Abstract
Arboviruses, belonging to the Simbu serogroup of the genus Orthobunyavirus, often cause congenital malformations and reproductive loss in cattle. The recent occurrences of such reproductive problems suggest that new arboviruses have emerged in Japan. However, there is no information on the presence of these viruses in South Korea. The aim of this study was to determine the presence of antibodies for Akabane, Aino, Peaton, Sathuperi, and Shamonda viruses in four regions, namely Gyeonggi, Jeollabuk, Jeollanam provinces, and Jeju Island of South Korea by a serum neutralization test. Antibody positivity against Akabane, Aino, Peaton, Sathuperi, and Shamonda viruses was detected in the country, with average seropositive rates of 10.4, 4.5, 1.1, 4.9, and 5.6%, respectively.
Keywords: Aino virus, Akabane virus, Peaton virus, Sathuperi virus, Shamonda virus
Arthropod-borne viruses (arboviruses) are transmitted among vertebrates by blood-sucking arthropods, such as mosquitoes, biting midges, and ticks [34, 35]. There are more than 520 known arboviruses, and about 25 of these are known to cause diseases in livestock [35].
Representatives of problematic arboviruses for cattle include Akabane virus (AKAV) and Aino virus (AINOV) belonging to the Simbu serogroup of the genus Orthobunyavirus. Arboviruses can infect any species or sexes of cattle; however, symptoms remain in apparent unless the cow is pregnant, in which the pregnancy often results in stillbirth, abortion, premature birth, or congenital malformation of calves. Thus, farmers suffer significant economic losses from such outbreaks [15].
AKAV infections have been reported in Australia [14], Japan [15, 33, 37], the Middle East [31], South Korea [18, 23], and Taiwan [19]. AINOV infections have also been reported in Japan [6, 40]. Vaccines have also been developed. Large-scale outbreaks of AKAV and AINOV infections no longer occur in vaccinated regions; however, their occurrence continues in other regions.
The arbovirus-infected arthropod vectors are spreading further north each year possibly due to global warming, resulting in the expansion of infected regions [34, 35]. Moreover, with diversification of international transportation and logistics, there is an increased opportunity for arthropod vectors to encounter livestock, and thereby cause the spread of arbovirus infections [5].
In Japan, mainly in the Okinawa and the Kyushu regions, new arboviruses were found in the blood of cattle and biting midges after the late 1990s, confirming their entry. Peaton virus (PEAV) [21] and Sathuperi virus (SATV) [38] were found in 1999, while Shamonda virus (SHAV) [41] was found in 2002. They all belong to the genus Orthobunyavirus and the family Bunyaviridae.
Pregnant cattle that are suspected to have PEAV has also been reported to undergo abnormal labor [30]. Although no damage to cattle by SHAV infection has been reported, genetic analyses have shown that Schmallenbergvirus (SBV) is a possible ancestor of SHAV [7] or is likely are assortment between SATV and SHAV [39]. SBV first emerged in Europe in 2011 and caused outbreaks in cattle, goats, and sheep, causing fever, stillbirth, abortion, fetal malformation, diarrhea, and reduced milk yield. It also increased the importance of Orthobunyavirus in livestock worldwide [4].
PEAV, SATV, and SHAV were isolated one after another in Japan, suggesting the extent of damage to livestock. However, PEAV, SATV, and SHAV have not yet been reported in Korea. In order to clarify the state of infiltration of new arboviruses in cattle, the first epidemiological survey of new arboviruses belonging to the genus Orthobunyavirus was performed in Korea through this study.
From October 2013 to February 2015, blood samples were collected from 268 cattle that were between 6 months and 14 years of age (unvaccinated) from 7 farms (A to G) in Gyeonggi, Jeollabuk, Jeollanam provinces, and Jeju Island in Korea (Fig. 1). Required sample size was calculated using EZR (Easy R; Saitama Medical Center, Jichi Medical University, Saitama, Japan), with 10% precision, expected prevalence of 15% based on our preliminary survey data 95% confidence interval (CI). Minimum sample size was 196. Blood samples were centrifuged at 2,000 rpm for 10 min and sera samples were stored at −20°C until use. AKAV strain JaGAr39 [26], AINOV strain JaNAr28, PEAV strain KSB-1/P/06, SATV strain KSB-2/C/08, and SHAV strain KSB-6/C/02 [11] were cultured in hamster lung (HmLu-1) cells and used for the serum neutralization test.
Fig. 1.
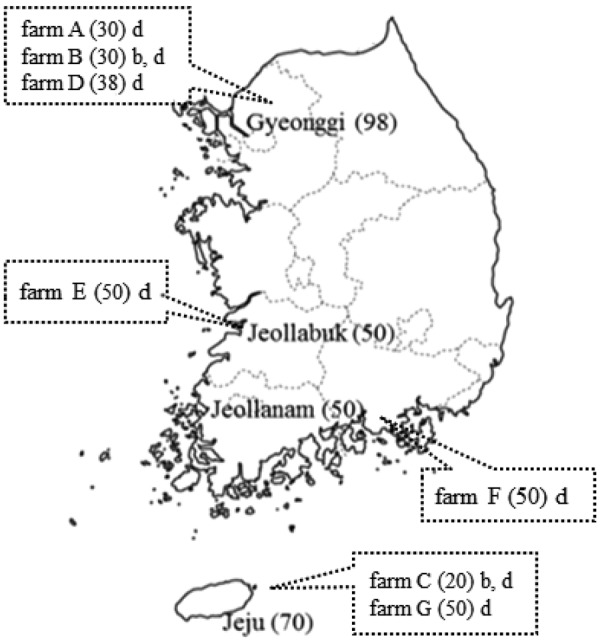
Sampling regions including farms, and the number of samples tested in South Korea. Blood samples were collected from dairy and beef cattle from October 2013 to February 2015. Number in parentheses represents sample size. b: beef cattle, d: dairy cattle.
Bovine sera were inactivated at 56°C for 30 min and serially diluted (two-fold) in Eagle’s minimal essential medium (EMEM; Nissui, Tokyo, Japan) supplemented with 2% fetal bovine serum (FBS) and a mixture of antibiotics in round-bottom 96-well microplates. Each serum dilution was mixed with equal volume of 200 TCID50/0.05 ml of virus and incubated at 37°C for 1 hr. Then 0.05 ml of the mixture was inoculated with HmLu-1 cells at 37°C for 1 hr. After inoculation, the mixture was removed and 0.1 ml of EMEM was added to each well. After incubation in a humidified 5% CO2 atmosphere at 37°C for 7 days, antibody titer was determined as the reciprocal of the highest serum dilution showing complete inhibition of cytopathic effect. Samples with titers of 1:4 or greater were considered to be positive [13].
Seroprevalence and their corresponding 95% CI and statistical analysis were calculated using EZR. χ2 test was used to analyze differences in seroprevalence between provinces, ages, sexes, production type, and rearing form. A P-value of less than 0.05 was considered statistically significant.
Serum neutralization test confirmed the presence of antibodies for AKAV, AINOV, PEAV, SATV, and SHAV in Gyeonggi, Jeollabuk, and Jeollanam provinces and Jeju Island. Their average seropositive rates were 10.4% (28/268, 95% CI: 7.1–14.7%), 4.5% (12/268, 95% CI: 2.3–7.7%), 1.1% (3/268, 95% CI: 0.2–3.2%), 4.9% (13/268, 95% CI: 2.6–8.2%), and 5.6% (15/268, 95% CI: 3.2–9.1%), respectively. Only the presence of anti-AKAV antibodies was confirmed in Gyeonggi province, with a positive rate of 2.0% (2/98, 95% CI: 0.2–7.2%). In Jeollabuk province, antibody positivity against AKAV, AINOV, and SATV was detected, with positive rates of 12.0% (6/50, 95% CI: 4.4–24.6%), 4.0% (2/50, 95% CI: 0.5–14.0%), and 2.0% (1/50, 95% CI: 0.0–10.9%), respectively. In Jeollanam province, antibody positivity against AKAV, AINOV, and SHAV was detected, with positive rates of 8.0% (4/50, 95% CI: 2.1–19.5%), 6.0% (3/50, 95% CI: 1.2–16.8%), and 4.0% (2/50, 95% CI: 0.5–14.0%), respectively. In contrast, in Jeju Island, antibodies against all the 5 viruses (AKAV, AINOV, PEAV, SATV, and SHAV) were detected, with positive rates of 22.9% (16/70, 95% CI: 13.6–34.6%), 10.0% (7/70, 95% CI: 4.1–19.7%), 4.3% (3/70, 95% CI: 0.9–12.1%), 17.1% (12/70, 95% CI: 9.1–28.2%), and 18.6% (13/70, 95% CI: 10.2–29.8%), respectively (Table 1). Infiltration rates of viruses tested were higher in Jeju Island than those in Gyeonggi, Jeollabuk, or Jeollanam province. The differences in seroprevalence among the 4 regions for each virus were investigated and found to be significant (P<0.05).
Table 1. Analysis for the association between potential risk factors and seroprevalence of arboviruses using χ2 test.
| Risk factor | AKAV | AINOV | PEAV | SATV | SHAV | |
|---|---|---|---|---|---|---|
| Region | ||||||
| GG | 2/98 (2.0%) | 0/98 (0.0%) | 0/98 (0.0%) | 0/98 (0.0%) | 0/98 (0.0%) | |
| JB | 6/50 (12.0%) | 2/50 (4.0%) | 0/50 (0.0%) | 1/50 (2.0%) | 0/50 (0.0%) | |
| JN | 4/50 (8.0%) | 3/50 (6.0%) | 0/50 (0.0%) | 0/50 (0.0%) | 2/50 (4.0%) | |
| JJ | 16/70 (22.9%) | 7/70 (10.0%) | 3/70 (4.3%) | 12/70 (17.1%) | 13/70 (18.6%) | |
| P-value | 2.29E-04 | 0.020 | 0.035 | 7.20E-07 | 7.41E-07 | |
| Sex | ||||||
| Male | 2/16 (12.5%) | 0/16 (0.0%) | 0/16 (0.0%) | 0/16 (0.0%) | 0/16 (0.0%) | |
| Female | 26/252 (10.3%) | 12/252 (4.8%) | 3/252 (1.2%) | 13/252 (5.2%) | 15/252 (6.0%) | |
| P-value | 1.000 | 0.787 | 1.000 | 0.740 | 0.657 | |
| Production type | ||||||
| Beef | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | |
| Dairy | 28/248 (11.3%) | 12/248 (4.8%) | 3/248 (1.2%) | 13/248 (5.2%) | 15/248 (6.0%) | |
| P-value | 0.227 | 0.657 | 1.000 | 0.611 | 0.531 | |
| Rearing form (Jeju only) | ||||||
| Feedlot | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | 0/20 (0.0%) | |
| Grazing | 16/50 (32.0%) | 7/50 (14.0%) | 3/50 (6.0%) | 12/50 (24.0%) | 13/50 (26.0%) | |
| P-value | 0.010 | 0.186 | 0.641 | 0.040 | 0.029 | |
GG: Gyeonggi province, JB: Jeollabuk province, JN: Jeollanam province, JJ: Jeju island.
In 4 out of 7 farms, serum antibody positivity for arbovirus was detected. The seropositive rates were 6.7% (2/30, 95% CI: 8.0–22.1%) for farm A, 18.0% (9/50, 95% CI: 8.6–31.4%) for farms E and F, and 66.0% (33/50, 95% CI: 51.2–78.8%) for farm G.
Samples were collected from dairy cattle Holsteins, beef cattle Hanwoo (Bostaurus coreanae), and black cow, and variation among production type and between sexes was investigated; however, no significant differences were noted. Samples used for the test were collected from cattle that were aged between 6 months and 14 years, and seropositive rate was investigated by age. Cattle were divided into those less than one year old and those over one year old, based on whether or not they had experienced their first summer after birth. When the rate of antibody positivity was compared between these two groups, a significant (P<0.05) difference was noted, except for PEAV. In cattle that were over one year old or had experienced summer at least once, we analyzed their sera by further dividing them into 4 stages: 1 year, 2 to 4 years, 5 to 7 years, and over 8 years of age. Antibodies against AKAV, AINOV, and SHAV were noted in all stages, and against PEAV and SATV were noted in the stages of 1 year, 2 to 4 years, and over 8years (Table 2). This suggests that the presence of new arboviruses in cattle is due to a recent infection in South Korea.
Table 2. Analysis for the association between cattle age and seroprevalence of arboviruses using χ2 test.
| Risk factor | AKAV | AINOV | PEAV | SATV | SHAV | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <1y | 3/88 (3.4%) | 0/88 (0.0%) | 0/88 (0.0%) | 0/88 (0.0%) | 0/88 (0.0%) | |
| 1y≤ | 25/180 (13.9%) | 12/180 (7.2%) | 3/180 (1.7%) | 13/180 (7.2%) | 15/180 (8.3%) | |
| P-value | 0.015 | 0.030 | 0.549 | 0.023 | 0.012 | |
| (Age stages 1y≤) | ||||||
| 1y | 11/51 (21.6%) | 7/51 (13.7%) | 1/51 (2.0%) | 8/51 (15.7%) | 1/51 (2.0%) | |
| 2–4y | 9/95 (9.5%) | 2/95 (2.1%) | 1/95 (1.1%) | 4/95 (4.2%) | 7/95 (7.4%) | |
| 5–7y | 3/25 (12.0%) | 1/25 (4.0%) | 0/25 (0.0%) | 0/25 (0.0%) | 4/25 (16.0%) | |
| 8y≤ | 2/9 (22.2%) | 2/9 (22.2%) | 1/9 (11.1%) | 1/9 (11.1%) | 3/9 (33.3%) | |
The cattle rearing form was also surveyed. Cattle were reared by feedlot in Gyeonggi, Jeollabuk, and Jeollanam provinces. They were reared by either feedlot or grazing in Jeju Island. Differences in the seropositive rates for the five arboviruses due to differences in the cattle rearing form were investigated only in Jeju Island. The rates for antibody positivity for AKAV, SATV, and SHAV were significantly (P<0.05) higher in cattle reared by grazing compared to that in cattle reared by feedlot (Table 1).
The newly emerged arboviruses, PEAV, SATV, and SHAV are classified into the Simbu serogroup of the genus Orthobunyavirus, similar to AKAV and AINOV. Antibody cross-reactivity exists for some arboviruses of the Simbu serogroup. However, cross reactivity among AKAV, AINOV, PEAV, and SHAV was not observed in cross-neutralization tests [28, 41], and that between SATV and SHAV has not been reported, suggesting that each antibody positivity against these viruses observed in the 4 regions was due to introduction by AKAV, AINOV, PEAV, SATV and SHAV, respectively.
Arboviruses were isolated in countries with a tropical or subtropical climate. PEAV was initially isolated in Australia in 1976 [28]. SATV was initially isolated in India in 1957 [3]. SHAV was initially isolated in Nigeria in 1965 [2]. It is difficult for adult midges to survive winter in countries that have four seasons, such as Japan and Korea. They do not become indigenous owing to interruption of their infection cycle. Therefore, it is assumed that they might have been brought in from tropical and subtropical regions [32, 36, 40]. Recently, it has been reported that SBV can overwinter, but the exact mechanism remains unknown [4].
Among the five arboviruses surveyed, only anti-AKAV antibody positivity was confirmed in Gyeonggi province, farm A. Gyeonggi province is located at 37° North latitude and 127° East longitude. It belongs to a cold temperate zone with a dry winter climate based on Koppen-Geiger climate classification. After 2000, 25cases caused by AKAV infection were reported in Gyeonggi province [1]. In Japan, AKAV infection-induced diseases occurred in Hokkaido and Tohoku regions [25, 29, 35] belonging to cold temperate zones. This suggests that the activity area of arbovirus-infected arthropod vectors has been expanding northward. From 2006 to 2011, outbreaks of AKAV encephalomyelitis have been reported in Korea and Japan [10, 15, 23]. Calves and breeding cows exhibited neurological signs, including locomotor ataxia, astasia, and hypersensitivity after postnatal infection. These are different from other typical AKAV infection symptoms, such as abortion, stillborn calves, and congenital malformation in pregnant cattle. They might have originated from a new virus that invaded Korea and Japan from overseas.
Biting midges are vectors of bovine arboviruses. However, their distributed type species differs among epidemic countries. In Japan, AKAV and SATV were isolated from Culicoides(C.) oxystoma [17], and PEAV was isolated from C. jacobsoni [12]. In Australia, PEAV was isolated from C. brevitarsis [28]. In South Africa, SHAV was isolated from C. imicola [22]. In Korea, the AKAV genome was detected in C. punctatus, C. arakawae, C. maculatus, and C. oxystoma [24]. In cattle farms in Jeju Island, where antibodies against AKAV, AINOV, PEAV, SATV, and SHAV were detected, biting midges were collected using a light trap, and C. homotomus and C. punctatus were captured. However, no arbovirus antigen was detected in these biting midges.
Recent outbreaks of arbovirus infection have been characterized by an increase in frequency and expansion of outbreak regions due to temperature elevation and diversification of the causative virus [35]. Since 2000, loss of cattle due to arbovirus infection, including AKAV, has occurred every year in Korea and Japan. It is known that the outbreak of AKAV infection tends to occur at intervals of 5-6years. It has also occurred in regions without previous outbreaks [1, 9].
Regarding arboviruses used in this survey, mainly in Okinawa and the Kyushu regions in Japan, PEAV was isolated in 1999, 2005, and 2006, SATV was isolated in 1999, 2006, 2007, and 2008, and SHAV was isolated in 2002 and 2007 [11]. Stillbirth and abortion in cattle with suspected PEAV has been reported over the past several years [20, 21, 30]. The pathogenicity of SHAV in ruminants has not been confirmed; however, malformation induction has been observed in an experiment using chick embryos [22], suggesting that more attention must be paid to this virus. Outbreaks of SBV have occurred in Europe and caused considerable damage to livestock farms rearing cattle, goats, and sheep [7, 39]. Therefore, it may be necessary to reinforce information collection on arbovirus infection occurring worldwide and conduct surveys of new arbovirus types.
Sera of the cattle were positive to one or more of 5 viruses, but not all. Some of the reasons we could speculate are due to very short period of viremia (AKAV, AINOV and SBV in cattle last for less than 1 week) and low prevalence of these viruses.
Antibody positivity against all 5 viruses was confirmed in Jeju Island. Jeju Island is located at 33° North latitude and 126° East longitude, in the southernmost region of Korea. Its climate is classified as temperate humid. From June to October of 2014, the average temperature in Jeju Island was between 19.1 and 25.1°C (average maximum temperature: 22.1–27.8°C, average minimum temperature: 16.3–23.5°C), and average humidity was 69.7 to 84.3% [16]. Insects such as mosquitos and biting midges can readily grow in large numbers in such temperate climates with high humidity. In addition, an increase in transportation has been observed due to a rise in foreign tourists visiting Jeju Island. Moreover, many farms are rearing cattle by grazing. All these factors can increase the chance of cattle encountering virus-carrying biting midges, thereby increasing the risk of arbovirus infection.
In our study, high seropositive rates for the arboviruses were detected in farm G, but not in farm C. Arbovirus can easily infect animals when all factors are met, including biting midges, immune state of host livestock, and viral properties. Therefore, it is difficult to conclude why there was no infection in farm C. We assume that one of the reasons is the influence of the geographical condition. Jeju Island has a big mountain called Hallasan in the central area, and farm C is located in the eastern part of the mountain. Adult midges might have been brought in from tropical and subtropical regions by eastbound monsoons. However, farm C may be less affected owing to its geographical advantage.
The outbreaks of AKAV infection in 1989–90 [8] and Chuzan virus (belonging to the family Reoviridae) infection in 1993 [27] have been reported in Jeju Island. However, no arbovirus infection has been reported in Jeju Island after that, according to Korea Animal Health Integration System (KAHIS) of the Animal and Plant Quarantine Agency [1]. Since the development of arbovirus vaccines in Korea, outbreaks of arbovirus infection have reduced significantly; thus, vaccination rates have decreased. Cattle that have acquired immune antibodies through vaccination will not be infected again. Due to the high outbreak risk in Jeju Island, it is essential to vaccinate young reproductive cattle that have not received prior vaccination in the entire southern region of the Korean peninsula, including Jeju Island.
Arboviruses can infect animals when all factors are conductive, including the distribution and activity of biting midges, population and immune state of host livestock, and viral properties [34, 35, 37]. Although no case of arbovirus infection has been reported in Jeju Island after 2000, it may be important to perform arbovirus-related surveys in Jeju Island, a temperate region in Korea, in order to predict the risk of infection in Korea. In addition to continuous survey of the distribution of biting midges in cattle farms with high positive rates, it may be necessary to isolate arboviruses and perform genetic identification for these viruses.
Acknowledgments
We would like to thank Dr. Makoto Yamakawa, Exotic Disease Research Station, National Institute of Animal Health, for advising this work. We also thank Dr. Tohru Yanase, Subtropical Disease Research Division of Kyushu Research Station, National Institute of Animal Health, for virus distribution and identification of biting midges. We also thank staff members at College of Veterinary Medicine, Konkuk University, for their help in blood sampling.
REFERENCES
- 1.Animal and Plant Quarantine Agency Korea Animal Health Integration System, http://kahis.go.kr/home/lkntscrinfo/selectLkntsStats.do [accessed on August 8, 2018].
- 2.Causey O. R., Kemp G. E., Causey C. E., Lee V. H.1972. Isolations of Simbu-group viruses in Ibadan, Nigeria 1964–69, including the new types Sango, Shamonda, Sabo and Shuni. Ann. Trop. Med. Parasitol. 66: 357–362. doi: 10.1080/00034983.1972.11686835 [DOI] [PubMed] [Google Scholar]
- 3.Dandawate C. N., Rajagopalan P. K., Pavri K. M., Work T. H.1969. Virus isolations from mosquitoes collected in North Arcot district, Madras state, and Chittoor district, Andhra Pradesh between November 1955 and October 1957. Indian J. Med. Res. 57: 1420–1426. [PubMed] [Google Scholar]
- 4.European Food Safety Authority2014. Schmallenberg virus: State of Art. EFSA J. 12: 3681. [Google Scholar]
- 5.Forman S., Hungerford N., Yamakawa M., Yanase T., Tsai H. J., Joo Y. S., Yang D. K., Nha J. J.2008. Climate change impacts and risks for animal health in Asia. Rev. - Off. Int. Epizoot. 27: 581–597. doi: 10.20506/rst.27.2.1814 [DOI] [PubMed] [Google Scholar]
- 6.Fukutomi T., Ouchi M., Okuda K., Maruno F., Tabayashi K.1991. An abnormal calf birth suggestive of an infection of Ainovirus in Okayama prefecture. Nippon Juishikai Zasshi 44: 17–19. [Google Scholar]
- 7.Goller K. V., Höper D., Schirrmeier H., Mettenleiter T. C., Beer M.2012. Schmallenberg virus as possible ancestor of Shamonda virus. Emerg. Infect. Dis. 18: 1644–1646. doi: 10.3201/eid1810.120835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyun K. J.1990. Occurrence of Akabane Disease and it’s Antibody Test in Cattle Raised in Cheju-do. Korean J. Vet. Serv. 13: 90–95. [Google Scholar]
- 9.Japanese Ministry of Agriculture Forestry and Fisheries. Annual Statistics of Notifiable Animal Infectious Diseases (1937–2014). http://www.maff.go.jp/j/syouan/douei/kansi_densen/pdf/h26_todokede_ruinen.pdf [accessed on August 8, 2018].
- 10.Kamata H., Inai K., Maeda K., Nishimura T., Arita S., Tsuda T., Sato M.2009. Encephalomyelitis of cattle caused by Akabane virus in southern Japan in 2006. J. Comp. Pathol. 140: 187–193. doi: 10.1016/j.jcpa.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Kato T., Matsumoto H., Hirashima Y., Shirafuji H., Yamakawa M., Yanase T.2013. Improvement of RT-PCR assay for detection of orthobunyaviruses islated in Japan. Bull. Natl. Inst. Anim. Health 119: 47–52. [Google Scholar]
- 12.Kato T., Shirafuji H., Tanaka S., Sato M., Yamakawa M., Tsuda T., Yanase T.2015. Bovine arboviruses in culicoides biting midges and sentinel cattle in Southern Japan from 2003 to 2013. Transbound. Emerg. Dis. 19: 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y. H., Oem J. K., Lee E. Y., Lee K. K., Kim S. H., Lee M. H., Park S. C.2015. Seroprevalence of five arboviruses in sentinel cattle as part of nationwide surveillance in South Korea, 2009–2012. J. Vet. Med. Sci. 77: 247–250. doi: 10.1292/jvms.14-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkland P. D., Barry R. D., Harper P. A., Zelski R. Z.1988. The development of Akabane virus-induced congenital abnormalities in cattle. Vet. Rec. 122: 582–586. doi: 10.1136/vr.122.24.582 [DOI] [PubMed] [Google Scholar]
- 15.Kono R., Hirata M., Kaji M., Goto Y., Ikeda S., Yanase T., Kato T., Tanaka S., Tsutsui T., Imada T., Yamakawa M.2008. Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet. Res. 4: 20. doi: 10.1186/1746-6148-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korea Meteorological Administration2014. Annual Climatological Report. 153–155. http://www.weather.go.kr/repositary/sfc/pdf/sfc_ann_2014.pdf [accessed on August 8, 2018].
- 17.Kurogi H., Akiba K., Inaba Y., Matumoto M.1987. Isolation of Akabane virus from the biting midge Culicoides oxystoma in Japan. Vet. Microbiol. 15: 243–248. doi: 10.1016/0378-1135(87)90078-2 [DOI] [PubMed] [Google Scholar]
- 18.Lee J. K., Park J. S., Choi J. H., Park B. K., Lee B. C., Hwang W. S., Kim J. H., Jean Y. H., Haritani M., Yoo H. S., Kim D. Y.2002. Encephalomyelitis associated with akabane virus infection in adult cows. Vet. Pathol. 39: 269–273. doi: 10.1354/vp.39-2-269 [DOI] [PubMed] [Google Scholar]
- 19.Liao Y. K., Lu Y. S., Goto Y., Inaba Y.1996. The isolation of Akabane virus (Iriki strain) from calves in Taiwan. J. Basic Microbiol. 36: 33–39. doi: 10.1002/jobm.3620360108 [DOI] [PubMed] [Google Scholar]
- 20.Matsumori Y., Ishimaru K., Ohashi S., Tsuda T.2002. Characterization and serological survey of the Peaton virus isolated from cattle in Nagasaki Prefecture. Nippon Juishikai Zasshi 55: 215–218. [Google Scholar]
- 21.Matsumori Y., Inai K., Yanase T., Ohashi S., Kato T., Yoshida K., Tsuda T.2002. Serological and genetic characterization of newly isolated Peaton virus in Japan. Brief report. Arch. Virol. 147: 401–410. doi: 10.1007/s705-002-8328-4 [DOI] [PubMed] [Google Scholar]
- 22.Meiswinkel R., Venter G., Nevill E., Coetzer J., Tustin R.2004. Vectors: Culicoides spp. Oxford University Press. 1: 93–136. [Google Scholar]
- 23.Oem J. K., Lee K. H., Kim H. R., Bae Y. C., Chung J. Y., Lee O. S., Roh I. S.2012. Bovine epizootic encephalomyelitis caused by Akabane virus infection in Korea. J. Comp. Pathol. 147: 101–105. doi: 10.1016/j.jcpa.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 24.Oem J. K., Chung J. Y., Kwon M. S., Kim T. K., Lee T. U., Bae Y. C.2013. Abundance of biting midge species (Diptera: Ceratopogonidae, Culicoides spp.) on cattle farms in Korea. J. Vet. Sci. 14: 91–94. doi: 10.4142/jvs.2013.14.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oike Y., Yoshida K., Minamoto K.1988. An epizootic of akabane disease in cattle in iwate prefecture from 1985 to 1986. Nippon Juishikai Zasshi 41: 246–250. [Google Scholar]
- 26.Oya A., Okuno T., Ogata T., Kobayashii, Matsuyama T.1961. Akabane, a new arbor virus isolated in Japan. Jpn. J. Med. Sci. Biol. 14: 101–108. doi: 10.7883/yoken1952.14.101 [DOI] [PubMed] [Google Scholar]
- 27.Park D. S., Lee B. K., An J. C., Moon S. H., Kim H. K., Son K. S.1993. An outbreak of Chuzan disease in Korea and the immunogenicity of binary ethylenimine-treated Chuzan virus vaccine in cattle. Kor. J. Vet. Publ. Hlth. 17: 301–305. [Google Scholar]
- 28.St George T. D., Standfast H. A., Cybinski D. H., Filippich C., Carley J. G.1980. Peaton virus: a new Simbu group arbovirus isolated from cattle and Culicoides brevitarsis in Australia. Aust. J. Biol. Sci. 33: 235–243. doi: 10.1071/BI9800235 [DOI] [PubMed] [Google Scholar]
- 29.Takamori H., Hino M., Takahashi K., Toyoshima T., Takeda Y., Takano Y., Tanaka S., Yamakawa M.2013. Pathological feature of Akabane disease occurring in Miyagi prefecture. Nippon Juishikai Zasshi 66: 39–44. [Google Scholar]
- 30.Takasaki M.2001. Deformity Case Suspected Peaton virus Participating. J. Vet. Clin. 48: 385–389. [Google Scholar]
- 31.Taylor W. P., Mellor P. S.1994. The distribution of Akabane virus in the Middle East. Epidemiol. Infect. 113: 175–185. doi: 10.1017/S0950268800051591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda T.2000. Congenital abnormalities of cattle caused by the arboviral infection. Yamaguchi Juigaku Zasshi 27: 1–18. [Google Scholar]
- 33.Uchida K., Murakami T., Sueyoshi M., Tsuda T., Inai K., Acorda J. A., Yamaguchi R., Tateyama S.2000. Detection of Akabane viral antigens in spontaneous lymphohistiocytic encephalomyelitis in cattle. J. Vet. Diagn. Invest. 12: 518–524. doi: 10.1177/104063870001200605 [DOI] [PubMed] [Google Scholar]
- 34.Weaver S. C., Reisen W. K.2010. Present and future arboviral threats. Antiviral Res. 85: 328–345. doi: 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamakawa M.2008. Characteristics of the Recent Prevalence of Arthropod-Borne Viruses in Japan. J. Vet. Epidemiol. 12: 4–6. doi: 10.2743/jve.12.4 [DOI] [Google Scholar]
- 36.Yamakawa M., Yanase T., Kato T., Tsuda T.2006. Chronological and geographical variations in the small RNA segment of the teratogenic Akabane virus. Virus Res. 121: 84–92. doi: 10.1016/j.virusres.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 37.Yanase T.2009. Arboviruses transmitted by Culicoides biting midges to live-stock. Med. Entomol. Zool. 60: 195–212. doi: 10.7601/mez.60.195 [DOI] [Google Scholar]
- 38.Yanase T., Fukutomi T., Yoshida K., Kato T., Ohashi S., Yamakawa M., Tsuda T.2004. The emergence in Japan of Sathuperi virus, a tropical Simbu serogroup virus of the genus Orthobunyavirus. Arch. Virol. 149: 1007–1013. doi: 10.1007/s00705-003-0266-7 [DOI] [PubMed] [Google Scholar]
- 39.Yanase T., Kato T., Aizawa M., Shuto Y., Shirafuji H., Yamakawa M., Tsuda T.2012. Genetic reassortment between Sathuperi and Shamonda viruses of the genus Orthobunyavirus in nature: implications for their genetic relationship to Schmallenberg virus. Arch. Virol. 157: 1611–1616. doi: 10.1007/s00705-012-1341-8 [DOI] [PubMed] [Google Scholar]
- 40.Yanase T., Kato T., Kubo T., Yoshida K., Ohashi S., Yamakawa M., Miura Y., Tsuda T.2005. Isolation of bovine arboviruses from Culicoides biting midges (Diptera: Ceratopogonidae) in southern Japan: 1985–2002. J. Med. Entomol. 42: 63–67. doi: 10.1093/jmedent/42.1.63 [DOI] [PubMed] [Google Scholar]
- 41.Yanase T., Maeda K., Kato T., Nyuta S., Kamata H., Yamakawa M., Tsuda T.2005. The resurgence of Shamonda virus, an African Simbu group virus of the genus Orthobunyavirus, in Japan. Arch. Virol. 150: 361–369. doi: 10.1007/s00705-004-0419-3 [DOI] [PubMed] [Google Scholar]


