Abstract
Background:
Community pharmacists have a key role to play in the management of allergic rhinitis (AR). Their role is especially important because the majority of medications used to treat AR are available for purchase over-the-counter (OTC), allowing patients to self-select their own medications and bypass the pharmacists. Patients’ self-selection often results in suboptimal treatment selection, undertreated AR and poor clinical outcomes. In order for pharmacists to optimise the care for AR patients in the pharmacy, pharmacists need to be able to identify patient cohorts who self-select and are at high risk of mismanagement.
Objectives:
This study aimed to compare the demographics, clinical characteristics and medication selected, between pharmacy customers who choose to self-select and those who speak with a pharmacist when purchasing medication for their AR in a community pharmacy and identify factors associated with AR patients’ medication(s) self-selection behaviour.
Methods:
A cross-sectional observational study was conducted in a convenience sample of community pharmacies from the Sydney metropolitan area. Demographics, pattern of AR symptoms, their impact on quality of life (QOL) and medication(s) selected, were collected. Logistic regressions were used to identify factors associated with participants’ medication self-selection behaviour.
Results:
Of the 296 recruited participants, 202 were identified with AR; 67.8% were female, 54.5% were >40 years of age, 64.9% had a doctor’s diagnosis of AR, and 69.3% self-selected medication(s). Participants with AR who self-select were 4 times more likely to experience moderate-severe wheeze (OR 4.047, 95% CI 1.155-14.188) and almost 0.4 times less likely to experience an impact of AR symptoms on their QOL (OR 0.369, 95% CI 0.188-0.727).
Conclusions:
The factors associated with AR patients’ self-selecting medication(s) are the presence of wheeze and the absence of impact on their QOL due to AR symptoms. By identifying this cohort of patients, our study highlights an opportunity for pharmacists to engage these patients and encourage discussion about their AR and asthma management.
Keywords: Rhinitis, Allergic, Seasonal, Self Medication, Quality of Life, Community Pharmacy Services, Professional Role, Pharmacies, Surveys and Questionnaires, Multivariate Analysis, Australia
INTRODUCTION
Community pharmacists have a key role in managing allergic rhinitis (AR), which is a chronic respiratory condition increasing in prevalence.1 It is classically characterised by nasal itching, sneezing, anterior/posterior rhinorrhoea and nasal congestion, however ocular symptoms may present (itchy or watery eyes) as well as itchy throat/palate.2 AR currently affects up to 30% of the world’s population1,3, with 19% of Australians self-reporting AR.4 The socioeconomic burden of AR in Australia has been measured to be up to AUD9.4 billion, due to absenteeism from the work place, reduce productivity at work and treatment cost.1,5
When left undertreated, AR can impact on the day-to-day activities of individuals with the condition1,2 or predispose the development or worsening asthma.6-10 Despite having up to 90% of patients dually affected by AR and asthma11, the majority under-recognise the impact of their AR symptoms and its impact on asthma control.12 In fact, a high proportion of patients who have uncontrolled asthma, experience more severe AR symptoms when compared to patients with well controlled asthma.12 The importance of optimal treatment for AR increases for patients with both AR and asthma, as uncontrolled AR increases asthma-related risk.13 With optimal AR treatment, patients with coexisting AR and asthma have a lower risk for asthma related events.9,14
Early detection and optimal management of AR allows patients to minimise the impact of AR on the patient. Diagnosis of AR is often a challenge for Health Care Professionals (HCPs) because patients underreport their AR symptoms and HCPs are not always equipped with resources to make the correct diagnosis of AR. Optimal management of AR is further compromised with patients’ bypassing the HCPs altogether15,16, with 70% self-selecting medication for their AR symptoms.12,16-18 Patients’ self-selection is suboptimal with only 15% selecting appropriate over-the-counter (OTC) medications19 from community pharmacies.15,16 The most commonly used medications are oral antihistamines, which are not deemed to be the most effective medication for moderate-severe AR symptoms.16,20 Therefore, despite the high dependence on medications, AR sufferers remain undertreated.12,19,21
With an increasing number of OTC medications being available from Australian community pharmacies15 and online, the choice of medication becomes more complicated. The availability of AR treatments OTC in Australia has occurred ahead of other countries, with implications for self-medication patterns in rhinitis (and other disease states). While pharmacists are ideally placed to meet the needs of AR patients, however research has suggested that pharmacists are not being consulted by patients who visits the pharmacy, they are not taking advice from pharmacists for their AR.16,19,22 Pharmacists play a crucial role in optimising the management of AR by regularly updating patients with the latest knowledge on AR management and ensure that they are managing their AR with appropriate medications. This is because it has been shown that patients lack medical knowledge about their condition and treatment, which has led to many misconceptions about AR medications.23 Currently, many are in search for medications that are more effective for their condition20,23,24, and pharmacist can the make most of this opportunity to engage with this cohort of patients.
Clearly, if the management of AR is to improve, it is critical that AR patients seek advice from pharmacists when in the community pharmacy, in a timely and regular manner. Currently, little is understood about why patients choose to self-manage, bypassing pharmacists. In order for pharmacists to optimise the management of AR, it is important to identify patient cohorts who self-select and are at high risk of mismanagement. Therefore, this study aimed to (i) compare the demographics, clinical characteristics and medication(s) selected between pharmacy customers who choose to self-select and those who interact with a pharmacist when purchasing AR medication(s) within the community pharmacy setting and to (ii) identify factors associated with AR patients’ medication self-selection behaviour.
METHODS
Study design
This research took the form of a cross-sectional observational study conducted on a sample of pharmacy customers purchasing medications to treat AR symptom(s) from community pharmacies. The study was approved by the University of Sydney Human Research Ethics Committee (Ref No. 2015/527).
Community pharmacies within the Sydney metropolitan area who expressed an interest in research or pharmacy services were engaged to participate in this research. A researcher stood in the pharmacy and approached all pharmacy customers who choose to self-select off the shelf from the pharmacy and those who spoke to the pharmacist in regard to a product request, a symptom request or a doctor’s prescription. These pharmacy customers were only included in the study if they were purchasing a product for AR-related symptoms, i.e. sneezing, rhinorrhoea, nasal congestion, itchiness in the nose, ears or palate, itchy/watery eyes and wheeze. The sample size was calculated to ensure that data were collected from a representative sample, based on an estimated proportion of 0.5 (50%) of people with AR self-selecting medication in a pharmacy.20 A sample of 200 AR participants was required.25
The pharmacy customers were invited to participate if they fulfilled the following inclusion criteria: independently self-selected OTC medication(s) to treat AR-related symptoms (i.e. sneezing, rhinorrhoea, nasal congestion, itchiness in the nose, ears or palate, itchy/watery eyes and wheeze) or interacted with a pharmacist for OTC and/or prescribed medication(s) for these symptoms. Pharmacy customers who selected medication(s) on behalf of others (parents of children less than 18 years old and partners) were also included if they were instructed to purchase a particular product by others and could complete the data collection process and did not violate the following exclusion criteria. The exclusion criteria included unable to complete the data collection process or expressed disinterest in participation (Figure 1). Pharmacy customers, younger than 18 years old were not approached, as adolescents are not old enough to give their own consent in participating in this study, but parents who accompanied them in the pharmacy were eligible to participate and answer on their behalf. Also, pharmacy customers who were purchasing on behalf of their partner were eligible, as in real life, people with AR trivialise their condition and people with AR may find it more convenient for others to purchase their AR medication for them. All participants gave verbal consent to participate prior to data collection.
Figure 1.
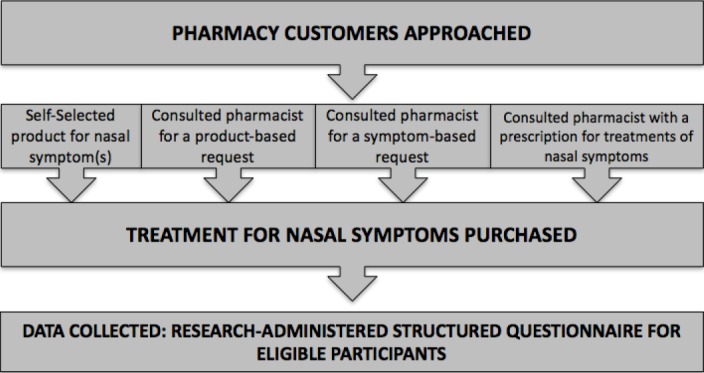
Study Design Overview
Participants were classified as having AR, NAR, or ‘other’. Classification was based on doctor’s diagnosis self-reported by participants or where a previous diagnosis was not present, determined by the expert panel of clinicians, pharmacists and researchers who applied the criteria for the diagnosis of AR according to the ARIA guidelines26, which is based on triggers, and symptoms reported. The triggers were reported in response to the question: “What brings on/makes your symptoms worse?” and “Is there, if any, a particular time of the year that these symptom(s) occur?”.16
Variables
Data were collected using a researcher administered survey (
). This included demographic characteristics, pattern of AR symptoms, their impact on quality of life (QOL), triggering factors and medication(s) selected (class of medications and reason for the selection). The survey was developed based on the empirical data and the framework of the international guidelines – Allergic Rhinitis and the Impact on Asthma (ARIA).26 The questions in the survey were based on patients’ symptoms and medication management of AR and the practicality for pharmacists to assess and manage patients with AR in the pharmacy.27 The survey was designed to facilitate quick and easy administration and reviewed by specialist clinical experts, i.e. a respiratory physician and clinical pharmacists. All responses were anonymised, and participants were de-identified.
Bias
Potential bias in this study may have arisen as a result of: convenience sample of pharmacies within a Sydney Metropolitan area; the collection of data during high allergy seasons; inability to collect data from people who have mild AR who are less likely to visit a pharmacy for treatment.
Quantitative variables
ARIA guidelines classify AR according to patients’ symptom(s) severity and impact on QOL experienced.26 There are four categories; mild or moderate-severe intermittent and mild or moderate-severe persistent.26 Symptoms that occurred less than four days per week or less than four weeks per year were classified intermittent, and symptoms that occurred more than four days per week and more than four weeks per year were classified persistent.26
Participants were asked to report the severity of their symptoms in the questionnaire, either none, mild, moderate or severe of their presenting symptoms, in accordance with Total Symptoms Score (TSS).28 The impact of their QOL on participants’ symptoms were also recorded. The impacts are related to whether they experienced an impact on their daily activities, performance at school or at work and/or disturb their sleep. Their symptoms were considered moderate-severe if they report their symptoms to be moderate or severe in the TSS table or if they report the presence of any impact on their QOL. The frequency of their symptom occurrence was also recorded in the questionnaire, as to whether they experienced symptoms less or more than four days per week and/or less or more than four weeks per year,
Statistical analysis
Data were analysed with SPSS version 24TM (SPSS-IBM, Chicago, IL, USA). Descriptive statistics were used, and data were compared between participants who self-selected and those who interacted with the pharmacist. Categorical variables were analysed using the Pearson chi-square test, and continuous variables were analysed using the independent sample t-test. A series of independent variables (participants’ demographics, reported moderate-severe symptoms, impact of AR symptoms on QOL, medications selected) were evaluated to see if it was associated with participants’ medication self-selection behaviour. These independent variables were statistically examined for suitability for inclusion in the multivariate logistic regression modelling using univariate logistic regression analysis to examine the presence of any binary correlations between participants who self-selected and each independent variable. Multivariate logistic regression analysis was performed on the univariate predictors, with p<0.05 used as the threshold for entry into the model, which was a value sufficiently significant to ensure potential interactions were not disregarded.29 A statistical approach to variable selection was chosen as this was an exploratory study and no prior assumptions of relationships between factors have been established.29 The goodness of fit of the logistic regression model was confirmed by the Hosmer and Lemeshow test. The final logistic regression model was determined with significance levels set at p<0.05.29
RESULTS
Data collection occurred in August-September, 2015 and April-July, 2016 (Australian Spring and Autumn respectively) from 8 community pharmacies, 6 hours/day and 4 days in each pharmacy. Each survey took an average of 5 minutes to administer for each participant. A flowchart of participants included and excluded are summarised in Figure 2. The 37 individuals who did not meet the inclusion criteria, were purchasing treatments other than for nasal symptoms or were unable to answer questions relating to the purchase of the product when purchasing for others.
Figure 2.

Participants Flowchart
Of the participants with AR, 1.5% (3/202) has mild intermittent, 1.5% (3/202) has mild persistent, 43.5% (88/202) has moderate-severe intermittent and 53.5% (108/202) has moderate-severe persistent.
Table 1 summarises participants’ demographic characteristics. Of the 202 participants identified as having AR (Figure 1), 54.5% (110/202) were aged >40 years, 67.8% (137/202) were female, 35.1% (71/202) had undiagnosed AR, and 69.3% (140/202) self-selected medication(s) (Table 1). There were no significant differences in age groups, gender and HCP diagnosis of AR between participants who chose to self-select and those who spoke with a pharmacist (Table 1).
Table 1.
Demographic, clinical characteristics and medication class selected of total sample and by those who self-selected (n=140) and those who speak with a pharmacist (n=62).
| Survey item | All participants (n=202) | Self-Selected | p-value | |
|---|---|---|---|---|
| Yes (n=140) | No (n=62) | |||
| Gender | ||||
| Female | 137 (67.8%) | 100 (71.4%) | 37 (59.7%) | 0.105 |
| Male | 65 (32.2%) | 40 (28.6%) | 25 (40.3%) | |
| Age | ||||
| < 18 years old | 15 (7.4%) | 12 (8.57%) | 4 (4.84%) | |
| 18-39 years old | 75 (37.1%) | 52 (37.1%) | 23 (37.1%) | >0.05 |
| > 40 years old | 110 (54.5%) | 77 (55.0%) | 33 (53.2%) | |
| HCP diagnosed AR | 131 (64.9%) | 91 (65.0%) | 40 (64.5%) | 1.000 |
| AR symptoms (moderate-severe) | ||||
| Sneezing | 128 (63.4%) | 86 (61.4%) | 42 (67.7%) | 0.431 |
| Rhinorrhoea | 139 (68.8%) | 91 (65.0%) | 48 (77.4%) | 0.100 |
| Nasal Congestion | 129 (63.9%) | 84 (60.0%) | 45 (72.6%) | 0.112 |
| Itchy/Watery Eyes | 118 (58.4%) | 81 (57.9%) | 37 (59.7%) | 0.877 |
| Itchy Nose | 63 (31.2%) | 48 (34.3%) | 15 (24.2%) | 0.188 |
| Itchy Ears/Palate | 45 (22.3%) | 33 (23.6%) | 12 (19.4%) | 0.585 |
| Wheeze | 27 (13.4%) | 24 (17.1%) | 3 (4.8%) | 0.023 |
| Frequency of AR symptoms | ||||
| Intermittent | 91 (45.0%) | 62 (44.3%) | 29 (46.8%) | 0.761 |
| Persistent | 111 (55.0%) | 78 (55.7%) | 33 (53.2%) | |
| Seasonal* | 124 (61.4%) | 84 (60.0%) | 40 (64.5%) | 0.639 |
| Identified at least a trigger that affected their AR symptoms | 149 (73.8%) | 108 (77.1%) | 41 (66.1%) | 0.119 |
| AR symptoms impacted on at least one aspect of QOL** | 122 (60.4%) | 75 (53.6%) | 47 (75.8%) | 0.003 |
| Class of medications selected | ||||
| Oral Antihistamine | 115 (56.9%) | 82 (58.6%) | 33 (53.2%) | 0.539 |
| Intranasal Antihistamine | 2 (0.5%) | 2 (1.4%) | 0 (0%) | 1.000 |
| Intranasal Corticosteroids | 63 (31.2%) | 34 (24.3%) | 29 (46.8%) | 0.003 |
| Intranasal Decongestant | 23 (11.4%) | 17 (12.1%) | 6 (9.7%) | 0.811 |
| Oral Decongestant | 4 (2.0%) | 2 (1.4%) | 2 (3.2%) | 0.589 |
| Saline | 17 (8.4%) | 9 (6.4%) | 8 (12.9%) | 0.168 |
Seasonal – participants reported that their symptoms occurred seasonally or all year round in response to the question “Is there, if any, a particular time of the year that these symptom(s) occur?”
Aspect of QOL includes Impact on daily activities, performance at school or at work, or sleep disturbance.
Table 1 also summarises participant’s clinical characteristics - pattern of symptoms, impact of AR symptoms on QOL, triggering factors, and classes of medications selected for the symptoms experienced. Moderate-severe rhinorrhoea was the most commonly experienced symptom overall, followed by nasal congestion and sneezing. Over two-thirds (136/202) of participants experienced nasal and ocular symptoms in combination with itchiness in the ears/palate, with 32.7% (66/202) experiencing nasal symptoms only. Oral antihistamines and intranasal corticosteroids were the most frequently selected medication classes (Table 1). Figure 3 summarises the impact of AR symptoms on QOL by participants who self-selected and those who interacted with a pharmacist. The majority of the participants in this study could identify at least a trigger (Table 1). Those who self-selected were more likely be experiencing a wheeze, (p=0.023), and less likely to have an impact of AR symptoms on QOL (p=0.003) and/or purchase of intranasal corticosteroids (p=0.003) (Table 1).
Figure 3.

Impact of allergic rhinitis (AR) symptoms on at least one aspect of quality of life (QOL) - daily activities, performance and sleep, and each domain individually of total sample (n=202) and by self-selected (n=140) and interacted with the pharmacist (n=62) groups.
Following univariate logistic regression analysis, two independent variables were significantly correlated with medication self-selection; presence of moderate-severe wheeze and AR symptoms impacting on at least one aspect of QOL (Table 2). There was no correlation between these two variables, therefore they were subsequently included for analysis in the multivariate logistic regression model. Classes of medication selected were not included in the model. These variables were statistically significant (chi-squared=15.546, df=2, p<0.001) (Table 2). Participants who self-selected were 4 times more likely to experience moderate-severe wheeze (OR 4.047, 95% CI 1.155-14.188) and almost 0.4 times less likely to experience AR symptoms impacting on their QOL (OR 0.369, 95% CI 0.188-0.727) (Table 2).
Table 2.
Analysis of factors associated with participants’ medication self-selection behaviour.
| Analysis | Predictors | B | S.E. | Wald | df | Sig. | Exp (B) | 95% C.I.for Exp(B) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Univariate | Moderate-severe wheeze | 1.403 | 0.633 | 4.917 | 1 | 0.027 | 4.069 | 1.177 | 14.067 |
| Impacted on Quality of Life | -0.999 | 0.342 | 8.555 | 1 | 0.003 | 0.368 | 0.189 | 0.719 | |
| Multivariate | Moderate-severe wheeze | 1.398 | 0.640 | 4.772 | 1 | 0.029 | 4.047 | 1.155 | 14.188 |
| Impacted on Quality of Life | -0.996 | 0.346 | 8.309 | 1 | 0.004 | 0.369 | 0.188 | 0.727 | |
DISCUSSION
It is well established that patients commonly and sub-optimally self-select treatment for their AR, whilst continue to live with symptoms which impact on their QOL. This study is the first to explore the factors that are associated with medication self-selection behaviour of patients with AR in a ‘real-life’ setting viz; primary care and community pharmacy. Currently, the research question in this study has not been addressed to date. Our study revealed that the majority of people with AR self-selected OTC medication(s) in the community pharmacy to treat AR symptoms without speaking to the pharmacist. This study also found significant differences between those who self-selected and those who interacted with the pharmacist. The differences were related to the presence of moderate-severe wheeze and impact of AR symptoms on at least one aspect of QOL. Interestingly, symptom severity was not a driving factor for participants to interact with the pharmacist, although a majority of patients with AR were experiencing moderate-severe symptoms. While significantly higher proportion of participants who interacted with the pharmacist were purchasing intranasal corticosteroids compared to those who self-selected medication(s), this medication class was not included in the logistic regression model as it was an outcome of the pharmacist interaction.
Participants who self-select their own medication were less likely to report an impact of their AR symptoms on their QOL. In this study, 60% of the patients reported having AR symptoms impacting on one or more QOL domains (daily activities, performance at work or school, or sleep disturbance). There was a disconnection between the QOL and the severity of the AR symptoms reported by the participants. This is not an uncommon perception, in fact this has occurred similarly with other diseases such as asthma. Patients with asthma also underperceive the severity of their condition.30 This suggests the patients can tolerate symptoms but when these symptoms impact on their QOL16, it begins to impact on their medication management behaviour. This kind of behaviour has been reported in previous literature.20,21,24,31,32 This might also reflect the concept of symptoms and patients’ perception. From the pharmacist’s perspective, these findings highlight that 1) patients who self-select are less likely to experience an impact of AR symptoms on their QOL and not speak to the pharmacist but pharmacists cannot assume that these patients have mild disease and are able to manage it without advice; 2) patients’ poor perceptions of their AR symptoms are barriers to optimal management of AR16 and pharmacists should not solely rely on patients’ perception to guide optimal treatment. Hence, in addressing this problem there are several possibilities/recommendations that we propose: 1) Pharmacists attempt/aim to approach every patient at least initially to assess their condition and follow up about their AR on the patients. 2) Pharmacy staff are encouraged to prompt patients to speak to the pharmacists before leaving the pharmacy. 3) Tools can be available for patients to self-evaluate their symptoms, such as the visual analogue scale, then prompted to speak to the pharmacist when appropriate. These tools are available through ARIA. It could be placed at the shelving where the AR medications are located for patients to evaluate their AR status.
In trying to determine whether participants had coexisting asthma, it was felt that asking the patient whether they experienced wheeze was the most non-judgemental and appropriate approach in this real-life scenario. In this study, the proportion of patients with co-existing wheeze was 13%, which is at the lower end of the range of the published prevalence of asthma amongst AR patients.2 Participants who self-selected were more likely to be also experiencing moderate-severe wheeze in addition to AR. While this was both an unexpected and counter-intuitive finding, the literature indicates that there are complexities associated with asthma patients who are known to overestimate their asthma control33 and underestimate the seriousness of their asthma.34 Possible explanations for this finding could be due to patients’ misinterpretation of the term ‘wheeze’ or because patients with asthma consider their AR a “minor” condition compared to wheeze. However, this study was not able to determine where patients place the importance of their wheeze, but it was able to clearly suggest that they do not associate their AR with their wheeze. It is important for pharmacists to be aware of this finding especially in light of the recent “Thunderstorm Asthma” events resulting in serious exacerbations and even death.35 Pharmacists should alert patients regarding these co-existing conditions, provide them with education36,37, and refer them to a general practitioner for a diagnosis, as it is critical that these patients treat their AR and co-existing conditions optimally. Pharmacists should recommend intranasal corticosteroids, as literature has shown that this medication does not only optimally controls AR symptoms but also reduces asthma symptoms.13
The majority of treatments for AR are available OTC. Although this allows for patients to purchase these medications OTC, it also provides opportunity for mismanagement of AR to occur. Therefore although 65% of patients with AR have had a diagnosis, it was possible for them to choose incorrect or suboptimal treatment options for their conditions. There are three possibilities for this situation, 1) patients might be recommended a treatment OTC by their doctor, which they may or may not take up or 2) patients might be prescribed a medication but chose to select their own medication OTC or 3) patients with follow up scripts from pharmacy. Nonetheless, while the terms suboptimally treated, undertreated AR and poor clinical outcomes of AR are similar, they are different. Suboptimal treatment selection refers to choosing a treatment that is not necessarily incorrect however it is not the optimal treatment for that patient, under treatment refers to a less than optimal amount of what might be an optimal treatment and poor clinical outcomes is not related to treatment but is describing the clinical feature/presentation.
The strengths of this research are the identification of opportunities for pharmacists to intervene in the current management of AR in the community pharmacy are identified; proper counselling and recommendation of medication selection, especially for patients with co-existing asthma. The limitations of this study are associated with the cross-sectional study design, non-randomised selection of pharmacies and the limited number of patients with mild AR approached.
CONCLUSIONS
In conclusion, the key factors associated with AR patients’ self-selecting medication(s) are the presence of moderate-severe wheeze and the absence of AR symptoms impacting on their QOL. This research highlights the need for pharmacists to assist every patient who self-selects OTC medications, because this study has demonstrated that some patients are likely to be experiencing coexisting asthma and maybe underestimating the impact of AR on their QOL. Pharmacists should engage their AR patients and ensure that a proper diagnosis is obtained, an evaluation for coexisting conditions made, impact of the condition on QOL assessed and the most appropriate treatment recommended. Pharmacists plays the important role in AR management and future research should focus on providing evidence for the role of the pharmacist in the management of AR. Pharmacy staff are encouraged to prompt patients to consult pharmacists about their AR before leaving the pharmacy. Tools, available through ARIA, can also be available for patients, at the shelving where AR medications are located, for patients to self-evaluate their symptoms, such as the visual analogue scale, then prompted to speak to the pharmacist when appropriate.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
CONFLICT OF INTEREST
Vicky Kritikos: Received honoraria from AstraZeneca, GlaxoSmithKline and Pfizer.
Kwok Yan: Received honoraria for speaking and consulting from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, Mundipharma and Pfizer.
Peter Smith: Has also been a speaker for Meda, GlaxoSmithKline, Novartis, Mundipharma and AstraZeneca.
David Price: A board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from UK National Health Service, British Lung Foundation, Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Zentiva, and Theravance; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Novartis and Mundipharma; payment for travel/accommodation/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals, and AstraZeneca; funding for patient enrolment or completion of research from Chiesi, Teva Pharmaceuticals, Zentiva, and Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK, and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; and is peer reviewer for grant committees of the Medical Research Council, Efficacy and Mechanism Evaluation programme, and Health Technology Assessment.
Sinthia Bosnic-Anticevich: A member of the Teva Pharmaceuticals Devices International Key Experts Panel; received research support from Research in Real Life; payment for lectures/speaking engagements and for developing educational presentations from Teva and Mundipharma; received Honoria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, for her contribution to advisory boards/key international expert forum.
Contributor Information
Rachel Tan, Quality Use of Respiratory Medicines Group, Woolcock Institute of Medical Research, University of Sydney. Sydney (Australia). stan6464@uni.sydney.edu.au.
Biljana Cvetkovski, Quality Use of Respiratory Medicines Group, Woolcock Institute of Medical Research, University of Sydney. Sydney, NSW (Australia). biljana.cvetkovski@sydney.edu.au.
Vicky Kritikos, Clinical Researcher Pharmacist. Quality Use of Respiratory Medicines Group, Woolcock Institute of Medical Research, University of Sydney; & Department of Respiratory Medicine, Royal Prince Alfred Hospital. Sydney, NSW (Australia). vicky.kritikos@sydney.edu.au.
Kwok Yan, Department of Respiratory Medicine, Royal Prince Alfred Hospital. Sydney, NSW (Australia). kwokyan@yansydney.com.
David Price, Academic Primary Care, University of Aberdeen, Aberdeen (United Kingdom). dprice@opri.sg.
Peter Smith, Institution: Clinical Medicine, Griffith University. Southport, QLD (Australia). pksm@mac.com.
Sinthia Bosnic-Anticevich, Professor and Principal Research Fellow. Quality Use of Respiratory Medicines Group, Woolcock Institute of Medical Research, University of Sydney; & Sydney Local Health District, Sydney, NSW (Australia). sinthia.bosnic-anticevich@sydney.edu.au.
REFERENCES
- 1.Pawankar R, Canonica G, Holgate S, Loceky R. World Allergy Organisation (WAO): White book on allergy. Wisconsin: World Allergy Organisation; [(accessed August 3, 2018)]. Available at: http://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web.pdf . [Google Scholar]
- 2.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, Correia de Sousa J, Cruz AA, Cuello-Garcia CA, Demoly P, Dykewicz M, Etxeandia-Ikobaltzeta I, Florez ID, Fokkens W, Fonseca J, Hellings PW, Klimek L, Kowalski S, Kuna P, Laisaar KT, Larenas-Linnemann DE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines –2016 Revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Passali D, Cingi C, Staffa P, Passali F, Muluk NB, Bellussi ML. The International Study of the Allergic Rhinitis Survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018;8(1):e7. doi: 10.5415/apallergy.2018.8e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AIHW. Australia's health 2016. Canberra, Australia: 2016. Contract No.: Cat. no. AUS 199. [Google Scholar]
- 5.Pawankar R, Canonica R, Holgate S, Lockey R, Blaiss M. World Allergy Organisation (WAO) White Book on Allergy: update 2013. Milwaukee: WAO; 2013. [Google Scholar]
- 6.Oka A, Matsunaga K, Kamei T, Sakamoto Y, Hirano T, Hayata A, Akamatsu K, Kikuchi T, Hiramatsu M, Ichikawa T, Nakanishi M, Minakata Y, Yamamoto N. Ongoing allergic rhinitis impairs asthma control by enhancing the lower airway inflammation. J Allergy Clin Immunol Pract. 2014 Mar-Apr;2(2):172–178. doi: 10.1016/j.jaip.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Omachi TA, Reddy SR, Chang E, Broder MS, Antonova J, Calhoun W. Allergic status is associated with increased number of asthma exacerbations. Am J Respir Crit Care Med. 2016;193:A4970. [Google Scholar]
- 8.de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67(7):582–587. doi: 10.1136/thoraxjnl-2011-201168. [DOI] [PubMed] [Google Scholar]
- 9.Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012;26(3):187–190. doi: 10.2500/ajra.2012.26.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Britten N. 'Strong medicine': an analysis of pharmacist consultations in primary care. Fam Pract. 2000;17(6):480–483. doi: 10.1093/fampra/17.6.480. [DOI] [PubMed] [Google Scholar]
- 11.Terreehorst I, Oosting AJ, Tempels-Pavlica Z, de Monchy JG, Bruijnzeel-Koomen CA, Hak E, van Wijk RG. Prevalence and severity of allergic rhinitis in house dust mite-allergic patients with bronchial asthma or atopic dermatitis. Clin Exp Allergy. 2002;32(8):1160–1165. doi: 10.1046/j.1365-2745.2002.01461.x. [DOI] [PubMed] [Google Scholar]
- 12.Bosnic-Anticevich S, Kritikos V, Carter V, Yan KY, Armour C, Ryan D, Price D. Lack of asthma and rhinitis control in general practitioner-managed patients prescribed fixed-dose combination therapy in Australia. J Asthma. 2018;55(6):684–694. doi: 10.1080/02770903.2017.1353611. [DOI] [PubMed] [Google Scholar]
- 13.Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002;109(1):57–62. doi: 10.1067/mai.2002.120554. [DOI] [PubMed] [Google Scholar]
- 14.Walker S, Sheikh A. Self reported rhinitis is a significant problem for patients with asthma. Prim Care Respir J. 2005;14(2):83–87. doi: 10.1016/j.pcrj.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guirguis LM. Mixed methods evaluation: pharmacists'experiences and beliefs toward an interactive communication approach to patient interactions. Patient Educ Couns. 2011;83(3):432–442. doi: 10.1016/j.pec.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Tan R, Cvetkovski B, Kritikos V, Price D, Yan K, Smith P, Bosnic-Anticevich S. Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon. Asthma Res Pract. 2017;3:8. doi: 10.1186/s40733-017-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62(9):1057–1063. doi: 10.1111/j.1398-9995.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 18.Canonica GW, Triggiani M, Senna G. 360 degree perspective on allergic rhinitis management in Italy: a survey of GPs, pharmacists and patients. Clin Mol Allergy. 2015;13:25. doi: 10.1186/s12948-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan R, Cvetkovski B, Kritikos V, Price D, Yan K, Smith P, Bosnic-Anticevich S. The burden of rhinitis and the impact of medication management within the community pharmacy setting. J Allergy Clin Immunol Pract. 2018;6(5):1717–1725. doi: 10.1016/j.jaip.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Williams A, Scadding G. Is reliance on self-medication and pharmacy care adequate for rhinitis patients? Int J Clin Pract. 2009;63(1):98–104. doi: 10.1111/j.1742-1241.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 21.Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med. 2006;100(2):354–362. doi: 10.1016/j.rmed.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi C, Musicco E, Rastrelli F, Bettoncelli G, Passalacqua G, Canonica GW. The patient with rhinitis in the pharmacy. A cross-sectional study in real life. Asthma Res Pract. 2015;1:4. doi: 10.1186/s40733-015-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cvetkovski B, Kritikos V, Yan K, Bosnic-Anticevich S. Tell me about your hay fever: a qualitative investigation of allergic rhinitis management from the perspective of the patient. NPJ Prim Care Respir Med. 2018;28(1):3. doi: 10.1038/s41533-018-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fromer LM, Blaiss MS, Jacob-Nara JA, Long RM, Mannion KM, Lauersen LA. Current Allergic Rhinitis Experiences Survey (CARES): Consumers'awareness, attitudes and practices. Allergy Asthma Proc. 2014;35(4):307–315. doi: 10.2500/aap.2014.35.3766. [DOI] [PubMed] [Google Scholar]
- 25.Hardon A, Hodgkin C. How to investigate the use of medicines by consumers. Geneva Switzerland: World Health Organization and University of Amsterdam; 2004. [Google Scholar]
- 26.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 27.Members of the Workshops. ARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy - Allergic rhinitis and its impact on asthma. Allergy. 2004;59(4):373–387. doi: 10.1111/j.1398-9995.2003.00468.x. [DOI] [PubMed] [Google Scholar]
- 28.Demoly P, Bousquet P, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013;43(8):881–888. doi: 10.1111/cea.12121. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: Wiley; 2005. Introduction to the logistic regression model. ISBN: 978-0-471-72214-4. [Google Scholar]
- 30.Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, Lin J, Muttalif AR, Perng DW, Tan TL, Yunus F, Neira G. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy. 2015;8:93–103. doi: 10.2147/JAA.S82633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price D, Scadding G, Ryan D, Bachert C, Canonica GW, Mullol J, Klimek L, Pitman R, Acaster S, Murray R, Bousquet J. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. doi: 10.1186/s13601-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafheutle EI, Cantrill JA, Nicolson M, Noyce PR. Insights into the choice between self-medication and a doctor's prescription: a study of hay fever sufferers. Int J Pharm Pract. 1996;4(3):156–161. [Google Scholar]
- 33.Bereznicki BJ, Chapman MP, Bereznicki LR. Factors associated with overestimation of asthma control: A cross-sectional study in Australia. J Asthma. 2017;54(4):439–446. doi: 10.1080/02770903.2016.1226899. [DOI] [PubMed] [Google Scholar]
- 34.Price D, Fletcher M, Van Der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Amato G, Vitale C, D'Amato M, Cecchi L, Liccardi G, Molino A, Vatrella A, Sanduzzi A, Maesano C, Annesi-Maesano I. Thunderstorm related asthma: what happens and why. Clin Exp Allergy. 2016;46(3):390–396. doi: 10.1111/cea.12709. [DOI] [PubMed] [Google Scholar]
- 36.Lourenço O, Calado S, Sá-Sousa A, Fonseca J. Evaluation of allergic rhinitis and asthma control in a Portuguese community pharmacy setting. J Manag Care Spec Pharm. 2014;20(5):513–522. doi: 10.18553/jmcp.2014.20.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36(2):235–248. doi: 10.1016/j.iac.2015.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


