Abstract
Parkinson disease (PD) compromises oropharyngeal swallowing, which negatively affects quality of life and contributes to aspiration pneumonia. Dysphagia often begins early in the disease process, and does not improve with standard therapies. As a result, swallowing deficits are undertreated in the PD population. The Pink1 −/− rat is used to model PD, and demonstrates widespread brainstem neuropathology in combination with early-onset sensorimotor dysfunction; however, to date, swallowing behaviors have not been evaluated. To test the hypothesis that Pink1 −/− rats demonstrate early-onset differences in swallowing, we analyzed within-subject oropharyngeal swallowing using videofluoroscopy. Pink1 −/− and wildtype (WT) controls at 4 (Pink1 −/− n = 16, WT = 16) and 8 (Pink1 −/− n = 12, WT = 12) months of age were tested. The average and maximum bolus size was significantly increased in Pink1 −/− rats at both 4 and 8 months. Bolus average velocity was increased at 8 months for all animals; yet, Pink1 −/− animals had significantly increased velocities compared to WT at 8 months. The data show a significant reduction in mastication rate for Pink1 −/− rats at 8 months suggesting the onset of oromotor dysfunction begins at this time point. Relationships among swallowing variables and neuropathological findings, such as increased alpha-synuclein protein in the nucleus ambiguus and reductions in noradrenergic cells in the locus coeruleus in the Pink1 −/− rats, were determined. The presence of early oropharyngeal swallowing deficits and relationships to brainstem pathology in Pink1−/− rat models of PD indicate that this may be a useful model of early swallowing deficits and their mechanisms. These findings suggest clinical implications for early detection and management of dysphagia in PD.
Keywords: Parkinson disease, Dysphagia, Deglutition, Videofluoroscopy, Rat, Alpha-synuclein, Pink1
Introduction
Parkinson disease (PD) affects at least 1% of the population over the age of 65 and 10% of individuals over the age of 80 [1, 2]. The neuropathology of PD is complex and progressive, leading to a wide range of sensorimotor deficits, including deficits in cranial sensorimotor function, resulting in dysphagia, dysphonia, and dysarthria [3–6]. These systems are linked by common brainstem pathways to peripheral nerves mediating swallowing; however, the specific pathology that correlates to behavioral deficits is unknown. Oropharyngeal swallowing problems in PD include delayed oral transit time, festinated tongue movement, uncontrolled bolus with premature loss to the pharynx, piecemeal deglutition, reduced movement of the hyolaryngeal complex, pharynx, tongue base, and epiglottis, as well as esophageal motility and reflux issues [7–14]. Complications of dysphagia in PD are severe and may lead to weight loss, diet change, and death from aspiration pneumonia, the leading cause of death in PD [15, 16]. Therefore, understanding the progression and mechanisms of PD will lead to improved treatment plans.
Individuals with PD are diagnosed with swallowing impairments in the later stages of PD, missing early dysfunction in the ‘preclinical’ or prodromal stages (before significant nigrostriatal dopamine loss) [17–19]. Consequently, studying humans in the prodromal period is often difficult due to methodological limitations. Thus, animal models of PD offer a means to circumvent these limitations in order to characterize underlying neuropathology, disease mechanisms, and identify potential treatments for dysphagia during the early stages of PD [20–22].
Mutations to the Pink1 (PTEN-induced putative kinase) gene in humans have been associated with both an autosomal recessive, early-onset form of PD as well sporadic cases [23–25]. Evidence indicates that associated deficits within the human Pink1 genetic form of PD may also include voice and swallow deficits similar to idiopathic PD, though this has not been well characterized in terms of onset, progression, and severity [26]. Pink1 −/− rats have been used to study early behavioral deficits [27, 28]. Specifically, Pink1 −/− rats develop sensorimotor deficits, including progressive vocalization and oromotor (tongue, chewing) deficits [27], as well as observable alpha-synuclein aggregations in brainstem regions (the periaqueductal gray and nucleus ambiguus) important in oromotor behaviors [27, 29]. However, oropharyngeal swallow function has not been evaluated in this model of PD.
Videofluoroscopy, the tool most commonly used to assess abnormalities in oropharyngeal swallowing in humans with PD [30], has also been used to assess animal models of swallowing in health and disease [31]. The primary aim of this study was to use videofluoroscopy to describe oropharyngeal swallowing in the Pink1 −/− rat and age-matched wildtype (WT) controls. The 4- and 8-month time points were chosen because past data suggest they are semi-analogous to the ages at which early-stage swallowing deficits are thought to be present in humans with PD [32]. We hypothesized that early-onset differences in oropharyngeal swallowing (bolus area, bolus velocity, and mastication rate) would be present at 4 months and worsen at 8 months of age in Pink1 −/− rats compared to WT. The second aim of this study was to interpret past immunohistochemistry findings of early-stage neuropathology, as they relate to the presence of early swallowing deficits in the Pink1 −/− model. We hypothesized that deficits would negatively correlate to tyrosine hydroxylase (TH)-labeled cells, in the locus coeruleus and substantia nigra. Because data suggest the presence of abnormal alpha-synuclein in the nucleus ambiguus [27], we also used western blotting to quantify alpha-synuclein protein levels with the specific hypothesis that Pink1 −/−rats would have an increased protein compared to WT.
Materials and Methods
Animals and Housing
Thirty-two male Long Evans rats (SAGE Laboratories, Sigma Aldrich) were tested at 4 months of age (Pink1 −/−n = 16, WT = 16) and 24 of these rats were tested at 8 months of age (Pink1 −/− n = 12, WT = 12). Seven to 8 months of age in the rat correspond to approximately 20 years for a human [33]. Mutations in the PARK gene family, including the Pink1 gene, lead to early-onset forms of the disease (approximately 20 years of age). While the clinical characteristics of Pink1-linked Parkinsonism are similar to sporadic Parkinson disease, the age of onset in Pink1-linked Parkinsonism is significantly earlier [34, 35]. Early ‘preclinical’ signs include deficits in vocalizations prior to the onset of classical motor signs. The 4- and 8-month time points in this rat model are analogous to those in humans with PARK mutations. Like humans, rats have significant vocalizations deficits and motor signs [27, 28]. All rats used in this study were also part of a larger study characterizing cranial and gross sensorimotor dysfunction in homozygous and heterozygous Pink1 −/−[27]. In the larger study, all rats underwent the following behavioral measures: ultrasonic vocalizations, tongue force, pasta biting, gross motor performance, and videofluoroscopy. The data from the videofluoroscopy assay are reported as the basis of this study and the remaining behavioral measures were reported in the previous Grant et al. (2015) study. A subset of rats were euthanized at 4 months (n = 4 per genotype) for immunohistochemical analysis. Please refer to Grant et al. [27] for further details. A separate cohort of animals was used for the alpha-synuclein immunoblotting (8 months of age, n = 4 per genotype).
All animals were housed in pairs in standard polycarbonate cages, on a 12:12-h reverse light cycle. Rats arrived at 6 weeks of age and were handled and acclimated to tasks before testing. Food and water were provided ad libitum, although food was restricted overnight for 18 h prior to videofluoroscopy. Body weight was monitored per animal care protocol requirements. All rats maintained standard weight/growth.
All procedures were approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee (IACUC; Protocol #M02505). Additionally, protocols used were in adherence with guidelines approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHEW Publication 80–23, Revised 8th Edition, 2011, Office of Science and Health reports, DRR/NIH Publication 80–23, Revised 8th Edition, 2011, Office of Science and Health reports, DRR/NIH, Bethesda, MD 20205).
Overview of Testing
To assess oropharyngeal swallowing behaviors, videofluoroscopy was performed at 4 and 8 months of age, following overnight food restriction. Each rat was isolated from its cagemate in its home cage, which was then placed in the radiographic field in the lateral plane, with a 1.8-cm disk placed in the field for calibration purposes. Each rat was given a 5 g peanut butter/5 mL barium sulfate (EZ-M Varibar Nectar) mixture (pudding consistency) positioned on an L-shaped platform secured to the interior of the cage, at the level of the mouth. Each rat was allowed to ingest the peanut butter-barium mixture ad libitum for a maximum of 5 min while images were obtained on a C-ARM fluoroscope model OEC 9800 (GE Medical Systems, Salt Lake City, UT) at a rate of 30 frames per second.
Videofluoroscopy Data Analysis
A customized software program in ImageJ (National Institutes of Health, Bethesda, MD) was used to analyze digitized videos in real-time and in a frame-by-frame manner to determine the following measures (described in detail below): bolus area (mm2), bolus velocity (mm/s), and mastication rate (cycles/s). Two research assistants, masked to condition, rated the videos. Each rater analyzed one-half of the data. For quantitative analysis purposes, three individual swallows were identified and selected as “clear swallows” for each rat. Criteria for clear swallows included superior radiographic quality, lateral view, and minimal or no obstruction of the swallow from the animal’s positioning. Given the frequent changing of position by the animals, identifying multiple swallows that met all criteria and allowed for accurate measurements was challenging. Regardless, the data obtained from a sample of three swallows are sufficient for fully assessing the oropharyngeal swallowing ability. Measurements were averaged for the three swallows for each rat for the variables listed below.
Bolus area (mm2) was measured after swallow initiation and before the head of the bolus reached C4; the average and maximum bolus area was calculated for each animal. Bolus velocity, defined as the average speed that the head of the bolus traveled from the initiation point (entrance to oropharynx) through five frames (roughly to C4), was calculated (mm/s). The distance measurement and time measurements were used to calculate average and maximum bolus velocity. Head movement that could affect oropharyngeal measures was accounted for by marking the initial and final head position (with a point placed on a stable location, the crown of the head) and adjusting with a calibrated correction factor. Mastication rate refers to the number of complete mandible opening and closing cycles over a period of time (cycles/s). Mastication rate was measured during the oral phase of the swallow, following procurement of the bolus. A frame count of 5 total opening and closings of the mandible was measured. The first five complete mandible opening and closing cycles were assessed, regardless of whether incisors or molars were used.
Tyrosine Hydroxylase Immunohistochemistry
Following 8 month testing, rats were euthanized; brains were collected and processed for immunohistochemical (ir) analysis; the full methods, details, and results are published in Grant et al. [27]. The numbers of TH-ir in both the locus coeruleus (cell count) and substantia nigra (optical density) were averaged for the two sections, and that value was used for correlation analysis (described below).
Alpha-Synuclein Immunoblotting
For a subset of animals (n = 4 per genotype), animals were deeply anesthetized with isoflurane and rapidly decapitated. The brains were dissected out and immediately frozen and stored at − 80 °C. Brains were sliced in the coronal plane on a cryostat to a 250 μm thickness at − 15 °C, and mounted on gelatin-coated glass slides. 1-mm tissue samples within the nucleus ambiguus (Bregma − 12.36 mm, approximately) were micropunched using the Brain Punch Set (Stoelting, Wood Dale, IL, USA) under a dissection microscope over dry ice. Tissue from the left and right hemispheres was taken, where the cerebellum and 4th ventricle were present [36]. Anatomically equivalent sections were used from each animal. Because of the size of the region of interest, samples for each genotype were pooled for adequate protein concentrations for analysis; pooled genotype samples were run in triplicate.
Supernatant was mixed with a pre-calculated volume of 2 × Laemmli buffer (Bio -Rad, #161–0737) with 2-mercaptoethanol. Extracted protein samples (35 μg of total protein from each animal as determined by BCA assay analysis) were pooled per genotype, denatured at 95 °C for 5 min, and lysates were resolved on a Criterion Precast Gel (4–20% gradient Tris–HCl-polyacrylamide gels, 1.0 mm, 12 × 2 Well Comb, Bio -Rad, #3450032). Prestained protein standards (Precision Plus Protein Dual Xtra Standards, Bio-Rad, #161–0377) were included on gels as molecular mass markers. Samples were subjected to electrophoresis in 10× Tris-buffered saline buffer with glycine (TBS, Bio-Rad, #161–0771) for 1:15 h at 125 V and then transferred in 10× TBS with glycine (Bio-Rad, #170–6435) with 20% methanol for 1.5 h at 100 V onto 0.2-μm nitro-cellulose membranes (Bio-Rad, #1620112). Membranes were blocked with filtered 5% Bovine Serum Albumin (BSA, Fisher, #BP-1600) in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1 h at 4 °C with constant agitation. Blots were probed with primary antibodies (anti-alpha-synuclein, 1:1500, Millipore #AB5038) and loading control (anti-actin, 1:1000, Millipore #MAB1501) in TBST containing filtered 5% BSA overnight (minimum 16 h) at 4 °C with constant agitation. Following primary antibody incubation, blots were washed in TBS-T 6 × 10 min and then probed with horseradish peroxidase-conjugated anti-rabbit IgG (1:10,000 dilution, Cell Signaling Technology Inc., #7074S) and anti-mouse IgG (1:10,000 dilution, Cell Signaling Technology Inc., #7076S)). Blots were washed in TBS-T 6 × 10 min and enhanced chemiluminescence substrate with Super Signal West Pico (Thermo Scientific, #34080) was used to develop immunoblots using a ChemiDoc-IT2 Imager (UVP, LLC). ImageJ (National Institutes of Health) was used to analyze grayscale band density normalized to actin internal controls.
Statistical Analysis
Two-way repeated measures ANOVA was used to examine comparisons for behavioral measures (bolus area, velocity, mastication rate) and the two independent variables, genotype (Pink1 −/−, WT) and age (4, 8 months). Main effects and interactions were examined. Post hoc analysis was performed with Holm-Sidak comparisons. Pearson correlation analysis was performed for the variables of swallowing behavior (bolus area, bolus velocity, mastication rate) presented in this study, and TH-ir in the substantia nigra and TH-ir cell counts in the locus coeruleus, which was published in the previous study. Critical level of significance was set a priori at 0.05. Data were rank transformed for mastication rate, but not bolus area or velocity, as data failed to conform to assumptions for ANOVA.
For immunoblotting, a Gel Analysis method outlined in the ImageJ documentation was used (http://rsb.info.nih. gov/ij/docs/menus/analyze.html#gels). Paired Students’ t test was used to evaluate alpha-synuclein protein concentration differences between genotypes. Interclass correlation coefficients were used to determine inter- and intra-rater reliability on 10% of the swallows. Prior studies in our lab have shown that re-analyzing 10% of collected data yields adequate data for assessing inter- and intra-rater reliability. The purpose of rater reliability measures is to ensure accuracy and this is accomplished by having a detailed methods protocol, intraclass correlations on the reliability data, and by spot-checking after analysis.
Results
Data for bolus area and bolus velocity at both 4 and 8 months are displayed in Fig. 1. For mastication rate (Fig. 2), one animal in the WT group did not exhibit five consecutive openings and closings of the mandible at 8 months, and thus data from this animal were excluded for a total of n = 11 for the WT group.
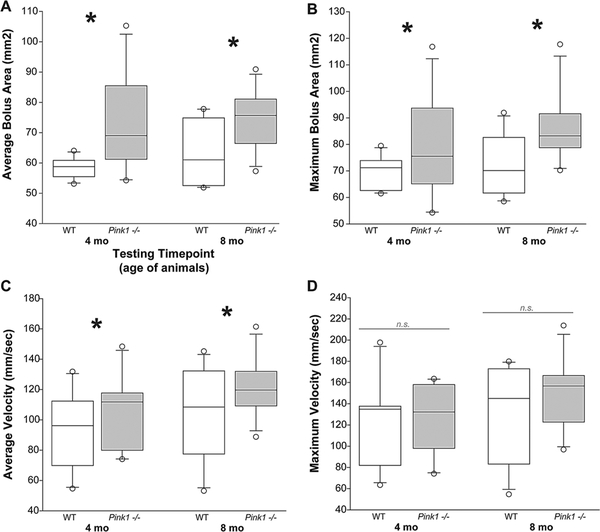
Fig. 1.
Bolus area and velocity in Pink1 −/− and wildtype rats. a Average bolus area (mm2) at 4 and 8 months of age/testing time point. b Maximum bolus area (mm2). c Average velocity (mm/s). d Maximum velocity (mm/s). White bar is wildtype (WT), and gray bar is Pink1 -/−. For box plots, the boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are indicated by open circles. Asterisks represents statistical significance between genotypes (*p < 0.05). Bars (n.s.) represent non-significant findings
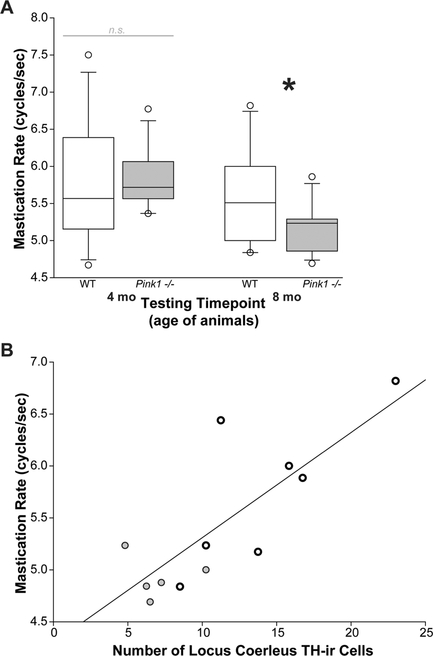
Fig. 2.
Mastication rate correlates to LC tyrosine hydroxylase. a Mastication rate (cycles/s) at 4 and 8 months of age/testing time point. At 8 months of age, positive correlation between the number of tyrosine hydroxylase (TH) immunoreactive in locus coeruleus (LC) versus mastication rate. Black/open circles are wildtype rats; black/gray circles are Pink1 −/− rats. Regression line indicates positive correlation (p < 0.01)
Bolus Area
There were no significant interactions between age and genotype for the average bolus area [F(1,40) = 0.66, p = 0.29] (Fig. 1a) or maximum bolus area [F(1,40) = 0.36, p = 0.55] (Fig. 1b). Pink1 −/− rats showed an increase in bolus area compared to WT; there was a main effect of genotype [F(1,40) = 14.33, p \ 0.001], regardless of age [F(1,40) = 1.17 p = 0.29]. Similarly, there was a significant main effect of genotype for maximum bolus area [F(1,40) = 7.69, p = 0.008], but not a main effect for age [F(1,40) = 2.29, p = 0.14].
Bolus Velocity
There were no significant age by genotype interactions for average bolus velocity [F(1,39) = 0.062, p = 0.81] (Fig. 1c) or maximum bolus velocity [F(1,39) = 0.24, p = 0.63] (Fig. 1d). There was a main effect of genotype for average bolus velocity; Pink1 −/− rats had significantly increased velocities compared to WT [F(1,39) = 4.23, p = 0.046]. There was no main effect of age for average bolus velocity [F(1,39) = 3.001; p = 0.091]. There was no main effect of genotype [F(1,39) = 0.69, p = 0.41] or age [F(1,39) = 1.65, p = 0.21] for maximum velocity.
Mastication Rate
For mastication rate, there was a significant interaction between age and genotype [F(1,21) = 4.88, p = 0.038] (Fig. 2a). Pink1 −/− rats had significantly slower mastication rates at 8 months compared to 4 months (p < 0.001). There was not a significant difference in mastication rate for the WT animals between 4 and 8 months (p = 0.536). There were also no significant differences in mastication rate between Pink1 −/− and WT at either 4 months (p = 0.225) or 8 months (p = 0.128). The non-significant finding between genotypes at 8 months may be due to the spread of variation within the WT animals, observable with the bar-whisker plots.
Immunohistochemistry Summary
A detailed report of immunohistochemistry findings can be found in Grant et al. [27]. To summarize, Pink1 −/− rats showed decreased TH-ir in the locus coeruleus, and decreased TH-ir in the substantia nigra at 8 months of age. The amount of TH-ir was not significantly reduced in the striatum at 8 months for either genotype. At 8 months, Pink1 −/− rats exhibited differences in alpha-synuclein aggregation as compared to WT rats. Pink1 −/− rats showed the densest aggregation in the periaqueductal gray, substantia nigra pars compacta, locus coeruleus, and nucleus ambiguus and no aggregation in the striatum. WT rats did not show alpha-synuclein aggregates in any of the regions examined [17].
Correlation Analysis
Pearson correlation analysis revealed a significant positive correlation between mastication rate and TH-ir counts in the locus coeruleus (df = 11; r = 0.77, p = 0.0046; Fig. 2b), but not the substantia nigra (df = 16; r = - 0.25, p = 0.35). Average bolus area and maximum bolus area did not correlate with TH-ir in the locus coeruleus (df = 11, r = - 0.26, p = 0.41; df = 11, r = - 0.61, p = 0.25) or the substantia nigra (df = 17, r = 0.05, p = 0.85; df = 17, r = - 0.03, p = 0.91) (data not displayed). Additionally, average and maximum bolus velocity was not correlated with TH-ir in the locus coeruleus (df = 11, r = - 0.03, p = 0.92; df = 11, r = 0.02, p = 0.96) or the substantia nigra (df = 17, r = - 0.33, p = 0.18; df = 17, r = - 0.24, p = 0.35) (data not displayed).
Alpha-Synuclein Immunoblotting
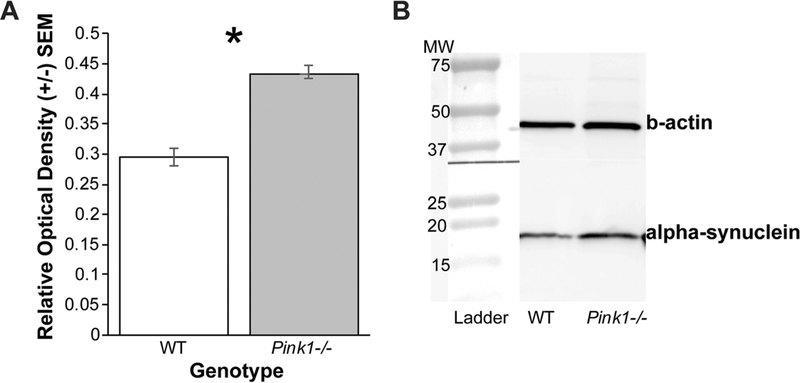
Pink1 −/− rats (mean = 0.43, SEM = 0.00063) had significant increased alpha-synuclein protein in the nucleus ambiguus compared to WT (mean = 0.29, SEM = 0.015) (t(2) = - 9.38, p = 0.011; Fig. 3).
Fig. 3.
Nucleus ambiguus alpha-synuclein western blot. a Pooled samples comparing protein concentrations in wildtype (WT) to Pink1 −/− in the nucleus ambiguus. Net protein concentrations were normalized to loading control (b-actin). Error bars are standard error of the mean. Asterisk demonstrates significant differences (p < 0.05). b Representative western blot bands of alpha-synuclein protein and beta-actin (b-actin) shown for each genotype. Molecular weight (MW) ladder shown at left for comparison
Rater Reliability
Rater reliability was determined with interclass correlation coefficients (ICC). For bolus velocity, the ICC was 0.85. Inter-rater reliability was found to be 0.99 for mastication rate. Intra-rater reliability for rater 1 was 0.99 and for rater 2 was 0.90 for mastication rate.
Discussion
PD significantly influences swallowing, which negatively affects quality of life and contributes to aspiration pneumonia [16, 37]; however, the underlying mechanisms for PD-related dysphagia are unknown. Dysphagia research using animal models, including the Pink1 −/− model of PD, has the potential to shape practice and treatment in the human population [22]. The primary purpose of this study was to use videofluoroscopy to determine whether early oropharyngeal swallowing impairments are evident in the Pink1 −/− genetic rat model of PD and to relate these deficits to early neuropathological changes. Additionally, data from a previously published study were used to examine brainstem regions important to oropharyngeal swallowing assayed for the presence of TH-ir [27]. Correlation analyses were used to determine whether immunohistochemical findings were related to behavioral swallowing deficits.
Previous reports show that mastication rates and bolus velocities decrease with age in rats [31]. Past studies have also shown that parkinsonian rats (severe unilateral lesion to the medial forebrain bundle) have significantly smaller bolus areas compared to aged rats, but increased bolus speeds [31]. Together, these data suggest that the combination of age and neurodegeneration plays a significant role in swallowing physiology. Compared to WT rats, Pink1 −/− rats exhibited a significant reduction in mastication rate from 4 to 8 months, suggesting early impairment in the oral stage of swallowing. We previously reported that Pink1 −/− rats also demonstrated more irregular and inconsistent biting patterns than the WT group in a pasta-biting task [27]. There were also significant genotype differences observed between groups for bolus area and bolus velocity. Specifically, Pink1 −/− rats took significantly larger bolus sizes and had increased oropharyngeal velocities. Similar to other behavioral phenotypes in this model, the Pink1 −/− rat may have deficits in grading fine motor patterns that lead to initial increases in measures of motor behavior [27]. With respect to the increased velocity seen, it may be that the increased bolus velocity observed in the Pink1 −/− rats represents the difficulty with bolus control and premature loss to the pharynx that is often seen in the human PD population. With a reduction in oral control, bolus transit may be impacted. This is clinically relevant to humans as this may contribute to premature loss of the bolus to the pharynx, resulting in aspiration before the swallow. The study also demonstrated variability in swallowing outcome measures at 4 months, which may be explained by increased within-individual variability often seen in motor tasks such as walking, finger tapping, speech, and swallowing [30, 38–40].
This study used the first three clearly observable swallows in a trial, and future studies should evaluate feeding over time. In general, this study is highly significant because oropharyngeal swallowing deficits have not yet been characterized in this progressive, genetic rodent model. Understanding the onset, progression, and nature of swallowing deficits can ultimately improve treatment for PD-related dysphagia.
We found that reductions in TH-ir in the locus coeruleus of Pink1 −/− rats were significantly correlated with reductions in mastication rate in Pink1 −/− rats. However, TH density in the substantia nigra was not linked to mastication rate. This finding suggests that brainstem nora-drenergic mechanisms may contribute to early swallowing dysfunction in PD, independent of loss of nigrostriatal dopamine, although this study did not directly test that hypothesis. Additionally, this finding is consistent with evidence that oropharyngeal dysphagia, and other cranial sensorimotor deficits, such as dysphonia and dysarthria, do not respond to treatments that target nigrostriatal dopamine depletion [41–43]. Given that the pathology in PD is widespread [44–46] and includes compromise of nondopaminergic brainstem regions and neurotransmitter systems [45, 46], the positive correlation between mastication rate and TH-ir suggests that compromise of the locus coeruleus contributes to early oropharyngeal swallowing deficits in the Pink1 −/− rat model of PD.
In addition to reductions in TH-ir, previous data have revealed evidence of additional pathology outside of the nigrostriatal dopamine system in Pink1 −/− rats at 8 months of age [27, 29]. Specifically, there is evidence of alpha-synuclein aggregation in the nucleus ambiguus and periaqueductal gray [27, 29], which may also contribute to the preclinical manifestation of cranial sensorimotor deficits. Here, we show quantified evidence of increased alpha-synuclein protein concentrations in the nucleus ambiguus of Pink1 −/− rats, compared to WT. These data are consistent with the published report of proteinase K-resistant alpha-synuclein aggregations in multiple brainstem regions of the Pink1 −/− rat [27]. The nucleus ambiguus contains the motor neurons that innervate muscles of the palate, pharynx, larynx, and striated esophagus associated with swallowing in the rat [47, 48]. These findings, coupled with evidence that functional swallowing is also affected, reveal the importance of further characterizing non-dopaminergic mechanisms such as neuronal loss in the locus coeruleus and alpha-synuclein aggregation in pertinent brainstem regions in the manifestation and treatment of cranial sensorimotor deficits. This rat model provides us with behavior in combination with the ability to evaluate pathology in tissues within a controlled environment, which is nearly impossible to attain in human subjects. Identifying preclinical signs including deficits in swallowing behavior and detecting the pathology/mechanisms underlying the dysfunction will ultimately lead to better treatments.
The role of nigrostriatal dopamine loss on oromotor dysfunction and oropharyngeal dysphagia in PD is unclear. Rodent neurotoxin models that cause significant nigrostriatal dopamine depletion certainly lead to swallow deficits [31, 49]. For example, Russell et al., using a medial fore-brain bundle 6-OHDA infusion to cause nigrostriatal dopamine depletion, found significant decreases in mastication rate and bolus size per swallow [31]. Other differences in aspects of swallowing between the 6-OHDA and genetic models have been reported elsewhere [27, 50], suggesting different manifestation of swallowing deficits depending upon the model. Importantly, Grant et al. [27] do not report significant differences in nigral or striatal TH-ir between genotypes. While it is clear that nigrostriatal dopamine does play a role in regulating muscle movements for swallowing, there are clearly other mechanisms associated with certain swallowing deficits, especially in the early stages of PD. Specifically, we show a significant decrease in mastication rate in the Pink1 −/− model between 4 and 8 month of age; this corresponds to the increased presence of alpha-synuclein aggregation in brainstem regions, including the nucleus ambiguus, important for vocalization and swallowing behaviors [27, 29]. However, while the inter- and intra-rater reliability measures were very high, a significant limitation of this work is the capture rate (30 frames/s) and resolution of the video used for analysis [51].
Swallowing is a complex motor behavior modulated and regulated by neural substrates throughout the entire brain, with both cortical and subcortical inputs [52–56]. As such, it is reasonable to postulate that in the early stages of PD, early degeneration of brain structures extraneous to the nigrostriatal pathways may lead to the early swallowing deficits currently seen in the human population [57, 58], which is consistent with our translational rodent model. The pathophysiology of PD is complex and the neural mechanisms underlying the manifestation and treatment of cranial sensorimotor deficits, such as oropharyngeal dysphagia, remain vastly understudied and, consequently, undertreated. However, the presence of significant early swallowing deficits in a genetic model of PD, in addition to early neuropathology identified in other areas of the brain important for swallowing, provides the impetus to explore neural mechanisms beyond striatal dopamine depletion.
Acknowledgements
We would like to thank Dr. Glen Leverson, PhD for his assistance with statistical analysis.
Funding
This work was funded by the Michael J. Fox foundation (Ciucci) and National Institutes of Health R01 DC014358 (Ciucci), F32 DC014399 (Kelm-Nelson), and R21 DC016135 (Kelm-Nelson).
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflict of interest to report.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Pankratz N, Wojcieszek J, Fouroud T. Parkinson disease overview. Gene Reviews, 2009. [Google Scholar]
- 2.de Lau L, Giesbergen P, de Rijk M. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–4. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15:14–20. [DOI] [PubMed] [Google Scholar]
- 4.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46:9–17. [DOI] [PubMed] [Google Scholar]
- 5.Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1998;11:131. [PubMed] [Google Scholar]
- 6.Ackermann H, Ziegler W. Articulatory deficits in parkinsonian dysarthria: an acoustic analysis. J Neurol Neurosurg Psychiatry. 1991;54:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer M, Barlow S. Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord. 1978;43:47–57. [DOI] [PubMed] [Google Scholar]
- 9.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12:246–69. [DOI] [PubMed] [Google Scholar]
- 10.Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, Ellis C, Rodriguez AD, Burkhead LM, Rosenbek JC. Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation. 2009;24:131–44. [DOI] [PubMed] [Google Scholar]
- 11.Leopold NA, Kagel MC. Laryngeal deglutition movement in Parkinson’s disease. Neurology. 1997;48:373–6. [DOI] [PubMed] [Google Scholar]
- 12.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–8 discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 13.Wintzen AR, Badrising UA, Roos RA, Vielvoye J, Liauw L. Influence of bolus volume on hyoid movements in normal individuals and patients with Parkinson’s disease. Can J Neurol Sci. 1994;21:57–9. [DOI] [PubMed] [Google Scholar]
- 14.Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson’s disease. Dysphagia. 1998;13:95–100. [DOI] [PubMed] [Google Scholar]
- 15.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing. 2006;35:614–8. [DOI] [PubMed] [Google Scholar]
- 16.Beyer MK, Herlofson K,Årsland D, Larsen JP. Causes of death in a community-based study of Parkinson’s disease. Acta Neurol Scand. 2001;103:7–11. [DOI] [PubMed] [Google Scholar]
- 17.Volonté MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurol Sci. 2002;23:s121–2. [DOI] [PubMed] [Google Scholar]
- 18.Muller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, Poewe W, Litvan I. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol. 2001;58:259–64. [DOI] [PubMed] [Google Scholar]
- 19.Rusz J,Čmejla R, Růžičková H, Klempíř J, Majerová V, Picmausová J, Roth J, Růžička E. Evaluation of speech impairment in early stages of Parkinson’s disease: a prospective study with the role of pharmacotherapy. J Neural Transm. 2013;120:319–29. [DOI] [PubMed] [Google Scholar]
- 20.Campos FL, Carvalho MM, Cristovao AC, Je G, Baltazar G, Salgado AJ, Kim YS, Sousa N. Rodent models of Parkinson’s disease: beyond the motor symptomatology. Front Behav Neurosci. 2013;7:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–9. [DOI] [PubMed] [Google Scholar]
- 22.German RZ, Crompton AW, Gould FD, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia. 2017;32:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J- F, Wang L, He D, Yang QHO, Duan Z-X, Zhang X-W, Nie L-L, Yan X-X, Tang B-S. Clinical features and [11C]-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurol Sci. 2011;32:35–40. [DOI] [PubMed] [Google Scholar]
- 24.Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005;64:1958–60. [DOI] [PubMed] [Google Scholar]
- 25.Bonifati V, Rohé CF, Breedveld GJ, Fabrizio E, De Mari M, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De Gaetano A, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Libera AD, Tinazzi M, De Pandis F, Fabbrini G, Goldwurm S, de Klein A, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA, Network TIPG. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. [DOI] [PubMed] [Google Scholar]
- 26.Bonifati V Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Supplement 1):S4–6. [DOI] [PubMed] [Google Scholar]
- 27.Grant LM, Kelm-Nelson CA, Hilby BL, Blue KV, Paul Rajamanickam ES, Pultorak JD, Fleming SM, Ciucci MR. Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 gene knockout rat model of Parkinson’s disease. J Neurosci Res. 2015;93:1713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer Iii RC, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MAS, Fiske BK, Sherer TB, Frasier MA. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70:190–203. [DOI] [PubMed] [Google Scholar]
- 29.Kelm-Nelson CA, Stevenson SA, Ciucci MR. Atp13a2 expression in the periaqueductal gray is decreased in the Pink1 −/− rat model of Parkinson disease. Neurosci Lett. 2016;621:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CA, Ciucci MR. Multimodal swallowing evaluation with high-resolution manometry reveals subtle swallowing changes in early and mid-stage Parkinson disease. J Parkinsons Dis. 2016;6:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluoro-graphic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2012;28:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciucci MR, Grant LM, Rajamanickam ESP, Hilby BL, Blue KV, Jones CA, Kelm-Nelson CA. Early identification and treatment of communication and swallowing deficits in Parkinson disease. Semin Speech Lang. 2013;34:185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta P The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4:624–30. [PMC free article] [PubMed] [Google Scholar]
- 34.Ibáñez P, Lesage S, Lohmann E, Thobois S, Michele GD, Borg M, Agid Y, Dürr A, Brice A, French Parkinson’s Disease Genetics Study Group. Mutational analysis of the PINK1 gene in early-onset parkinsonism in Europe and North Africa. Brain. 2006;129:686–94. [DOI] [PubMed] [Google Scholar]
- 35.Kumazawa R, Tomiyama H, Li Y, Imamichi Y, Funayama M, Yoshino H, Yokochi F, Fukusako T, Takehisa Y, Kashihara K, Kondo T, Elibol B, Bostantjopoulou S, Toda T, Takahashi H, Yoshii F, Yoshikuni M, Hattori N. Mutation analysis of the PINK1 gene in 391 patients with Parkinson disease. Arch Neurol. 2008;65:802–8. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson G. The rat brain in stereotaxic coordinates 5th ed Burlington: Elsevier; 2005. [Google Scholar]
- 37.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, Rosenbek JC. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias P, Robles-Garcia V, Espinosa N, Corral Y, Cudeiro J. Validity of the finger tapping test in Parkinson’s disease, elderly and young healthy subjects: is there a role for central fatigue? Clin Neurophysiol. 2012;123:2034–41. [DOI] [PubMed] [Google Scholar]
- 39.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24:1815–20. [DOI] [PubMed] [Google Scholar]
- 40.Skodda S Aspects of speech rate and regularity in Parkinson’s disease. J Neurol Sci. 2011;310:231–6. [DOI] [PubMed] [Google Scholar]
- 41.Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord. 2000;15:1132–8. [DOI] [PubMed] [Google Scholar]
- 42.Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, Pollak P, Gentil M. Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study. Brain. 2004;127:602–15. [DOI] [PubMed] [Google Scholar]
- 43.Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord. 2000;33:59–88. [DOI] [PubMed] [Google Scholar]
- 44.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:79–84. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34. [DOI] [PubMed] [Google Scholar]
- 47.Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res. 1985;57:256–63. [DOI] [PubMed] [Google Scholar]
- 48.Jean A Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69. [DOI] [PubMed] [Google Scholar]
- 49.Ciucci MR, Connor NP. Dopaminergic influence on rat tongue function and limb movement initiation. Exp Brain Res. 2009;194:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciucci MR, Russell JA, Schaser AJ, Doll EJ, Vinney LM, Connor NP. Tongue force and timing deficits in a rat model of Parkinson disease. Behav Brain Res. 2011;222:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, Lukasik SL, German RZ. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia. 2013;28:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14:77–86. [DOI] [PubMed] [Google Scholar]
- 53.Martin RE. Neuroplasticity and swallowing. Dysphagia. 2009;24:218–29. [DOI] [PubMed] [Google Scholar]
- 54.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–71. [DOI] [PubMed] [Google Scholar]
- 57.Sung HY, Kim J-S, Lee K-S, Kim Y-I, Song I-U, Chung S-W, Yang D-W, Cho YK, Park JM, Lee IS, Kim SW, Chung I-S, Choi M-G. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord. 2010;25:2361–8. [DOI] [PubMed] [Google Scholar]
- 58.Rusz J,Čmejla R, Růžičková H, Klempíř J, Majerová V, Picmausová J, Roth J, Růžička E. Acoustic assessment of voice and speech disorders in Parkinson’s disease through quick vocal test. Mov Disord. 2011;26:1951–2. [DOI] [PubMed] [Google Scholar]