Abstract
Purpose
While GOLD classification has been revised, its clinical impacts on outcomes of COPD patients have not been widely evaluated in real-world cohorts.
Materials and methods
According to 2007, 2013, and 2017 GOLD classifications, distribution and clinical characteristics of group-shifted patients and the risk of acute exacerbation were analyzed in combined Korean COPD cohorts. Future risk for annual moderate-to-severe exacerbation was estimated as incidence rate ratio (IRR) and compared by groups.
Results
Among 1,880 COPD patients, in GOLD 2017 classification, groups B and A were increased to 61.2% and 22.2% of total population, while group C was shrunken to 2.2% and patients with higher risk were decreased (16.6% in GOLD 2017 vs 44.7% in GOLD 2013). The kappa coefficient of agreement of both systems was 0.581 (agreement 71.7%). Groups B and D showed higher IRR of moderate-to-severe exacerbation than group A (IRR 2.4 and 5.3 respectively, P<0.001), whereas group C was not different from group A. When groups C and D were combined, the IRR for acute exacerbation for each group showed good linear trends (2.5 [1.6–3.7] for group B and 4.8 [3.0–7.7] for combined group [C+D], P<0.001).
Conclusions
In the revised GOLD 2017 system, COPD patients with higher risk were much decreased in Korean cohorts, and group C was negligible in size and clinical impacts on expecting future exacerbation. Serial increase in the risk for exacerbation was more concrete and predictable when group C was combined with group D.
Keywords: pulmonary disease, chronic obstructive/classification, pulmonary disease, chronic obstructive/diagnosis, chronic obstructive/epidemiology, risk factors, severity of illness index
Introduction
COPD is a complex disease with various manifestations in symptoms and performance status.1 GOLD 2007 suggested classification according to airflow obstruction level and evaluated severity.2 However, the correlation between airflow limitation and the patient’s symptoms or risk of acute exacerbation was not clear3 and its use was limited.
In GOLD 2013, patients were divided into four groups of A, B, C, and D considering previous exacerbation history and patient’s symptom score (COPD Assessment Test [CAT] and mMRC scale) as well as the airflow limitation severity.4 Following GOLD 2011, it was revised to be classified as high risk of acute exacerbation when there was one hospital admission in GOLD 2013. Despite the complexity of classification, there has been controversy on predicting clinical outcomes with the classification.5,6
According to the newly suggested GOLD 2017 classification,7 the history of acute exacerbation of COPD (AECOPD) was the only variable for assessing future risk on this assessment tool. This new classification system will be tested for its usefulness in real fields. Recently, a study of PLATINO showed no clear change in the GOLD 2017 classification and difference in mortality prediction.8 However, until now, reports on the usefulness of this new classification tool are limited.
This study was aimed to evaluate the clinical implication of GOLD 2017 classification system in real fields’ cohorts in terms of distribution of patients, distribution changes of grouping after annual follow-up, risk prediction of future AECOPD, and changes in symptom scores.
Materials and methods
Study design and population
Data of three prospective observational cohorts in South Korea, namely Korean COPD Subtype Study (KOCOSS; NCT02800499),9 The Korean Obstructive Lung Disease Cohort Study (KOLD),10 and Seoul National University airway registry (SNU airway registry; NCT02527486), were combined and analyzed. As some patients were overlapped in each cohort, duplicated cases were controlled. This study was approved by the Institutional Review Board of each hospital. All patients provided written informed consent form at enrollment. We obtained the approval for using patients’ data from each center, and confidentiality of patients was maintained. The institutions participating in this study were as follows: Gachon University Hospital; Gangnam Severance Hospital, Kyung Hee University Hospital, Gangdong Kyung Hee University Hospital, Gangbuk Samsung Hospital, Kangdong Sacred Heart Hospital, Kangbuk Samsung Hospital, Kangwon National University Hospital, Konkuk University Chungju Hospital, Kyungbuk University Hospital, Konkuk University Medical Center, Gyeongsang University Hospital, Korea University Hospital, National Medical Center, Dongguk University Hospital, Dong-A University Hospital, Pusan National University Hospital, Bucheon St Mary’s Hospital, Soon Chun Hyang University Bucheon Hospital, Pochon CHA University Hospital, Eulji General Hospital, Korea University Anam Hospital, Samsung Medical Center, Sungkyunkwan University Hospital, Seoul National University Hospital, Seoul National University Bundang Hospital, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Ulsan University Asan Hospital, Catholic University of Korea St Paul’s Hospital, St Vincent’s Hospital, Soon Chun Hyang University Seoul Hospital, Ajou University School of Medicine Hospital, Yeungnam University Hospital, Ilsan Dongguk University Hospital, Ilsan Paik Hospital, Yonsei University Wonju Hospital, Uijeongbu St Mary’s Hospital, Incheon St Mary’s Hospital, Inha University Hospital, Inje University Hospital, Ewha Womans University Medical Center, Chonbuk National University, Jeju National University Hospital, Soon Chun Hyang University Cheonan Hospital, Hallym University Sacred Heart Hospital, and Hanyang University Guri Hospital. All patients provided written informed consent form. Also, we obtained the approval for using patients’ medical records from each center, and confidentiality of patients was maintained.
Data collection
Epidemiologic data including age, sex, body mass index, and pack-years of smoking at initial visit were collected. We included patients’ data from standardized spirometry at initial visit and 1-year follow-up visit. Post-bronchodilator tests were conducted pre- and post-200 µg of salbutamol administration. FEV1, FVC, and FEV1/FVC records were collected. Quality of life of patients at initial visit and at 1-year follow-up visit was measured using St George’s Respiratory Questionnaire (SGRQ), CAT, and mMRC. Use of medications including inhalers such as inhaled corticosteroid/long-acting beta agonist (ICS/LABA), short-acting beta agonist, long-acting muscarinic antagonist (LAMA), LAMA/LABA, and oral roflumilast was reviewed for 1 year before the first visit.
Definition and classification of patients
COPD was defined as a patient with irreversible airway obstruction, with post-bronchodilator FEV1/FVC <70%. Moderate-to-severe AECOPD was defined by using antibiotics in outpatient clinics, emergency room admission, or admission due to an increased quantity or purulent changes of sputum, or aggravation of dyspnea.
We stratified patients according to the GOLD 2007 staging and GOLD 2013 and GOLD 2017 classifications. According to GOLD 2007 criteria, patients were classified into stage I (mild airflow limitation; FEV1 ≥80%), stage II (moderate airflow limitation; FEV1 ≥50% and <80%), stage III (severe airflow limitation; FEV1 ≥30% and <50%), stage IV (very severe airflow limitation; FEV1 <30%). According to GOLD 2013 classification, patients were stratified into four groups. Group A was defined as patients with less symptoms (mMRC 0 or 1, CAT <10), low risk (mild/moderate airflow limitation or 0–1 exacerbations per year), group B was defined as patients with more symptoms (mMRC ≥2, CAT ≥10), low risk (mild/moderate airflow limitation or 0–1 exacerbations per year), group C was defined as patients with less symptoms (mMRC 0 or 1, CAT <10), high risk (severe or very severe airflow limitation or ≥2 exacerbations per year or ≥1 hospitalized exacerbation per year), and group D was defined as patients with more symptoms (mMRC ≥2, CAT ≥10), high risk (severe or very severe airflow limitation or ≥2 exacerbations per year or ≥1 hospitalized exacerbation per year). By contrast, the GOLD 2017 classification excludes classification by airflow limitation grade. The differences in COPD classifications are summarized in Figure S1.
Statistical analyses
Statistical analyses were performed by using Stata 13.0 (StataCorp 2013, Stata Statistical Software: Release 13, College Station, TX: StataCorp LP).
Categorical variables were described as absolute numbers (n) and relative frequencies (%) and continuous variables as the mean ± SD. We applied Pearson’s chi-square test and Fisher’s exact test for categorical variables and Student’s t-test for continuous variable. Multivariate logistic regression analysis was used to predict AECOPD in each group. The incidence rate ratio (IRR) of AECOPD was acquired with negative binomial regression analysis. Annual change of total SGRQ and CAT scores across GOLD 2013 and 2017 groups were compared by using ANOVA test.
Results
Among the 2,037 patients enrolled in KOLD, KOCOSS, and SNU airway registry, 1,880 patients were included in this analysis (Figure 1). Mean age was 69.2 (SD 8.9) and percentage of males was 93.9%. Baseline mean FEV1% predicted was 56.6 (SD 18.6), CAT score was 15.0 (SD 7.9), and SGRQ score was 33.2 (SD 18.7). The mean number of AECOPDs in the year prior to enrollment was 0.5 (SD 1.5). Frequently prescribed medications in this population were LAMA, ICS/LABA, and LABA in order. However, when we compared the three cohorts incorporated in this study population, there was heterogeneity of baseline characteristics such as age, sex, and patient-reported outcomes including symptom scores and exacerbation rate because of the difference in inclusion criteria and recruitment period of each cohort (Table 1).
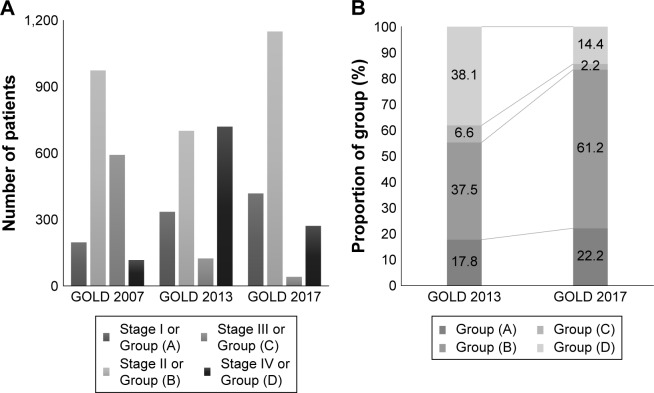
Figure 1.
Patient selection flow.
Abbreviations: CAT, COPD Assessment Test; KOCOSS, Korean COPD Subtype Study; KOLD, The Korean Obstructive Lung Disease Cohort Study; SNUH registry, Seoul National University airway registry.
Table 1.
Baseline characteristics of study population
| Variables | Total population (N=1,880) | KOCOSS (n=1,500) | KOLD (n=236) | SNUH airway registry (n=144) | P-value |
|---|---|---|---|---|---|
|
| |||||
| Age | 69.2±8.9 (n=1,662) | 69.3±9.3 (n=1,294) | 67.9±7.4 | 70.2±7.9 (n=132) | 0.028 |
| Sex, male (%) | 1,629/1,734 (93.9%) (n=1,734) | 1,273/1,368 (93.1%) | 216/222 (97.3%) | 140/144 (97.2%) | 0.009 |
| Smoking pack-years | 44.5±25.4 (n=1,736) | 44.2±24.9 (n=1,365) | 45.0±26.8 | 45.9±25.6 (n=135) | 0.721 |
| Body mass index | 22.8±3.3 (n=1,876) | 22.9±3.4 (n=1,500) | 22.8±3.2 (n=232) | 22.1±3.3 (n=143) | 0.041 |
| FEV1 (% pred) | 56.6±18.6 | 56.5±18.5 | 55.8±17.9 | 58.7±20.5 | 0.309 |
| Post-BDR FEV1/FVC | 49.0±11.7 | 49.4±11.7 | 47.6±11.2 | 46.7±12.5 | 0.006 |
| Positive BDR (>12% and 200 mL) | 68/1,870 (3.6%) (n=1,870) | 49/1,498 (3.3%) | 19/236 (8.1%) | 0/135 (0%) | <0.001 |
| mMRC | 1.5±0.9 (n=1,842) | 1.5±0.9 | 1.5±1.1 | 1.7±0.9 | 0.004 |
| CAT total | 15.0±7.9 (n=1,670) | 15.4±7.9 | 9.6±7.6 | 14.5±6.4 | <0.001 |
| SGRQ total | 33.2±18.7 (n=1,825) | 33.9±18.8 | 29.4±18.8 | 31.3±16.5 | 0.002 |
| Medication for COPD at baseline | |||||
| LAMA | 937/1,671 (56.1%) | 864/1,292 (66.9%) | 71/235 (30.2%) | 2/144 (1.4%) | <0.001 |
| LABA | 237/1,353 (14.9%) | 233/1,211 (19.2%) | 4/235 (1.7%) | 0/144 (0%) | <0.001 |
| LAMA/LABA | 96/412 (23.3%) | 33/33 (100.0%) | 63/235 (26.8%) | 0/144 (0%) | <0.001 |
| ICS/LABA | 616/1,617 (38.1%) | 568/1,238 (45.9%) | 4/235 (1.7%) | 42/144 (29.2%) | <0.001 |
| Roflumilast | 76/1,545 (4.9%) | 74/1,092 (6.4%) | 2/235 (0.9%) | 0/144 (0%) | <0.001 |
| Total moderate-to-severe exacerbation rate in the year prior to enrollment | 0.5±1.5 (n=1,873) | 0.5±1.4 (n=1,498) | 0.7±2.2 (n=235) | 0.2±0.7 (n=140) | 0.004 |
Note: Continuous variables are presented as mean value ± SD.
Abbreviations: BDR, bronchodilator response; CAT, COPD Assessment Test; ICS, inhaled corticosteroid; KOCOSS, Korean COPD Subtype Study; KOLD, The Korean Obstructive Lung Disease Cohort Study; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; SGRQ, St George’s Respiratory Questionnaire; SNUH registry, Seoul National University airway registry.
Distribution of COPD patients according to GOLD classification systems
The study population was classified with the criteria according to GOLD 2007 stages, GOLD 2013 and GOLD 2017 groups, and the distribution is described in Table 2 and Figure 2.
Table 2.
Comparison of patients’ distribution between GOLD 2007, GOLD 2013, and GOLD 2017
| Total (N=1,880) | GOLD 2013 | GOLD 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (A) | (B) | (C) | (D) | |||
| 335 (17.8%) | 701 (37.5%) | 124 (6.6%) | 720 (38.1%) | 418 (22.2%) | 1,150 (61.2%) | 41 (2.2%) | 271 (14.4%) | |||
| GOLD 2007 | I | 197 (10.5%) | 65 (19.4%) | 116 (16.6%) | 4 (3.2%) | 12 (1.7%) | 65 (15.6%) | 116 (10.1%) | 4 (9.8%) | 12 (4.4%) |
| II | 974 (51.8%) | 270 (80.6%) | 585 (83.5%) | 28 (22.6%) | 91 (12.6%) | 270 (64.6%) | 585 (50.9%) | 28 (68.3%) | 91 (33.6%) | |
| III | 592 (31.5%) | 0 | 0 | 86 (69.4%) | 506 (70.3%) | 78 (18.7%) | 380 (33.0%) | 8 (19.5%) | 126 (46.5%) | |
| IV | 117 (6.2%) | 0 | 0 | 6 (4.8%) | 111 (15.4%) | 5 (1.2%) | 69 (6.0%) | 1 (2.4%) | 42 (14.4%) | |
Note: Data are presented as number of patients (percent).
Figure 2.
Changes in distribution of COPD patients with new GOLD 2017 classification.
Notes: (A) Distribution of patients by GOLD classification. (B) Comparison of subgroup proportion of GOLD 2013 and 2017 classifications.
In GOLD 2007 staging, most patients showed mild-to-moderate airflow limitation with FEV1 >50% (n=1,171, 62.3%), while the patients with very severe airflow limitation was only 6.2%. In GOLD 2013 classification, group D patients were most common (720 patients, 38.1%) followed by group B, group A, and group C.
In the new classification system of GOLD 2017, COPD patients in group B were predominantly common and group C was shrunken to 2.2% of total population. As a result, COPD patients with high risk were decreased to 16.6% in GOLD 2017 from 44.7% of population in GOLD 2013. The kappa coefficient of agreement for subject classification by the two grouping (GOLD 2013 vs GOLD 2017) was 0.581 (agreement 71.7%).
By applying new classification of GOLD 2017, 83.4% of patients of the total population were re-classified as group A and B, the low-risk group, while 55.3% of the population was in the low-risk group according to GOLD 2013 classification.
Comparison of clinical characteristics between patients in group B of GOLD 2013 classification and patients newly migrated to group B by GOLD 2017 classification
We compared clinical characteristics of patients in group B of GOLD 2013 and patients newly recruited in group B of GOLD 2017 (initially classified as group D in GOLD 2013 and abbreviated as group B′; Table 3).
Table 3.
Comparison of baseline clinical characteristics between patients in group B of GOLD 2013 and patients newly migrated to group B by GOLD 2017 classification
| Group (B) in GOLD 2013 (n=701) | Group (B) newly migrated in 2017; group B′ (n=449) | P-value | |
|---|---|---|---|
|
| |||
| Age | 69.8±9.1 (n=629) | 68.3±8.0 (n=387) | 0.008 |
| Sex, male (%) | 601/650 (92.5%) | 390/404 (96.5%) | 0.007 |
| Smoking pack-years | 43.7±24.3 (n=642) | 44.8±25.9 (n=421) | 0.490 |
| Body mass index | 23.5±3.1 (n=698) | 21.8±3.4 (n=448) | <0.001 |
| FEV1(% pred) | 67.9±14.0 | 38.1±7.5 | <0.001 |
| Post-BDR FEV1/FVC | 54.6±9.2 | 39.3±9.2 | <0.001 |
| Positive BDR (>12% and 200 mL) | 23/697 (3.3%) | 14/446 (3.1%) | 0.881 |
| mMRC | 1.5±0.8 (n=684) | 1.9±0.9 (n=44) | <0.001 |
| CAT total | 16.4±6.1 (n=664) | 18.2±6.9 (n=402) | <0.001 |
| SGRQ total | 32.6±15.6 (n=677) | 39.7±19.1 (n=439) | <0.001 |
| Medication for COPD at baseline | |||
| LAMA | 337/614 (54.9%) | 242/406 (59.6%) | 0.136 |
| LABA | 108/599 (18.0%) | 42/381 (11.0%) | 0.003 |
| LAMA/LABA | 26/118 (22.0%) | 17/94 (18.1%) | 0.478 |
| ICS/LABA | 186/600 (31.0%) | 207/402 (51.5%) | <0.001 |
| Roflumilast | 5/572 (0.9%) | 35/381 (9.2%) | <0.001 |
| Total moderate-to-severe exacerbation rate in the year prior to enrollment | 0.07±0.26 | 0.09±0.29 | 0.221 |
Note: Continuous variables were presented as mean value ± SD.
Abbreviations: BDR, bronchodilator response; CAT, COPD Assessment Test; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; SGRQ, St George’s Respiratory Questionnaire.
As expected, there was no statistically significant difference between groups B and B′ in total moderate-to-severe exacerbation rate or incidence. Mean age of group B′ was statistically significantly lower than group B (P=0.008). But, FEV1% predicted and FEV1/FVC ratio were poorer in group B′, and patient-reported symptom scores including mean mMRC score, total CAT score, and total SGRQ score were more severe in group B′. Exposure to ICS/LABA and roflumilast during a year before cohort enrollment was much higher in group B′ than in group B (51.5% vs 31.0% for ICS/LABA, P<0.001; 9.2% vs 0.9% for roflumilast, P<0.001; Table 3). Though there is no difference in total moderate-to-severe exacerbation rate or incidence between two groups, prospective annual experience of moderate-to-severe exacerbation was higher in group B′ (102/372 [27.4%] vs 100/260 [38.5%], P=0.003).
In Table S1, we compare the characteristics of patients in group A of GOLD 2013 and patients in group A of GOLD 2017 (abbreviated as group A′). Group A′ showed significantly more severe airflow limitation (FEV1) and symptoms (total CAT and SGRQ scores). Also, prospective total annual moderate-to-severe exacerbation rate was higher in group A′.
Comparison of group shifting in 1-year follow-up according to GOLD classification systems
Among the 1,880 COPD patients, 1,096 patients could be reevaluated with GOLD 2013 and 2017 classifications at 1-year follow-up visit. At 1-year follow-up visit, 29 patients (2.7%) were improved in airflow limitation above the criteria for COPD. Overall, the proportion of group C was very similar at both time points, while patients in the higher risk groups were shifted to less severe group probably with treatment for 1 year. The trends were prominent when groups C and D were combined (Table 4; P for trends <0.001).
Table 4.
Comparison of group shifting among GOLD classification systems in 1-year follow-up
| GOLD classification | Group at enrollment | Group in 1-year follow-up | Difference | P for trend |
|---|---|---|---|---|
|
| ||||
| GOLD 2013 | ||||
| Group (A) | 335 (17.8%) | 234 (21.4%) | 3.6 | <0.001 |
| Group (B) | 701 (37.3%) | 424 (38.7%) | 1.4 | |
| Group (C) | 124 (6.6%) | 72 (6.6%) | 0 | |
| Group (D) | 720 (38.3%) | 337 (30.8%) | −7.5 | |
|
| ||||
| GOLD 2017 | ||||
| Group (A) | 418 (22.2%) | 276 (25.2%) | 3.0 | <0.001 |
| Group (B) | 1,150 (61.2%) | 6,408 (58.4%) | −2.8 | |
| Group (C) | 41 (2.2%) | 30 (2.7%) | 0.5 | |
| Group (D) | 2,714 (14.4%) | 121 (11.0%) | −3.4 | |
|
| ||||
| Reclassified GOLD 2017 with combined group | ||||
| Group (A) | 418 (22.2%) | 276 (25.2%) | 3.0 | <0.001 |
| Group (B) | 1,150 (61.2%) | 640 (58.4%) | −2.78 | |
| Group (C+D) | 312 (16.6%) | 151 (13.8%) | −2.8 | |
Based on the classification of GOLD 2013, 140 patients (12.8%) migrated to lower risk groups. Similar changes were observed in the view of GOLD 2017; 273 patients (24.9%) moved to higher risk groups and 161 patients (14.7%) improved to lower risk groups (Tables 4 and S2).
Impact of GOLD classifications on the risk of acute exacerbations
For the patients followed regularly for more than 1 year, the associations with acute exacerbations and GOLD classifications were analyzed (Table 5). In GOLD 2013 and GOLD 2017 classification systems, the OR for the risk of moderate-to-severe AECOPD was reversed between groups B and C. Furthermore, in GOLD 2017, group C did not show any statistical significance for the risk of exacerbation compared with group A (Table 5, OR 1.0, 95% CI 0.3–3.8, P-value 0.997). When groups C and D were combined, the OR for the risk of AECOPD was linearly and significantly increased from group A to combined group (Table 5 and Figure 3).
Table 5.
Univariate and multivariate analyses for impact of GOLD classifications on the risk of AECOPD in 1-year follow-up period
| GOLD classification | Univariate analysis
|
Multivariate analysisa
|
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
|
| ||||
| GOLD 2007 | ||||
| Stage I | Reference | Reference | ||
| Stage II | 2.3 (1.2–4.2) | 0.010 | 2.1 (1.0–4.3) | 0.043 |
| Stage III | 3.6 (1.9–6.8) | <0.001 | 3.9 (1.9–8.1) | <0.001 |
| Stage IV | 3.8 (1.8–8.0) | 0.001 | 4.4 (1.9–10.7) | 0.001 |
| GOLD 2013 | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.0 (1.2–3.1) | 0.005 | 2.5 (1.4–4.4) | 0.001 |
| Group (C) | 2.0 (1.1–3.7) | 0.032 | 2.3 (1.1–4.8) | 0.033 |
| Group (D) | 4.0 (2.6–638) | <0.001 | 5.8 (3.4–10.1) | <0.001 |
| GOLD 2017 | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.0 (1.4–2.9) | <0.001 | 2.4 (1.5–3.6) | <0.001 |
| Group (C) | 1.7 (0.7–4.1) | 0.234 | 1.0 (0.3–3.8) | 0.997 |
| Group (D) | 4.5 (2.9–6.9) | <0.001 | 5.9 (3.5–10.0) | <0.001 |
| GOLD combined group | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.0 (1.4–2.9) | <0.001 | 2.4 (1.5–3.6) | <0.001 |
| Group (C+D) | 4.0 (2.6–6.1) | <0.001 | 5.0 (3.0–8.3) | <0.001 |
Note:
Adjusted for age, sex, body mass index, and pack-years.
Abbreviation: AECOPD, acute exacerbation of COPD.
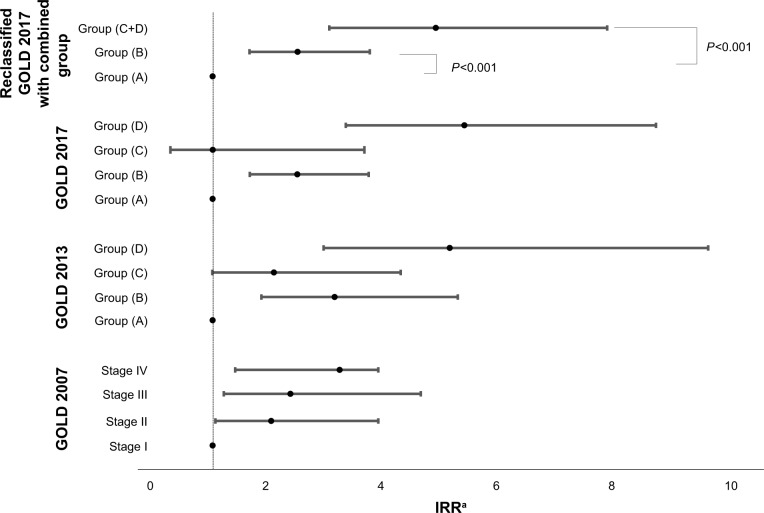
Figure 3.
Associations between GOLD classifications and prospective total moderate-to-severe annual exacerbation rate.
Note: aAdjusted for age, sex, body mass index, and pack-years.
Abbreviation: IRR, incidence rate ratio.
Stages II, III, and IV showed higher IRR for moderate-to-severe exacerbations than stage I in GOLD 2007 staging. IRRs for exacerbation in group C by GOLD 2013 and GOLD 2017 were not statistically different from that of group A (IRR =1.0, 95% CI 0.3–3.6, P=0.997).
Associations between GOLD classifications and symptomatic score change in 1-year follow-up period
In Table 6, total SGRQ and CAT scores change during follow-up for 1 year was calculated; statistically significant difference was observed between groups A, C (less symptomatic groups) and B, D (more symptomatic groups) in both GOLD 2013 and 2017 classifications.
Table 6.
Associations between GOLD classifications and symptomatic score (SGRQ and CAT) changesa in 1-year follow-up
| GOLD classification | Total SGRQ score | P-value | Total CAT score | P-value |
|---|---|---|---|---|
|
| ||||
| GOLD 2007 | ||||
| Stage I | −4.8 (14.3) (n=96) | 0.105 | −1.1 (6.7) (n=66) | 0.085 |
| Stage II | −1.5 (13.4) (n=611) | −0.8 (6.4) (n=507) | ||
| Stage III | −0.6 (16.3) (n=404) | 0.1 (7.2) (n=340) | ||
| Stage IV | −1.1 (20.7) (n=81) | −1.8 (7.4) (n=70) | ||
| GOLD 2013 | ||||
| Group (A) | 0.6 (9.5) (n= 183) | 0.017 | 1.7 (4.7) (n= 121) | <0.001 |
| Group (B) | −2.8 (14.9) (n=447) | −1.6 (6.8) (n=393) | ||
| Group (C) | 1.8 (13.8) (n=86) | 3.3 (6.1) (n=66) | ||
| Group (D) | −1.5 (16.9) (n=488) | −0.9 (7.0) (n=415) | ||
| GOLD 2017 | ||||
| Group (A) | 1.0 (11.1) (n=243) | 0.023 | 2.3 (5.4) (n=166) | <0.001 |
| Group (B) | −1.8 (16.1) (n=749) | −1.2 (7.0) (n=655) | ||
| Group (C) | 0.6 (10.9) (n=26) | 2.5 (4.9) (n=21) | ||
| Group (D) | −3.1 (15.3) (n=186) | −1.3 (6.6) (n= 153) | ||
| Reclassified GOLD 2017 with combined group | ||||
| Group (A) | 1.0 (11.1) (n=243) | 0.017 | 2.3 (5.4) (n=166) | <0.001 |
| Group (B) | −1.8 (15.9) (n=7,495) | −1.2 (7.0) (n=655) | ||
| Group (C+D) | −2.6 (15.4) (n=212) | −0.8 (6.6) (n= 174) | ||
Note:
Mean change (SD).
Abbreviations: CAT, COPD Assessment Test; GOLD, global initiative for chronic obstructive lung disease; SGRQ, St George’s Respiratory Questionnaire.
Discussion
Based on the controversy on the role of severity of airflow limitation in determining strategy for medical treatment, new GOLD 2017 suggested a separate approach to evaluate airflow limitation and risk of AECOPD. In this study, we tried to figure out the distribution of GOLD 2017 classification and how the new system affects the future risk of exacerbation. With this study, we could describe the distribution of COPD patients under new classification system and show the clinical difference in patients of old and new group B.
At first, we should consider the distribution change of COPD patients in real fields under new classification. Compared with the COPDgene study in which group A was 33.6%, B was 20.5%, C was 7.9%, and D was 38.0%,11 the distribution of higher risk group (group C and D) was similar while the symptomatic lower risk group was larger in this study population. Patients with high risks migrated to groups A and B and group B became the dominant group occupying 61.5% of all COPD patients. The change is similar to that of the recent report from the PLATINO study.8 Our study conducted in a different area than the PLATINO study, patients were from Latin America, and the COPDGene Cohort study, patients were from USA, respectively, the change in group reorganization of GOLD 2017 among Korean COPD patients was similar to the other large cohorts.
Another important finding is that group C was shrunk to only 2.2% under GOLD 2017 classification. Even in GOLD 2013 classification, the proportion of group C was variable but small with a proportion of 4%–23% according to previous COPD cohorts. Considering the specific risk factor used to determine category assignment in the higher risk groups of GOLD 2013, namely, C1 (FEV1 only) group was 74.2% in this study (Table S3) which is similar to other large COPD cohorts.3,5,11
In the new classification, group B was dominant but might be heterogeneous. When we compared the characteristics of patients in group B of GOLD 2013 and patients migrated to group B under the new classification (ie, group B′), we found that patients in group B′ were more symptomatic (higher score in mMRC, CAT, and SGRQ) and used ICS and roflumilast more frequently despite the exacerbation rate in previous year was not different. The differences were extended to the association with the higher risk of prospective acute exacerbation in group B′ than in group B. The findings suggest that GOLD 2017 may undervalue the risk of acute exacerbations in patients with poor symptom score and lung function. Although the prior exacerbation history is the major and important determinant of future exacerbation, mMRC and airflow limitation were also risk factors for exacerbation.12–14 Therefore, these limitations and combining heterogeneous subpopulation with a simple criteria of exacerbation frequency may be hurdles to utilization of the new GOLD criteria by clinicians.
In this study, prospective acute exacerbation risk and incidence rate were sequentially increased according to severity of airflow limitation as shown in previous studies.15 However, group C was not different from group A in expecting future risk of exacerbation (Tables 5 and 7). Interestingly, the limitations of GOLD 2017 were minimized, and the linear association by groups with clinical outcomes including trends in group shifting with follow-up, exacerbation risk for 1-year follow-up, and annual exacerbation risk was more consistent. Korean COPD guideline stratified the groups with either FEV1 (<60% or ≥60%) or exacerbation history (0–1 vs ≥2) in the previous year resulting in low-risk or high-risk group (group “da”). Group “da” included the 23.3% of low-risk group assigned in GOLD 2013 as a higher group, and 15.3% of GOLD B included in Korean group “da” had experienced exacerbation the following year which was similar to 17% of GOLD D included in Korean group “da”.16
Table 7.
Associations between GOLD classifications and prospective total moderate-to-severe annual exacerbation rate
| GOLD classification | Univariate analysis
|
Multivariate analysisa
|
||
|---|---|---|---|---|
| IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
|
| ||||
| GOLD 2007 | ||||
| Stage I | Reference | Reference | ||
| Stage II | 2.3 (1.3–4.1) | 0.0069 | 2.0 (1.0–3.8) | 0.036 |
| Stage III | 2.9 (1.6–5.4) | <0.001 | 2.3 (1.2–4.6) | 0.014 |
| Stage IV | 3.8 (1.8–7.9) | <0.001 | 3.2 (1.4–7.3) | 0.006 |
| GOLD 2013 | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.8 (1.7–4.4) | <0.001 | 3.1 (1.8–5.2) | <0.001 |
| Group (C) | 2.3 (1.3–4.3) | 0.008 | 2.0 (1.0–4.2) | 0.052 |
| Group (D) | 4.6 (2.9–7.2) | <0.001 | 5.1 (3.0–8.4) | <0.001 |
| GOLD 2017 | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.5 (1.6–3.4) | <0.001 | 2.4 (1.6–3.5) | <0.001 |
| Group (C) | 2.3 (1.0–5.3) | 0.060 | 1.0 (0.3–3.6) | 0.997 |
| Group (D) | 5.1 (3.3–7.9) | <0.001 | 5.3 (3.3–8.6) | <0.001 |
| Reclassified GOLD 2017 with combined group | ||||
| Group (A) | Reference | Reference | ||
| Group (B) | 2.3 (1.6–3.4) | <0.001 | 2.5 (1.6–3.7) | <0.001 |
| Group (C+D) | 4.7 (3.1–7.2) | <0.001 | 4.8 (3.0–7.7) | <0.001 |
Note:
Adjusted for age, sex, body mass index, and pack-years.
Abbreviation: IRR, incidence rate ratio.
Considering the results of this study, we suggest that the future classification algorithm might be necessary to be modified to combine group C and D, and incorporating the baseline FEV1.
Potential limitations of this study are related to patient enrollment and data sampling. Our cohorts are not a population-based cohort and recruited patients in tertiary hospital. Therefore, 37.6% of patients in this study had moderate-to-severe airflow limitation, higher than a previous report from South Korea (6.4%).17 Nevertheless, the largest cohort, KOCOSS, is a nation-wide cohort and it may attenuate the selection bias. Also there was limitation in analyzing mortality, and pulmonary function change was not included because of short history of cohorts. At the enrollment, about 20% of patients in group A and 30% in group B according to GOLD reported ICS/LABA use. The overtreatment could affect prospective result of AECOPD in this study.
Conclusion
In conclusion, this study used a comprehensive database of nationwide COPD cohorts in South Korea and showed that in new GOLD 2017, greater portion of COPD patients were identified as being at lower risk of acute exacerbations than the previous GOLD 2013 (83.4% vs 55.3%). In the new classification, Group C was shrunk to a minor group and it did not show any benefit in predicting acute exacerbation compared with group A. Combining groups C and D showed advantage in predicting exacerbations.
Clinical trial registration
KOrea COpd Subgroup Registry and Subtype Research (KOCOSS); https://ClinicalTrials.gov/ct2/show/NCT02800499, NCT02800499.
Seoul National University Airway Registry; https://ClinicalTrials.gov/ct2/show/NCT02527486, NCT02527486.
Supplementary materials
Changes in COPD classifications.
Abbreviations: CAT, COPD Assessment Test; GOLD, global initiative for chronic obstructive lung disease.
Table S1.
Comparison of demographical and clinical characteristics between Class A in GOLD 2013 and 2017
| Class (A) in GOLD 2013(n=335) | Class (A) migrated in 2017 (C→A; n=83) | P-value | |
|---|---|---|---|
|
| |||
| Age | 68.5±9.1 (n=292) | 69.0±9.2 (n=78) | 0.584 |
| Sex, male (%) | 308/320 (96.3%) | 73/79 (92.4%) | 0.140 |
| Smoking pack-years | 43.6±23.3 (n=312) | 47.8±30.4 (n=70) | 0.201 |
| Body mass index | 23.6±3.1 (n=334) | 22.1 ±3.6 | <0.001 |
| Baseline FEV1 (% pred) | 68.7± 13.2 | 40.6±6.3 | <0.001 |
| Baseline FEV1/FVC | 55.5±8.7 | 41.1±9.4 | <0.001 |
| Positive bronchodilator response (>12% and 200 mL) | 15/334 (4.5%) | 7/82 (8.5%) | 0.166 |
| mMRC | 0.7±0.5 | 0.8±0.4 | 0.046 |
| Baseline cat total | 5.7±2.5 | 5.8±2.4 | 0.789 |
| Baseline Sgrq total | 18.9± 12.0 | 22.7± 13.4 | 0.012 |
| Initial LAMA use | 118/293 (40.3%) | 40/73 (54.8%) | 0.025 |
| Initial LABA use | 45/275 (16.4%) | 10/70 (14.3%) | 0.672 |
| Initial LAMA/LABA use | 28/112 (25.0%) | 8/29 (27.6%) | 0.776 |
| Initial ICS/LABA | 60/271 (22.1%) | 22/69 (20.3%) | 0.091 |
| Initial roflumilast use | 3/266 (1.1%) | 3/67 (4.5%) | 0.098 |
| Total moderate-to-severe exacerbation rate in the year prior to enrollment | 0.2±0.7 | 0.5± 1.0 | 0.028 |
Note:Continuous variables were presented as mean value ± SD.
Abbreviations: CAT, COPD Assessment Test; GOLD, global initiative for chronic obstructive lung disease; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; SGRQ, St George's Respiratory Questionnaire.
Table S2.
Comparison of group shifting among GOLD classification systems in 1-year follow-up
| Group at enrollment | Group in 1 year
|
||||
|---|---|---|---|---|---|
| By GOLD 2013
| |||||
| Group (A) | Group (B) | Group (C) | Group (D) | Group without airflow limitation | |
|
| |||||
| Group (A) | 96 (41.0%) | 35 (8.3%) | 13 (18.1%) | 9 (5.6%) | 8 (27.6%) |
| Group (B) | 79 (33.8%) | 237 (56.2%) | 11 (15.3%) | 58 (14.7%) | 11 (37.9%) |
| Group (C) | 16 (6.8%) | 18 (4.3%) | 25 (34.7%) | 14 (18.2%) | 4 (13.8%) |
| Group (D) | 43 (18.4%) | 132 (31.3%) | 23 (31.9%) | 256 (55.7%) | 6 (20.7%) |
|
| |||||
|
By GOLD 2017
|
|||||
| Group (A) | Group (B) | Group (C) | Group (D) | Group without airflow limitation | |
|
| |||||
| Group (A) | 128 (46.4%) | 59 (9.2%) | 12 (40.0%) | 6 (5.0%) | 10 (34.5%) |
| Group (B) | 128 (46.0%) | 462 (72.2%) | 10 (33.3%) | 74 (61.2%) | 14 (48.3%) |
| Group (C) | 7 (2.5%) | 11 (1.7%) | 3 (10.0%) | 0 (0%) | 2 (6.9%) |
| Group (D) | 14 (5.1%) | 108 (16.9%) | 5 (16.7%) | 41 (33.9%) | 3 (10.3%) |
|
| |||||
|
By reclassified GOLD 2017 with combined group
|
|||||
| Group (A) | Group (B) | Group (C+D) | Group without airflow limitation | ||
|
| |||||
| Group (A) | 128 (46.4%) | 59 (9.2%) | 18 (11.9%) | 10 (34.5%) | |
| Group (B) | 127 (46.0%) | 462 (72.2%) | 84 (55.6%) | 14 (48.3%) | |
| Group (C+D) | 21 (7.3%) | 119 (18.6%) | 49 (32.5%) | 5 (17.2%) | |
Table S3.
Comparison of patients’ distribution between GOLD 2007, GOLD 2013, and GOLD 2017
| Total (N=1,880) | GOLD 2013 | GOLD 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C3) | (D3) | (A) | (B) | (C) | (D) | |||
| 335 (17.8%) | 704 (37.5%) | 124 (6.6%) | 720 (38.1%) | 418 (22.2%) | 1,157 (61.5%) | 41 (2.2%) | 271 (14.4%) | |||
| GOLD 2007 | I | 197 (10.5%) | 65 (19.4%) | 116 (16.6%) | 4 (3.2%) | 12 (1.7%) | 65 (15.6%) | 117 (10.1%) | 4C2 (9.8%) | 12D2 (4.6%) |
| II | 974 (51.8%) | 270 (80.6%) | 587 (83.5%) | 27 (22.6%) | 91 (12.6%) | 270 (64.6%) | 587 (50.7%) | 28C2 (68.3%) | 91D2 (33.7%) | |
| III | 592 (31.5%) | 0 | 0 | 86 (69.4%) | 506 (70.3%) | 78C1 (18.7%) | 380D1 (33.1%) | 8C3 (19.5%) | 124D3 (47.0%) | |
| IV | 117 (6.2%) | 0 | 0 | 6 (4.8%) | 111 (15.4%) | 5C1 (1.2%) | 69D1 (6.1%) | 1C3 (2.4%) | 429D3 (14.4%) | |
Notes: C1, C2, C3, D1, D2, D3: classified by GOLD 2013; C1, D1: by FEV1; C2, D2: by exacerbation; C3, D3: by both.
Acknowledgments
The authors would like to thank the research participants of KOLD, KOCOSS, and SNU airway registry.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. DKK introduced the study concept and drafted the manuscript. JHS contributed to data analysis and drafting of the manuscript. DKK, CHL, SJU, YBP, KHY, KSJ, SDL, YMO, and EKK participated in the enrollment of patients, acquisition of data, and critical revision of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wedzicha JA. The heterogeneity of chronic obstructive pulmonary disease. Thorax. 2000;55(8):631–632. doi: 10.1136/thorax.55.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 5.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 6.Soriano JB, Alfageme I, Almagro P, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013;143(3):694–702. doi: 10.1378/chest.12-1053. [DOI] [PubMed] [Google Scholar]
- 7.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. [Accessed June, 2017]. Available from: http://goldcopd.org.
- 8.Menezes AM, Wehrmeister FC, Perez-Padilla R, et al. The PLATINO study: description of the distribution, stability, and mortality according to the Global Initiative for Chronic Obstructive Lung Disease classification from 2007 to 2017. Int J Chron Obstruct Pulmon Dis. 2017;12:1491–1501. doi: 10.2147/COPD.S136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JY, Chon GR, Rhee CK, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: The Korea COpd Subgroup Study Team Cohort. J Korean Med Sci. 2016;31(4):553–560. doi: 10.3346/jkms.2016.31.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park TS, Lee JS, Seo JB, et al. Study design and outcomes of Korean Obstructive Lung Disease (KOLD) Cohort Study. Tuberc Respir Dis. 2014;76(4):169–174. doi: 10.4046/trd.2014.76.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natori H, Kawayama T, Suetomo M, et al. Evaluation of the Modified Medical Research Council Dyspnea Scale for predicting hospitalization and exacerbation in Japanese patients with chronic obstructive pulmonary disease. Intern Med. 2016;55(1):15–24. doi: 10.2169/internalmedicine.55.4490. [DOI] [PubMed] [Google Scholar]
- 14.Mcgarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, Mcknight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109(2):228–237. doi: 10.1016/j.rmed.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang YI, Park YB, Oh YM, et al. Comparison of Korean COPD guideline and GOLD initiative report in term of acute exacerbation: a validation study for Korean COPD guideline. J Korean Med Sci. 2014;29(8):1108–1112. doi: 10.3346/jkms.2014.29.8.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DS, Kim YS, Jung KS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med. 2005;172(7):842–847. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in COPD classifications.
Abbreviations: CAT, COPD Assessment Test; GOLD, global initiative for chronic obstructive lung disease.