Abstract
Purpose
To evaluate timing and duration differences in airway protection and esophageal opening after oral intubation and mechanical ventilation for acute respiratory distress syndrome (ARDS) survivors versus age-matched healthy volunteers.
Methods
Orally intubated adult (≥18 years old) patients receiving mechanical ventilation for ARDS were evaluated for swallowing impairments via a videofluoroscopic swallow study (VFSS) during usual care. Exclusion criteria were tracheostomy, neurological impairment, and head and neck cancer. Previously recruited healthy volunteers (n=56) served as age-matched controls. All subjects were evaluated using 5-ml thin liquid barium boluses. VFSS recordings were reviewed frame-by-frame for the onsets of 9 pharyngeal and laryngeal events during swallowing.
Results
Eleven patients met inclusion criteria, with a median (interquartile range [IQR]) intubation duration of 14 (9, 16) days, and VFSSs completed a median of 5 (4, 13) days post-extubation. After arrival of the bolus in the pharynx, ARDS patients achieved maximum laryngeal closure a median (IQR) of 184 (158, 351) ms later than age-matched, healthy volunteers (p<0.001) and longer to achieve laryngeal closure with a median (IQR) difference of 151 (103, 217) ms (p<0.001), although there was no significant difference in duration of laryngeal closure. Pharyngoesophageal segment opening was a median (IQR) of −116 (−183, 1) ms (p=0.004) shorter than in age-matched, healthy controls.
Conclusions
Evaluation of swallowing physiology after oral endotracheal intubation in ARDS patients demonstrates slowed pharyngeal and laryngeal swallowing timing, suggesting swallow-related muscle weakness. These findings may highlight specific areas for further evaluation and potential therapeutic intervention to reduce post-extubation aspiration.
Keywords: deglutition, deglutition disorders, dysphagia, intubation, mechanical ventilation, acute respiratory distress syndrome, fluoroscopy
Critical illness requiring orotracheal intubation with mechanical ventilation occurs in approximately 13–20 million patients globally1 and continues to grow annually.2–6 During mechanical ventilation, muscle wasting and weakness occurs commonly and early after the onset of critical illness.7–9 With oropharyngeal swallowing requiring a synergistic activation of more than 30 muscles,10–12 there is great opportunity for dysfunction and poor patient outcomes as a result of critical illness with intubation and mechanical ventilation.13–16 Weakness during swallowing, leading to dysfunction and altered timings of the coordination of these nerves and muscles, may result in dysphagia with or without aspiration and worse patient outcomes.15–18 Aspiration, one consequence of dysphagia, can lead to pulmonary infection (e.g., aspiration pneumonia) that results in increased morbidity, longer hospital stay, increased hospital charges, and death.19–22 Surgical patients with aspiration pneumonia, for example, have a 4-fold increased odds of admission to the ICU and a 7-fold increased odds of inpatient mortality during their hospital stay.21
After critical illness, dysphagia is also an important issue. For instance, within 48 hours of oral endotracheal tube extubation from mechanical ventilation for acute respiratory failure, up to 56% of patients have dysphagia.13,23–27 However, referral to speech-language pathologists for a swallowing evaluation is relatively infrequent,28 often delayed, and highly variable in clinical practice.29 We previously reported a 33% referral rate for post-extubation swallowing assessment across 13 ICUs at 4 hospitals in a prospective study of acute respiratory distress syndrome (ARDS) patients.28 In patients surviving ARDS, an archetypical example of critical illness,30 resolution of dysphagia symptoms often takes 3–6 months, and in some cases up to 5 years.31
Despite its importance, there is limited understanding of changes in swallowing physiology after extubation and mechanical ventilation in critically ill patients. A systematic review of the literature demonstrated that existing research had heterogeneous and/or small patient samples, unclear/undefined outcomes, and inconsistent assessment methods.13 Moreover, existing studies largely address a single outcome of dysphagia—aspiration. Although early identification of aspiration is important, identifying the impaired swallowing physiology that leads to aspiration is valuable for considering targeted treatment plans, helping inform prognosis, and enhancing the ability of patients to work toward their recovery goals.32–34 Hence, the objective of this study was to evaluate the timing and duration of key swallowing events in ARDS patients after oral intubation and mechanical ventilation compared to age-matched, healthy volunteers. The swallowing events of primary interest were those associated with airway protection and pharyngoesophageal segment (PES) opening, with a secondary objective of comparing temporal patterns of 8 distinct pharyngeal and laryngeal swallowing events with respect to initiation of the pharyngeal swallow.
Methods
Participants
Data were available from two groups for comparison: 1) patients ≥21 years old with ARDS35 who were orally intubated with mechanical ventilation in an ICU, and 2) healthy volunteers. Eligible ARDS patients were part of a prospective cohort study, consecutively screened from 4 ICUs in 1 teaching hospital between 2004 and 2007, who had a videofluoroscopic swallow study (VFSS) completed as a part of routine clinical care, with ≥1 administration of 5-ml liquid barium. As part of pre-existing eligibility criteria for the prospective cohort study, ARDS patients were excluded from enrollment if they met any of the following criteria: 1) >5 days of mechanical ventilation before ARDS onset; 2) communication/language barrier or pre-existing cognitive impairment; 3) pre-existing ARDS of >24 hours duration before being transferred into the study site hospital; 4) physician order for limitations in life support (e.g., no use of vasopressors permitted) at study eligibility; 5) life expectancy of <6 months due to pre-existing illness (e.g., terminal cancer); and 6) tracheotomy during their ICU stay. This study was approved by the Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from each patient or his/her proxy if the patient was incapable of consent.
Healthy volunteers were participants in a previous prospective cohort study.36,37 Inclusion criteria were: 1) consumption of liquids and solid foods as part of a regular diet, and 2) no swallowing complaints. Exclusion criteria were: 1) presence of a known swallowing disorder, 2) gastroesophageal symptoms and/or disease associated with dysphagia, 3) upper aerodigestive tract surgical procedures, 4) pulmonary, head and neck, and/or neurologic disease, 5) current medications with known effects on swallowing or breathing, and 6) use of any tobacco products within the past 10 years.
Instrumentation and Procedures
Swallowing physiology data was acquired using VFSS in a similar manner for both ARDS patients and healthy volunteers as described herein. All VFSS imaging used thin liquid barium (liquid Barosperse® barium sulfate suspension). VFSSs for ARDS patients were originally recorded on 1/2-inch videocassettes using an S-VHS videocassette recorder that were later converted to digital recordings at 29.97 frames per second. Healthy volunteers were recorded directly to digital media at 30 frames per second and analyzed using the Digital Swallowing Workstation, model 7200 manufactured by Kay PENTAX Corp., Lincoln Park, NJ (currently PENTAX Medical), with details previously published.36,37
All participants (i.e., ARDS patients and healthy volunteers) were positioned in lateral projection with ARDS patients seated and healthy volunteers standing. The field of view was often intentionally limited in the ARDS patients as a standard procedure in our facility to reduce radiation exposure to the eyes, yielding a field of view that often included the oral cavity posterior to the incisors, whereas healthy volunteers had an anterior limit of patients’ lips for the field of view. Aside from this difference in anterior limits of the field of view, both groups had similar views that included the posterior pharyngeal wall posteriorly, nasal cavity superiorly, and upper esophagus inferiorly.38–40
The number of administrations of 5-ml liquid barium was variable, depending on whether the patient safely swallowed the first 5-ml or whether subsequent administrations were required to determine effectiveness of implemented swallowing strategies.38–40 The first 5-ml bolus of liquid barium was analyzed in ARDS patients to be comparable with the healthy volunteers. In the healthy volunteers, 5-ml liquid barium was self-administered via a 30-ml medicine cup (1 cup per trial) in 2 trials, with no statistically significant variability between trialsl;36,37 hence, for this analysis, only the first trial was evaluated. Both ARDS patients and healthy volunteers were evaluated in the lateral viewing plane and asked to hold the 5-ml bolus in their mouths before being asked to swallow. After loading the bolus, the fluoroscope was turned on and the subject was asked to swallow. The movement of the barium was visually followed from the oral cavity, through the pharynx, and through the PES as the bolus entered the esophagus. The fluoroscope was turned off at the completion of the swallow.
Data Analysis
All pharyngeal swallowing events analyzed for the current study were referenced from the swallowing events previously published.36,37 Throughout this study, the first video frame corresponding to each identified event was established as the event’s onset. All onsets were identified using the slow motion, freeze-frame, and frame advance functions of the digital video players. Five blinded reviewers completed all kinematic assessments, with 3 reviewers for each swallowing event. Intraclass correlation coefficient was 0.99 across all events.
Nine swallowing events were evaluated in every subject (Table 1). For our primary objective, 6 of these 9 swallowing events (Table 2) were evaluated to analyze 3 pharyngeal duration measures associated with aspiration risk:41–45 1) time to achieve maximum laryngeal closure (lc - mlc), 2) duration of laryngeal closure (mlc - lr), and 3) duration of PES opening (peso - lpeso). Analyses for the secondary objective examined the temporal pattern of the pharyngeal swallow considering all 9 swallowing events (Table 1), with initiation of the pharyngeal swallow, identified as onset of hyoid excursion (he), as the reference point. Onsets for all 9 events for ARDS patients were identified from the videos and recorded as a video frame number using VirtualDub (version 1.10.4, Avery Lee, virtualdub.org). The difference in number of video frames from he to each of the 8 remaining events, multiplied by 0.3336 per video frame for each of the ARDS patients and 0.03333 ms per video frame for each of the heathy controls, determined the time-to-onset for each swallowing event from initiation of the pharyngeal swallow.
Table 1.
Operational definitions for swallowing event onsetsa
| Event onsets | Definition | Event typeb |
|---|---|---|
| Hyoid excursion (he) | Onset of superior and anterior movement of hyoid bone | Physiologic |
| Bolus at the ramus of the mandible (br) | Bolus head arrival at radiographic posterior angle of the ramus of mandible | Bolus flow |
| Laryngeal closing (lc) | Onset of forward displacement of arytenoid cartilages toward base of the epiglottis | Physiologic |
| PES opening (peso) | Onset of anterior-to-posterior widening of the area containing the cricopharyngeus located between C4 and C6 | Physiologic |
| Maximum laryngeal closure (mlc) | Full apposition of arytenoid cartilages to base of the epiglottis | Physiologic |
| Maximum hyoid excursion (mhe) | Most antero-superior position of the hyoid bone | Physiologic |
| Laryngeal reopening (lr) | Separation of arytenoid cartilages from base of the epiglottis | Physiologic |
| Last PES opening (lpeso) | Last frame when PES is open before closing post-swallow | Physiologic |
| Hyoid return to rest (hrr) | Frame in which hyoid has returned (from its most superior and anterior position) to a stable, relaxed position | Physiologic |
PES, pharyngoesophageal segment; t0, reference time point
Event type distinguishes between anatomical movements producing a physiological response in the swallow (physiologic) and flow of the bolus relative to an anatomical landmark (bolus flow).
Table 2.
Operational definitions for selected duration measures.
| Duration Measure | Definition |
|---|---|
| Time to achieve laryngeal closure | Laryngeal closing to maximum laryngeal closure (lc - mlc) |
| Duration of laryngeal closure | Maximum laryngeal closure to laryngeal reopening (mlc - lr) |
| Duration of PES opening | PES opening to last PES opening (peso - lpeso) |
lc, laryngeal closure; lpeso, last PES opening; lr, laryngeal reopening; mlc, maximum laryngeal closure; PES, pharyngoesophageal segment; peso, PES opening (see Table 1 for definitions)
Statistical Analyses
ARDS patients were age-matched to all available healthy volunteers using the original age categories of the healthy volunteers: 1) 21 – 40 years, 2) 41 – 60 years, and 3) 61 – 80 years.36 No difference in swallowing kinematics has been demonstrated between the sexes; thus, no sex matching was performed.46 The median and interquartile ranges of differences between ARDS patients and healthy volunteers were calculated and compared using Wilcoxon rank sum tests. Given the 3 statistical comparisons planned for the primary objective, statistical significance was defined as p<0.017, based on a Bonferroni correction (i.e., α=0.05 divided by 3 comparisons). Statistical analyses were completed using Stata/IC version 15.1 (Stata Corporation, College Station, TX).
Results
Demographics
Of a total of 191 ARDS patients, 26 (14%) completed a VFSS via a physician order, based on a speech-language pathologist recommendation. Of these 26 patients, 11 (42%) met all eligibility criteria and were included in the final analysis (Figure 1). These 11 patients, 8 (73%) female, had a median (interquartile range [IQR]) age of 53 (44, 67) years (Table 3). The median (IQR) duration of oral endotracheal intubation was 14 (9, 16) days, with a time to VFSS of 5 (4, 13) days after extubation.
Figure 1.
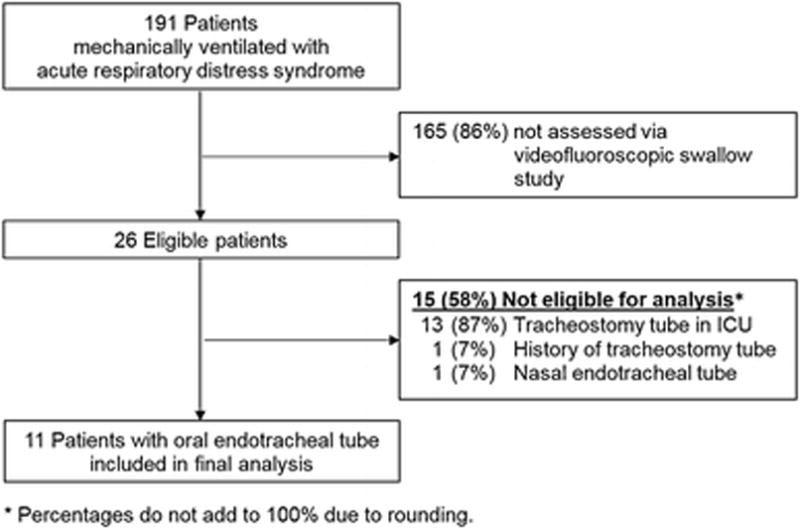
Table 3.
Participant characteristics.
| ARDS Patients (n = 11) |
|
|---|---|
| Demographics | |
| Female, no. (%) | 8 (73) |
| Age, median (IQR) years | 53 (44, 67) |
| Caucasian, No. (%) | 6 (55) |
| Baseline Health Status Before Admission | |
| Charlson Comorbidity Index, Median (IQR) score | 5 (2, 6) |
| Neurological disease, No. (%) | 3 (27) |
| Upper gastrointestinal disease, No. (%) | 5 (45) |
| ICU Admission Diagnosis Category, No. (%) | |
| Respiratory, including pneumonia | 7 (64) |
| Non-pulmonary sepsis | 1 (9) |
| Other | 3 (27) |
| ICU Factors | |
| APACHE II severity of illness score, median (IQR) | 29 (22, 42) |
| SOFA organ failure score, median (IQR) | 8 (4, 11) |
| Intubation duration, median (IQR) days | 14 (9, 16) |
| Reintubated, No. (%) | 2 (18) |
| ICU length of stay, median (IQR) days | 19 (12, 23) |
| VFSS after extubation, median (IQR) days | 5 (4, 13) |
| Hospital length of stay, median (IQR) days | 45 (23, 52) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; VFSS, videofluoroscopic swallow study
Pharyngeal Duration Measurements
Swallowing duration measurements for ARDS patients versus healthy volunteers are presented in Table 4. ARDS patients took longer to achieve laryngeal closure, with a median (IQR) of 151 (103, 217) ms longer (p<0.001) than healthy controls. Compared with healthy controls, pharyngoesophageal segment opening during the swallow was maintained a median (IQR) of −116 (−183, 1) ms shorter for ARDS patients. There was no significant difference (p=0.987) between groups for duration of laryngeal closure. One ARDS patient (9%) demonstrated aspiration during the VFSS.
Table 4.
Selected pharyngeal swallowing durationsa
| Time to achieve laryngeal closure (lc - mlc) | Duration of laryngeal closure (mlc - lr) | Duration of PES opening (peso - lpeso) | |
|---|---|---|---|
| ARDS Patients, median (IQR) msec | 334 (267, 467) | 401 (333, 467) | 401 (367, 534) |
| Healthy Volunteers, median (IQR) msec | 184 (133, 267) | 416 (350, 500) | 542 (484, 646) |
| Age-matched Difference, median (IQR) msec | 151 (103, 217) (p<0.001)b |
13 (−83, 42) (p=0.987) |
−116 (−183, 1) (p=0.004)b |
IQR, interquartile range; lc, laryngeal closure; lpeso, last PES opening; lr, laryngeal reopening; mlc, maximum laryngeal closure; PES, pharyngoesophageal segment; peso, PES opening; (see Table 1 for definitions).
All times are calculated relative to the time of hyoid excursion (he), with a negative time representing events occurring before hyoid excursion.
Statistically significant at p<0.017 after Bonferroni correction for multiple comparisons
Swallowing Events
Mean onsets of 8 distinct swallowing events and differences between ARDS patients and healthy volunteers are presented in Table 5. There were large differences between the groups in 4 swallowing events from the time of initiation of the pharyngeal swallow: 1) time to achieve maximum laryngeal closure (mlc), 2) maximum hyoid excursion, 3) laryngeal reopening after completion of the pharyngeal swallow (lr), and 4) hyoid return to rest (hrr), i.e., total duration of the pharyngeal swallow. After initiation of the pharyngeal swallow, the time to achieve laryngeal closure (mlc), the time to achieve maximum hyoid excursion (mhe), and the time to reopen the larynx after the pharyngeal swallow (lr) were a median (IQR) of 118 (17, 218) ms (p=0.002), 83 (0, 216) ms (p=0.006) and 101 (68, 202) ms (p<0.001) longer in ARDS patients, respectively. These 3 events contributed greatly to lengthening the pharyngeal swallow. Total duration of the pharyngeal swallow for ARDS patients was a median (IQR) of 635 (303, 968) ms longer (p<0.001) compared to healthy volunteers (Figure 2).
Table 5.
Timing of onset of 9 swallowing eventsa
| br | lc | peso | mlc | mhe | lr | lpeso | hrr | |
|---|---|---|---|---|---|---|---|---|
| ARDS Patients, median | −0 (67, 100) | 0 (0, 100) | 200 (133, 300) | 300 (267, 467) | 367 (300, 500) | 734 (734, 868) | 667 (601, 801) | 1502 (1168, 1902) |
| Healthy Volunteers median | 17 (−33, 176) | 8 (0, 49) | 182 (124, 217) | 217 (166, 283) | 284 (233, 358) | 633 (576, 700) | 700 (658, 815) | 865 (750, 958) |
| Age-matched Difference, median | −1 (−234, 66) (p=0.338) |
−17 (−17, 83) (p=0.706) |
50 (−50, 109) (p=0.145) |
118 (17, 218) (p=0.002) |
83 (0, 216) (p=0.006) |
101 (68, 202) (p<0.001) |
−50 (−116, 67) (p=0.145) |
651 (303, 968) (p<0.001) |
br, bolus at the ramus; IQR, interquartile range; lc, laryngeal closing; mlc, maximum laryngeal closure; peso, pharyngoesophageal segment (PES) opening; mhe, maximum hyoid excursion; lr, laryngeal reopening; lpeso; last PES opening; hrr, hyoid return to rest (see Table 1 for definitions)
All times are calculated relative to the time of hyoid excursion (he), with a negative time representing events occurring before hyoid excursion.
Figure 2.
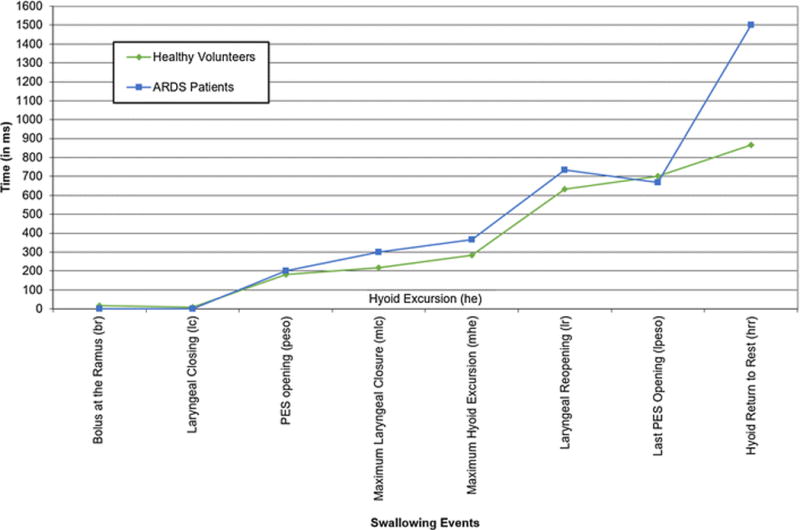
Swallowing event onsets, relative to initiation of the pharyngeal swallow (i.e., hyoid excursion), in patients with acute respiratory distress syndrome vs. healthy volunteers.
Note: Variability is indicated in Table 5.
Discussion
This study evaluated a prospective cohort of ARDS patients who were orally intubated and mechanically ventilated compared to an existing dataset of age-matched, healthy volunteers on 3 temporal measurements associated with aspiration risk during 5-ml thin liquid barium swallows using VFSS. To our knowledge, this is the first study to characterize the temporal relationships of swallowing events in a post-extubated ARDS patient population. We observed that ARDS patients had slow closure of the larynx during the swallow, lasting a median of 151 ms. This duration was nearly double that of healthy volunteers, placing patients at increased risk for entrance of liquids and solid food into the airway before the swallow begins and as the swallow continues through full closure of the larynx. Notably, only 1 (9%) of the 11 ARDS patients aspirated the 5-ml thin liquid bolus, suggesting that factors other than timing of swallowing are associated with aspiration in ARDS patients after extubation (e.g., sensation,47 respiratory-swallow coordination48,49). Although there was no difference in the duration of laryngeal closure, ARDS patients were delayed in re-opening their airway after the swallow. This potential protection, however, may be offset by their reduced duration of PES opening leading to greater retention of portions of the bolus after the swallow. Our sample size of ARDS patients was small and only 1 (9%) patient aspirated during the VFSS. As such, no conclusions may be made regarding the altered timings found in this study; however, our study raises the hypothesis that altered timing of swallowing events may not increase risk of aspiration in post-extubated ARDS patients.
Delayed initiation of the pharyngeal swallow has been attributed to altered and/or reduced sensation.12,50,51 For orally intubated ARDS patients, this change in sensation may arise from the extended duration of an endotracheal tube and/or minimal stimulation in the oral cavity and/or pharynx throughout the duration of intubation.15,16,52–54 Another possible cause may be depressed swallowing responses from sedation medications and sedation status.55–57 Given that an endotracheal tube and mechanical ventilation are often needed for survival of ARDS patients, altered sensation may be a consequence of intubation that becomes a target for rehabilitation. In isolation, entry of the bolus into the pharynx prior to the initiation of the pharyngeal swallow (i.e., hyoid excursion) is a normal variant.58 Although there was no apparent delay in the initiation of the pharyngeal swallow, the lengthy period required to close the larynx requires some attention.
Oropharyngeal swallowing is performed by skeletal muscles and is, therefore, vulnerable to atrophy during critical illness.7 In patients who are critically ill and require intubation with mechanical ventilation, dysphagia may be manifested as slowed execution of swallowing movements.53 Swallowing during intubation is initially eliminated when the neuromuscular block is administered during placement of the endotracheal tube. After the neuromuscular block wears off, typically in 30 minutes, swallowing may resume, depending on sedation, but frequency of swallowing during intubation is currently unknown. Healthy adolescents and young adults swallow an average of 300 times per hour during meals, 30 – 40 times per hour during a restful activity such as reading, and 8 times per hour while sleeping.59 Comparatively, non-intubated patient populations swallow less frequently, but are highly variable.60 The median duration of intubation was 14 days in these ARDS patients. Absent of mealtime stimuli to swallow, and given the severity of critical illness, we expected to see substantive changes in the temporal coordination of swallowing, specifically in movements associated with hyolaryngeal excursion, airway closure, and PES opening due to the presence of an endotracheal tube and its influence of restricting movement of these structures.
The association of dysphagia with duration of endotracheal intubation with mechanical ventilation in critically ill patients is controversial.13 Patients who are critically ill, especially those who are intubated with mechanical ventilation, frequently experience muscle weakness, at least partially related to bedrest and disuse of skeletal muscles.61–63 Moreover, some data suggest that ICU length of stay is associated with dysphagia.64,65 If this were true, it would follow that dysphagia is less common in patients who are critically ill but do not require intubation with mechanical ventilation, a hypothesis that requires further research.
We cannot overlook the possibility that the extended time required for ARDS patients to close their larynx during swallowing may be the result of the reduced or altered sensation during intubation ultimately affecting sensorimotor muscle response, muscle weakness, or both. It has been suggested that residual sedation may be responsible for post-extubation dysphagia.54 Patients in this study were evaluated by VFSS a median of 5 (IQR: 4, 13) days after extubation, sufficiently long enough for residual sedation to resolve. Moreover, 9 of the 11 patients had been discharged from the ICU at the time of the VFSS, and all patients were appropriately alert for completion of the VFSS as documented in the medical record and determined by the speech-language pathologist who completed the VFSS. The 2 patients still admitted to the ICU at the time of the VFSS were 4 and 5 days post-extubation, respectively. In the end, with an endotracheal tube removed, sedatives eliminated, and an alert patient, muscle weakness appears to be a plausible explanation.
Our secondary objective was to describe the temporal pattern of 8 distinct pharyngeal and laryngeal swallowing events in ARDS patients vs. healthy volunteers. The ARDS patients demonstrated 4 swallowing events that were substantially different: 1) longer time to achieve laryngeal closure, 2) longer time to achieve hyoid excursion, 3) longer time before the larynx reopens after the pharyngeal swallow, and 4) a longer duration of the pharyngeal swallow. The onsets of all other swallowing events demonstrated little difference between groups. These findings are aligned with previous muscle physiology studies in animals post-anesthesia, demonstrating little disturbance in the patterning but with overall lengthening of the swallow.12,66
Of note, the temporal pattern changed for two events, with closure of the PES preceding laryngeal reopening in patients. This small adjustment in sequencing, however, may be explained by the larger variability for these time points observed in our patients. However, if this temporal shift in events remains robust in future studies, the previously stated conclusion that this shift in time allows for delayed reopening and protects the airway is tenuous. In fact, the risk of aspiration occurring after the swallow increases with the presence of residue remaining in the pharynx.
Limitations
Several limitations exist with this study. First, the sample size of ARDS patients was small, leading to reduced precision of the results and being underpowered to detect potentially clinically important associations and differences between groups of participants. Second, the recording media and equipment for recording were different between the ARDS patients and healthy volunteers. Although there is a minor frame rate loss when comparing the ARDS patients using a frame rate of 29.97 frames per second vs. the 30 frames per second in the healthy controls, the loss is nominal. Third, only ARDS patients who were intubated with oral endotracheal tubes were studied. These results may not be generalizable to other groups of ARDS patients who may not have been sufficiently concerning to clinicians and were not referred for a VFSS. These results also may not be generalizable to other types of critical illnesses requiring oral endotracheal intubation in the ICU. Despite these potential limitations, this study offers new insights into the physiology of impaired swallowing and potential targets for assessment of post-extubation dysphagia in critically ill patients recovering from ARDS.
Conclusions
This observational study found that this group of patients with ARDS who were orally intubated with mechanical ventilation and clinically referred for VFSS has significant changes in the timing of pharyngeal swallowing events. These preliminary findings may indicate specific areas for potential therapeutic intervention to aid in the recovery of dysphagia from critical illness.
Acknowledgments
The authors thank Lisa Aronson Friedman, Sc.M. for her statistical assistance and Therese Cole, M.A., CCC-SLP, BCS-S and Nicole Langton-Frost M.A. CCC-SLP, BCS-S for their contributions in analyzing video event timings. The authors also thank Bonnie Martin-Harris, Ph.D., CCC-SLP, BCS-S for the healthy volunteers data set.
Funding
This study was funded by the National Institutes of Health.
Research was supported by the National Institutes of Health (Grants: P050HL73994, R01HL088045, and 5K23DC013569).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
All authors declare that there is no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(10):2787–2793. e2781–2789. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA. Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 4.Higgins TL, Kramer AA, Nathanson BH, Copes W, Stark M, Teres D. Prospective validation of the intensive care unit admission Mortality Probability Model (MPM0-III) Crit Care Med. 2009;37(5):1619–1623. doi: 10.1097/CCM.0b013e31819ded31. [DOI] [PubMed] [Google Scholar]
- 5.Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: Implications for healthcare delivery. Crit Care Med. 2008;36(5):1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- 6.Zilberberg MD, de Wit M, Shorr AF. Accuracy of previous estimates for adult prolonged acute mechanical ventilation volume in 2020: Update using 2000–2008 data. Crit Care Med. 2012;40(1):18–20. doi: 10.1097/CCM.0b013e31822e9ffd. [DOI] [PubMed] [Google Scholar]
- 7.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 8.Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 9.Dres M, Dube BP, Mayaux J, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195(1):57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 10.Bass NH. The Neurology of Swallowing. In: Groher ME, editor. Dysphagia: Diagnosis and Management. Boston: Butterworth-Heinemann; 1997. pp. 7–36. [Google Scholar]
- 11.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 12.Miller AJ. Deglutition. Physiol Rev. 1982;62(1):129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- 13.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: A systematic review. Chest. 2010;137(3):665–673. doi: 10.1378/chest.09-1823. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky MB, Gellar JE, Dinglas VD, et al. Duration of oral endotracheal intubation is associated with dysphagia symptoms in acute lung injury patients. J Crit Care. 2014;29(4):574–579. doi: 10.1016/j.jcrc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macht M, King CJ, Wimbish T, et al. Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit Care. 2013;17(3):R119. doi: 10.1186/cc12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care. 2011;15(5):R231. doi: 10.1186/cc10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: How important is dysphagia? Dysphagia. 1998;13(2):69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 18.Martin BJ, Corlew MM, Wood H, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9(1):1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560–566. doi: 10.1378/chest.68.4.560. [DOI] [PubMed] [Google Scholar]
- 20.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 21.Kozlow JH, Berenholtz SM, Garrett E, Dorman T, Pronovost PJ. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937. doi: 10.1097/01.CCM.0000069738.73602.5F. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE. Pulmonary aspiration syndromes. Current Opinion in Pulmonary Medicine. 2011;17(3):148–154. doi: 10.1097/MCP.0b013e32834397d6. 110.1097/MCP.1090b1013e32834397d32834396. [DOI] [PubMed] [Google Scholar]
- 23.Ajemian MS, Nirmul GB, Anderson MT, Zirlen DM, Kwasnik EM. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: Implications for management. Arch Surg. 2001;136(4):434–437. doi: 10.1001/archsurg.136.4.434. [DOI] [PubMed] [Google Scholar]
- 24.Barker J, Martino R, Reichardt B, Hickey EJ, Ralph-Edwards A. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg. 2009;52(2):119–124. [PMC free article] [PubMed] [Google Scholar]
- 25.Barquist E, Brown M, Cohn S, Lundy D, Jackowski J. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: A randomized, prospective trial. Crit Care Med. 2001;29(9):1710–1713. doi: 10.1097/00003246-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 26.El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of Severe Aspiration Pneumonia in Institutionalized Elderly. Am J Respir Crit Care Med. 2003;167(12):1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 27.Tolep K, Getch CL, Criner GJ. Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest. 1996;109(1):167–172. doi: 10.1378/chest.109.1.167. [DOI] [PubMed] [Google Scholar]
- 28.Brodsky MB, González-Fernández M, Mendez-Tellez PA, Shanholtz C, Palmer JB, Needham DM. Factors associated with swallowing assessment after oral endotracheal intubation and mechanical ventilation for acute lung injury. Ann Am Thorac Soc. 2014;11(10):1545–1552. doi: 10.1513/AnnalsATS.201406-274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macht M, Wimbish T, Clark BJ, et al. Diagnosis and treatment of post-extubation dysphagia: Results from a national survey. J Crit Care. 2012;27(6):578–586. doi: 10.1016/j.jcrc.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herridge MS, Angus DC. Acute lung injury–affecting many lives. N Engl J Med. 2005;353(16):1736–1738. doi: 10.1056/NEJMe058205. [DOI] [PubMed] [Google Scholar]
- 31.Brodsky MB, Huang M, Shanholtz C, et al. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5-year longitudinal study. Ann Am Thorac Soc. 2017;14(3):376–383. doi: 10.1513/AnnalsATS.201606-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. Am J Speech Lang Pathol. 2005;14(1):61–70. doi: 10.1044/1058-0360(2005/008). [DOI] [PubMed] [Google Scholar]
- 33.Steele CM, Cichero JA. Physiological factors related to aspiration risk: A systematic review. Dysphagia. 2014 doi: 10.1007/s00455-014-9516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia. 2015;30(1):2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Archives of Otolaryngology - Head and Neck Surgery. 2005;131(9):762–770. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94(5):1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 38.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. Am J Roentgenol. 1990;154(5):953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 39.Logemann JA. Evaluation and treatment of swallowing disorders. 2nd. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- 40.Palmer JB, Kuhlemeier KV, Tippett DC, Lynch C. A protocol for the videofluorographic swallowing study. Dysphagia. 1993;8(3):209–214. doi: 10.1007/BF01354540. [DOI] [PubMed] [Google Scholar]
- 41.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95(1):52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 42.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4(1):8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 43.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97(6):1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 44.Dodds WJ, Logemann JA, Stewart ET. Radiologic assessment of abnormal oral and pharyngeal phases of swallowing. Am J Roentgenol. 1990;154(5):965–974. doi: 10.2214/ajr.154.5.2108570. [DOI] [PubMed] [Google Scholar]
- 45.Logemann JA, Kahrilas PJ, Cheng J, et al. Closure mechanisms of laryngeal vestibule during swallow. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1992;262(2):G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 46.Molfenter SM, Steele CM. Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia. 2013;28(2):226–233. doi: 10.1007/s00455-012-9437-6. [DOI] [PubMed] [Google Scholar]
- 47.Clayton NA, Carnaby GD, Peters MJ, Ing AJ. Impaired laryngopharyngeal sensitivity in patients with COPD: The association with swallow function. Int J Speech Lang Pathol. 2014;16(6):615–623. doi: 10.3109/17549507.2014.882987. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Harris B. Optimal patterns of care in patients with chronic obstructive pulmonary disease. Semin Speech Lang. 2000;21(4):311–321. doi: 10.1055/s-2000-8384. quiz 320–311. [DOI] [PubMed] [Google Scholar]
- 49.Gross RD, Prigent H. Chronic obstructive pulmonary disease and occult aspiration: A review of the recent literature. Curr Phys Med Rehabil Rep. 2015;3(4):280–286. [Google Scholar]
- 50.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9(2):90–95. doi: 10.1007/BF00714593. [DOI] [PubMed] [Google Scholar]
- 51.Miller AJ. Significance of sensory inflow to the swallowing reflex. Brain Res. 1972;43(1):147–159. doi: 10.1016/0006-8993(72)90280-6. [DOI] [PubMed] [Google Scholar]
- 52.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112(2):338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 53.Burgess GE, III, Cooper JR, Jr, Marino RJ, Peuler MJ, Warriner RA., III Laryngeal competence after tracheal extubation. Anesthesiology. 1979;51(1):73–77. doi: 10.1097/00000542-197907000-00016. [DOI] [PubMed] [Google Scholar]
- 54.de Larminat V, Montravers P, Dureuil B, Desmonts JM. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit Care Med. 1995;23(3):486–490. doi: 10.1097/00003246-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 55.McKay RE, Malhotra A, Cakmakkaya OS, Hall KT, McKay WR, Apfel CC. Effect of increased body mass index and anaesthetic duration on recovery of protective airway reflexes after sevoflurane vs desflurane. Br J Anaesth. 2010;104(2):175–182. doi: 10.1093/bja/aep374. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki CT, Yu Z, Xu J, Hundal J, Rosenblatt W. Effects of altered consciousness on the protective glottic closure reflex. Ann Otol Rhinol Laryngol. 2006;115(10):759–763. doi: 10.1177/000348940611501008. [DOI] [PubMed] [Google Scholar]
- 57.Nishino T, Honda Y, Kohchi T, Shirahata M, Yonezawa T. Effects of increasing depth of anaesthesia on phrenic nerve and hypoglossal nerve activity during the swallowing reflex in cats. British Journal of Anaesthesia. 1985;57(2):208–213. doi: 10.1093/bja/57.2.208. [DOI] [PubMed] [Google Scholar]
- 58.Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. J Speech Lang Hear Res. 2007;50(3):585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 59.Lear CS, Flanagan JB, Jr, Moorrees CF. The frequency of deglutition in man. Arch Oral Biol. 1965;10(1):83–100. doi: 10.1016/0003-9969(65)90060-9. [DOI] [PubMed] [Google Scholar]
- 60.Seidl RO, Nusser-Müller-Busch R, Ernst A. The influence of tracheotomy tubes on the swallowing frequency in neurogenic dysphagia. Otolaryngol Head Neck Surg. 2005;132(3):484–486. doi: 10.1016/j.otohns.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 61.Lee CM, Fan E. ICU-acquired weakness: What is preventing its rehabilitation in critically ill patients? BMC Med. 2012;10:115. doi: 10.1186/1741-7015-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Jonghe B, Lacherade J-C, Sharshar T, Outin H. Intensive care unit-acquired weakness: Risk factors and prevention. Crit Care Med. 2009;37(10):S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 63.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Solh A, Okada M, Bhat A, Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29(9):1451–1455. doi: 10.1007/s00134-003-1870-4. [DOI] [PubMed] [Google Scholar]
- 65.Hogue CW, Jr, Lappas GD, Creswell LL, et al. Swallowing dysfunction after cardiac operations. Associated adverse outcomes and risk factors including intraoperative transesophageal echocardiography. J Thorac Cardiovasc Surg. 1995;110(2):517–522. doi: 10.1016/S0022-5223(95)70249-0. [DOI] [PubMed] [Google Scholar]
- 66.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]


