,176 |
| Mean age, yrs | 52.3 | 52.7 | 52.6 | 52.7 | 53.2 | 54.1 | 53.9 | 53.7 |
| ≥65 yrs, % | 27.6 | 28.2 | 27.9 | 28.1 | 29.1 | 31.6 | 31.4 | 30.9 |
| Female, % | 49.4 | 48.5 | 47.8 | 47.5 | 47.4 | 47.2 | 46.8 | 46.2 |
| Medicare Advantage, % | 7.4 | 8.9 | 10.0 | 11.7 | 13.9 | 18.7 | 18.6 | 17.7 |
| Plan type, % | ||||||||
| HMO | 31.0 | 28.4 | 27.3 | 24.8 | 24.2 | 23.2 | 23.6 | 24.5 |
| PPO | 68.7 | 69.8 | 69.3 | 69.3 | 68.6 | 70.1 | 69.3 | 67.5 |
| CDHP | 0.4 | 1.8 | 3.4 | 5.9 | 7.2 | 6.6 | 7.1 | 7.9 |
| Residence region, % | ||||||||
| Northeast | 21.1 | 20.5 | 18.1 | 17.8 | 18.1 | 17.6 | 17.8 | 17.6 |
| Midwest | 31.1 | 31.1 | 32.6 | 32.0 | 32.8 | 34.3 | 34.3 | 34.6 |
| South | 34.0 | 31.8 | 32.0 | 32.2 | 31.1 | 31.2 | 30.7 | 31.2 |
| West | 13.9 | 16.7 | 17.3 | 18.1 | 18.0 | 16.8 | 17.1 | 16.6 |
| Type 2 diabetes | ||||||||
| Patients, N | 346,486 | 390,055 | 400,625 | 400,804 | 410,234 | 406,655 | 397,693 | 373,858 |
| Mean age, yrs | 60.3 | 61.1 | 61.1 | 61.5 | 62.0 | 62.8 | 63.1 | 63.3 |
| ≥65 yrs, % | 36.0 | 39.0 | 39.1 | 40.0 | 41.4 | 44.8 | 46.1 | 46.7 |
| Female, % | 47.1 | 47.1 | 47.0 | 46.9 | 46.9 | 47.0 | 46.8 | 46.8 |
| Medicare Advantage, % | 9.2 | 11.4 | 12.8 | 14.6 | 17.1 | 22.1 | 21.9 | 21.5 |
| Plan type, % | ||||||||
| HMO | 30.5 | 27.5 | 25.7 | 23.5 | 23.0 | 22.3 | 22.6 | 24.0 |
| PPO | 69.3 | 71.1 | 71.6 | 71.9 | 70.7 | 71.5 | 70.8 | 68.5 |
| CDHP | 0.3 | 1.3 | 2.7 | 4.6 | 6.3 | 6.2 | 6.6 | 7.6 |
| Residence region, % | ||||||||
| Northeast | 19.9 | 19.0 | 17.2 | 17.3 | 17.3 | 17.2 | 16.9 | 16.6 |
| Midwest | 31.0 | 32.3 | 33.7 | 32.6 | 33.4 | 34.9 | 36.2 | 36.5 |
| South | 34.3 | 31.6 | 31.6 | 32.0 | 31.4 | 31.6 | 30.8 | 31.6 |
| West | 14.8 | 17.1 | 17.5 | 18.1 | 17.9 | 16.3 | 16.1 | 15.4 |
Patients who did not satisfy the study criteria for type 1 or type 2 diabetes, as well as patients with gestational diabetes, were excluded.
Across our study population of patients diagnosed with diabetes, the overall prevalence of diabetes consistently increased from 5.2% in 2007 to 6.7% in 2014, which was mainly driven by the increasing prevalence of type 2 diabetes from 4.9% in 2007 to 6.3% in 2014. The prevalence of type 1 diabetes was much lower, and relatively stable, with fluctuations between 0.3% and 0.4% between 2007 and 2014.
Type 2 Diabetes
Across the study period, the average age of patients with type 2 diabetes increased by approximately 3 years, from 60.3 years in 2007 to 63.3 years in 2014. Approximately 47% of the patients with type 2 diabetes were female. The proportion of patients with type 2 diabetes aged ≥65 years increased from 36.0% in 2007 to 46.7% in 2014, which was reflected in the increased share of Medicare Advantage plan members.
The incidence of hypertension increased over the years (from 72.8% in 2007 to 80.9% in 2014) as did dyslipidemia (from 69.4% in 2007 to 78.9% in 2014) among patients with type 2 diabetes; the increase was consistently almost 2-fold greater than the incidence among the controls without diabetes (Supplemental Table 1, available at www.AHDBonline.com). Among the microvascular complications of type 2 diabetes, the incidence of retinopathy fluctuated between 12.8% and 13.6% across the study duration, whereas the rates of nephropathy (from 9.8% in 2007 to 15.0% in 2014) and neuropathy (from 16.3% in 2007 to 20.8% in 2014) increased considerably. All the microvascular and macrovascular complications typically related to type 2 diabetes were much less common in the control cohorts (Supplemental Table 1).
Biguanides (specifically metformin) were consistently used by more than 50% of the patients with type 2 diabetes (Supplemental Figure 1, available at www.AHDBonline.com). The use of sulfonylureas decreased substantially over time (from 35.8% in 2007 to 25.6% in 2014) as did thiazolidinediones (from 29.0% in 2007 to 5.5% in 2014) among patients with type 2 diabetes, whereas the use of dipeptidyl peptidase (DPP) inhibitors increased markedly, from 6.4% in 2007 to 15.0% in 2014. The use of glucagon-like peptide-1 agonists remained steady over time, at approximately 5.2% (Supplemental Figure 1). The use of any insulin increased slightly over time (from 14.8% in 2007 to 15.8% in 2014), with the use of analog short-acting insulin increasing by 30% (from 5.7% in 2007 to 7.4% in 2014) and analog basal insulin increasing by 18% (from 9.3% in 2007 to 11.0% in 2014) as shown in Supplemental Figure 2 (available at www.AHDBonline.com).
As shown in Supplemental Table 2 (available at www.AHDBonline.com), the all-cause hospitalization rate among patients with type 2 diabetes decreased slightly, from 17.0% in 2007 to 15.8% in 2014, whereas the rate of all-cause emergency department visits increased from 17.7% in 2007 to 20.1% in 2014. More than 50% of hospitalizations and emergency department visits were related to type 2 diabetes. The all-cause hospitalization and emergency department visit rates were much higher among the patients with type 2 diabetes than among the controls. Using the HEDIS metrics, there were slight increases in the proportion of patients receiving at least 1 HbA1c test (from 67.5% in 2007 to 71.3% in 2014), diabetic retinal examination (from 36.0% in 2007 to 38.0% in 2014), LDL testing (from 63.6% in 2007 to 64.6% in 2014), or nephropathy laboratory test (from 30.5% in 2007 to 36.3% in 2014) across the duration of the study.
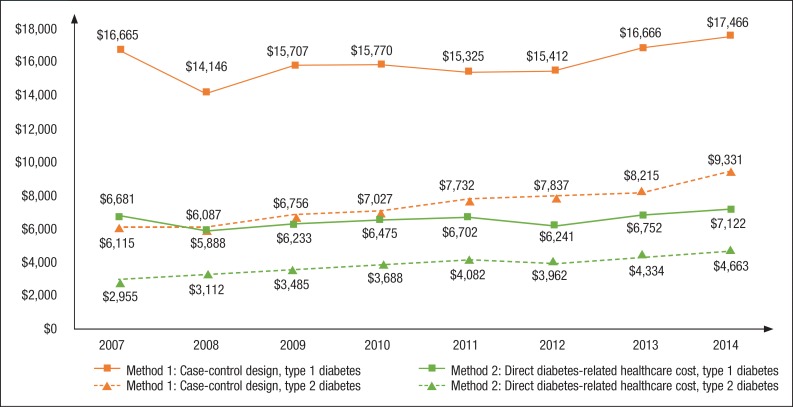
For the all-cause healthcare costs (Method 1), the inflation-adjusted total annual costs incurred by patients with type 2 diabetes fluctuated between $14,184 and $15,716 per patient across the study years, whereas the annual costs per patient for the controls ranged between $7833 and $8963 (Supplemental Table 3, available at www.AHDBonline.com). In Method 1, the difference in all-cause healthcare costs between the patients with type 2 diabetes and the controls without diabetes varied between $5888 and $7122 across the study duration (Figure 2 and Supplemental Table 3). The largest cost drivers were hospitalizations, outpatient services, and pharmacy prescription costs (Supplemental Table 3).
Figure 2. Adjusted Costs of Care for Types 1 and 2 Diabetes Estimated by 2 Cost-Estimation Methods in the United States, 2007–2014.
NOTE: Case-control design was used to estimate the cost difference in Method 1, where diabetes-related cost is the all-cause healthcare cost of diabetes minus the all-cause healthcare costs of their matched controls without diabetes; a code-based cost approach was used in Method 2, where diabetes-related cost is captured as the cost of healthcare services with diagnosis codes or pharmacy codes for diabetes and diabetes medications, respectively.
With Method 2, the direct costs attributable to type 2 diabetes increased from $2955 in 2007 to $4663 in 2014, which was driven mainly by increasing pharmacy prescription and outpatient services costs over time (Figure 2 and Supplemental Table 3). The type 2 diabetes–attributable costs based on Method 1 were almost 2-fold greater than Method 2 in most years in the study.
Type 1 Diabetes
The average age of patients with type 1 diabetes remained steady, at approximately 53 years, and there were slightly more males across the study period (Table 1). Consistent with the growing share of patients aged ≥65 years in the overall study population, the proportion of enrollees with Medicare Advantage plans increased from 7.4% in 2007 to 17.7% in 2014. More than 50% of the patients with type 1 diabetes were diagnosed with hypertension (55.3% in 2007 and 61.8% in 2014) or dyslipidemia (from 54.0% in 2007 to 65.5% in 2014), which was almost 2-fold higher than the incidence among the controls (Supplemental Table 1). The incidence of these 2 conditions steadily increased over the study years in patients with type 1 diabetes and in the controls.
Among the microvascular complications of patients with type 1 diabetes, the rates of retinopathy fluctuated between 31.9% and 34.2% across the study, whereas the rates for nephropathy (from 19.4% in 2007 to 22.0% in 2014) and neuropathy (from 28.6% in 2007 to 32.4% in 2014) marginally increased. All the microvascular and macrovascular complications of type 1 diabetes were less common in the controls, and most of the rates of these complications were <5% (Supplemental Table 1).
The all-cause hospitalization rate among patients with type 1 diabetes was 23.7% in 2007, and then gradually decreased to 20.8% in 2014, whereas the rate of all-cause emergency department visits remained relatively unchanged, fluctuating between 23.2% and 24.4% across the study period (Supplemental Table 2). The majority of the hospitalization and emergency department visits were related to type 1 diabetes.
The all-cause hospitalization and emergency department visit rates were much higher among the patients with type 1 diabetes than among the controls without type 1 diabetes. Based on HEDIS metrics, there were increases in the proportion of patients receiving at least 1 HbA1c test (from 72.4% in 2007 to 79.7% in 2014), diabetic retinal examination (from 44.3% in 2007 to 46.5% in 2014), LDL test (from 59.7% in 2007 to 64.4% in 2014), or nephropathy laboratory test (from 41.9% in 2007 to 48.4% in 2014) across the duration of the study.
For the all-cause healthcare costs (Method 1), the inflation-adjusted total annual costs incurred by patients with type 1 diabetes slightly increased from $23,261 per patient in 2007 to $25,855 in 2014, whereas the annual costs per patient for the controls ranged from $6996 to $9925 (Supplemental Table 3). In Method 1, the difference in all-cause healthcare costs between the patients with type 1 diabetes and the controls ranged between $14,146 and $17,466 across the study duration (Figure 2 and Supplemental Table 3).
Hospitalizations, outpatient services, and pharmacy prescription costs were the largest cost drivers; however, all-cause healthcare costs in every category increased slightly over the study period (Supplemental Table 3). With Method 2, the costs directly attributable to type 1 diabetes increased from $6115 in 2007 to $9331 in 2014, which was driven mainly by increasing pharmacy prescription and outpatient services costs over time (Figure 2). The costs attributable to type 1 diabetes based on Method 1 were more than 2-fold greater than the estimates from Method 2 in most years over the study duration.
In 2014, the projected total number of patients with diabetes who received treatment in the United States was 19.34 million, consisting of 18.26 million patients with type 2 diabetes and 1.08 million with type 1 diabetes (Table 2). That year, the total projected direct cost of care for patients with diabetes was $314.8 billion, including $287 billion for type 2 diabetes and $27.8 billion for type 1 diabetes. The projected incremental cost attributed to type 2 diabetes (Method 1) was $130.1 billion in 2014, whereas the type 2 diabetes–related direct healthcare cost (Method 2) was projected to be $85.2 billion. For 2014, the projected incremental costs attributable to type 1 diabetes were $18.8 billion using Method 1, and $10 billion using Method 2.
Table 2.
Projected US Population and Healthcare Costs for Patients with Diabetes, 2007–2014
| Projections | 2007 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|
| US population with diabetes, N, in millions | 17.52M | 18.35M | 18.61M | 19.30M | 19.30M | 19.27M | 19.34M |
| Type 1, N, in millions | 1.08M | 1.13M | 1.15M | 1.16M | 1.14M | 1.13M | 1.08M |
| Type 2, N, in millions | 16.44M | 17.22M | 17.46M | 18.14M | 18.16M | 18.14M | 18.26M |
| Overall costs incurred by patients with diabetes, $, in billions | 263.7B | 288.4B | 297.5B | 312.5B | 292.7B | 309.4B | 314.8B |
| Type 1, $, in billions | 25.0B | 27.6B | 27.9B | 29.2B | 27.9B | 29.1B | 27.8B |
| Type 2, $, in billions | 238.6B | 260.8B | 269.6B | 283.3B | 264.8B | 280.3B | 287.0B |
| Incremental cost attributable to diabetes, Method 1, $, in billions | 127.8B | 125.1B | 131.2B | 139.3B | 131.0B | 141.3B | 148.8B |
| Type 1, $, in billions | 17.9B | 17.8B | 18.1B | 17.7B | 17.6B | 18.8B | 18.8B |
| Type 2, $, in billions | 109.9B | 107.3B | 113.1B | 121.6B | 113.3B | 122.5B | 130.1B |
| Direct diabetes-related cost, Method 2, $, in billions | 55.2B | 67.7B | 72.5B | 83.0B | 80.9B | 87.9B | 95.2B |
| Type 1, $, in billions | 6.6B | 7.6B | 8.1B | 8.9B | 9.0B | 9.3B | 10.0B |
| Type 2, $, in billions | 48.6B | 60.0B | 64.4B | 74.0B | 71.9B | 78.6B | 85.2B |
Discussion
Diabetes-related costs continue to increase as a result of increasing in the overall disease prevalence and the high cost of managing the disease.22,23 In an era of tightening healthcare resources, it is imperative for disease-related costs to be estimated accurately to ensure that limited financial resources are optimally located. Our population-based, real-world study is based on one of the largest nationally representative administrative claims data repositories in the United States.
The use of a multipanel, cross-sectional design over an 8-year period enabled us to examine the prevalence, treatment patterns, care quality measures, healthcare resource utilization, and costs for the management of patients with type 1 or type 2 diabetes during much of the past decade. This is especially important because our analysis allowed for the inclusion of an unprecedented number of new classes of diabetes medications, with attendant price increases23 and changes in treatment guidelines.24
Cost management relies on rational and accurate estimation methods. In our study, we used 2 estimation approaches to assess the cost of care, including all-cause case-control estimation (Method 1) and direct diabetes-related cost estimation (Method 2). These 2 methods have been developed in previous research.17
The case-control estimation used in Method 1 is significantly more complex to execute than the diabetes-related direct cost-estimation in Method 2, and, as such, it may not be applied as often. The results from Method 2 were consistently lower than those from Method 1 by severalfold. This was consistent with the results of the earlier study by Tunceli and colleagues, who used similar cost-estimation methods.17 Such differences were also reported in an earlier study that used 2 slightly different cost-estimation approaches for diabetes.25
Several reasons may account for the differences reported in previous studies.17,25 Method 2 in our study aggregated the costs of drugs and medical procedures that were solely attributable to diabetes. This is a reliable approach that is facilitated well by administrative claims data, but the use of diabetes-specific diagnosis codes excludes chronic microvascular and macrovascular complications and multiple comorbidities closely associated with diabetes, which could be more debilitating and costly than diabetes itself, depending on the disease severity.6,7,26 In addition, Method 2 relies on the complete and accurate coding of diagnoses, treatments, and procedures, which can be problematic in administrative claims databases, because their completeness and accuracy are not typically assessed for payment purposes.
Method 1 included all relevant diabetes-related costs and took into account the aggregated costs for treating comorbidities along with the underlying diabetes. Although patients with diabetes were matched in a 1:1 ratio with controls by age, sex, state of residence, and health plan type, the comorbidities and microvascular and macrovascular complications typically associated with diabetes occurred at a severalfold greater rate in patients with diabetes than in the controls. This presents a persuasive rationale for including in cost calculations conditions closely associated with diabetes for an accurate picture of the disease-attributable costs.
A knowledge gap exists in the current literature in recognizing that different methods can produce vastly disparate results, even when calculating costs for the same condition, within the same population. The differences in costs identified by the 2 methods in our study raise an important alert for users of cost data in healthcare policy, care management, and budget evaluation.
Caution is required when selecting cost-estimation methods to evaluate disease burden, health policy, intervention implementation, and budgeting to ensure that costs are adequately captured. This also relates to reviewing previously published data, and, in particular, to reviewing the cost-estimation methodology, because the method used can substantially affect the overall applicability of the results. Our results also indicate signs of improving quality of care measured by HEDIS criteria. The proportion of patients with diabetes who received the recommended testing gradually increased across the study duration; however, these levels were still not optimal, and the engagement of providers by payers to evaluate the quality of care and performance-centric payment models are warranted to promote further improvements.
Also evolving during our study period were value-based payments; quality initiatives, such as the Centers for Medicare & Medicaid Services Quality Measures; and commercial insurer programs in patient-centered medical homes and accountable care organizations. These initiatives contain specific measures that support better glycemic control in patient populations, and may have influenced medication prescribing practices, as well as healthcare utilization and costs, during the period.
Several important findings are noteworthy with regard to the use of antidiabetes medications. First, the use of metformin remained consistent across each of the 8 years of the study, although the utilization rates for metformin were barely more than 50% of the population. Sulfonylurea and thiazolidinedione use decreased over the years, as would be expected after the introduction of newer medications with potentially improved side-effect profiles; however, the use of one of the newer medication classes, DPP-4, substantially increased. The use of analog short-acting insulin and basal insulin also increased during the study period.
Trends in prescribing should continue to be monitored as new antidiabetes medication classes, such as sodium-glucose transport protein 2 inhibitors, and new data on existing drugs (eg, improved cardiovascular outcomes) are introduced to the marketplace.
In this study, we used a linear projection methodology18 to project the national prevalence and cost of care for diabetes based on administrative claims data, which provides another source of evidence to cross-validate the estimates from the national surveys.4,14 The overall projected prevalence of diabetes and the total cost of care in our study are consistent with the estimates of patients diagnosed with diabetes in those national surveys.4,14
Limitations
This study has some limitations. Our claims data may not have adequately accounted for all individuals with type 2 diabetes in the HealthCore Integrated Research Database nor all the care they accessed from sources outside their health plan; this could be less substantial in the commercially insured population in our study than in the broader US population, including the uninsured.
Information on potential confounders, such as smoking status and lifestyle factors, are not available in claims data. Although cross-sectional snapshots of the outcomes within specific years were evaluated, the outcomes in any one year may not be meaningfully compared with outcomes in another year.
In this study, the same group of patients was not followed from year to year, nor across the study duration. Patients were only required to have continuous health plan enrollment for 2 full calendar years, not the full 8-year study period. Consequently, any inferences related to the overall study period should be made with caution. Because this study only used claims data to estimate the rates of HEDIS measures attainment, comparisons with published HEDIS rates that include additional data sources should be done with care. The projection methodology used in this study was adjusted for age, sex, and residence region, because these are the only categories available in administrative claims and in the US Census American Community Survey data. In addition, socioeconomic status, education level, and other variables are not available in claims data and, therefore, could not be used for adjustment. Finally, the generalizability of the study results may be limited to US commercially insured patients and Medicare Advantage members.
Conclusions
Using projections to the entire US population in 2014, our findings suggest that the direct costs of care for patients with diabetes exceeded $300 billion that year. Variations in cost estimates may exert considerable influence on the interpretation and allocation of resources in healthcare and health policy. Our findings show that the healthcare costs attributed to diabetes differed greatly during the study period, depending on the estimation method used. Faced with high and steadily increasing expenditures for diabetes management, estimation methods must be selected carefully to obtain a comprehensive picture of the economic burden of diabetes mellitus.
Our results can be instructive in the generation of hypotheses for future studies, and they may offer immediate use in shaping policy questions by payers and providers, as well as provide updated information on diabetes prevalence and the increasing cost of care.
Acknowledgment
Bernard B. Tulsi, MSc, Senior Medical Writer at HealthCore, provided writing support for this article.
Appendix
Funding Source
Support for this study was provided by Novo Nordisk.
Author Disclosure Statement
Dr Willey and Dr Deshpande are employees of HealthCore, which received funding from Novo Nordisk to conduct this study; Dr Kong is an employee of Novo Nordisk; Dr Hobbs is an employee and a stockholder of Novo Nordisk; Dr Sakurada is a stockholder of Novo Nordisk and was an employee of Novo Nordisk at the time of the study; Dr Raval, Mr Wu, and Dr Tunceli were employees of HealthCore at the time of the study; Dr Windsheimer and Mr Bouchard were employees of Novo Nordisk at the time of the study.
Contributor Information
Vincent J. Willey, Staff Vice President, HealthCore, Wilmington, DE.
Sheldon Kong, Executive Director, Health Economics and Outcomes Research, Novo Nordisk, Plainsboro, NJ.
Bingcao Wu, Employees of HealthCore at the time of the study.
Amit Raval, Employees of HealthCore at the time of the study.
Todd Hobbs, Vice President and Chief Medical Officer, Diabetes & Obesity, Novo Nordisk.
Andrea Windsheimer, Employees of Novo Nordisk at the time of the study.
Gaurav Deshpande, Senior Researcher, HealthCore.
Ozgur Tunceli, Employees of HealthCore at the time of the study.
Brian Sakurada, Employees of Novo Nordisk at the time of the study.
Jonathan R. Bouchard, Employees of Novo Nordisk at the time of the study.
References
- 1. Zhuo X, Zhang P, Barker L, et al. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37:2557–2564. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed August 8, 2018.
- 3. American Diabetes Association. Statistics about diabetes. Updated July 19, 2017. www.diabetes.org/diabetes-basics/statistics/. Accessed August 8, 2018.
- 4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rowley WR, Bezold C, Arikan Y, et al. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. [DOI] [PubMed] [Google Scholar]
- 7. Druss BG, Marcus SC, Olfson M, et al. Comparing the national economic burden of five chronic conditions. Health Aff (Millwood). 2001;20:233–241. [DOI] [PubMed] [Google Scholar]
- 8. Laiteerapong N, Huang ES, Chin MH. Prioritization of care in adults with diabetes and comorbidity. Ann N Y Acad Sci. 2011;1243:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. [DOI] [PubMed] [Google Scholar]
- 10. Pantalone KM, Hobbs TM, Wells BJ, et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3:e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Potts JE, Gray LJ, Brady EM, et al. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS One. 2015;10:e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eliaschewitz FG, Barreto T. Concepts and clinical use of ultra-long basal insulin. Diabetol Metab Syndr. 2016;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Standl E, Owen DR. New long-acting basal insulins: does benefit outweigh cost? Diabetes Care 2016;39(Suppl 2):S172–S179. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. National Diabetes Statistics Report. Estimates of Diabetes and its Burden in the United States 2014. 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 2, 2016.
- 15. Centers for Disease Control and Prevention. Crude and age-adjusted percentage of civilian, noninstitutionalized adults with diagnosed diabetes, United States, 1980–2010. 2012. www.cdc.gov/diabetes/statistics/prev/national/figage.htm. Accessed November 5, 2016.
- 16. Dall TM, Zhang Y, Chen YJ, et al. The economic burden of diabetes. Health Aff (Millwood). 2010;29:297–303. [DOI] [PubMed] [Google Scholar]
- 17. Tunceli O, Wade R, Gu T, et al. Cost of diabetes: comparison of disease-attributable and matched cohort cost estimation methods. Curr Med Res Opin. 2010;26:1827–1834. [DOI] [PubMed] [Google Scholar]
- 18. Wasser T, Wu B, Yčas JW, Tunceli O. Applying weighting methodologies to a commercial database to project US Census demographic data. Am J Accountable Care. 2015:33–38. [Google Scholar]
- 19. American Community Survey Multiyear Accuracy of the Data (5-year 2010–2014). www2.census.gov/programs-surveys/acs/tech_docs/accuracy/MultiyearACSAccuracyofData2014.pdf. Accessed November 5, 2016.
- 20. National Committee for Quality Assurance. Healthcare Effectiveness Data and Information Set (HEDIS) & Quality Measurement. www.ncqa.org/hedis-quality-measurement. Accessed August 8, 2018.
- 21. US Bureau of Labor Statistics. Consumer Price Index (CPI) for medical care. http://data.bls.gov. Accessed August 14, 2014.
- 22. Centers for Disease Control and Prevention. At a glance 2016: diabetes: working to reverse the US epidemic. www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2016/diabetes-aag.pdf. Accessed November 5, 2016.
- 23. Peter P, Lipska K. The rising cost of diabetes care in the USA. Lancet Diabetes Endocrinol. 2016;4:479–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Standards of Medical Care in Diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4–S5. [DOI] [PubMed] [Google Scholar]
- 25. Honeycutt AA, Segel JE, Hoerger TJ, Finkelstein EA. Comparing cost-of-illness estimates from alternative approaches: an application to diabetes. Health Serv Res. 2009;44:303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maddigan SL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with diabetes and comorbidities in a Canadian national population health survey. Qual Life Res. 2005;14:1311–1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.