Abstract
Objectives:
There are no population-based estimates of the incidence or risk factors for acute cardiac manifestations in children with systemic lupus erythematosus (SLE) to guide screening and diagnostic imaging practices. We estimated the incidence and prevalence of acute cardiac manifestations of child-onset SLE compared to adult-onset SLE and identified factors associated with cardiac diagnoses.
Methods:
We identified children (5–17 years) and adults (18–64) with incident SLE (≥3 ICD-9 codes 710.0, >30 days apart) using Clinformatics® DataMart (OptumInsight, Eden Prairie, MN) de-identified U.S. administrative claims (2000–2013). We calculated incidence and prevalence of three outcomes: ≥1 diagnosis code for 1) pericarditis and/or myocarditis, 2) endocarditis or 3) valvular insufficiency. Negative binomial regression was used to identify characteristics associated with cardiac diagnoses in children and determine whether SLE onset in childhood versus adulthood was independently associated with cardiac involvement.
Results:
There were 297 children and 6927 adults with new-onset SLE. 17.8% of children had ICD-9 codes for acute cardiac diagnoses, the incidence of which were highest in the first year after SLE diagnosis (12.2 per 100 person-years). African American race (incidence rate ratio (IRR) 6.6, 95% CI [2.9, 15.0], p<0.01) and nephritis (IRR 7.0, 95% CI [2.6, 18.6], p<0.01) were associated with acute cardiac diagnoses in children. Child-onset disease was independently associated with a 4.4-fold higher rate of pericarditis or myocarditis compared to adult-onset SLE after adjustment for other disease and demographic characteristics (95% CI [2.4, 8.0], p<0.01).
Conclusion:
This study establishes baseline estimates of the incidence and prevalence of pericarditis and myocarditis in child-onset SLE, which is substantially higher than that of adultonset SLE. Prospective echocardiographic evaluations are needed to validate incidence measures and characterize the natural history of acute cardiac manifestations in child-onset SLE, as well as identify risk factors for poor cardiac outcomes to inform screening and management.
Keywords: Pediatric systemic lupus erythematosus, Systemic lupus erythematosus, Cardiovascular diseases, Epidemiology
Introduction
Child-onset systemic lupus erythematosus (SLE) is a heterogeneous, multi-systemic autoimmune condition with the potential for a variety of acute cardiac manifestations, including pericarditis, myocarditis, non-bacterial endocarditis, and valvular insufficiency. Compared to their adult counterparts, children and adolescents with SLE have a higher mortality rate and greater cumulative disease damage,1,2 yet there is a paucity of data on cardiac outcomes in this high risk group. The overall burden and natural history of cardiac manifestations in this population is not well characterized—as a result there is little consensus on if or when to screen for these conditions.
Acute cardiac manifestations have been reported to occur in up to half of adults with SLE on echocardiogram and are frequently asymptomatic.3,4 Estimates of the prevalence of pericarditis in child-onset SLE range from 12–43% 5,6 but have limited generalizability due to small numbers and single-center designs, and there are no published estimates of endocarditis or valvular insufficiency in child-onset SLE. It is also unknown what risk factors are associated with the development of acute cardiac manifestations. Pericardial effusions can result in lifethreatening cardiac tamponade,7 and severe valvular disease carries a high risk of stroke and death,8 thus it is important to characterize both the burden of and risk factors for these acute inflammatory lesions during the initial stages of disease. Furthermore, acute myopericardial involvement and valvular insufficiency may be initial insults preceding chronic myocardial and endocardial damage.9,10 Understanding the baseline characteristics of cardiac involvement in children with SLE has important implications for the development of diagnostic and management strategies in this high-risk population.
This study leverages a large administrative database to assess cardiac diagnoses in a large, nationally representative sample of both children and adults with an incident diagnosis of SLE. The objectives were to: 1) estimate the incidence and prevalence of acute cardiac manifestations of children with new-onset SLE, 2) identify other patient characteristics associated with a higher incidence of cardiac involvement, and 3) compare the rates of cardiac manifestations in childonset versus adult-onset SLE. We hypothesized that there is a significant burden of myopericarditis and valvular disease in children with SLE that is at least comparable to that identified in adults, and there are additional demographic and disease characteristics associated with a higher frequency of acute cardiac involvement.
Patients and Methods
Study Design.
Retrospective population-based cohort study.
Data Source and Subjects.
Children ages 5–17 years inclusive with an incident diagnosis of SLE were identified using the Clinformatics® DataMart (OptumInsight, Eden Prairie, MN) deidentified U.S. administrative claims data from 2000 through 2013. For comparison, adults age 18–64 with incident diagnoses of SLE during the same time period were also included. OptumInsight data is derived from a national commercial health insurance and Medicare Advantage (C and D) database that contains patient and encounter-level data for approximately 20% of US residents, including demographics, medical diagnoses specified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes, procedure codes, prescription drug claims, and provider specialty. An exemption for this study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia (IRB #17–013682).
The diagnosis of SLE was defined using a previously validated algorithm requiring at least 3 hospital or physician visit claims with an ICD-9 diagnosis code for SLE (710.0), each recorded at least 30 days apart.11,12 Incident SLE cases had at least 12 months of claims data with no SLE codes in any position preceding the first primary or secondary SLE diagnosis code, as previously described.13 The date of the first SLE code was considered the date of SLE diagnosis. In order to capture cardiac diagnoses occurring immediately before SLE diagnosis, the index date for yearly incidence calculations was defined as 30 days prior to SLE diagnosis. Subjects with cardiac diagnoses preceding the index date by up to 11 months were censored for incidence estimates. Prevalence estimates included cardiac diagnosis codes up to 12 months before SLE diagnosis and until the end of enrollment. Individuals with pre-existing ICD-9 codes for congenital heart disease, bacterial endocarditis or rheumatic fever were excluded.
Study Measures.
The primary outcomes were the first ICD-9 diagnosis code for the following acute cardiac manifestations of SLE: 1) Pericarditis and/or myocarditis, defined as at least one ICD-9 diagnosis code for pericarditis (423.1, 423.9 and 420.xx)14–16 or myocarditis (422.9x, 429.0); 2) endocarditis (424.91); or 3) valvular insufficiency (424.0–424.3, excluding rheumatic heart disease (394.1) and bacterial endocarditis (421.0–421.9)).17 Secondary outcomes included diagnosis codes for cardiac tamponade (423.3) and procedure codes for pericardial drainage (ICD9-PCS 37.0, CPT 33010, 33011, 33015, 33025).
Covariates of interest included the following: demographic characteristics (age and calendar year at diagnosis, sex, race/ethnicity, geographic region, highest household education); hospitalization within 30 days of SLE diagnosis as a proxy for disease severity; and disease characteristics present within 12 months of the index date (nephritis, major central nervous system (CNS) involvement as defined by seizure or stroke, any psychiatric disorder, venous thromboembolism or antiphospholipid antibody syndrome (APS)). Medication use was determined by ≥1 prescription claim for glucocorticoids, cyclophosphamide, other immunosuppressants, and antimalarials. Age was categorized into child-onset (age 5–17) or adult-onset (18–64) for the primary analyses, and the adult-onset group was further stratified into younger adults (age 18–44) and older adults (45–64) for secondary comparisons. SLE nephritis was identified using a validated algorithm,11,12 and we have previously described methods for categorization of demographic characteristics and identification of other disease characteristics in this database.13,18
Statistical Analysis.
Demographic and disease characteristics among children and adults with SLE were assessed using standard descriptive statistics and compared using Pearson’s chi-square tests. Prevalence rates for each cardiac manifestation were calculated using Poisson regression, with the outcome being at least one cardiac diagnosis code between 12 months before SLE diagnosis and the end of enrollment, and total SLE cases as the offset. Prevalence rates in children were compared to all adults, younger adults, and older adults in the Poisson models. Annual incidence rates stratified by age were calculated separately for each year after the index date, with personyears as the offset. Subjects contributed to person-time from the index date until censored at the time of the first cardiac diagnosis or the end of that observation year. For subjects with more than one cardiac manifestation, only the first diagnosis was used for incidence calculations of the composite outcome of any cardiac diagnosis. In estimates of pericarditis/myocarditis and valvular insufficiency as separate outcomes, subjects could be counted in both models with censoring on the date of the first diagnosis code relevant to each outcome. Tests of trend in annual incidence by calendar year were performed using Cuzick Wilcoxon rank sum methods.
Negative binomial regression with stepwise selection was used to identify demographic and disease characteristics associated with the incidence of cardiac manifestations in children with SLE. Covariates determined a priori to be of clinical significance were forced into the model and retained if coefficients of interest changed by more than 15%. Since regional differences in echocardiography use influence cardiac disease detection rates, clustering by U.S. census region was accounted for.18
Comparisons of unadjusted incidence rates were performed using mid-P exact tests. Separate negative binomial regression models adjusted for race/ethnicity, sex, non-cardiac organ involvement, and antiphospholipid antibodies/venous thromboembolism were used to determine whether age of SLE-onset is independently associated with myopericardial involvement or valvular disease. Goodness-of-fit was assessed by deviance of the residuals, which compares predicted values of the fitted model with those of the saturated model.
Several sensitivity analyses were performed. As disease manifestations often precede recognition of SLE, the index date for the annual incidence calculations was varied from the date of the first SLE claim to 30, 90 and 180 days prior. To assess the outcome definitions, we separately tested published coding algorithms for pericarditis in adults with SLE15 and inflammatory bowel disease, which differed by inclusion of 420.0 and 423.1.16 The outcome definition of valvular insufficiency was also altered to require a billing code for an echocardiogram in the 6 months preceding the first valvular insufficiency code. Lastly, to assess for potential differences in billing practices between pediatric and adult providers, rates of cardiac diagnosis codes among adolescents cared for by pediatric versus adult rheumatologists were compared. All data analyses were performed using STATA 15.0 (STATA Corp, College Station TX) statistical software.
Results
Prevalence of cardiac manifestations of child-onset SLE
There were a total of 297 children with an incident diagnosis of SLE. Average continuous enrollment was 6.0 years, and average follow-up time after the index date was 2.9 years. Demographic and disease characteristics are shown in Table 1. The total prevalence of any acute cardiac diagnosis was 17.8% (95% CI [13.6 – 23.3]).10.8% of children with SLE had a diagnosis of pericarditis or myocarditis, of which pericarditis comprised the vast majority of diagnoses, 9.1% had a diagnosis code for valvular insufficiency and only 1.0% had diagnosis codes for endocarditis (Table 2). Of those with myocardial or pericardial involvement, 21% also had diagnosis codes for valvular insufficiency.
Table 1.
Demographic and disease characteristics by age group
| Child-onset n = 297 | Adult-onset n = 6927 | p-value | |
|---|---|---|---|
| Age in years, mean (SD) | 14.0 (2.7) | 43.7 (11.4) | - |
| Female, n (%) | 256 (86%) | 6203 (90%) | 0.07 |
| Race | |||
| White | 162 (55%) | 4296 (62%) | <0.01 |
| Black | 52 (18%) | 1038 (15%) | |
| Hispanic | 38 (13%) | 712 (10%) | |
| Other | 25 (8%) | 218 (3%) | |
| Unknown/Missing | 20 (7%) | 663 (10%) | |
| Region | |||
| Northeast | 34 (11%) | 778 (11%) | 0.68 |
| Midwest | 79 (27%) | 1667 (24%) | |
| West | 48 (16%) | 1017 (15%) | |
| South | 136 (46%) | 3462 (50%) | |
| Unknown/Missing | 0 (0%) | 3 (<1%) | |
| Highest household education | |||
| High school or less | 80 (27%) | 2045 (30%) | 0.60 |
| Bachelor Degree or more | 200 (67%) | 4470 (64%) | |
| Unknown/Missing | 17 (6%) | 412 (6%) | |
| Disease Characteristicsa | |||
| Nephritis | 82 (28%) | 585 (9%) | <0.01 |
| Cerebrovascular disease | 11 (4%) | 626 (9%) | <0.01 |
| Seizure | 29 (10%) | 330 (5%) | <0.01 |
| sychiatric disorder | 63 (21%) | 2099 (30%) | <0.01 |
| aPLS / VTEb | 15 (5%) | 523 (8%) | 0.11 |
| Hospitalization at diagnosisc | 90 (30%) | 763 (11%) | <0.01 |
| Medication Used | |||
| Glucocorticoids, oral | 250 (84%) | 5566 (80%) | 0.10 |
| Cyclophosphamide | 20 (7%) | 145 (2%) | <0.01 |
| Other immunosuppressante | 150 (51%) | 2432 (35%) | <0.01 |
| Mycophenolate | 92 (31%) | 825 (12%) | <0.01 |
| Azathioprine | 47 (16%) | 929 (13%) | 0.23 |
| Methotrexate | 45 (15%) | 1337 (19%) | 0.08 |
| Hydroxychloroquine | 242 (81%) | 5310 (77%) | 0.05 |
Disease characteristics assessed within one year of index date
Antiphospholipid antibody syndrome or venous thromboembolism
Hospitalization within 30 days of first SLE diagnosis code
Medication use any time during continuous enrollment
Includes mycophenolate, azathioprine, methotrexate, calcineurin inhibitors, and leflunomide
Table 2.
Prevalence of acute cardiac manifestations of SLE by age of disease onset
| Age 18–44 (N = 297) Prevalence [95% CI] |
Age 18–44 (N = 3498) Prevalence [95% CI] |
Age 45–64 (N = 3387) Prevalence [95% CI] |
P* | ||||
|---|---|---|---|---|---|---|---|
| Myo-/pericarditis | 10.8% | [7.6 – 15.2] | 6.2% | [5.4 – 7.0] | 5.3% | [4.6 – 6.2] | <0.01 |
| Pericarditis | 10.4% | [7.3 – 14.8] | 6.2% | [5.3 – 7.0] | 5.1% | [4.4 – 6.0] | <0.01 |
| Myocarditis | 1.0% | [0.3 – 3.1] | 0.3% | [0.2 – 0.6] | 0.3% | [0.1 – 0.5] | 0.12 |
| Endocarditis | 1.0% | [0.3 – 3.1] | 1.0% | [0.7 – 1.4] | 0.9% | [0.6 – 1.2] | 0.87 |
| Valve Insufficiency | 9.1% | [6.2 – 13.3] | 16.9% | [15.6 – 18.3] | 22.9% | [21.4 – 24.6] | <0.01 |
p-values for differences in prevalence across age categories from Poisson regression models
Incidence and timing of cardiac manifestations of child-onset SLE
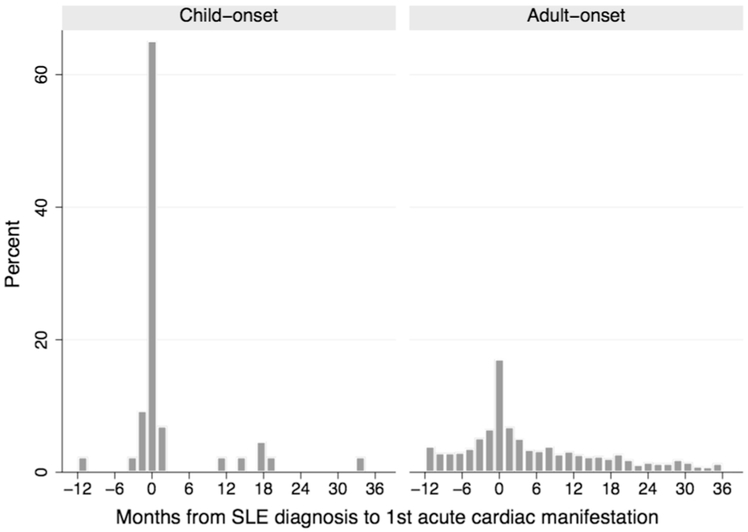
The majority of acute cardiac diagnoses occurred near the time of SLE diagnosis, and 19% preceded the first SLE code by up to one year (Figure 1). For cardiac diagnosis codes preceding SLE diagnosis, median time from first cardiac diagnosis to the first SLE code was 32 days (IQR 6, 64). For cardiac diagnoses made on or after SLE diagnosis, median time from SLE diagnosis to the first cardiac manifestation was 14 days (IQR 1, 524). Accordingly, the estimated annual incidence for any acute cardiac manifestation was highest in the first year after the index date (12.1 per 100 person-years, 95% CI [8.6, 17.1]) (Table 3). There was no significant trend by calendar year (p = 0.69). Varying the index date between the first SLE code up to 180 days prior to the first SLE code resulted in a similar range of estimates for the annual incidence in the first year (10.7–13.0 per 100 person-years). Using the more limited coding definition for pericarditis 15 did not significantly change the results, nor did restricting the definition of valvular insufficiency to require a preceding echocardiogram, as all children with diagnoses of valvular insufficiency in the first year had preceding echocardiograms.
Figure 1.
Time from first SLE diagnosis code to the first acute cardiac diagnosis (pericarditis, myocarditis, endocarditis or valvular insufficiency) in months, stratified by age of SLE onset.
Table 3.
Annual incidence of acute cardiac manifestations by year after SLE diagnosis
| Child-onset | Adult-onset | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person -years |
Events | IR* | 95% CI | Person -years |
Events | IR* | 95% CI | IRR | p¶ | |
| First year§ | ||||||||||
| Total | 263 | 32 | 0.1 22 | [0.086, 0.172] | 6910 | 541 | 0.0 87 | [0.080, 0.094] | 1.40 | 0.07 |
| Myo-/pericarditis | 275 | 21 | 0.076 | [0.050, 0.117] | 6721 | 170 | 0.025 | [0.022, 0.029] | 3.02 | <0.01 |
| Pericarditis | 276 | 20 | 0.072 | [0.047, 0.112] | 7421 | 169 | 0.025 | [0.022, 0.029] | 2.88 | <0.01 |
| Myocarditis | 294 | 2 | 0.007 | [0.002, 0.027] | 7642 | 5 | 0.001 | [0.0003, 0.002] | 9.41 | 0.03 |
| Endocarditis | 296 | 1 | 0.003 | [.0005, 0.024] | 7628 | 23 | 0.003 | [0.002, 0.005] | 1.01 | 0.89 |
| Valve insufficiency | 280 | 13 | 0.046 | [0.027, 0.080] | 7000 | 432 | 0.068 | [0.062, 0.074] | 0.69 | 0.17 |
| Second year | ||||||||||
| Total | 258 | 4 | 0.0 16 | [0.006, 0.041] | 6502 | 205 | 0.0 34 | [0.030, 0.039] | 0.45 | 0.09 |
| Myo-/pericarditis | 271 | 4 | 0.015 | [0.006, 0.039] | 6648 | 47 | 0.007 | [0.005, 0.009] | 2.11 | 0.19 |
| Endocarditis | 296 | 0 | - | - | 7611 | 10 | 0.001 | [0.001, 0.003] | - | 0.66 |
| Valve insufficiency | 279 | 0 | - | - | 6707 | 181 | 0.029 | [0.025, 0.034] | - | <0.01 |
Incidence rates per person-year
First year with SLE following index date
p-value for mid-P exact test of unadjusted incidence rate ratio
Among the different types of cardiac manifestations, the annual incidence was highest for pericarditis, followed by valvular insufficiency (Table 3). There were no incident diagnoses of valvular insufficiency in year two, however 7 children received their first valvular insufficiency diagnosis between 3–10 years after the index date. Of the 3 children with endocarditis, one was identified at the time of SLE diagnosis and two were identified 6 and 11 years later.
Factors associated with acute cardiac diagnoses in child-onset SLE
African American race was independently associated with a 6.6-fold higher incidence of any cardiac manifestation (95% CI [2.9, 15.0], p <0.01) (Table 4). CNS involvement and renal disease also remained independently associated with a significantly greater incidence of any cardiac involvement, while female sex and Asian race were associated with a lower incidence. Similarly, African American race and nephritis were the most strongly associated with myopericarditis (adjusted IRR 8.8, 95% CI [1.9, 40.8], p <0.01 and IRR 15.4, 95% CI [2.2, 110.1], p <0.01, respectively).
Table 4.
Factors associated with any cardiac manifestation of child-onset SLE in the first year
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Age | 1.0 | [0.9, 1.2] | 0.78 | - | - | - |
| Female | 0.4 | [0.2, 0.7] | <0.01 | 0.3 | [0.1, 0.7] | 0.01 |
| Race/Ethnicity | ||||||
| White | - | - | - | - | - | - |
| Black | 3.2 | [2.3, 4.5] | <0.01 | 6.6 | [2.9, 15.0] | <0.01 |
| Hispanic | 2.2 | [0.4, 12.6] | 0.37 | 4.4 | [0.2, 96.0] | 0.34 |
| Asian | 0.4 | [0.01, 13.7] | 0.59 | 0.02 | [0.01, 0.2] | <0.01 |
| Higher household educationa | 2.2 | [0.7, 6.7] | 0.17 | 6.6 | [0.6, 69.0] | 0.12 |
| Nephritis | 4.9 | [2.8, 8.5] | <0.01 | 7.0 | [2.6, 18.6] | <0.01 |
| Cerebrovascular disease | 3.7 | [2.3, 6.1] | <0.01 | 18.6 | [5.8, 60.2] | <0.01 |
| Seizure | 3.2 | [1.7, 5.9] | <0.01 | 20.6 | [2.3,185.3] | 0.01 |
| Psychiatric disorder | 0.6 | [0.1, 3.5] | 0.57 | - | - | - |
| aPLS / VTEb | 0.5 | [0.02, 16.4] | 0.72 | 0.1 | [0.01, 2.3] | 0.14 |
| Hospitalization ±30 daysc | 30.3 | [8.8, 104.1] | <0.01 | - | - | - |
Univariable and multivariable negative binomial regression models (N=292) of disease and demographic factors associated with incidence of cardiac diagnoses among children with SLE within one year after the index date
Bachelor degree or more
Antiphospholipid antibody syndrome or venous thromboembolism
Hospitalization within 30 days of first SLE diagnosis code not included in multivariable analysis due to collinearity with other disease manifestations
Comparison of child-onset with adult-onset SLE
There were 6927 adults under age 65 with an incident diagnosis of SLE during the same period with an average continuous enrollment of 6.1 years and average follow-up time of 3.3 years. Compared to adult-onset SLE, child-onset disease was associated with a higher frequency of African American race, nephritis, seizures, hospitalization at diagnosis, and prescriptions for immunosuppressants other than glucocorticoids at any time during enrollment (Table 1).
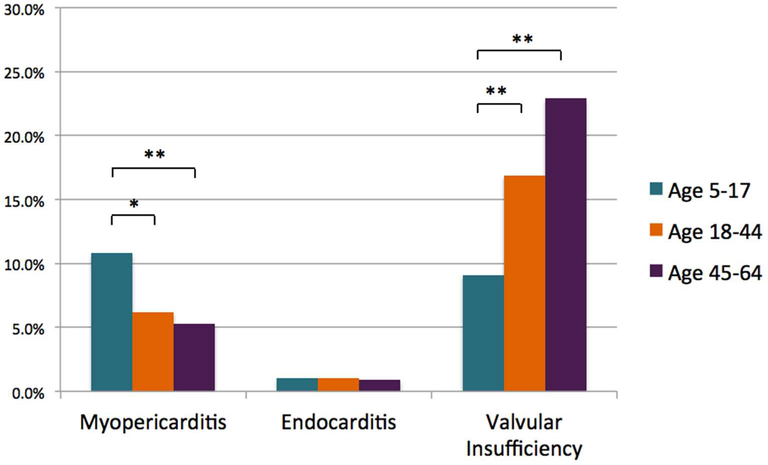
The prevalence of pericarditis and/or myocarditis was significantly higher in children compared to all adults (p < 0.01), younger adults only (p < 0.01) and older adults only (p < 0.01) (Figure 2). The converse was true for valvular insufficiency, which increased in prevalence with age category (p < 0.01). There were no differences in the prevalence of pericarditis/myocarditis or valvular insufficiency codes among children seen by pediatric rheumatologists (n=120) compared to those seen only by adult rheumatologists (n = 146) to suggest that differences in billing patterns influenced the rates of cardiac diagnoses (10.8% versus 11.0%, p 0.97 and 9.2% versus 9.6%, p 0.90, respectively).
Figure 2.
Prevalence of acute cardiac manifestations of SLE by age of onset. Bars represent the percent of children, compared to young adults (age 18–44) and older adults (age 45–64), with at least one acute cardiac diagnosis during the period 12 months prior to SLE diagnosis until end of enrollment. * = p-value <0.01; ** = p-value ≤ 0.001.
Compared to adults, children with SLE had a significantly higher incidence of pericarditis and myocarditis in the first year (unadjusted IRR 3.0, p <0.01) (Table 3), although the timing of initial cardiac diagnoses was similar (Figure 1). After adjustment for demographic characteristics and non-cardiac disease manifestations, children with SLE had an over 4-fold higher incidence of pericarditis/myocarditis compared to adults (IRR 4.4, 95% CI [2.4, 8.0], p < 0.01) (Supplemental Table 1). In contrast, children had a 2-fold lower adjusted incidence of valvular insufficiency (IRR 0.5, 95% CI [0.4, 0.6], p < 0.01). The presence of diagnosis codes for antiphospholipid antibodies and venous thromboembolic events was independently associated with a higher incidence of valvular insufficiency (IRR 3.7, 95% CI [2.1, 6.5], p < 0.01) (Supplemental Table 1).
Lastly, four children with a diagnosis of pericarditis (two of which also had a diagnosis of tamponade) required a pericardial drainage procedure. While these complications were rare, codes for pericardial drainage and tamponade occurred significantly more frequently in children compared to adults with SLE (p < 0.01 and p = 0.04, respectively).
Discussion
This study provides initial population-based estimates of the incidence and prevalence of cardiac involvement in child-onset SLE, and there are several important findings that increase awareness of cardiac disease in this population and may help inform the approach to screening, diagnosis and management. First, there is a higher incidence and prevalence of acute cardiac manifestations of SLE among those with child-onset SLE compared to adult-onset disease, which parallels other markers of overall disease severity. Second, the first occurrences of cardiac involvement were largely noted within months of SLE diagnosis, which has important implications for the use and timing of cardiac diagnostic tests. Third, although the incidence and prevalence of valvular disease are lower in children, at least 9% of children in this cohort were found to have valvular insufficiency after clinically obtained echocardiography, and therefore routine screening would likely identify even higher rates of disease. Lastly, African American race was independently associated with a nearly 7-fold increase in the incidence of cardiac manifestations among children with SLE, and therefore the racial composition of each patient population should be considered in the approach to determining indications for diagnostic testing.
In this study, both the incidence and prevalence of myopericarditis were significantly higher in child-onset SLE compared to adult-onset SLE. Although there are no previously published incidence estimates for comparison, the prevalence estimates of pericarditis in this study are similar to the 12% reported prevalence from the largest North American cohort of children with SLE,6 but lower than that of a smaller, predominantly African American cohort (39%).5 The higher rates of pericarditis and myocarditis observed in child versus adult-onset SLE could be due to a more aggressive overall disease phenotype in children. Several studies have demonstrated that child-onset SLE is associated with higher rates of nephritis, greater cumulative damage, and higher mortality.1,2,19,20 However, most of these studies have focused on renal disease and steroid toxicity. There have been conflicting results regarding the comparison of rates of serositis,20–22 and no prior studies have specifically compared rates of pericarditis or myocarditis. In this cohort, children had significantly higher rates of hospitalization at SLE diagnosis compared to adults. As a result, higher rates of cardiac diagnoses could reflect greater overall disease severity, true phenotypic differences in organ involvement, higher utilization of diagnostic testing in inpatient settings, or practice variation between pediatric and adult centers. Additional research is needed to determine reasons for the difference in rates of cardiac involvement among child-onset versus adult-onset SLE, and clinicians should maintain awareness that adult guidelines to image only symptomatic or antiphospholipid antibody positive patients23 may not be appropriate for child-onset disease.
To our knowledge, this is the first study to estimate the incidence and the largest study to estimate the prevalence of valvular disease in child-onset SLE. Although the study relied on diagnostic codes, the estimated 9% prevalence of valvular insufficiency is within the range of previously published estimates from single-center echocardiographic studies, in which 3 of 19 (16%),24 and 2 of 31 (6%) children had mild to moderate valvular insufficiency.5 The estimates from this study likely represent the lower limit of the true incidence and prevalence, as only children who underwent imaging would have been identified, and diagnostic codes may not reflect all echocardiographic abnormalities. In prospective echocardiographic studies of adults with SLE, the prevalence of valvular abnormalities ranges from 9 to 50%.4,10,25 Valvular disease in the general adult population increases sharply with age,26 therefore the higher rate of valvular insufficiency in adult-onset SLE observed likely reflects the contribution of age-related valvular disease in addition to true valvulitis. While valvular disease is frequently asymptomatic, epidemiologic studies demonstrate that the presence of moderate or severe valvular heart disease is associated with a significant decrease in overall survival among the general U.S. population,26 and a higher risk of stroke and death among patients with SLE.8 Severity of valvular lesions cannot be assessed using administrative data, and therefore larger longitudinal studies of childonset SLE with echocardiographic and clinical data are needed to fully characterize the natural history and clinical implications of valvular disease in this population, particularly in the setting of other comorbidities such as hypertension.
There has been little consensus on if and when to use routine echocardiography to screen for cardiac involvement in children with SLE, as carditis and valvulitis are frequently subclinical.3,24,25,27 This study provides some initial insights into the timing of and risk factors for cardiac involvement that could inform future guidelines. First, cardiac involvement was most commonly identified at or near the time of SLE diagnosis, which was consistent with findings from an inception cohort of 31 children with SLE.5 Thus, if any routine diagnostic testing were to be implemented it would best be performed at the time of initial SLE diagnosis. Secondly, we identified several correlates to incident cardiac manifestations, including the presence of CNS involvement, nephritis, male sex, and African American race, which may each be markers of overall disease severity. Of note, the prior pediatric study with the highest prevalence estimate of cardiac manifestations of child-onset SLE was in a largely African American cohort.5 Therefore clinicians should maintain an even higher suspicion for cardiac involvement in African American children. Future studies evaluating these potential risk factors for cardiac involvement in an unselected population of children with SLE undergoing echocardiography can help determine which patients, if any, warrant routine cardiac screening.
There are several strengths of this study. To our knowledge, this is the first study to estimate the incidence of acute cardiac manifestations in a nationally representative cohort of children with SLE. It is also the first study to compare rates of myopericarditis and valvular insufficiency between child-onset and adult-onset SLE, adding to the body of literature demonstrating clinically important differences in these two groups. There are also several limitations to our study inherent to administrative data. There are no validated codes for cardiac manifestations of SLE, and the de-identified dataset precluded review of physician records to validate diagnoses or grade severity. To address this, we searched the literature for published codes and performed sensitivity analyses using different coding algorithms. In addition, to assess potential differences in billing practices, we compared codes for older adolescents cared for by adult versus pediatric providers and did not find a significant difference. Future studies with echocardiographic data will be needed to validate and determine the clinical relevance of these diagnosis codes. Lastly, the majority of individuals in this database are commercially insured and fewer African American and Hispanic individuals are represented compared to federal insurance databases. Studies suggest lower socioeconomic status and African American race are associated with greater SLE disease severity.28,29 As a result, we may be underestimating the rate of acute cardiac manifestations. Of note however, the prevalence of lupus nephritis in this cohort was only slightly lower than the reported prevalence of 37% in a large Medicaid population using the same coding algorithm,11 and therefore the estimates are still likely to be generalizable.
In summary, this study establishes baseline estimates of the incidence, prevalence and timing of acute cardiac manifestations of child-onset SLE, which is one of the first steps in determining when children with SLE should undergo echocardiography or other cardiac diagnostic testing. At initial presentation, children with SLE have higher rates of major organ involvement, including myopericarditis. Clinicians should therefore maintain a high suspicion for cardiac involvement in this population, in particular in children who are African American, male, or have other major organ involvement. Future serial evaluations of cardiac abnormalities and their sequelae are needed to better characterize the natural history of cardiac manifestations in children with SLE, and to identify predictors of poor cardiac outcomes.
Supplementary Material
Adjusted association between age of SLE onset and incidence of cardiac manifestations
Acknowledgments
Funding:
Support for J.C. from NIH 5T32 HL007915
Footnotes
Disclosure Statement:
The listed authors have no conflicts of interest to report.
References
- 1.Hersh AO, Trupin L, Yazdany J, et al. Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res 2010; 62: 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner HI, Gladman DD, Ibañez D, et al. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008; 58: 556–562. [DOI] [PubMed] [Google Scholar]
- 3.Crozier IG, Li E, Milne MJ, et al. Cardiac involvement in systemic lupus erythematosus detected by echocardiography. Am J Cardiol 1990; 65: 1145–1148. [DOI] [PubMed] [Google Scholar]
- 4.Doria A, Iaccarino L, Sarzi-Puttini P, et al. Cardiac involvement in systemic lupus erythematosus. Lupus 2005; 14: 683–686. [DOI] [PubMed] [Google Scholar]
- 5.Oshiro AC, Derbes SJ, Stopa AR, et al. Anti-Ro/SS-A and anti-La/SS-B antibodies associated with cardiac involvement in childhood systemic lupus erythematosus. Ann Rheum Dis 1997; 56: 272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraki LT, Benseler SM, Tyrrell PN, et al. Clinical and laboratory characteristics and longterm outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr 2008; 152: 550–556. [DOI] [PubMed] [Google Scholar]
- 7.Maharaj SS, Chang SM. Cardiac tamponade as the initial presentation of systemic lupus erythematosus: a case report and review of the literature. Pediatr Rheumatol 2015; 13: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Villa F, Font J, Azqueta M, et al. Severe valvular regurgitation and antiphospholipid antibodies in systemic lupus erythematosus: A prospective, long-term, followup study. Arthritis Rheum 2005; 53: 460–467. [DOI] [PubMed] [Google Scholar]
- 9.Wijetunga M, Rockson S. Myocarditis in systemic lupus erythematosus. Am J Med 2002; 113: 419–423. [DOI] [PubMed] [Google Scholar]
- 10.Roldan CA, Shively BK, Crawford MH. An Echocardiographic Study of Valvular Heart Disease Associated with Systemic Lupus Erythematosus. N Engl J Med 1996; 335: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 11.Hiraki LT, Feldman CH, Liu J, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 2012; 64: 2669–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010; 19: 741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JC, Mandell DS, Knight AM. High Health Care Utilization Preceding Diagnosis of Systemic Lupus Erythematosus in Youth. Arthritis Care Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idowu RT, Carnahan R, Sathe NA, et al. A systematic review of validated methods to capture myopericarditis using administrative or claims data. Vaccine 2013; 31 Suppl 10: K34–40. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol 2000; 27: 1408–1413. [PubMed] [Google Scholar]
- 16.Bernstein CN, Wajda A, Blanchard JF. The Clustering of Other Chronic Inflammatory Diseases in Inflammatory Bowel Disease: A Population-Based Study. Gastroenterology 2005; 129: 827–836. [DOI] [PubMed] [Google Scholar]
- 17.Ismailov RM, Weiss HB, Ness RB, et al. Blunt cardiac injury associated with cardiac valve insufficiency: Trauma links to chronic disease? Injury 2005; 36: 1022–1028. [DOI] [PubMed] [Google Scholar]
- 18.Chang JC, Knight AM, Xiao R, et al. Use of echocardiography at diagnosis and detection of acute cardiac disease in youth with systemic lupus erythematosus. Lupus 2018; 961203318772022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker LB, Menon S, Schaller JG, et al. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol 1995; 34: 866–872. [DOI] [PubMed] [Google Scholar]
- 20.Tucker LB, Uribe AG, Fernández M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2008; 17: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sassi RH, Hendler JV, Piccoli GF, et al. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin Rheumatol 2017; 36: 89–95. [DOI] [PubMed] [Google Scholar]
- 22.Bundhun PK, Kumari A, Huang F. Differences in clinical features observed between childhood-onset versus adult-onset systemic lupus erythematosus: A systematic review and meta-analysis. Medicine (Baltimore) 2017; 96: e8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saric M, Armour AC, Arnaout MS, et al. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J Am Soc Echocardiogr 2016; 29: 1–42. [DOI] [PubMed] [Google Scholar]
- 24.Guevara JP, Clark BJ, Athreya BH. Point prevalence of cardiac abnormalities in children with systemic lupus erythematosus. J Rheumatol 2001; 28: 854–859. [PubMed] [Google Scholar]
- 25.Badui E, Garcia-Rubi D, Robles E, et al. Cardiovascular Manifestations in Systemic Lupus Erythematosus. Prospective Study of 100 Patients. Angiology 1985; 36: 431–441. [DOI] [PubMed] [Google Scholar]
- 26.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a populationbased study. The Lancet 2006; 368: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 27.Leung W-H, Wong K-L, Lau C-P, et al. Cardiac abnormalities in systemic lupus erythematosus: a prospective M-mode, cross-sectional and Doppler echocardiographic study. Int J Cardiol 1990; 27: 367–375. [DOI] [PubMed] [Google Scholar]
- 28.Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2003; 18: 2039–2046. [DOI] [PubMed] [Google Scholar]
- 29.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus. Identification of racial and socioeconomic influences. Arch Intern Med 1990; 150: 849–853. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adjusted association between age of SLE onset and incidence of cardiac manifestations