ABSTRACT
In late September 2016, the Americas became the first region in the world to have eliminated endemic transmission of measles virus. Several other countries have also verified measles elimination, and countries in all six World Health Organization regions have adopted measles elimination goals. The public health strategies used to respond to measles outbreaks in elimination settings are thus becoming relevant to more countries. This review highlights the strategies used to limit measles spread in elimination settings: (1) assembly of an outbreak control committee; (2) isolation of measles cases while infectious; (3) exclusion and quarantining of individuals without evidence of immunity; (4) vaccination of susceptible individuals; (5) use of immunoglobulin to prevent measles in exposed susceptible high-risk persons; (6) and maintaining laboratory proficiency for confirmation of measles. Deciding on the extent of containment efforts should be based on the expected benefit of reactive interventions, balanced against the logistical challenges in implementing them.
KEYWORDS: Control measures, elimination, immunoglobulin, measles, outbreaks, social distancing, vaccine
Background
While only one region of the world, the Americas, has so far successfully eliminated measles, an increasing number of countries have been verified to have eliminated measles, and the momentum towards measles eradication is growing. The work of maintaining measles control, however, does not stop once measles elimination is verified. Measles elimination has significant implications for any public health system, which needs to sustain physician awareness, high immunization coverage, and elimination-standard surveillance in the face of almost no disease, as long as measles is still endemic anywhere in the world. The efforts required to sustain elimination and respond to cases of measles may not meet any of the normal cost-effectiveness criteria applied in other areas of public health. Such efforts emphasize the priority of achieving near-perfect immunization coverage to minimize the impact of imported measles. When no endemic measles virus is circulating, it can be challenging to convince parents to get their children vaccinated and to prompt clinicians to test febrile rash illnesses for measles. It can also be difficult to maintain laboratory proficiency and sustain resources for an immunization program.
In this context, sharing the experience of using different containment strategies in countries that have eliminated measles for some time is increasingly relevant to a greater number of countries. This article reviews the use of these strategies in selected countries, by examining the measles surveillance guidelines these are based on, as well as the authors' experience. Specifically, we reviewed national measles surveillance guidelines from Australia,1 Canada,2 the United States,3 and the measles elimination field guide prepared by the Pan American Health Organization for the Region of the Americas,4 and published reports on measles outbreaks from these countries. These settings were selected because they have more than 60 years of combined experience being free of endemic measles.5-8 We discuss the evidence base for the strategies, the challenges faced when applying them, and the lessons learned on how to successfully implement them.
Outbreak response activities
In elimination settings, a single measles case is a public health priority, and prompt identification and investigation of measles is important to help expedite outbreak control strategies. The following key activities need to be implemented as part of outbreak control, often simultaneously, necessitating coordinated responses by public health agencies (Box 1 and Figure 1).1-4
Box 1. Key public health activities in response to a measles outbreak.
• Assemble an outbreak control team or response committee
• Determine coverage in affected and surrounding areas
• Enhance surveillance, i.e., active case-finding for additional cases
• Inform the public and other appropriate health authorities
• Educate case-patients and their contacts about the mode of transmission and on measures to minimize measles spread
• Proper case management, including administration of vitamin A as indicated
• Obtain specimens for laboratory confirmation and viral detection
• Implement control activities to limit virus transmission
○ Provide measles vaccine to unvaccinated persons
○ Assess immunity of contacts of cases, offer post-exposure prophylaxis (vaccine, immunoglobulin) to those susceptible
○ Implement isolation, quarantine, exclusion in households as needed
• Collect detailed data on cases and outbreak response
• Analyze and summarize outbreak, including other available surveillance and measles vaccine coverage data, to determine whether there is evidence of population immunity gaps that require public health action; disseminate these findings to pertinent stakeholders
Note: Adapted from “Steps in response to a measles outbreak” in reference 4.
Figure 1.
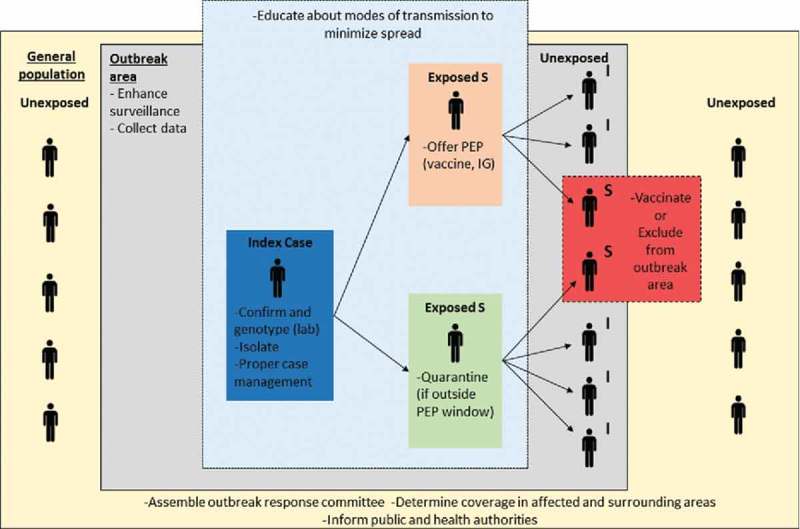
Measles outbreak control strategies to limit measles virus transmission.
Measles virus transmission and measles disease burden can be mitigated through vaccination of susceptible persons, administration of post-exposure prophylaxis (vaccine and immunoglobulin), and social distancing techniques (isolation, quarantine, and exclusion). In elimination settings, where general population immunity is high, outbreak response is prioritized in areas with high-risk of transmission or among persons at risk of severe disease. This simplified schematic is not meant to depict all complexities related to measles virus transmission or to public health interventions during measles outbreaks. Abbreviations: I = Immune; S = Susceptible; IG = Immunoglobulin; PEP = Post-exposure prophylaxis.
Because outbreak response is a broad logistical undertaking that requires considerable planning, preemptive or early assembly of a rapid response team or outbreak control committee is needed.9-12 Depending on the context and potential extent of the outbreak, these teams are composed of experts and stakeholders from local, regional, and national health departments and laboratories, local hospitals, and affected institutions or facilities. As with all emergency response teams, establishing and maintaining partnerships among members of the committee through routine training and emergency planning activities can help the committee work effectively once an outbreak occurs.11 At the outset, based on the capacity of the public health infrastructure (e.g., quality of surveillance, laboratory capacity), these committees can help determine the need for additional resources. The committees also assign responsibilities (e.g., identify a team leader for case investigations), decide on the implementation of containment strategies, and update local authorities and each other regularly on control activities.3 Local personnel who are adequately trained in reporting and investigating outbreaks and are familiar with the affected population, or who have established relationships with leaders in the community, can contribute greatly to the decision-making process.10,12,13
Once a case is detected, the risk of further transmission needs to be determined. Identifying exposed individuals at risk for severe disease who may benefit from post-exposure prophylaxis—including infants, unvaccinated pregnant women, and severely immunocompromised individuals—is a priority. A rapid assessment of factors that could contribute to virus spread at the local level, principally an evaluation of available vaccination coverage data in the affected population and in surrounding communities, should also be completed. Immunization registry data, for example, has recently been used to identify areas and/or groups with low measles vaccine uptake.13-15 When coverage data are unavailable, the Pan American Health Organization (PAHO) recommends use of Rapid Coverage Monitoring (RCM), an assessment tool that helps local managers identify areas where vaccination may be suboptimal.16 Other factors that might influence the risk for an outbreak include the size and population density of the affected community, and other contextual issues such as acceptability of vaccination in certain groups. Both statistical and mathematical modeling tools that leverage demographic, coverage, and case-based surveillance data have been developed to detect at-risk areas, either preemptively, or in the early stages of outbreaks.17-19
Surveillance should be augmented to search for additional cases and to assure the timely diagnosis of measles. The primary approach to enhancing surveillance is to increase awareness of local transmission. Commonly, physicians, emergency departments, laboratories, and schools/other educational facilities serving the affected community are alerted to the possibility of further cases, engaged to be part of active surveillance, and encouraged to notify suspected and confirmed cases to local health departments. Providers can be notified directly in person or via phone calls, or more broadly through epidemiological alerts that are sent by national, state, or local public health authorities via e-mail distribution systems. Previously unreported cases may be identified by reviewing emergency room attendance logs and electronic medical or laboratory records.20 Active case-finding may also be conducted in the community, preferably using a photo of a measles rash, to facilitate recognition. Both institutional and community case-finding are important in areas with low vaccination coverage and where underreporting may occur. Active surveillance is continued for at least one maximum incubation period after rash onset in the last case, at which time the outbreak may be defined as over (e.g., 18 days after rash onset in the last case, see Table 1).1-4
Table 1.
General guidance for measles outbreak control in four elimination settings.
| Strategy | United States | Canada | Australia | PAHO |
|---|---|---|---|---|
| Isolation of case-patients | • Through 4 days after rash onset | • Through 4 days after rash onset | • From onset of symptoms through 4 days after rash onset | • Through 5 days after rash onset |
| • Self-isolate at home | • Self-isolate at home | • At home | ||
| Quarantining (at home) of exposed susceptible contacts | Consider: Immune status and level of risk of person, setting (unvaccinated or high-risk population) | NS | NS | NS |
| • Voluntary | ||||
| “Exclusion” of exposed susceptible contacts from specific high-risk settings | • Through 21 days after rash onset in last case | • ≥5 days after first to ≤21 days after last exposure | • Through 18 days after last exposure | NS |
| • Affected institution (e.g., school, daycare) | • Childcare, schools, and post-secondary educational institutions; other | • Early childhood education and care services, and primary schools; other | ||
| Exclusion from outbreak area of non-exposed susceptible persons | • Through 21 days after rash onset in last case | NS | • Through 14 days after rash onset in last case | NS |
| • Early childhood education and care services, and primary schools | ||||
| Monitoring for compliance with isolation, quarantining, exclusion | • At discretion of health department | NS | • Daily phone call | ΝS |
| Post-exposure prophylaxis (PEP) of susceptible contacts | ||||
| -Vaccine | • ≤72 hours of first exposure, | • ≤72 hours of exposure, | • ≤72 hours of first exposure, | • ≤72 hours of exposure |
| -Immunoglobulin (IG) administration | • ≤6 days of exposure | • >72 hours to ≤6 days of exposure | • >72 hours to ≤6 days of exposure | NS |
| Community-wide non-targeted vaccination | • Rarely indicated | • Extent based on objective (e.g., limit secondary cases or spread in community), feasibility, level of risk | • To minimize ongoing transmission in defined groups of exposed susceptible people, where it is too late or not feasible to identify individuals who need PEP | • Target pockets of susceptible infants and children (all susceptible children aged 1–15 years) |
| • Targeted clinics to reach affected population preferred | • The largest possible area should be covered | |||
| Vaccination of infants aged 6–11 months as an outbreak control measure | • If many cases among infants aged <12 months | NS | NS | • If many cases among infants aged <12 months |
| Prioritization of IG for susceptible contacts at high risk of complications, and for whom vaccine is contraindicated | • Infants, pregnant women, severely immunocompromised individuals | • Infants, pregnant women, severely immunocompromised individuals | • Infants, pregnant women, immunocompromised individuals, healthcare workers, close personal (e.g., household) contacts | NS |
| May return after timely vaccine PEP | • To childcare, school, work | • To childcare, schools, and post-secondary educational institutions; other | • To early childhood education and care services, and primary schools | NS |
| May return after timely IG PEP | • To childcare, school, work; consider the immune status and intensity of contact in the setting, and presence of high risk individuals | • To childcare, schools, and post-secondary educational institutions; other | • To early childhood education and care services, and primary schools | NS |
| Laboratory confirmation | • Both IgM and PCR recommended | • Both IgM and PCR recommended | • Both IgM and PCR recommended | • Both IgM and PCR recommended |
| Preferred specimen and timing | • Serum at first contact | • Serum at first contact | • Serum <1 week after rash onset | • Serum at first contact |
| • Nasopharyngeal (NP) or throat swab ≤72 hours after rash onset | • NP swabs ≤4 after rash onset | • NP aspirate or throat swab and first catch urine <1 week after rash onset | • Throat swab, NP swab/aspirate at first contact | |
| Genotyping | • To distinguish between wild-type and vaccine strains in recently vaccinated (within 21 days) with fever/rash | • To distinguish between wild-type and vaccine strains in recently vaccinated (within 2–3 weeks) with fever/rash | • To distinguish between wild-type and vaccine strains in recently vaccinated with fever/rash | • ≥1 case in each transmission chain |
| • Representative cases of all outbreaks | • ≥1 case in each transmission chain | |||
| Active surveillance, outbreak cessation | • 42 days after rash onset in last case | 32 days after rash onset in last case | 18 days after rash onset in last case | • 21 days after rash onset in last case |
Note: NS = Not Specified. Information comes from References 1–4.
Guidance may vary at the state/local or provincial/territorial levels.
MMR vaccine may be recommended for infants aged 6 months through 11 months as post-exposure prophylaxis if administered within 72 hours of exposure in place of IG.
If MMR is given prior to 12 months of age, two additional doses separated by at least 4 weeks must be administered after 12 months of age.
MMR vaccine is recommended for infants aged ≥9 months.
I.e., lowering the age of vaccination and as opposed to giving vaccine as post-exposure prophylaxis.
Regardless of immunologic or vaccination status, because they might not be protected by the vaccine.
IG is given to infants <6 months if the mother contracts measles or is known to be non-immune.
IG is given to infants from birth to 5 months of age if mother has <2 MMR vaccine doses and no history of past measles infection, or tests negative for IgG (otherwise, no IG), and to infants aged 6 to 8 months.
These individuals cannot return to health care settings.
Immunocompromised children or staff should be excluded regardless of measles vaccination status or receipt of IG until 14 days after rash onset in the last case.
A second serum should be collected >72 hours after rash onset if a negative result is obtained from serum collected within 72 hours after rash onset.
Three weeks prior to illness onset implied.
Two maximum incubation periods (21 days from exposure to rash).
Two incubation periods (14 days from exposure to rash) and the maximum period of communicability (4 days post-rash).
Communicating with the public and other health authorities is essential to raise awareness of the risk of measles. For example, when exposures occur in large venues (such as restaurants, malls, or cinemas) or on public transport, the number exposed and the level of risk is uncertain. Thus, in lieu of individual contact tracing, informing the public about a potential exposure may improve case-finding.3 The public can be updated on the outbreak status and alerted of potential exposures in a variety of ways, including press briefings, media releases, notices on health department websites, forums involving community leaders, flyers posted at exposure sites, advertisements, or social media posts.1-4 Public communication should aim to provide consistent and clear information that is timely and frequent.11 Most of the time, the affected jurisdiction is responsible for leading this communication. When cases or contacts have the potential to involve multiple jurisdictions, including neighboring states or countries through travel, cross border notifications are sent to corresponding health authorities so that appropriate follow-up is done; these may involve international health regulation (IHR) notifications.1-4
Where appropriate, cases or their caregivers should be educated about the mode of transmission, infectious period, and measures to minimize the spread of measles. Preferably this advice is given as printed materials (fact sheets are often developed for this purpose).1 Dedicated measles phone lines may be set up to provide guidance to the public.11,12 Exposed persons are counseled to be watchful for measles compatible symptoms, and are given steps to follow if illness develops (e.g., how and where to seek medical evaluation without unnecessarily exposing other community members, including calling ahead of coming to a healthcare facility so that they can be isolated on arrival). More generally, measles outbreaks serve as a reminder of the risk of not vaccinating, and can be used by health authorities to promote vaccination and increase coverage.
Efforts need to be made to obtain clinical specimens for confirmation of disease in all suspected cases and for viral molecular detection and genotyping. The latter is an essential activity after elimination, because molecular epidemiology can help identify the origin of the outbreak (i.e., the source region/country from where the virus was imported), differentiate among separate chains of measles virus transmission, and distinguish between wild-type virus and the vaccine strain in recently vaccinated persons presenting with rash.
Measures to curtail the spread of measles include offering vaccination to non-immune individuals and post-exposure prophylaxis (vaccine or immunoglobulin) to susceptible contacts. Consideration should be given to the availability of immunoglobulin and vaccine and on the need for procurement. It is desirable to offer prophylaxis to those persons exposed in all settings visited by a case, although it is usually necessary to prioritize based on the level of risk and the potential for severe disease. There should be discussions regarding the locations where vaccination and post-exposure prophylaxis may be administered, and strategies on contacting hard-to-reach individuals, e.g., vaccination clinics at the health department versus door-to-door vaccination. Social distancing (isolation, quarantining, and exclusion)a and symptom monitoring might also be employed. During outbreaks, jurisdictions may consider postponing social or religious events that may propagate the disease, or use these gatherings as an opportunity to educate the public about the occurrence of measles and its associated risks.2
Pertinent demographic, clinical, and epidemiological data must be gathered during interviews of cases (or their parents or caregivers). Interviews are recommended to be completed within the first 48 hours after case identification.4 Optimally, case data is collected using measles investigation forms,1-4 and periodically entered into a database. Use of investigation forms allow for the systematic collection of key surveillance variables (e.g., the vaccination status of measles cases). Use of a logbook (e.g., an electronic spreadsheet) listing all confirmed, suspected, and discarded cases, and their corresponding information and pending actions, is also recommended to keep track of contact investigations.3 Thorough field investigation of cases and contacts is pivotal for identification of transmission networks and patterns of spread, and it helps determine the scope of vaccination and rapid response activities. Careful documentation of the number of vaccine doses and courses of immunoglobulin given, the total number of contacts per case, and information on the isolation and quarantine measures implemented during the response, is essential for assessing the effectiveness and impact of control measures.
An analysis of outbreak notification and response data should be done at the end of each outbreak. Much can be learned from measles outbreaks, especially in describing pockets of under-immunized people that may require targeted preventive efforts,27 and in documenting response strategies that were successful in limiting measles spread. Data from elimination settings indicate that the characteristics of unvaccinated populations are diverse; they may be faith-based groups,12 ethnic subpopulations,14,28 and certain age-cohorts29 or members of a socioeconomic strata30 that experienced lower immunization coverage historically. As such, preventive and response measures should be tailored to each population to be effective. Characterizing susceptible communities and response strategies can help pinpoint vulnerable groups, narrow measles immunity gaps, and optimize public health interventions. Such analysis can be strengthened greatly by having both numerator and denominator data on the number vaccinated. This enables coverage to be calculated for specific communities, such as groups that are under vaccinated due to religious or philosophical reasons,14 and efforts should be made to develop such data as these data are not widely available. Costs incurred by the public health sector during the response, or even a full economic evaluation, are also of interest.30-32 Finally, data from social media and search engines have been used to measure public measles vaccine confidence and the effectiveness of communication strategies during measles outbreaks,23-26 and might also be helpful for the early detection of outbreaks and to monitor disease spread, as has been done for other pathogens.21,22 Findings from these analyses should be disseminated to relevant stakeholders, e.g., frontline clinicians involved in the response, state or national public health authorities responsible for identifying and closing immunity gaps, and policy makers.
Containment strategies — guidance, evidence, challenges and special considerations
Isolation of measles cases while infectious
Guidance
A strategy to prevent further transmission of measles virus from a suspected case is isolation of the infected individual until he/she is either no longer contagious or until measles has been ruled out. The recommended length of time during which a person infected with measles should be isolated is based on the period of communicability of the virus, and is described as the number of days before and after the date of rash onset, when the amount of measles virus present in respiratory secretions is expected to be highest. This is generally accepted to be from the fourth day before rash onset (or 24 hours prior to the onset of prodromal symptoms), until at least the fourth day after rash onset, with the date of rash onset considered as day zero.1-4
Guidelines from elimination settingsb recommend that individuals with measles (including suspected cases) self-isolate at home, i.e., remain in their residence and away from non-household contacts through the fourth (or fifth) day after rash onset (Table 1).1-4 During isolation, household visits are generally discouraged and are restricted to vaccinated persons, if inevitable.1
Evidence
No studies have directly quantified the effectiveness of isolation during outbreaks. A simulation study, however, suggests that voluntary isolation and home quarantine were particularly important in reducing secondary transmissions from index cases and the risk of an outbreak in an elimination setting.33
Challenges
In elimination settings, at least in theory, prompt isolation of each imported case, combined with rapid and thorough follow up (and quarantining or exclusion) of those exposed before the imported case was recognized, could prevent outbreaks altogether. However, this is complicated by the fact that measles virus transmission occurs before appearance of the typical rash, and invariably requires a high index of suspicion for measles among health practitioners, as well as high-quality contact investigations and surveillance to capture all cases in each transmission chain.
At times, despite careful epidemiological investigations, the source patient (i.e., the imported case) is never identified, indicating exposures occurred before the index (or first-identified) cases were recognized.13,34,35 Although challenging, identification of each measles imported case—at least retrospectively—is of utmost importance in elimination settings. When the source of an outbreak is not detected, the number of generations of spread prior to identification of the index case(s) may be unknown.13,34 Detection of measles virus introductions is a requirement for verification of elimination status34 and is a key indicator of the adequacy of a measles surveillance system (WHO's target is for ≥80% of confirmed cases to have the source of infection identified).36
Although attempts are made during isolation to avoid contact with susceptible family members (e.g., infants and unvaccinated adults), this is often not possible, as exposure might have already occurred by the time measles is suspected or confirmed in the household. In a recent outbreak in Switzerland, for example, six occurrences of secondary transmission from 50 isolated cases were limited to household contacts.37 However, timely quarantine of exposed susceptible family members of a case can reduce the risk that measles will spread outside the home.
Additional challenges with isolating cases while contagious relate to issues of compliance and costs, e.g., loss of income, work absences. These are discussed in more detail in the context of exclusion and quarantining below.
Special considerations
A few specific scenarios related to the isolation of infectious persons need to be considered.
First, it is possible that a previously vaccinated person could nevertheless become infected with measles virus, e.g., they did not respond immunologically to the vaccine (primary vaccine failure) or their antibodies might have waned over time (secondary vaccine failure). The latter usually occurs during outbreaks in intense contact settings, and these cases may be partially protected from disease, often have a milder presentation, and may be difficult to diagnose.38,39 Their ability to transmit virus, however, is thought to be greatly diminished, as subsequent spread of measles from a person with prior immunity has rarely been documented.38,40,41 Because primary versus secondary vaccine failure cases cannot be readily distinguished without specialized testing and cases with a past immunologic response to measles might be contagious,38 any person with confirmed measles, regardless of vaccination status, is recommended to be isolated for the duration of the infectious period.1-4 (Of note, transmission from previously immune individuals is of great interest and has important implications for sustaining elimination; studying such transmission requires clinical specimens and specialized laboratory investigations.)
Second, persons with an underlying condition that results in a compromised immune state may have severe and prolonged disease, and may shed the virus for a longer period of time; measles RNA has been detected in specimens obtained more than 30 days after rash onset in children infected with HIV.42 Additional precautions are thus considered for immunocompromised persons, including maintaining isolation for the duration of their illness.2
Third, when an infectious or potentially infectious person requires medical attention (e.g., a susceptible contact in quarantine who develops measles-like symptoms), it is advised that either a home visit be arranged or that the person call ahead before visiting a clinic or emergency department. This ensures appropriate precautions are in place before the medical encounter to prevent infection of others in healthcare facilities.1
Exclusion and quarantining of individuals without presumptive evidence of immunity
Guidance
Exclusion of susceptible individuals from outbreak settings is used to protect those individuals from potential exposure to a disease and to reduce the risk they become infected themselves and subsequently transmit the disease to others. Similarly, quarantining aims to limit disease transmission by separating and restricting the movement of asymptomatic individuals who are exposed to a disease and are expected to become infectious (i.e., are susceptible).43,44 In measles elimination settings, the extent to which exclusion and/or quarantining of non-immune persons is recommended, legislated, and employed to contain measles outbreaks varies (Table 1).1-3 Quarantining of exposed persons, for example, is only explicitly mentioned in U.S. and Australian measles outbreak control guideline;s1,3 national Canadian guidelines discuss “isolation” of susceptible exposed persons,2 and this is legislated in some Canadian provinces and territories (e.g., in Ontario).45 Australian, Canadian, and U.S. control guidelines, as well as public health legislation in certain Australian states, emphasize ‘exclusion’ (as opposed to quarantining) of susceptible contacts from specific high-risk settings (e.g., school, child care, and healthcare facilities), where they could transmit the virus, including to individuals at risk of severe disease (e.g., infants, immunocompromised people). Exclusion of susceptible persons from an outbreak area, generally until one incubation period after the onset of rash in the last case, is advised in U.S., Canadian, and Australian control guidelines.
Quarantine is typically recommended for individuals exposed to measles who do not receive post-exposure prophylaxis and who cannot provide adequate evidence of presumptive immunity (i.e., documentation of vaccination, laboratory evidence of immunity [i.e., a positive serologic test for measles-specific IgG], birth before their respective country's measles vaccination program was initiated, or laboratory confirmation of disease).1-3 For measles, quarantine implies that a person should remain at home (or other location, but separated from others) with no non-immune visitors, for the duration of an incubation period,1-3 or until evidence of immunity can be produced. During the quarantine period, health officials may periodically monitor the individual(s) for symptoms via phone and/or home visits or instruct them to report any symptoms compatible with measles to local health departments (Table 1). Quarantine may come in the form of a mandated legal order or, more often, as a recommendation for voluntary quarantine at home. Voluntary quarantine is better aligned with the concept of modern quarantine, which recognizes the importance of respecting civil liberties, as well as the use of the least restrictive means necessary to achieve a public health goal.44,46 Alternatively, other social distancing strategies—such as avoidance of public places, limiting contact with others, and excluding non-immune persons from outbreak and/or specific high risk settings—are less restrictive than quarantine and may reduce the risk of transmission.1-3,44,46 Measles control guidelines in elimination settings discuss quarantine and exclusion as tools that can be used and enforced at the country's or jurisdiction's discretion.1-3,47
Evidence
Few reports have assessed the impact of quarantine, exclusion, and other social distancing strategies on measles outbreak control, and it is often difficult to quantify their individual effectiveness due to lack of specific data, or because of the confounding effects of other concurrent interventions like vaccination.11,12,14,30,48-50 Limited evidence suggests that using quarantine for non-immune close contacts of cases may considerably reduce the number of secondary cases from these contacts. During an outbreak in Geneva, an 18-day quarantine recommendation resulted in 6 secondary cases from 50 quarantined cases, compared to 81 secondary cases from 173 non-quarantined cases (relative risk: 0.26; 95% CI: 0.06–0.65).37 A modeling study simulating a measles outbreak in a synthetic population, which mimicked the demographic and socioeconomic characteristics of a highly vaccinated county in California, implies that home quarantine and voluntary isolation, when combined with post-exposure vaccine or immunoglobulin administration, had the largest impact in reducing measles transmission during an outbreak.33
Challenges
Exclusion and quarantine are theoretically ideal containment strategies for measles, because they immediately reduce the number of contacts with susceptible individuals that each ill individual makes. Their real-world application, however, comes with significant logistical challenges and costs.
First, the crucial task of determining who is not immune is complicated. Susceptibility to measles is easy to identify in certain groups, such as infants, persons who have religious or philosophical reasons for not being vaccinated and those who are medically contraindicated to receive measles vaccine. Similarly, older adults born before vaccine introduction, when measles was still endemic, are likely to have been infected naturally and therefore are presumed to be immune; this assumption works fairly well for measles control in eliminations settings.1-4 However, many other adults who could be immune might lack verifiable vaccine information51 and might not be able to obtain records quickly. These individuals might not understand why they are in quarantine, especially if they are not experiencing symptoms.1,46
Second, verifying compliance and monitoring individuals for symptoms while in quarantine (or in isolation) is resource-intensive for health authorities.3,49 This can be particularly challenging in some close-knit communities, where some parents believe that their children may benefit from natural exposure to measles52; they may consider having “measles parties” to increase the risk of transmission from infectious to vulnerable children. Laws concerning quarantine differ between countries and regions, and some jurisdictions may not have the legal authority to serve or enforce a quarantine order.1 Those that can enforce mandated quarantine orders occasionally require the aid of law enforcement,48,49 which drives cost even further. Logistical challenges may arise when the jurisdiction issuing the order is legally obligated to provide essential services (food, shelter, access to medical care, and medications) or other provisions during the quarantine period. The quarantine of individuals living in homeless shelters or communal living facilities presents an additional challenge to health authorities, as shared living spaces make quarantine compliance nearly impossible.14 In certain circumstances, enforcing quarantine may be counterproductive, in that it may discourage disease reporting and erode public confidence.
Third, quarantine is also expensive for families, who may experience psychological distress, loss of income due to work absences, and/or the additional cost of child or dependent care.30,53 During a measles outbreak in California in 2008, for example, families incurred an estimated cost of $775 per quarantined child.30
Special considerations
The use of exclusion and quarantine of non-immune individuals is likely effective in limiting the spread of measles during outbreaks in elimination settings.33,37 As with other measles control measures, when considering the use of these strategies, the risk of transmission in the community (e.g., measles vaccine coverage, degree of contact among individuals), as well as the risk for severe disease (e.g., among immunocompromised individuals and infants) should be assessed and balanced against the high monetary cost and the ethical and logistical challenges inherent in these interventions (Table 2).46
Table 2.
Factors to consider when deciding on the extent of public health interventions during measles outbreaks in elimination settings.
| What is the public health objective? |
| • Abort or modify the clinical course of the illness (e.g., post-exposure prophylaxis) |
| • Limit spread in the community (e.g., community-wide vaccination campaign, use of isolation, quarantining) |
| Considerations for tailoring response to the particular outbreak |
| • Feasibility of the intervention |
| ○ Community engagement, acceptability |
| ○ Healthcare infrastructure, public health capacity |
| ○ Availability of resources (vaccine, cold chain, promotional materials) |
| ○ Cost |
| • Risk of spread in affected (and surrounding) communities |
| ○ Size of the community |
| ○ Baseline vaccination coverage (within and surrounding the affected community) |
| ○ Population density, rates of contact (rural vs. urban, closed populations) |
| ○ Patterns of movement/travel |
| • Risk to persons prone to severe disease |
| ○ Unvaccinated infants, susceptible pregnant women, severely immunocompromised individuals |
| Specifics of the intervention |
| • Timeliness: Prompt case recognition, reporting, investigation, and vaccination of susceptible contacts can limit spread |
| • Target coverage (e.g., vaccination of >80% of target population) |
| • Target age range: |
| ○ Age groups with highest attack rates vs. all ages |
| ○ If burden is high among infants <12 months of age, measles vaccination of infants as young as 6 months of age should be considered |
| • Selective versus non-selective: |
| ○ Unvaccinated only vs. all, regardless of vaccination status |
| ○ Exposed only vs. exposed and non-exposed |
| • Spatial scale |
| ○ High-risk areas (households, healthcare institutions, schools/colleges, churches, border areas other populated/peri-urban settings) vs. entire community |
| • Outreach: |
| ○ Referral to healthcare provider or local hospital for vaccination or immunoglobulin |
| ○ Vaccination clinics at health departments |
| ○ Community outreach (e.g., door-to-door vaccination) |
Vaccination of susceptible individuals
Guidance and evidence
Vaccination of non-immune individuals is considered the key strategy in limiting the spread of measles during outbreaks. At the individual level, administration of measles vaccine within 72 hours of initial exposure may avert or modify the clinical course of the illness, and is generally recommended as a preventive tool in elimination settings (Table 1).1-4 Recent studies have confirmed a benefit from this intervention, with effectiveness of post-exposure immunization ranging between 91%–100%,54,55 corroborating observations made in the pre-elimination era.56-59 Two studies showing no protection from vaccine prophylaxis were limited by a small sample size60 or by delayed vaccine administration in relation to measles exposure.61
Wider use of this control measure may also affect disease transmission at the community level, by directly reducing the number of secondary measles cases and by increasing immunity to levels that impede sustained spread. Although this has not been evaluated systematically, evidence supporting supplementary vaccination activities during outbreaks is accumulating.62 In studies of varying design and in diverse settings, broad and early implementation of vaccination has been associated with shorter outbreak durations,63 smaller outbreak sizes as determined by mathematical models,64,65 alterations in the shape of epidemic curves and reductions in incident cases,12,66,67 lower than expected morbidity and mortality,66,68 and partial or complete avoidance of outbreaks in closed populations.69,70 Yet, in evaluating measles virus transmissibility during outbreaks, it is often difficult to disentangle the relative effects of reactive immunization versus a depletion of susceptible persons from natural infection, or the effects of other control measures (isolation of cases, quarantining or exclusion of susceptible contacts) and of community behavior (e.g., staying home due to illness). Likewise, more studies are needed to evaluate the starting conditions that may influence the success of vaccination efforts; these conditions might include the size and density of the susceptible group, measles immunization coverage within and surrounding the affected community, the timing of interventions, and the age cohorts targeted.
Challenges
When vaccination is directed to individuals known to have been exposed to measles, the primary challenge is the timely administration of the vaccine to those susceptible contacts, since measles cases can be infectious for four days before the characteristic rash develops and measles is recognized.54 Thus, successful implementation of this strategy requires a close working relationship between healthcare providers and public health specialists and the rapid identification and reporting of cases through active surveillance.54,55
Importantly, wider non-selective immunization, as in a mass vaccination campaign, implies that vaccination may reach at-risk individuals before potential exposure, as has been suggested in recent outbreaks.12,71 As expected, doses of measles vaccine given during outbreaks have been shown to be more effective when administered during pre-exposure compared with post-exposure periods (effectiveness of 79% vs. 50%, respectively).71 Also, children vaccinated more than 14 days before rash onset, i.e., before or around the time of exposure, have lower rates of complications and death.72 Finally, community-wide vaccination helps ensure susceptible groups are up to date with vaccine requirements, closing immunity gaps and increasing herd immunity.1-3
Special considerations
While non-selective immunization activities are recommended by WHO in countries with mortality reduction goals (if the risk of a large outbreak is high and capacity is sufficient),9 such activities have not been as strongly endorsed in elimination settings, where baseline vaccination levels are high and outbreaks occur in defined pockets of under-immunization (Table 1).1-4 A few studies suggest that, in highly vaccinated populations with low measles incidence, targeted campaigns (e.g., campaigns aimed at low coverage areas within a given population, or towards age groups with the highest number of cases) may be of greater benefit.73,74 Per U.S. and PAHO guidelines, for example, lowering the age of the first dose to 6 months of age as an outbreak control measure is dictated by whether or not there are cases among infants aged <12 months of age (Table 1).
Because vaccination campaigns are costly and resource intensive for public health agencies,31,75 deciding on the extent of immunization efforts in these settings should be based on several factors, including the strength of the healthcare infrastructure, the overall risk in the affected subpopulation, and the receptiveness of the community to such an intervention (Table 2). In the latter, delivering outbreak response strategies through culturally suitable approaches (e.g., involving community and spiritual leaders, interpreters, and local public health advisors) is key for their success.11-13,76,77
Use of immunoglobulin to prevent measles in exposed susceptible persons
Guidance
Human immunoglobulin (IG) is prepared from plasma pools derived from thousands of donors and provides passive protection via antibodies against measles. When IG is administered to susceptible persons within six days of initial exposure, it may provide protection or modify the clinical course of the disease (Table 1). Priority is given to individuals without evidence of immunity for whom the risk of severe disease or measles complications is highest; this includes immunocompromised persons, pregnant women, and infants too young to be vaccinated (Table 1). IG prophylaxis can also be considered for other non-immune persons who were exposed through intense or prolonged contact (e.g., in a household, daycare, school, or hospital).1,3
Severely immunocompromised persons are recommended to receive IG regardless of previous vaccination history, since they may still be at risk for developing measles and/or its complications. Infants as young as 6–11 months can be given measles vaccine in place of IG, as long as it is administered within 72 hours of exposure.78 Due to the presence of circulating maternal antibodies, infants <6 months of age may be at lower risk of disease compared to older infants aged 6–11 months. This differential risk, however, may no longer be as evident, given that women of childbearing age now develop immunity almost exclusively from vaccination, which results in lower levels of protective antibodies when compared to immunity following natural infection.1-3
The potency of different IG products varies by country, thus country-specific guidelines should be consulted when determining dosage regimens.79 Typically recommended doses for IG administered intramuscularly (IGIM) range from 0.2 mL to 0.5 mL/kg body weight.1-3 The maximum volume recommended for IGIM is 15mL, thus IGIM may provide less protection if administered to older children or adults who weigh more than 30 kg.78 In part due to these volume limitations, U.S. guidelines recommend IG be administered intravenously (IGIV) to severely immunocompromised persons and susceptible pregnant women exposed to measles.78 The recommended dose of IGIV is 400 mg/kg body weight.78 Of note, patients already receiving subcutaneous immunoglobulin (IGSC) or IGIV therapy are considered protected if, respectively, at least 200 mg/kg body weight was administered for two consecutive weeks, and 400 mg/kg body weight was administered within 3 weeks, before measles exposure.78
Evidence
There is some evidence regarding the effectiveness of IG for disease prevention. Of 13 studies included in a recent meta-analysis, two non-randomized control trials compared gamma globulin to no treatment (the remaining studies used other IG products such as convalescent or adult sera, or had control groups with interventions such as vaccine or other IG products). The combined risk ratio of these two studies was 0.17 (95% CI: 0.08–0.36), demonstrating an 83% decreased risk for measles among persons who receive IG compared with no treatment.80
Assessments of the effectiveness of IG prophylaxis during outbreaks have been possible given the narrow administration window and the prioritization of high-risk individuals, which means that some susceptible contacts will inevitably not receive IG. In recent outbreaks in the United States and Canada, the effectiveness of IG in preventing clinical disease was estimated to be 100% and 69%, respectively, when administered within six days of exposure.81,82 The benefit of IG prophylaxis in preventing measles among (almost all) recipients has also been demonstrated after exposures in various healthcare settings (e.g., in a waiting room, hospital, neonatal intensive care unit, and general pediatric and obstetric wards),83-86 where effective preventive strategies are crucial.
Challenges
Although data supports the use of IG as post-exposure prophylaxis, the effectiveness of IG varies by the potency of the IG lot, with higher antibody levels correlating with greater decrease of measles risk.87 In elimination settings, where there is limited exposure to wild-type measles virus, antibody levels in donor pools are primarily driven by vaccination, and measles-specific antibody concentrations have been decreasing over the years. This, combined with the volume restrictions of IGIM, may make administration of IGIM insufficient for older children and adults, and has important implications for optimal dosing recommendations. In 2013, for example, the U.S. Advisory Committee on Immunization Practices increased the recommended dose of IGIM to 0.5 mL/kg (from 0.25 mL/kg), because of lower antibody concentrations in IGIM in the post-elimination era.78 A potential need to increase the recommended IGIM prophylaxis dose has also been suggested in other elimination settings.82
As with vaccine, timely administration of IG as post-exposure prophylaxis within the six-day window is a challenge. In addition, persons who received IG may still develop measles, although the incubation period might be prolonged and their illness presentation may be unusual. Maintaining a high index of suspicion in individuals who received IG as post-exposure prophylaxis is necessary, and extending the monitoring period (e.g., to 28 days after exposure) is considered in some settings.3
Currently there is a global shortage of plasma-derived products. Where available, IG therapy can be expensive, requiring cold chain and sterile materials for storage and administration.88 An infusion of IGIV additionally requires hospitalization and monitoring of the patient's clinical status, which increases costs further. Finally, unlike the manufacturing practices that are applied to vaccines, there are no WHO quality standards for IG as a product that can be used by regulators.
Special considerations
IG does not confer long-lasting immunity against measles, so IG recipients should be vaccinated to be protected against subsequent measles exposures (provided the vaccine is not otherwise contraindicated, and the person is age-appropriate). Because IG interferes with the immune response to vaccination, immunization is delayed according to country-specific guidelines. In Australia, vaccination is postponed for at least 5 months depending on the dose of IG administered,1 and in the United States, vaccination is delayed for at least 6 months after IGIM and 8 months after IGIV.78 Canadian guidelines recommend different time-periods depending on the dose as well as the type of product and route of administration.89
Outbreak response guidelines from elimination settings recommend the routine use of IG as a measles prevention strategy for susceptible contacts, but in contrast to vaccination, IG is not recommended to control the spread of measles during outbreaks.78
Exclusion measures among exposed individuals who received appropriate post-exposure prophylaxis (vaccine or immunoglobulin)
Deciding on the exclusion of persons who receive post-exposure vaccination or immunoglobulin appropriately—i.e., within the recommended time periods of three and six days, respectively—is challenging. These individuals may still develop disease and become infectious, yet they may be less contagious. In general, lifting of quarantine or exclusion measures is acceptable if these individuals are returning to settings where population immunity is high and where there risk of transmission to individuals at risk for severe disease is low. Often, allowing persons to return to different settings serves as an incentive for these persons to receive post-exposure prophylaxis. Australian and Canadian guidelines, for example, allow the return of persons who received timely post-exposure prophylaxis (both vaccine or immunoglobulin) to early childhood care and education services, including primary schools (Table 1).1 U.S. guidelines are stricter, in that persons are permitted to return to childcare, school, or work after vaccine post-exposure prophylaxis, but the setting's immunity levels, intensity of contact, and the presence of populations at risk need to be considered before allowing persons to return after immunoglobulin post-exposure prophylaxis (Table 1).3 In addition, Canadian and U.S. guidelines specifically recommended susceptible persons not return to healthcare settings after receipt of either vaccine or immunoglobulin.2,3 Irrespective of the decision, persons who receive post-exposure prophylaxis should be monitored for signs and symptoms consistent with measles, and recommended to self-isolate at home from the onset of prodromal symptoms as soon as measles is suspected.1,3
Laboratory confirmation of measles during outbreaks
Guidance
In low-incidence settings, it is vital to pursue laboratory confirmation of all suspected cases of measles. Both a serum sample and a sample for virologic detection should be collected at first contact with every suspected case and forwarded to the laboratory as soon as possible (Table 1). The most commonly used methods to confirm a measles virus infection are detection of measles-specific immunoglobulin M (IgM) in serum by enzyme immunoassay (EIA) and detection of measles RNA by real-time polymerase chain reaction (RT-PCR). The preferred specimens for RT-PCR are throat or nasopharyngeal swabs, but urine samples are also acceptable (Table 1). These assays can usually be performed in 3–4 hours, and a positive result for detection of measles IgM or RNA, plus a clinically compatible illness, confirms a measles case.1,3,4
Other laboratory tests that can confirm a measles virus infection are seroconversion or demonstration of a fourfold rise in IgG titers in paired serum samples and isolation of measles virus in cell culture. The former is used less frequently because of the logistical challenges of collecting multiple serum samples. Virus isolation requires a laboratory that is equipped for cell culture and results from virus isolation may not be available for several weeks.
If the RT-PCR test is positive, it is usually possible to determine the genotype of the measles virus associated with the case. Genotype and sequencing information can help track transmission pathways, link or unlink cases and outbreaks, and identify the source of the virus. Sequence data are submitted to a global sequence database, MeaNS,90 which allows rapid tracking of lineages (referred to as named strains) of measles virus between and among countries.91
Challenges
In areas with low measles disease burden, serologic testing has a poor positive predictive value and confounds the interpretation of measles-specific IgM testing.2,92 In the absence of clinically compatible symptoms or a clear epidemiological link to a laboratory-confirmed case, false-positive IgM results are common.2,92 Thus, it is important to restrict laboratory testing to persons likely to have measles (e.g., those with a febrile rash illness and risk factors such as travel or being unvaccinated) and to obtain routinely specimens for RT-PCR testing along with serological samples.1-3 In this context, when confirming a diagnosis of measles with a positive IgM, Canada has specified the requirement of an appropriate exposure (epidemiological-link to another case or travel history), in addition to measles-compatible symptoms.2,92 Conversely, because measles is rare in elimination settings, suspected cases with a positive IgM necessitate detailed epidemiological investigations for an unrecognized exposure, and they may require additional diagnostic testing before being ruled out.2,4,92
Appropriately-timed laboratory testing is a challenge when case burden is high, but is essential for disease confirmation during outbreaks. Measles IgM antibodies appear in serum within 1–4 days after rash onset and can be detected up to 6–8 weeks after rash. Depending on the sensitivity of the assay used, a proportion of serum samples collected within 72 hours after rash onset may give negative results in an individual with measles. If a negative result is obtained from serum collected within 72 hours after rash onset, it is recommended that a second serum be collected >72 hours after rash onset (Table 1).1-4 As opposed to IgM, RNA detection is more likely to be successful when specimens are collected within three days after rash onset (Table 1), although RNA can be detected as late as 10–12 days after rash onset in some cases. While detection of measles virus RNA confirms a diagnosis of measles, a negative RT-PCR result does not rule out measles because the sensitivity of the method is greatly affected by the timing of specimen collection and by the quality of specimen processing, handling, and shipping.3
During outbreaks, potentially exposed individuals may be vaccinated as part of the outbreak response; approximately 5% will develop rash and fever from vaccination. Because serologic testing is unable to determine whether antibodies were induced by infection or vaccination, determination of the measles genotype provides the only means to distinguish between wild-type virus infection and a rash caused by recent measles vaccination. Since all measles vaccines are genotype A, a genotype that is no longer circulating, RT-PCR followed by sequence analysis can confirm the presence of wild-type or vaccine measles virus. In these situations, genotyping is generally recommended when vaccine was given within 2–3 weeks before rash onset (Table 1).1-3 RT-PCR and sequencing typically take 24–48 hours to complete, but recently, a new real-time RT-PCR assay has been introduced that can identify vaccine viruses in 3–4 hours.93 Of note, human-to-human transmission of the measles vaccine virus has not been documented.94
Special considerations
In elimination settings, most measles cases are in unvaccinated individuals, although some confirmed cases occur among vaccinated or presumptively immune individuals; in recent years in the United States and Canada, for example, 5% and 8% of measles cases had received 1 dose and 5% and 9% had received 2 doses of a measles-containing vaccine, respectively (74% and 63% were unvaccinated).95,96 Suspected measles cases among vaccinated individuals may require additional laboratory testing for confirmation and/or classification. The IgM response in measles-infected vaccinated persons may be brief and/or diminished, and thus a negative IgM result does not rule out measles; detection of measles RNA by RT-PCR may be the best method to confirm these cases.3 Confirmed measles in a previously vaccinated individual can be classified as a primary vaccine failure by measurement of low-avidity measles IgG antibody.38 Individuals with confirmed measles and a prior immunologic response to measles (i.e., reinfection cases) can be identified by the presence of high-avidity measles IgG antibody. A reinfection case in an individual who had measurable specific antibodies after documented vaccination constitutes a secondary vaccine failure. In some reinfection cases, results from the IgM EIA or RT-PCR may be unavailable or inconclusive. If these cases have high-avidity measles IgG, a diagnosis of reinfection is supported by measuring neutralizing antibody concentrations of ≥40,000 mIU/mL.97
The presence of measles-specific immunoglobulin G (IgG) indicates measles immunity; thus, IgG antibody testing before vaccination is sometimes considered for contacts who have an unknown vaccination history. Most often, however, serologic screening of contacts during an outbreak is not recommended, since it is generally not feasible to obtain the results in a timely manner without delaying post-exposure prophylaxis.1,3 In addition, vaccination is safe in individuals who are immune.1 If IgG testing is performed, the results can inform need for a second vaccine dose.3 Serologic screening to determine immunity is particularly discouraged following exposures in healthcare settings by U.S. guidelines,3 however, Canadian guidelines recommend IgG testing for the management of measles contacts in healthcare settings (Table 3).2 Overall, rapid IgG testing is considered more suitable prior to immunoglobulin administration,3 particularly for immunocompromised persons with uncertain immunization histories,1 and as long as immunoglobulin administration is not delayed. IgG testing may also be useful for persons who do not have written documentation of vaccination but who believe they were previously vaccinated and prefer not to be given another dose; a positive result would allow them to return to school or other setting where an outbreak is occurring.
Table 3.
General guidance for measles outbreak control in healthcare settings in three elimination settings.
| Strategy | United States | Canada | Australia |
|---|---|---|---|
| Exposure | • Closed settings | • Room or enclosed space | • Shared defined air-space |
| • ≤2 hours after infectious case left | • ≤2 hours after infectious case left | • ≤30 minutes after infectious case left | |
| Isolation of case-patients while in hospital | • Airborne precautions | • Airborne precautions | • Airborne precautions |
| • Through 4 days after rash onset | • Onset of symptoms to ≤4 days after rash onset | • Through 4 days after rash onset | |
| Caring for isolated case-patient | • Only staff who are immune | • Only staff who are immune | • Only staff who are immune |
| • N95 respirator even if immune | • No additional precautions (respirators) needed | ||
| Transporting infectious case-patient | • Should wear a mask | • NS | • Should wear a mask |
| Isolation (quarantine) of exposed susceptible patients while in hospital | • Airborne precautions | • Airborne precautions | • Airborne precautions |
| • Through 21 days after exposure | • ≥5 days to ≤21 days after last exposure, | • Through 18 days after last exposure | |
| Exclusion of case-staff from facility | • Through 4 days after rash onset | • Through 4 days after rash onset | • Through 4 days after rash onset |
| Exclusion of exposed susceptible staff from facility and patient contact | • ≥5 days to ≤21 days after exposure | • ≥5 days to ≤21 days after last exposure, | • Through 18 days after last exposure |
| Post-exposure prophylaxis (PEP) of susceptible contacts | |||
| -Vaccine | • ≤72 hours of first exposure | • ≤72 hours of first exposure (implied) | • ≤72 hours of first exposure |
| -Immunoglobulin (IG) | • ≤6 days of exposure | • ≤6 days of exposure (implied) | • ≤6 days of exposure |
| Return of isolated patients to floor after timely PEP | • Allowed in hospital settings (implied) | • Not allowed in hospital settings | • Allowed in hospital settings (implied) |
| Return of excluded staff to work after timely PEP | • Not allowed in hospital settings | • Not allowed in hospital settings | • Allowed in hospital settings (implied) |
Note: NS = Not specified; Information comes from References 1–3, 93–94.
E.g., waiting area, assessment room, ward.
Negative-pressure room; if unavailable, a single room with the door closed and away from susceptible contacts.
Regardless of whether they received post-exposure prophylaxis (vaccine or immunoglobulin).
In Canada, healthcare workers and patients with no documented doses of a measles-containing vaccine, no other evidence of immunity, or with 1 documented dose, are recommended to be tested for measles IgG antibody, receive one dose of MMR vaccine, and excluded from work (staff) or isolated (patients) pending results. If IgG results are positive, healthcare workers and patients are allowed to return; if negative, healthcare workers and patients should be vaccinated with a second dose (28 days after the first dose), and excluded (staff) or isolated (patients) regardless of whether they received post-exposure prophylaxis.
Did not receive vaccine within 72 hours or immunoglobulin within 6 days.
Use of oral fluid testing for seroprevalence studies98 and for diagnostic and genotyping purposes99–104 has proven valuable in a several European countries over the last decade. Collection of oral fluid specimens is less invasive than collection of serum and self-collection of oral fluid is possible.105 Another advantage is that oral fluid is a good sample for detection of measles viral RNA by RT-PCR. Therefore, oral fluid may play an increasing role for laboratory confirmation of measles, and in monitoring population immunity and identifying subpopulations at risk for measles in other settings.106 Use of oral fluid for detection of measles-specific IgM in a point-of-care test may also prove valuable for the early recognition of and response to measles cases and outbreaks.107,108
Containment strategies in healthcare settings
Because healthcare workers are at a higher risk of both being exposed to measles and of transmitting the virus to persons at risk of severe disease (e.g., immunocompromised persons), guidelines for measles control in healthcare facilities are generally stricter.109,110 Ideally, providers and administrative staff of healthcare facilities should be fully vaccinated or have other presumptive evidence of immunity.1–4,110 Documenting evidence of immunity to measles is recommended for all persons working in healthcare settings who have potential for exposure to patients and/or infectious materials.1–3 In some settings, vaccination may be a condition of employment.2 During healthcare facility measles outbreaks in elimination settings, the following control measures and procedures are undertaken (details are included in Table 3).
Immediate review of evidence of measles immunity among staff to ensure compliance with recommendations.1,3,110 To expedite control measures, staff immunization records should be readily available in computerized form at the facility for easy access.3
Vaccination of healthcare personnel without evidence of immunity.1–3,110
Identification and follow-up of potentially exposed persons (e.g., patients, healthcare personnel). Exposures may occur in waiting areas, emergency departments, wards, patient rooms, and laboratory or radiology areas. Healthcare personnel include but aren't limited to physicians, nurses, nursing assistants, technicians, volunteers, trainees, clerical staff, and environmental services staff1,3,110 Some exposed patients may have been discharged, and other exposed persons may be visitors.1
Provision of post-exposure prophylaxis (vaccine or immunoglobulin) to susceptible contacts. Vaccination includes giving a second dose to healthcare workers that have received only one dose of a measles-containing vaccine.1,2
Isolation of case-patients and of exposed susceptible patients in airborne infection isolation rooms.1,2,109
Exclusion of exposed susceptible healthcare personnel and those with known or suspected measles from the facility.1,2
Active surveillance, including prompt testing of patients and staff with prodromal symptoms; suspected cases are treated as confirmed pending laboratory results.1
Implementation of control measures within the facility. Hospitals usually have the main responsibility for implementing these measures in their facility;1 these may be coordinated by occupational health in consultation with local health departments.2
Conclusions
Until measles is eradicated globally, importations of measles virus will relentlessly challenge herd immunity and public health systems in all countries that have achieved or are close to achieving elimination of endemic measles transmission. Responding to measles outbreaks can be enormously expensive and disruptive to health services and society. To achieve maximal impact from reactive outbreak response strategies to limit the scale of outbreaks—in terms of case numbers, morbidity, and generations of transmission—it is critical to have sensitive public health surveillance operating reliably and universally to rapidly detect and vigorously respond to every suspected measles case. The relatively short incubation period of measles, the remarkable infectiousness of the virus, and the reality that transmission is occurring for four days before typical rash onset, demand immediate investigation, action, and resourcing akin to responding to a public health emergency.
However, although necessary, outbreak management is often insufficient to control measles virus transmission. Despite even formidable responsive efforts, the measles virus is adroit at discovering permissive transmission environments, with effective reproduction numbers approaching or exceeding one, and sleuthing out any existing immunity gaps. The only truly foolproof means to limit the extent of measles outbreaks and the contingent morbidity, mortality, and economic burden posed by measles importations in all countries is to maintain, via high immunization coverage, robust herd immunity throughout the population. It is thus essential to interrogate every outbreak and patterns of outbreaks, so as to pinpoint communities with geographical or shared socio-cultural features that are consistently missing out on the benefits of measles vaccination, or to identify settings allowing a greater opportunity for measles transmission.111 Targeting vaccination strategies to fill these immunity gaps can be a valuable legacy of thorough outbreak investigations.
Footnotes
Isolation: Separation of ill persons known or suspected to be infectious to limit the spread of disease to others; quarantine: Separation or restriction of movement of potentially exposed susceptible persons who are well but who might become ill and infectious in order to limit the spread of disease to others; exclusion: Restriction of susceptible persons (exposed or unexposed) from specific outbreak settings to protect them from exposure to a disease or to reduce the risk of spreading the disease to others in those settings.
Here, and in the rest of the document, we refer specifically to national measles control guidelines from Australia, Canada, the United States, and the Region of the Americas. Of note, guidance may vary at the state/local or provincial/territorial levels.
Abbreviations
- EIA
enzyme immunoassay
- IHR
International Health Regulations
- IG
immunoglobulin
- IGIM
immunoglobulin administered intramuscularly
- IGIV
immunoglobulin administered intravenously
- IGSC
immunoglobulin administered subcutaneously
- IgM
immunoglobulin M
- IgG
immunoglobulin G
- PAHO
Pan American Health Organization
- RT-PCR
real-time polymerase chain reaction
- RCM
Rapid Coverage Monitoring
- WHO
World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Ellen Laine, Kathryn Como-Sabetti, and Jayne Griffith from the Minnesota Department of Health.
Funding
The authors have indicated that they have no financial relationships relevant to this article.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.Measles Communicable Diseases Network Australia National Guidelines for Public Health Units. February 2015 2015. http://www.health.gov.au/internet/main/publishing.nsf/Content/BD2AD79FD34BFD14CA257BF0001D3C59/$File/Measles-SoNG-final-April2015.pdf (accessed January132017).
- 2.Guidelines for the prevention and control of measles outbreaks in Canada. An Advisory Committee Statement (ACS) Measles and Rubella Elimination Working Group (MREWG). October 2013 2013. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-3/assets/pdf/meas-roug-eng.pdf (accessed January132017). [DOI] [PMC free article] [PubMed]
- 3.CDC Manual for the Surveillance of Vaccine-Preventable Diseases. Chapter 7: Measles. April 1, 2014 2013 http://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.pdf (accessed September222015).
- 4.PAHO Measles Elimination Field Guide. 2005. http://www1.paho.org/hq/dmdocuments/2010/FieldGuide_Measles_2ndEd_e.pdf (accessed April232016).
- 5.Heywood AE, Gidding HF, Riddell MA, McIntyre PB, MacIntyre CR, Kelly HA. Elimination of endemic measles transmission in Australia. Bull World Health Organ 2009;87(1):64–71. doi: 10.2471/BLT.07.046375. PMID:19197406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papania MJ, Wallace GS, Rota PA, Icenogle JP, Fiebelkorn AP, Armstrong GL, Reef SE, Redd SB, Abernathy ES, Barskey AE, et al.. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr 2014;168(2):148–55. doi: 10.1001/jamapediatrics.2013.4342. PMID:24311021 [DOI] [PubMed] [Google Scholar]
- 7.PAHO Region of the Americas is declared free of measles. 2016. http://www.paho.org/hq/index.php?option = com_content&view = article&id = 12528:region-americas-declared-free-measles&Itemid = 1926&lang = en (accessed October25, 2016 2016).
- 8.King A, Varughese P, De Serres G, Tipples GA, Waters J, Working group on measles E. Measles elimination in Canada. J Infect Dis 2004;189 Suppl 1: S236–42. doi: 10.1086/378499. PMID:15106117 [DOI] [PubMed] [Google Scholar]
- 9.WHO Response to measles outbreaks in measles mortality reduction settings: Immunization, Vaccines and Biologicals. Geneva: WHO Press; 2009. [PubMed] [Google Scholar]
- 10.Chatterji M, Baldwin AM, Prakash R, Vlack SA, Lambert SB. Public health response to a measles outbreak in a large correctional facility, Queensland, 2013. Commun Dis Intell Q Rep. 2014;38(4):E294–7. PMID:25631590 [DOI] [PubMed] [Google Scholar]
- 11.Kershaw TSV, Simmonds K, St. Jean T. Outbreak of measles in a non-immunizing population. Alberta 2013. Canada Communicable Disease Report CCDR; 2014; 40–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gastanaduy PA, Budd J, Fisher N, Redd SB, Fletcher J, Miller J, McFadden DJ 3rd, Rota J, Rota PA, Hickman C, et al.. A measles outbreak in an underimmunized amish community in Ohio. N Engl J Med 2016;375(14):1343–54. doi: 10.1056/NEJMoa1602295. PMID:27705270 [DOI] [PubMed] [Google Scholar]
- 13.Hall V, Banerjee E, Kenyon C, et al.. Measles Outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep 2017;66(27):713–7. doi: 10.15585/mmwr.mm6627a1. PMID:28704350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahr P, DeVries AS, Wallace G, Miller C, Kenyon C, Sweet K, Martin K, White K, Bagstad E, Hooker C, et al.. An outbreak of measles in an undervaccinated community. Pediatrics 2014;134(1):e220–8. doi: 10.1542/peds.2013-4260. PMID:24913790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nic Lochlainn LM, Woudenberg T, van Lier A, Zonnenberg I, Philippi M, de Melker HE, Hahné SJM. A novel measles outbreak control strategy in the Netherlands in 2013–2014 using a national electronic immunization register: A study of early MMR uptake and its determinants. Vaccine 2017;35(43):5828–34. doi: 10.1016/j.vaccine.2017.09.018. PMID:28923422 [DOI] [PubMed] [Google Scholar]
- 16.Luman ET, Cairns KL, Perry R, Dietz V, Gittelman D. Use and abuse of rapid monitoring to assess coverage during mass vaccination campaigns. Bull World Health Organ 2007;85(9):651. doi: 10.2471/BLT.07.045328. PMID:18026614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonnesbeck CJ, Shea K, Carran S, Cassio de Moraes J, Gregory C, Goodson JL, Ferrari MJ. Measles outbreak response decision-making under uncertainty: a retrospective analysis. J R Soc Interface 2018;15(140). doi: 10.1098/rsif.2017.0575. PMID:29563241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam E, Schluter WW, Masresha BG, Teleb N, Bravo-Alcántara P, Shefer A, Jankovic D, McFarland J, Elfakki E, Takashima Y, et al.. Development of a district-level programmatic assessment tool for risk of measles virus transmission. Risk Anal. 2017;37(6):1052–62. doi: 10.1111/risa.12409. PMID:25976980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemos DR, Franco AR, de Oliveira Garcia MH, et al.. Risk analysis for the reintroduction and transmission of measles in the post-elimination period in the Americas. Rev Panam Salud Publica 2017;41:e121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung LF, Lurie P, Dayan G, Eduardo E, Britz PH, Redd SB, Papania MJ, Seward JF. A limited measles outbreak in a highly vaccinated US boarding school. Pediatrics 2005;116(6):1287–91. doi: 10.1542/peds.2004-2718. PMID:16322148 [DOI] [PubMed] [Google Scholar]
- 21.Alessa A, Faezipour M. A review of influenza detection and prediction through social networking sites. Theor Biol Med Model 2018;15(1):2. doi: 10.1186/s12976-017-0074-5. PMID:29386017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah MP, Lopman BA, Tate JE, Harris J, Esparza-Aguilar M, Sanchez-Uribe E, Richardson V, Steiner CA, Parashar UD. Use of Internet Search Data to Monitor Rotavirus Vaccine Impact in the United States, United Kingdom, and Mexico. J Pediatric Infect Dis Soc 2018;7(1):56–63. doi: 10.1093/jpids/pix004. PMID:28369477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broniatowski DA, Hilyard KM, Dredze M. Effective vaccine communication during the disneyland measles outbreak. Vaccine 2016;34(28):3225–8. doi: 10.1016/j.vaccine.2016.04.044. PMID:27179915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deiner MS, Fathy C, Kim J, Niemeyer K, Ramirez D, Ackley SF, Liu F, Lietman TM, Porco TC. Facebook and Twitter vaccine sentiment in response to measles outbreaks. Health Informatics J. 2017: 1460458217740723. PMID:29148313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollema L, Harmsen IA, Broekhuizen E, Clijnk R, De Melker H, Paulussen T, Kok G, Ruiter R, Das E. Disease detection or public opinion reflection? Content analysis of tweets, other social media, and online newspapers during the measles outbreak in The Netherlands in 2013. J Med Internet Res 2015;17(5):e128. doi: 10.2196/jmir.3863. PMID:26013683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radzikowski J, Stefanidis A, Jacobsen KH, Croitoru A, Crooks A, Delamater PL. The measles vaccination narrative in twitter: A quantitative analysis. JMIR Public Health Surveill. 2016;2(1):e1. doi: 10.2196/publichealth.5059. PMID:27227144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durrheim DN. Measles Elimination – Using outbreaks to identify and close immunity gaps. N Engl J Med. 2016;375(14):1392–3. doi: 10.1056/NEJMe1610620. PMID:27705259 [DOI] [PubMed] [Google Scholar]
- 28.Najjar Z, Hope K, Clark P, Nguyen O, Rosewell A, Conaty S. Sustained outbreak of measles in New South Wales, 2012: risks for measles elimination in Australia. Western Pac Surveill Response J. 2014;5(1):14–20. doi: 10.5365/wpsar.2013.4.4.001. PMID:25635228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson N, Andrews R, Riddell M, Leydon J, Lynch P. Outbreak investigation t. A measles outbreak among young adults in Victoria, February 2001. Commun Dis Intell Q Rep. 2002;26(2):273–8. PMID:12206382 [DOI] [PubMed] [Google Scholar]
- 30.Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, Lebaron CW. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747–55. doi: 10.1542/peds.2009-1653. PMID:20308208 [DOI] [PubMed] [Google Scholar]
- 31.Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, Wallace GS. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine 2014;32(11):1311–7. doi: 10.1016/j.vaccine.2013.10.012. PMID:24135574 [DOI] [PubMed] [Google Scholar]
- 32.Parker AA, Staggs W, Dayan GH, Ortega-Sánchez IR, Rota PA, Lowe L, Boardman P, Teclaw R, Graves C, LeBaron CW. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355(5):447–55. doi: 10.1056/NEJMoa060775. PMID:16885548 [DOI] [PubMed] [Google Scholar]
- 33.Enanoria WT, Liu F, Zipprich J, Harriman K, Ackley S, Blumberg S, Worden L, Porco TC. The effect of contact investigations and public health interventions in the control and prevention of measles transmission: A simulation study. PLoS One 2016;11(12):e0167160. doi: 10.1371/journal.pone.0167160. PMID:27941976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas S, Hiebert J, Gubbay JB, Gournis E, Sharron J, Severini A, Jiaravuthisan M, Shane A, Jaeger V, Crowcroft NS et al.. Measles outbreak with unique virus genotyping, Ontario, Canada, 2015. Emerg Infect Dis. 2017;23(7):1063–9. doi: 10.3201/eid2307.161145. PMID:28628461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkat H, Kassem AM, Su CP, Hill C, Timme E, Briggs G, Komatsu K, Robinson S, Sunenshine R, Patel M, et al.. Notes from the field: Measles Outbreak at a United States immigration and customs enforcement facility – Arizona, May-June 2016. MMWR Morb Mortal Wkly Rep 2017;66(20):543–4. doi: 10.15585/mmwr.mm6620a5. PMID:28542125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO WHO-recommended standards for surveillance of selected vaccine-preventable diseases 2003. http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf.
- 37.Delaporte E, Wyler Lazarevic CA, Iten A, Sudre P. Large measles outbreak in Geneva, Switzerland, January to August 2011: descriptive epidemiology and demonstration of quarantine effectiveness. Euro Surveill. 2013;18(6):18–25. PMID:23410259 [PubMed] [Google Scholar]
- 38.Rosen JB, Rota JS, Hickman CJ, Sowers SB, Mercader S, Rota PA, Bellini WJ, Huang AJ, Doll MK, Zucker JR, et al.. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin Infect Dis. 2014;58(9):1205–10. doi: 10.1093/cid/ciu105. PMID:24585562 [DOI] [PubMed] [Google Scholar]
- 39.Oya M, Hakozaki Y, Kubota T. Modified Measles. Intern Med 2017;56(7):885. doi: 10.2169/internalmedicine.56.7639. PMID:28381764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohil SK, Okubo S, Klish S, Dickey L, Huang SS, Zahn M. Healthcare workers and post-elimination era measles: Lessons on acquisition and exposure prevention. Clin Infect Dis 2016;62(2):166–72. doi: 10.1093/cid/civ802. PMID:26354971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rota JS, Hickman CJ, Sowers SB, Rota PA, Mercader S, Bellini WJ. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J Infect Dis. 2011;204 Suppl 1: S559–63. doi: 10.1093/infdis/jir098. PMID:21666213 [DOI] [PubMed] [Google Scholar]
- 42.Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183(4):532–8. doi: 10.1086/318533. PMID:11170977 [DOI] [PubMed] [Google Scholar]
- 43.CDC Quarantine and Isolation. https://www.cdc.gov/quarantine/index.html. (accessed February222017).
- 44.Institute of Medicine (IOM). Ethical and Legal Considerations in Mitigating Pandemic Disease, Workshop Summary. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 45.Ontario Infectious Diseases Protocol Appendix A. Chapter Measles 2014. http://www.health.gov.on.ca/en/pro/programs/publichealth/oph_standards/docs/measles_chapter.pdf.
- 46.Yang YT, Silverman RD. Social distancing and the unvaccinated. N Engl J Med. 2015;372(16):1481–3. doi: 10.1056/NEJMp1501198. PMID:25806793 [DOI] [PubMed] [Google Scholar]
- 47.CDC Control of Communicable Diseases. A Rule by the Health and Human Services Department. 2017. https://www.federalregister.gov/documents/2017/01/19/2017-00615/control-of-communicable-diseases. (accessed March222017).
- 48.Centers for Disease C, Prevention Postexposure prophylaxis, isolation, and quarantine to control an import-associated measles outbreak–Iowa, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(41):969–71. PMID:15496826 [PubMed] [Google Scholar]
- 49.Collier MG, Cierzniewski A, Duszynski T, Munson C, Wenger M, Beard B, Gentry R, Duwve J, Kutty PK, Pontones P. Measles outbreak associated with international travel, Indiana, 2011. J Pediatric Infect Dis Soc. 2013;2(2):110–8. doi: 10.1093/jpids/pis132. PMID:26619458 [DOI] [PubMed] [Google Scholar]
- 50.Dayan GH, Ortega-Sanchez IR, LeBaron CW, Quinlisk MP, Iowa Measles Response T. The cost of containing one case of measles: the economic impact on the public health infrastructure–Iowa, 2004. Pediatrics. 2005;116(1):e1–4. doi: 10.1542/peds.2004-2512. PMID:15995008 [DOI] [PubMed] [Google Scholar]
- 51.Clemmons NS, Wallace GS, Patel M, Gastanaduy PA. Incidence of Measles in the United States, 2001–2015. JAMA. 2017;318(13):1279–81. doi: 10.1001/jama.2017.9984. PMID:28973240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poethko-Muller C, Ellert U, Kuhnert R, Neuhauser H, Schlaud M, Schenk L. Vaccination coverage against measles in German-born and foreign-born children and identification of unvaccinated subgroups in Germany. Vaccine. 2009;27(19):2563–9. doi: 10.1016/j.vaccine.2009.02.009. PMID:19428862 [DOI] [PubMed] [Google Scholar]
- 53.Reynolds DL, Garay JR, Deamond SL, Moran MK, Gold W, Styra R. Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiol Infect. 2008;136(7):997–1007. doi: 10.1017/S0950268807009156. PMID:17662167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barrabeig I, Rovira A, Rius C, Muñoz P, Soldevila N, Batalla J, Domínguez A. Effectiveness of measles vaccination for control of exposed children. Pediatr Infect Dis J. 2011;30(1):78–80. doi: 10.1097/INF.0b013e3181f7001c. PMID:20844460 [DOI] [PubMed] [Google Scholar]
- 55.Sheppeard V, Forssman B, Ferson MJ, Moreira C, Campbell-Lloyd S, Dwyer DE, McAnulty JM. The effectiveness of prophylaxis for measles contacts in NSW. N S W Public Health Bull. 2009;20(5-6):81–5. doi: 10.1071/NB08014. PMID:19552854 [DOI] [PubMed] [Google Scholar]
- 56.Brody JA, Haseley R. A measles epidemic in an Alaskan boarding school. Northwest Med. 1965;64(12):938–41. PMID:5848890 [PubMed] [Google Scholar]
- 57.Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr. 1978;93(1):43–6. doi: 10.1016/S0022-3476(78)80597-6. PMID:650343 [DOI] [PubMed] [Google Scholar]
- 58.Watson GI. Protection after exposure to measles by attenuated vaccine without gamma-globulin. Br Med J. 1963;1(5334):860–1. doi: 10.1136/bmj.1.5334.860. PMID:13999209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krugman S, Giles JP, Jacobs AM, Friedman H. Studies with a further attenuated live measles-virus vaccine. Pediatrics 1963;31: 919–28. PMID:13927100 [PubMed] [Google Scholar]
- 60.Rice P, Young Y, Cohen B, Ramsay M. MMR immunisation after contact with measles virus. Lancet. 2004;363(9408):569–70. doi: 10.1016/S0140-6736(04)15553-0. PMID:14975626 [DOI] [PubMed] [Google Scholar]
- 61.King GE, Markowitz LE, Patriarca PA, Dales LG. Clinical efficacy of measles vaccine during the 1990 measles epidemic. Pediatr Infect Dis J. 1991;10(12):883–8. PMID:1766702 [DOI] [PubMed] [Google Scholar]
- 62.Cairns KL, Perry RT, Ryman TK, Nandy RK, Grais RF. Should outbreak response immunization be recommended for measles outbreaks in middle- and low-income countries? An update. J Infect Dis. 2011;204 Suppl 1: S35–46. doi: 10.1093/infdis/jir072. PMID:21666184 [DOI] [PubMed] [Google Scholar]
- 63.Guris D, Auerbach SB, Vitek C, Maes E, McCready J, Durand M, Cruz K, Iohp K, Haddock R, Rota J, et al.. Measles outbreaks in Micronesia, 1991 to 1994. Pediatr Infect Dis J. 1998;17(1):33–9. doi: 10.1097/00006454-199801000-00008. PMID:9469392 [DOI] [PubMed] [Google Scholar]
- 64.Grais RF, Conlan AJ, Ferrari MJ, Djibo A, Le Menach A, Bjørnstad ON, Grenfell BT. Time is of the essence: exploring a measles outbreak response vaccination in Niamey, Niger. J R Soc Interface. 2008;5(18):67–74. doi: 10.1098/rsif.2007.1038. PMID:17504737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonacic Marinovic AA, Swaan C, Wichmann O, van Steenbergen J, Kretzschmar M. Effectiveness and timing of vaccination during school measles outbreak. Emerg Infect Dis. 2012;18(9):1405–13. doi: 10.3201/eid1809.111578. PMID:22931850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sniadack DH, Moscoso B, Aguilar R, Heath J, Bellini W, Chiu MC. Measles epidemiology and outbreak response immunization in a rural community in Peru. Bull World Health Organ. 1999;77(7):545–52. PMID:10444877 [PMC free article] [PubMed] [Google Scholar]
- 67.Alberti KP, King LA, Burny ME, Ilunga BK, Grais RF. Reactive vaccination as an effective tool for measles outbreak control in measles mortality reduction settings, Democratic Republic of Congo, 2005–2006. Int Health. 2010;2(1):65–8. doi: 10.1016/j.inhe.2009.12.009. PMID:24037053 [DOI] [PubMed] [Google Scholar]
- 68.Goodson JL, Wiesen E, Perry RT, Mach O, Kitambi M, Kibona M, Luman ET, Cairns KL. Impact of measles outbreak response vaccination campaign in Dar es Salaam, Tanzania. Vaccine. 2009;27(42):5870–4. doi: 10.1016/j.vaccine.2009.07.057. PMID:19656496 [DOI] [PubMed] [Google Scholar]
- 69.Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med. 1963;269: 75–7. doi: 10.1056/NEJM196307112690204. PMID:13970852 [DOI] [PubMed] [Google Scholar]
- 70.Al Wahaibi SE-BH, Al-Sulaiman MA. Measles outbreak in Riyadh city, 1997. Saudi Epidemiol Bull. 1997;4: 4–5. [Google Scholar]
- 71.Hales CM, Johnson E, Helgenberger L, Papania MJ, Larzelere M, Gopalani SV, Lebo E, Wallace G, Moturi E, Hickman CJ, et al.. Measles outbreak associated with low vaccine effectiveness among adults in Pohnpei State, Federated States of Micronesia, 2014. Open Forum Infect Dis. 2016;3(2):ofw064. doi: 10.1093/ofid/ofw064. PMID:27186587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mgone JM, Mgone CS, Duke T, Frank D, Yeka W. Control measures and the outcome of the measles epidemic of 1999 in the Eastern Highlands Province. P N G Med J. 2000;43(1-2):91–7. PMID:11407624 [PubMed] [Google Scholar]
- 73.Minetti A, Bopp C, Fermon F, François G, Grais RF, Grout L, Hurtado N, Luquero FJ, Porten K, Sury L, et al.. Measles outbreak response immunization is context-specific: insight from the recent experience of Medecins Sans Frontieres. PLoS Med. 2013;10(11):e1001544. doi: 10.1371/journal.pmed.1001544. PMID:24223523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minetti A, Hurtado N, Grais RF, Ferrari M. Reaching hard-to-reach individuals: Nonselective versus targeted outbreak response vaccination for measles. Am J Epidemiol. 2014;179(2):245–51. doi: 10.1093/aje/kwt236. PMID:24131555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the Postelimination Era, 2001–2014. Clin Infect Dis 2015;61(4):615–8. doi: 10.1093/cid/civ387. PMID:25979309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahta L, Ashkir A. Addressing MMR Vaccine Resistance in Minnesota's Somali Community. Minn Med 2015;98(10):33–6. PMID:26596077 [PubMed] [Google Scholar]
- 77.Kennedy AM, Gust DA. Measles outbreak associated with a church congregation: a study of immunization attitudes of congregation members. Public Health Rep 2008;123(2):126–34. doi: 10.1177/003335490812300205. PMID:18457065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease C, Prevention . Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1–34. PMID:23760231 [PubMed] [Google Scholar]
- 79.Young MK, Bertolini J, Kotharu P, Maher D, Cripps AW. Do Australian immunoglobulin products meet international measles antibody titer standards? Hum Vaccin Immunother. 2017;13(3):607–12. doi: 10.1080/21645515.2016.1234554. PMID:27763809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014; (4):CD010056. PMID:24687262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65:1843–7. doi: 10.1093/cid/cix639. [DOI] [PubMed] [Google Scholar]
- 82.Bigham M, Murti M, Fung C, Hemming F, Loadman S, Stam R, Van Buynder P, Lem M. Estimated protective effectiveness of intramuscular immune serum globulin post-exposure prophylaxis during a measles outbreak in British Columbia, Canada, 2014. Vaccine. 2017;35(20):2723–7. doi: 10.1016/j.vaccine.2017.03.069. PMID:28392140 [DOI] [PubMed] [Google Scholar]
- 83.Lejeune A, Martin L, Santibanez S, Thee S, Gratopp A, Späth P, Mankertz A, Kallinich T, von Bernuth H. Postexposure prophylaxis with intravenous immunoglobulin G prevents infants from getting measles. Acta Paediatr. 2017;106(1):174–7. doi: 10.1111/apa.13634. PMID:27748542 [DOI] [PubMed] [Google Scholar]
- 84.Tapisiz A, Polat M, Kara SS, Tezer H, Simsek H, Aktas F. Prevention of measles spread on a paediatric ward. Epidemiol Infect. 2015;143(4):720–4. doi: 10.1017/S0950268814001344. PMID:24877882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Charlier C, Hourrier S, Leruez-Ville M, Zahar JR, Floret D, Salomon LJ, Toubiana J, Lecuit M, Lapillonne A, Lortholary O, et al.. Polyvalent immunoglobulins in neonates after perinatal exposure to measles: Benefits and long-term tolerance of immunoglobulins. J Infect. 2015;71(1):131–4. doi: 10.1016/j.jinf.2015.01.010. PMID:25620798 [DOI] [PubMed] [Google Scholar]
- 86.Ayscue P, Kamali A, Rutledge J, et al. Measles Exposure in a Neonatal Intensive Care Unit and Labor and Delivery Ward— California, January 2015. Open Forum Infect Dis. 2015;2:1171–1171. [Google Scholar]
- 87.Endo A, Izumi H, Miyashita M, Taniguchi K, Okubo O, Harada K. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. J Pediatr. 2001;138(6):926–8. doi: 10.1067/mpd.2001.113710. PMID:11391343 [DOI] [PubMed] [Google Scholar]
- 88.Plemper RK, Snyder JP. Measles control–can measles virus inhibitors make a difference? Curr Opin Investig Drugs. 2009;10(8):811–20. PMID:19649926 [PMC free article] [PubMed] [Google Scholar]
- 89.Guidelines for the interval between administration of immune globulin (Ig) preparations or blood products and measles-mumps-rubella (MMR), measles-mumps-rubella-varicella (MMRV) or univalent varicella vaccine to maximize immunization effectiveness https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-11-blood-products-human-immune-globulin-timing-immunization.html#p1c10t1.
- 90.Genetic diversity of wild-type measles viruses and the global measles nucleotide surveillance database (MeaNS). Wkly Epidemiol Rec. 2015;90(30):373–80. PMID:26211016 [PubMed] [Google Scholar]
- 91.Rota PA, Brown KE, Hubschen JM, Muller CP, Icenogle J, Chen MH, Bankamp B, Kessler JR, Brown DW, Bellini WJ, et al.. Improving global virologic surveillance for measles and rubella. J Infect Dis. 2011;204 Suppl 1: S506–13. doi: 10.1093/infdis/jir117. PMID:21666207 [DOI] [PubMed] [Google Scholar]
- 92.Bolotin S, Lim G, Dang V, Crowcroft N, Gubbay J, Mazzulli T, Schabas R. The utility of measles and rubella IgM serology in an elimination setting, Ontario, Canada, 2009–2014. PLoS One. 2017;12(8):e0181172. doi: 10.1371/journal.pone.0181172. PMID:28850604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roy F, Mendoza L, Hiebert J, McNall RJ, Bankamp B, Connolly S, Lüdde A, Friedrich N, Mankertz A, Rota PA, et al.. Rapid Identification of Measles Virus Vaccine Genotype by Real-Time PCR. J Clin Microbiol. 2017;55(3):735–43. doi: 10.1128/JCM.01879-16. PMID:27852670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenwood KP, Hafiz R, Ware RS, Lambert SB. A systematic review of human-to-human transmission of measles vaccine virus. Vaccine. 2016;34(23):2531–6. doi: 10.1016/j.vaccine.2016.03.092. PMID:27083423 [DOI] [PubMed] [Google Scholar]
- 95.De Serres G, Desai S, Shane A, Hiebert J, Ouakki M, Severini A. Measles in Canada Between 2002 and 2013. Open Forum Infect Dis. 2015;2(2):ofv048. doi: 10.1093/ofid/ofv048. PMID:26110163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fiebelkorn AP, Redd SB, Gastanaduy PA, Clemmons N, Rota PA, Rota JS, Bellini WJ, Wallace GS. A comparison of postelimination measles epidemiology in the United States, 2009–2014 Versus 2001–2008. J Pediatric Infect Dis Soc. 2017;6(1):40–8. PMID:26666559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sowers SB, Rota JS, Hickman CJ, Mercader S, Redd S, McNall RJ, Williams N, McGrew M, Walls ML, Rota PA, et al.. High concentrations of measles neutralizing antibodies and high-avidity measles IgG accurately identify measles reinfection cases. Clin Vaccine Immunol. 2016;23(8):707–16. doi: 10.1128/CVI.00268-16. PMID:27335386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vainio K, Samdal HH, Anestad G, Wedege E, Skutlaberg DH, Bransdal KT, Mundal R, Aaberge IS. Detection of measles- and mumps-specific IgG antibodies in paired serum and oral fluid samples from Norwegian conscripts. Eur J Clin Microbiol Infect Dis. 2008;27(6):461–5. doi: 10.1007/s10096-008-0460-3. PMID:18293018 [DOI] [PubMed] [Google Scholar]
- 99.Hahne SJ, Nic Lochlainn LM, van Burgel ND, Kerkhof J, Sane J, Yap KB, van Binnendijk RS. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis. 2016;214(12):1980–6. doi: 10.1093/infdis/jiw480. PMID:27923955 [DOI] [PubMed] [Google Scholar]
- 100.Carr MJ, Conway A, Waters A, Moran J, Hassan J, Hall WW, Connell J. Molecular epidemiology of circulating measles virus in Ireland 2002–2007. J Med Virol. 2009;81(1):125–9. doi: 10.1002/jmv.21365. PMID:19031456 [DOI] [PubMed] [Google Scholar]
- 101.Warrener L, Slibinskas R, Chua KB, Nigatu W, Brown KE, Sasnauskas K, Samuel D, Brown D. A point-of-care test for measles diagnosis: detection of measles-specific IgM antibodies and viral nucleic acid. Bull World Health Organ 2011;89(9):675–82. doi: 10.2471/BLT.11.088427. PMID:21897488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Michel Y, Saloum K, Tournier C, Quinet B, Lassel L, Pérignon A, Grimprel E, Carbajal R, Vabret A, Freymuth F, et al.. Rapid molecular diagnosis of measles virus infection in an epidemic setting. J Med Virol. 2013;85(4):723–30. doi: 10.1002/jmv.23515. PMID:23364811 [DOI] [PubMed] [Google Scholar]
- 103.Moore C, Cottrell S, Hoffmann J, Carr M, Evans H, Dunford L, Lawson H, Brown KE, Jones R. Self-collected buccal swabs and rapid, real-time PCR during a large measles outbreak in Wales: Evidence for the protective effect of prior MMR immunisation. J Clin Virol. 2015;67: 1–7. doi: 10.1016/j.jcv.2015.03.012. PMID:25959148 [DOI] [PubMed] [Google Scholar]
- 104.OR B, Carr MJ, Connell J, Dunford L, Hall WW, Hassan J. Seroepidemiology and phylogenetic characterisation of measles virus in Ireland, 2004–2013. J Clin Virol. 2014;60(4):374–80. doi: 10.1016/j.jcv.2014.05.010. PMID:24929750 [DOI] [PubMed] [Google Scholar]
- 105.Dimech W, Mulders MN. A review of testing used in seroprevalence studies on measles and rubella. Vaccine. 2016;34(35):4119–22. doi: 10.1016/j.vaccine.2016.06.006. PMID:27340096 [DOI] [PubMed] [Google Scholar]
- 106.Centers for Disease C , Prevention. Recommendations from an ad hoc Meeting of the WHO Measles and Rubella Laboratory Network (LabNet) on use of alternative diagnostic samples for measles and rubella surveillance. MMWR Morb Mortal Wkly Rep. 2008;57(24):657–60. PMID:18566565 [PubMed] [Google Scholar]
- 107.Moss WJ. Measles. Lancet. 2017;390(10111):2490–502. doi: 10.1016/S0140-6736(17)31463-0. PMID:28673424 [DOI] [PubMed] [Google Scholar]
- 108.Shonhai A, Warrener L, Mangwanya D, Slibinskas R, Brown K, Brown D, Featherstone D, Samuel D. Investigation of a measles outbreak in Zimbabwe, 2010: potential of a point of care test to replace laboratory confirmation of suspected cases. Epidemiol Infect. 2015;143(16):3442–50. doi: 10.1017/S0950268815000540. PMID:25865645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory C . 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control 2007;35(10 Suppl 2):S65–164. doi: 10.1016/j.ajic.2007.10.007. PMID:18068815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Advisory Committee on Immunization P, Centers for Disease C, Prevention Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(RR-7):1–45. [PubMed] [Google Scholar]
- 111.Durrheim DN, Crowcroft NS, Strebel PM. Measles – The epidemiology of elimination. Vaccine. 2014;32(51):6880–3. doi: 10.1016/j.vaccine.2014.10.061. PMID:25444814 [DOI] [PubMed] [Google Scholar]


