Abstract
Basal cell carcinoma (BCC) is the most frequent human cancer and is becoming an important health problem in an ageing population. Based on their clinical and histological characteristics, thick BCC are typically divided into low-risk nodular and high-risk infiltrative subtypes, although the underlying mechanisms are poorly understood. We have identified molecular mechanisms that explain the aggressiveness of high-risk infiltrative BCC, with a potential direct clinical impact. In this study, we first show that fibroblasts, TGFβ and fibronectin are found preferentially in infiltrative human BCC. This allowed us to develop in vivo models for the study of infiltrative BCC, which in turn let us confirm the role of TGFβ in inducing peritumoral fibronectin deposition and tumor infiltration. We then show that fibronectin promotes adhesion and migration of BCC cell lines through integrin α5β1-mediated phosphorylation of focal adhesion kinase (FAK). Fittingly, inhibition of integrin α5β1 and phospho-FAK both prevent fibronectin-induced migration of BCC cells in vitro as well as BCC infiltration in vivo. Altogether our results open important insights into the pathogenesis of aggressive infiltrative BCC, and identify integrin α5β1 or FAK inhibition as promising strategies for the treatment of advanced BCC.
INTRODUCTION
Basal cell carcinoma (BCC) is the most common skin cancer, affecting more than 50% of Caucasians during their lifetime (Kasper et al., 2012). Virtually 100% of BCC, independently of their clinical or histological subtype, depend on a deregulated Hedgehog (Hh) signaling pathway ultimately leading to the activation of Gli transcription factors (Epstein, 2008, Rubin and de Sauvage, 2006). However, despite this molecular homogeneity, BCC can display very different morphological aspects and have varying clinical prognoses (Kasper et al., 2012, Telfer et al., 2008). Whereas superficial, micro-nodular and even thick nodular BCC are considered as low-risk tumors, infiltrative BCC are associated with higher tumor recurrence after surgery and lower response to radiotherapy (Breuninger and Dietz, 1991, Caccialanza et al., 2014, Piccinno et al., 2017). Consequently, a better understanding of the functional determinants of nodular and infiltrative phenotypes is of high clinical relevance. Interestingly, some insights into the pathogenesis of BCC subtypes were obtained from a large genomic analysis of human BCC, which identified additional genetic mutations in MYCN, PPP6C and PTPN14 in infiltrative BCC (Bonilla et al., 2016). However, it must be noted that the impact of these mutations was neither validated in vitro nor in vivo. Recently, accumulating evidences point out the role of the tumor-stroma interactions in BCC infiltration. Tumor-associated macrophages (TAM) were shown to act on BCC progression through paracrine activation of cyclo-oxygenase 2 (COX-2) (Tjiu et al., 2009). Similarly, carcinoma-associated fibroblasts (CAF) were reported to promote BCC invasion through paracrine activation of c-MET in BCC tumor cells (Marsh et al., 2008, Tjiu et al., 2009). Chiu et al. showed the implication of CXCR4-induced angiogenesis in infiltrative BCC (Chu et al., 2009). Altogether, these observations support the role of the microenvironment in BCC progression, although functional validation is currently lacking, as there is no appropriate in vivo mouse model of infiltrative BCC.
The tumor stroma is composed of a bio-active network of proteins forming the extra-cellular matrix (ECM). Importantly, low-risk nodular BCC present histologically as well-defined tumor nests in a loose myxoid stroma. They mostly display a continuous basement membrane and split retraction at the tumor-stroma interface. On the contrary, high-risk infiltrative BCC are defined histologically by irregular tumor strands in a densely-packed fibrotic stroma, in which basement membrane and split retraction are mostly absent (Barsky et al., 1987, Crowson, 2006, Kallioinen et al., 1984). These histological observations suggest that ECM modifications may play a role in BCC subtype and progression. Modifications of the ECM network actually appear early during cancer progression and are associated with tumor progression (Barcellos-Hoff et al., 2013, Butcher et al., 2009, Levental et al., 2009, Lorusso and Ruegg, 2008) and poor prognosis (Conklin et al., 2011). Because the impact of the ECM on BCC progression has not yet been studied, we focused our work on this important field.
In this study, we show that TGFβ and fibronectin are highly expressed in the peritumoral stroma of infiltrative BCC. Using two different BCC allograft models, we show that TGFβ induces peritumoral fibronectin and confers infiltrative features to BCC. Consistently, fibronectin promotes the migration of BCC tumor cells in vitro through integrin α5β1 binding and downstream phosphorylation of focal adhesion kinase (FAK). Both integrin α5β1 and FAK inhibition prevent fibronectin–induced migration. Consistently, silencing of FAK expression in tumor cells, inhibition of phospho-FAK with the tyrosine kinase inhibitor PF-562271 or the integrin α5β1 inhibitor K34C all prevent TGFβ-induced infiltration in vivo. Altogether, these results reveal the pivotal role of TGFβ-induced peritumoral fibronectin in BCC progression and suggest that FAK inhibitors, available in the clinics, may be used for the treatment of advanced infiltrative BCC.
RESULTS
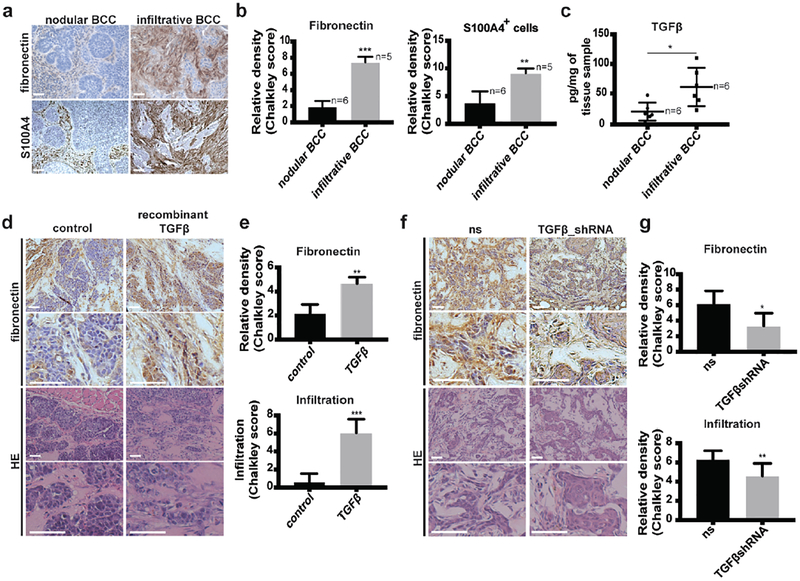
TGFβ is associated with peritumoral fibronectin deposition and the transition from nodular to infiltrative phenotype in human BCC samples and mouse models.
Peritumoral fibrosis has been shown to confer poor prognosis in several tumor types (Barcellos-Hoff et al., 2013, Cirri and Chiarugi, 2011). Regarding skin tumors, peritumoral fibrotic stroma is observed in advanced and aggressive infiltrative BCC (Crowson, 2006), suggesting that modifications of the ECM may be responsible for BCC progression. Among the various ECM components of the fibrotic response, fibronectin has been reported as the most strongly expressed upon fibroblast activation in chronic fibrosis (Wynn, 2008). In order to determine the relationship between fibrosis and BCC infiltration, human BCC samples were collected and stained for fibronectin and S100A4, a marker for carcinoma-associated fibroblasts (Kalluri, 2016). Haematoxylin-eosin (HE) stains were performed on adjacent slides to determine BCC subtype. Higher levels of fibronectin and higher density of S100A4+ fibroblasts were found at the periphery of infiltrative compared to nodular BCC (Figures 1a and 1b). Importantly, previously-treated or -biopsied tumor samples were excluded from our study. Hence, secondary modification of fibronectin expression due to inflammation or tissue repair after treatment can be safely excluded. To get an insight into the molecular drivers of peritumoral fibrosis in BCC, human samples were tested by quantitative real-time PCR for various secreted cytokines reported to promote fibronectin deposition. Among the various cytokines tested, TGFβ was found to be significantly higher in infiltrative compared to nodular BCC, both at the mRNA and protein levels (Supplementary Figure S1a and Figure 1c). These results suggest that TGFβ may be a key regulator of fibronectin induction and tumor infiltration in BCC.
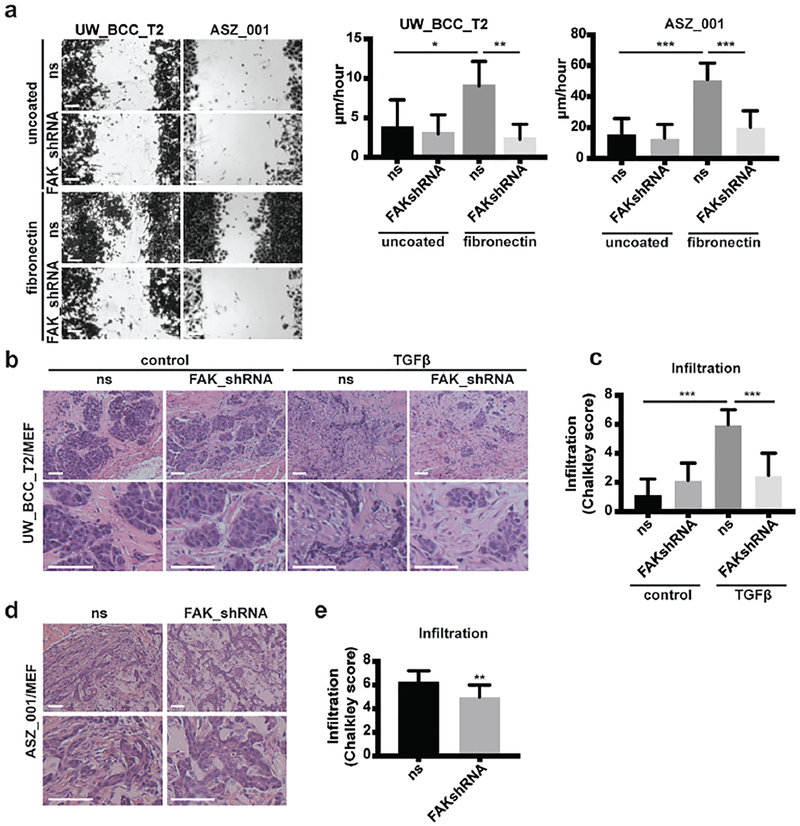
Figure 1. TGFβ is associated with peritumoral fibronectin deposition and the transition from nodular to infiltrative phenotype in human BCC samples and mouse models.
(a) Fibronectin and S100A4 stainings of nodular and infiltrative human BCC tumors.
(b) Quantification of the stainings shown in (a).
(c) Quantification of TGFβ at the protein level measured in nodular and infiltrative human BCC.
(d) Fibronectin and HE stainings of UW_BCC_T2/MEF tumors injected either with PBS (control) or recombinant TGFβ (400ng/injection, 3×/week).
(e) Quantification of fibronectin and infiltration in UW_BCC_T2/MEF tumors shown in (d).
(f) Fibronectin and HE stainings of ASZ_001_ns/MEF and ASZ_001_TGFβshRNA/MEF tumors.
(g) Quantification of fibronectin and infiltration in tumors shown in (f).
Scale bars indicate 100μm. Columns and error bars represent the mean ± SD for n ≥ 5 samples or mice per group. *p < 0.05, **p < 0.01 and ***p < 0.001.
To test this hypothesis in vivo, we induced “nodular-like” BCC by co-injecting the human UW_BCC_T2 cell line with mouse embryonic fibroblasts (MEFs) subcutaneously into NODSCID immunosuppressed mice. In this model, recombinant TGFβ was injected repeatedly into UW_BCC_T2 growing tumors (Supplementary Figure 1b). At the time of sacrifice, no difference in tumor growth (volume) was observed (Supplementary Figure 1c). However, higher levels of fibronectin as well as morphological changes reminiscent of human infiltrative BCC were found in tumors injected with recombinant TGFβ compared to controls. (Figures 1d and 1e). Additional stainings for Ki67 as well as cleaved Caspase 3 did not show any difference in proliferation and apoptosis levels, respectively (Supplementary Figure 1d). Reduced E-cadherin, without associated N-cadherin enhancement upon TGFβ suggested a possible incomplete epithelial-to–mesenchymal transition (EMT) in this model (Supplementary Figure 1e). Interestingly, murine ASZ_001 cells co-injected with MEFs in NOD-SCID mice spontaneously formed “infiltrative-like” BCC. In this model, we neutralized TGFβ1 in tumor cell using lentiviral-mediated expression of TGFβ1-targeting short hairpin (sh)-RNA (Supplementary Figure 1f). When co-injected wit MEF (Supplementary Figure 1b), TGFβ1-silencing reduced tumor growth, peritumoral fibronectin and partially restored a “nodular-like” phenotype (Figures 1f and 1g; Supplementary Figure S1c). Taken together, these results strongly suggest that TGFβ plays a key role in peritumoral fibronectin deposition as well as in the switch from a nodular to an infiltrative phenotype. Moreover, they help to address the current lack of in vivo models for the study of infiltrative BCC.
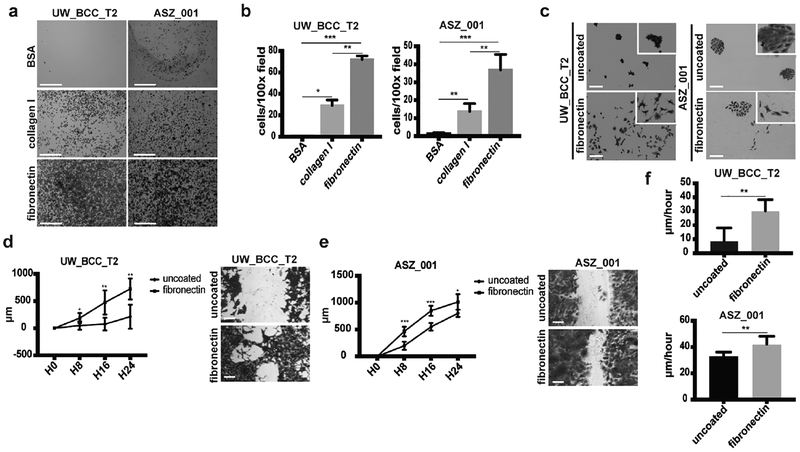
Increased BCC adhesion and migration on fibronectin is mediated by integrin α5β1.
ECM proteins have been shown to provide a scaffold that favors tumor cell migration and invasion (Pickup et al., 2014). We therefore decided to explore whether BCC cells may adhere to deposited fibronectin in vitro. Using an adhesion assay, we found that both the human UW_BCC_T2 and murine ASZ_001 BCC cell lines are able to adhere very efficiently to fibronectin when compared to control (uncoated) or collagen I, the most abundant ECM component (Figures 2a and 2b). Interestingly, long-term cultures on fibronectin revealed phenotypic modifications such as reduced cell-to-cell contacts and longer cytoplasmic extensions, suggestive of greater cell motility (Figure 2c). To address this question, we used the previously described wound-healing assay (Liang et al., 2007, Polacheck et al., 2013) either on uncoated or fibronectin-coated dishes. For both UW_BCC_T2 and ASZ_001 cell lines, fibronectin-coating promoted cell migration (Figures 2d–f).
Figure 2. Fibronectin promotes BCC adhesion and migration.
(a)Crystal violet staining of adhesion assays performed with UW_BCC_T2 and ASZ_001 on BSA, collagen I or fibronectin. Scale bars indicate 1mm.
(b) Quantification of adhesion assays shown in (a).
(c) Crystal violet staining of long-term clonogenic assays performed with UW_BCC_T2 and ASZ_001 on uncoated or fibronectin-coated plates.
(d) Timecourse and crystal violet staining of migration assays performed with UW_BCC_T2 on uncoated or fibronectin-coated plates.
(e) Timecourse and crystal violet staining of migration assays performed with ASZ_001 on uncoated or fibronectin-coated plates.
(f) Tumor cell velocity measured from migration assays performed in (d) and (e).
Scale bars indicate 100μm (unless specified). Columns and error bars represent the mean ± SD for n ≥ 3 per group. *p < 0.05, **p < 0.01 and ***p < 0.001.
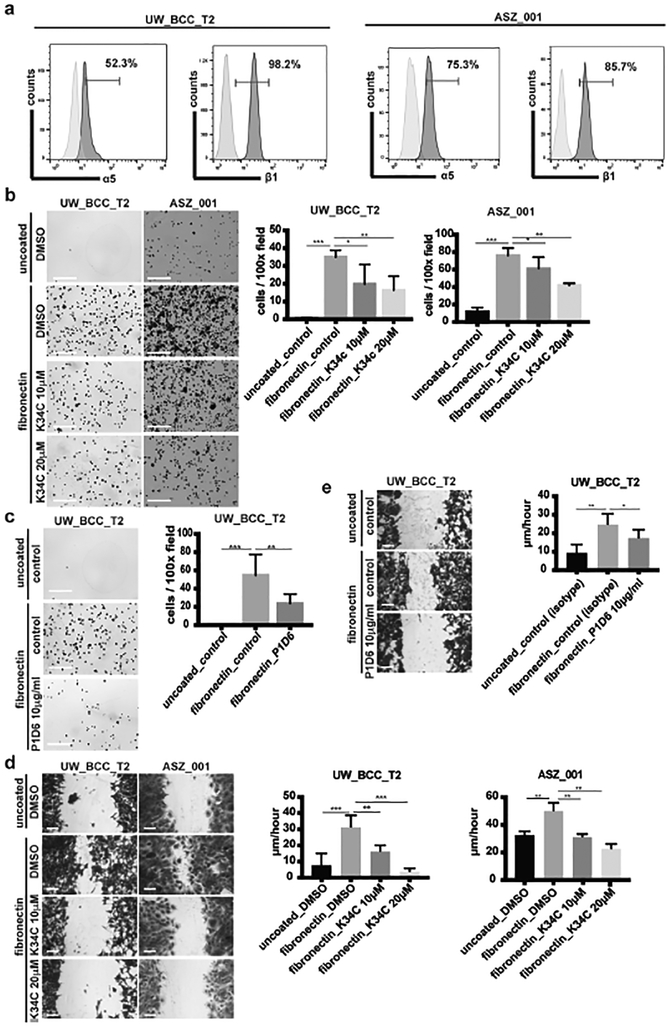
Integrin α5β1 is a main fibronectin receptor, binding to its RGD domain (Schaffner et al., 2013). Interestingly, we observed a significant expression of integrin α5β1 at the mRNA (not shown), and more importantly at the protein level in both UW_BCC_T2 and ASZ_001 cell lines (Figure 3a). To test the functional implication of integrin α5β1, we used both K34C, a recently developed pharmacologic inhibitor of integrin α5β1 (Ray et al., 2014) and P1D6, an anti-human integrin α5β1 blocking antibody. K34C significantly reduced UW_BCC_T2 and ASZ_001 cells adhesion on fibronectin in a dose-dependent manner (Figure 3b). Similar results were obtained using P1D6 blocking antibody on UW_BCC_T2 cells (Figure 3c). Consistently, K34C inhibitor prevented fibronectin-induced migration of UW_BCC_T2 and ASZ_001 cells (Figure 3d). Similar results were obtained using P1D6 blocking antibody on UW_BCC_T2 cells (Figure 3e). Migration time-courses are shown in Supplementary Material (Supplementary Figures S2a–c). Importantly, neither K34C nor P1D6 did affect UW_BCC_T2 and ASZ_001 cell growth on fibronectin (Supplementary Figures S2d–f), suggesting a specific effect of integrin α5β1 in mediating fibronectin-induced cell migration. Taken together, these results show that fibronectin promotes efficiently the migration of BCC cells through a mechanism implicating integrin α5β1 binding.
Figure 3. BCC adhesion and migration on fibronectin is mediated by integrin α5β1.
(a) Flow cytometry analysis of integrin α5 and β1 subunits on UW_BCC_T2 and ASZ_001.
(b) Crystal violet staining and quantification of UW_BCC_T2 and ASZ_001 adhesion to fibronectin when treated with DMSO or K34C.
(c) Crystal violet staining and quantification of UW_BCC_T2 adhesion to fibronectin when treated with isotype (control) or P1D6 antibodies.
(d) Crystal violet staining and quantification of UW_BCC_T2 and ASZ_001 migration on fibronectin when treated with DMSO or K34C.
(e) Crystal violet staining and quantification of UW_BCC_T2 migration on fibronectin when treated with isotype (control) or P1D6 antibodies.
Scale bars indicate 1mm in (b) and (c), and 100μm in (d) and (e). Columns and error bars represent the mean ± SD for n ≥ 3 per group. *p < 0.05, **p < 0.01 and ***p < 0.001.
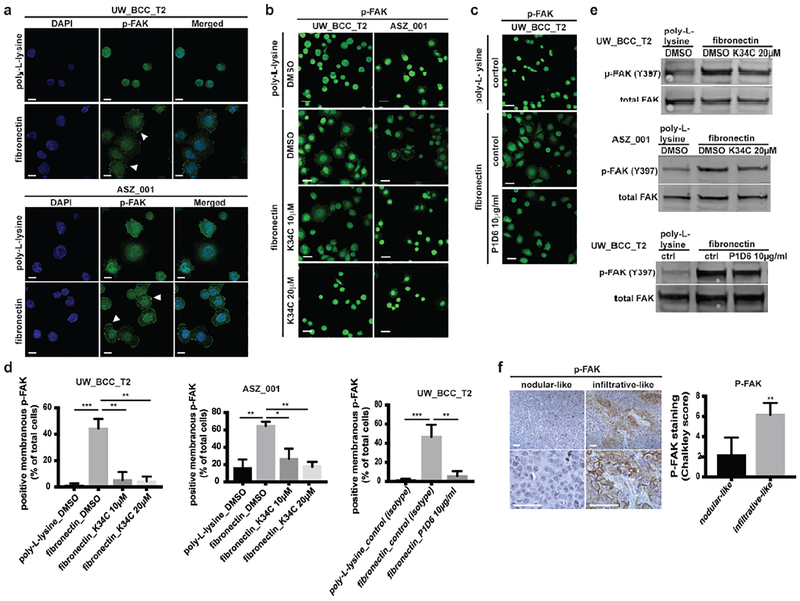
Fibronectin adhesion to integrin α5β1 induces focal adhesion kinase (FAK) phosphorylation both in vitro and in vivo.
FAK activation has been observed in various types of cancers and is widely associated with poor prognosis (Gabarra-Niecko et al., 2003). It is a key mediator between extracellular adhesion to ECM and intracellular regulation of actin-cytoskeleton organization and cell motility. Integrin binding to ECM typically activates FAK through autophosphorylation at tyrosine 397 (Y397), providing a binding site for downstream activating molecules implicated in proliferation, survival and motility (Tilghman and Parsons, 2008). We suspected that this mechanism could also be at play in BCC, and checked the phosphorylation status of FAK at Y397 in BCC cells lines, upon adhesion to fibronectin. As predicted by our model, we observed high levels of phosphorylated FAK (phospho-FAK) at the cell membrane of BCC growing on fibronectin but not on those growing on poly-l-lysine (Figure 4a). Consistently, K34C (Figures 4b and 4d) and P1D6 antibody (Figures 4c and 4d), both efficiently prevented FAK phosphorylation, bringing proof that this event is mediated by α5β1 integrin. Reduced FAK phosphorylation upon K34C and P1D6 treatments were confirmed by western blot (Figure 4e and Supplementary Figures S3a and S3b). Importantly, we observed preferential FAK phosphorylation in infiltrative-like areas of BCC injected with TGFβ (Figure 4f).
Figure 4. Fibronectin adhesion to integrin α5β1 induces FAK phosphorylation.
(a) Phospho-FAK immunostainings of UW_BCC_T2 and ASZ_001 on poly-L-lysine or fibronectin.
(b) Phospho-FAK immunostainings of UW_BCC_T2 and ASZ_001 on fibronectin treated with DMSO or K34C.
(c) Phospho-FAK immunostainings of UW_BCC_T2 on fibronectin treated with isotype (control) or P1D6 antibodies.
(d) Quantification of membranous phospho-FAK shown in (b) and (c).
(e) Western blots for phospho(Y397)- and total-FAK in UW_BCC_T2 and ASZ_001 on fibronectin treated with DMSO or K34C, and UW_BCC_T2 on fibronectin treated with isotype (control) or P1D6 antibodies.
(f) Phospho-FAK stainings and quantification in nodular- compared to infiltrative-like areas of UW_BCC_T2 tumors injected with TGFβ. Scale bars indicate 100μm.
White arrowheads indicate membranous phospho-FAK. Scale bars indicate 20μm (unless specified). Columns and error bars represent the mean ± SD for n ≥ 3 per group. *p < 0.05, **p < 0.01 and ***p < 0.001.
FAK mediates fibronectin-induced migration in vitro and TGFβ-induced infiltration in vivo.
To test the functional role of FAK in fibronectin-induced migration, we silenced FAK expression in tumor cells through lentiviral-mediated expression of FAK-targeting shRNA. Significant reduction in FAK level was confirmed at both the mRNA (not shown) and protein levels in both UW_BCC_T2 and ASZ_001 cell lines (Supplementary Figures S4a and S4b). In both cell lines, FAK silencing efficiently prevented fibronectin-induced migration in vitro (Figure 5a and Supplementary Figure S4c). In contrast to integrin α5β1 inhibition, FAK silencing also significantly reduced tumor cell growth in vitro (Supplementary Figure S4d). This difference may be explained by the implication of phospho-FAK in mediating tumor cell growth through alternative integrins (Desgrosellier and Cheresh, 2010). We then tested the effect of FAK silencing in vivo (Supplementary Figure S4e). Consistently with the in vitro observations, FAK silencing in UW_BCC_T2 significantly prevented both tumor growth and TGFβ-induced infiltration in vivo (Figures 5b and 5c; Supplementary Figure S4f). Similarly, FAK silencing in ASZ_001 prevented both tumor growth and spontaneous infiltration in vivo (Figures 5d and 5e; Supplementary Figure S4g).
Figure 5. FAK mediates fibronectin-induced migration in vitro and TGFβ-induced infiltration in vivo.
(a) Crystal violet staining and quantification of migration assays performed with UW_BCC_T2_ns or UW_BCC_T2_FAKshRNA and ASZ_001_ns or ASZ_001_FAKshRNA cells on uncoated or fibronectin-coated plates.
(b) HE stainings of UW_BCC_T2_ns/MEF or UW_BCC_T2_FAKshRNA/MEF tumors injected either with PBS or recombinant TGFβ (400ng/injection, 3×/week).
(c) Quantification of infiltration in tumors shown in (b).
(d) HE stainings of ASZ_001_ns/MEF or ASZ_001_FAKshRNA/MEF tumors.
(e) Quantification of infiltration in tumors shown in (d).
Scale bars indicate 100μm. Columns and error bars represent the mean ± SD for n ≥ 5 per group. *p < 0.05, **p < 0.01 and ***p < 0.001.
Taken together, these results identify integrin α5β1-dependent FAK phosphorylation as a key regulator of BCC cell adhesion and migration on fibronectin in vitro as well as infiltration in vivo.
Phospho-FAK inhibitor PF-562271 prevents fibronectin-induced migration in vitro and TGFβ-induced infiltration in vivo.
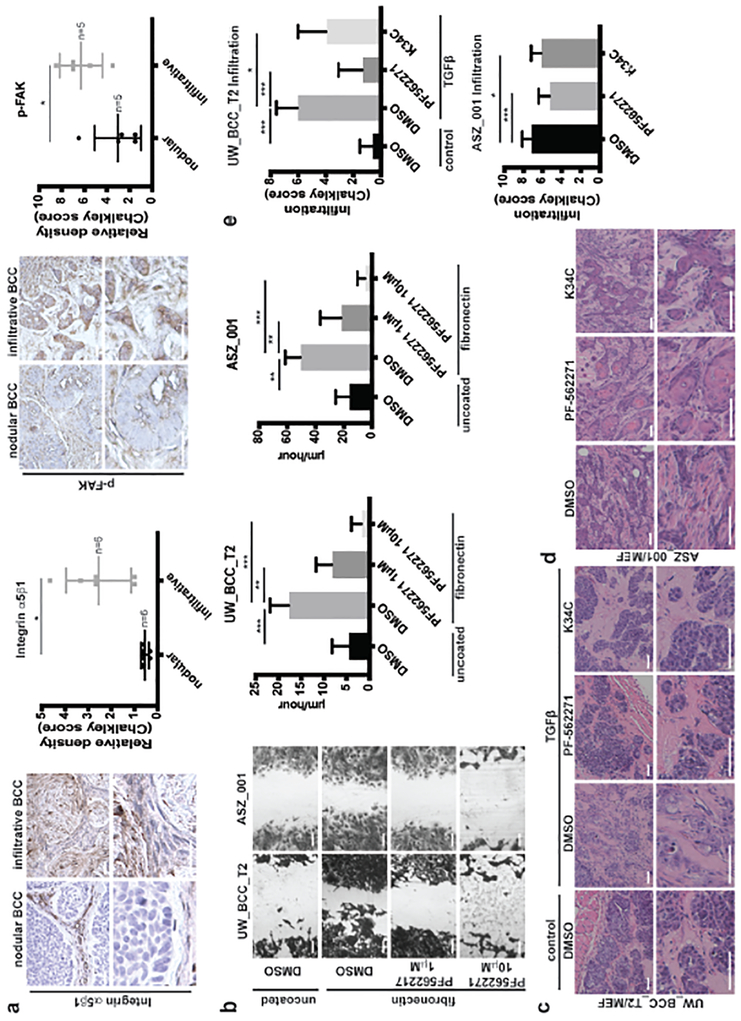
Having identified FAK as a key factor driving BCC invasiveness, we aimed to determine if this finding could have clinical implications. To support integrin α5β1 / phospho-FAK implication in human BCC progression, both were quantified in human BCC samples using immunohistochemistry. As expected, whereas integrin α5β1 was barely expressed in nodular BCC, significant expression was found in infiltrative BCC (Figure 6a). Similarly, phospho-FAK was found preferentially in highly infiltrative BCC (Figure 6a). Taken together with our previous findings, this strongly suggested that phospho-FAK targeting may prevent BCC infiltration in human.
Figure 6. Phospho-FAK inhibition prevents fibronectin-induced migration in vitro and TGFβ-induced infiltration in vivo.
(a) Pictures and quantification of integrin α5β1 and phospho-FAK stainings in human nodular and infiltrative BCC.
(b) Crystal violet staining and quantification of UW_BCC_T2 and ASZ_001 migration on fibronectin treated with DMSO or PF-562271.
(c) HE stainings of UW_BCC_T2/MEF tumors injected either with PBS or recombinant TGFβ (400ng/injection, 3×/week) and simultaneously treated with either DMSO, PF-562271 (50mg/kg, 1×/day) or K34C (50μl at 20μM).
(d) HE stainings of ASZ_001/MEF tumors treated with either DMSO, PF-562271 (50mg/kg, 1×/day) or K34C (50μl at 20μM).
(e) Quantification of infiltration in tumors shown in (c) and (d).
Scale bars indicate 100μm. Horizontal bars or columns and error bars represent the mean ± SD for n ≥ 5. *p < 0.05, **p < 0.01 and ***p < 0.001.
Efficient inhibitors of FAK activity are available (Golubovskaya, 2014, Slack-Davis et al., 2007) and tested successfully in Phase I clinical trials for patients with solid advanced tumors (Infante et al., 2012). We therefore hypothesized that they could also prevent expansion of aggressive BCC. We started by testing the effect of PF-562271, an enzymatic phospho-FAK inhibitor (Infante et al., 2012), in vitro and observed that it could efficiently prevent fibronectin-induced migration of BCC cells (Figure 6b and Supplementary Figures S5a). Similarly to FAK silencing, PF-562271 also significantly reduced tumor cell growth on fibronectin in vitro (Supplementary Figures S5b). Stimulated by these results we set up to test whether FAK inhibition also impinged tumor migration in vivo, by monitoring the effect of PF-562271 in our allograft models of invasive BCC (Supplementary Figure S5c). Mice bearing UW_BCC_T2/MEF tumors were injected intratumorally with recombinant TGFβ and simultaneously treated with or without the inhibitor. While PF-562271 did impinge on the growth of tumors (Supplementary Figure S5d), it also significantly prevented TGFβ-induced infiltrative phenotype (Figures 6c and 6e). Similarly, PF-52271 efficiently prevented both tumor growth (Supplementary Figure S5e) and spontaneous infiltration of ASZ_001/MEF tumors (Figures 6d and 6e). The lack of biovailability data regarding integrin α5β1 inhibitor K34C unfortunately precludes from systemic administration at the time being. Nevertheless, as a proof-of-concept, we performed repeated intratumoral injection of K34C (Supplementary Figure S5c) that partially prevented TGFβ-induced infiltration in UW_BCC_T2/MEF tumors as well as spontaneous infiltration in ASZ_001/MEF tumors, without impacting tumor growth (Figures 6c–e; Supplementary Figures 5d and 5e). Altogether, these results implicate TGFβ, fibronectin, and FAK phosphorylation through integrin α5β1 as a molecular pathway driving a phenotypic switch from nodular BCC to invasive BCC, with potential direct clinical benefits.
DISCUSSION
Here, we bring important insights in the field of aggressive BCC, setting up in vivo models for the study of infiltrative BCC and revealing the role of TGFβ-induced fibronectin in promoting tumor cell motility through integrin α5β1-mediated FAK phosphorylation.
The first key finding is the central implication of TGFβ-induced fibronectin as a functional link between fibrotic stroma and infiltrative phenotype during BCC progression. The dense peritumoral network induced upon TGFβ injection in our murine model is consistent with the previous report of TGFβ as the most potent activator of chronic fibrosis (Meng et al., 2016). Importantly, chronic fibrosis is a self-amplifying process, in which remodeled ECM sustains myofibroblast differentiation and release/activation of latent TGFβ (Annes et al., 2003, Munger and Sheppard, 2011). By analogy, initiation and maintenance of peritumoral fibrosis upon tumor-derived TGFβ expression may benefit from a self-amplifying loop (Kojima et al., 2010). Once activated, stromal TGFβ-induced remodeling may induce secretion of various ECM components and ECM-remodeling molecules that potentially contribute to tumor progression (Ueha et al., 2012). Our work definitively contributes to define TGFβ-induced fibronectin as a key functional connector between fibrotic stroma and BCC tumor progression, resulting in the concomitant transition from a loose myxoid towards a dense fibrotic stroma and from a nodular towards an infiltrative tumor phenotype (Crowson, 2006). As fibronectin is considered one of the most upregulated protein upon TGFβ stimulation (Rybinski et al., 2014, White and Muro, 2011), it is not surprising to find high levels of fibronectin in various chronic fibrotic processes as excessive scarring (keloids), systemic sclerosis, chronic radiodermatitis or desmoplastic tumors (Berman and Duncan, 1990, Dooley et al., 2010, Kelsh et al., 2015, Roberts et al., 1988, Tien et al., 2009, Walker, 2001). Importantly, several fibrotic skin events (like scars and chronic radiodermatitis among others) increase the risk to develop BCC (Handa et al., 2003, Kowal-Vern and Criswell, 2005, Serrano-Ortega et al., 2002). Further functional studies will be needed to link fibrotic events with the increased susceptibility to develop BCC.
As epithelial-to-mesenchymal transition (EMT) is reported in wound healing process and fibrosis (Li et al., 2007), one intriguing question is whether EMT is required during the nodular to infiltrative transition. Our data support the hypothesis that EMT may act as a driver of BCC progression, as TGFβ, fibronectin and integrin α5β1 were all implicated in EMT (Cicchini et al., 2008, Maschler et al., 2005, Massague, 2008, Park and Schwarzbauer, 2014). Moreover, despite the absence of N-cadherin detection, we demonstrated reduced E-cadherin in vivo upon TGFβ injection. Similarly, Papanikolaou et al. recently observed reduced E-cadherin levels as well as increased membranous integrin-linked kinase (ILK) at the invasive front of infiltrative BCC (Papanikolaou et al., 2010), resulting in a shift from cell-to-cell contacts to cell-to-stroma interactions that is mediated by integrins. Further studies will be needed to study these intriguing observations.
The second key finding is the functional implication of phospho-FAK downstream of integrin α5β1 activation. In fact, we showed that phospho-FAK inhibition recapitulates the effect of integrin α5β1 inhibition in term of fibronectin-induced migration in vitro and TGFβ-induced infiltration in vivo. However, phospho-FAK inhibition also prevented tumor cell proliferation on fibronectin in vitro, as well as tumor growth in vivo, whereas integrin α5β1 inhibition did not. Most integrins bind and activate FAK through their β subunit. Some however activate specific Src-family kinases (SFK) through their α subunit (as α1, α5 or αV) or exert a positional control over adjacent receptor tyrosine kinases (RTKs), which in turn affect FAK activity (Alavi et al., 2003, Giancotti and Tarone, 2003, Hood et al., 2003, Miranti and Brugge, 2002, Wary et al., 1996, Wary et al., 1998). These complex and specific interactions between integrins, SFK, adjacent RTK and various cofactors modulate FAK activation and dictate specific downstream effector pathways, as cell proliferation, cell survival or cell migration (Desgrosellier and Cheresh, 2010, Guo and Giancotti, 2004). Our data suggest that FAK is required for both cell proliferation and migration through different activation routes in BCC. While integrin α5β1 specifically mediates fibronectin-induced migration, further studies will be needed to decipher the mechanisms of FAK-mediated proliferation. Altogether, our data identify FAK inhibition as a promising therapy for advanced BCC. Importantly, various phospho-FAK inhibitors have been recently developed, some with proven clinical success (Golubovskaya, 2014, Infante et al., 2012, Slack-Davis et al., 2007). The precise place of FAK inhibitors along surgery, radiotherapy or Smoothened-inhibitors naturally remains to be clarified.
In conclusion, this work suggests that TGFβ, fibronectin, integrin α5β1 and phospho-FAK act together as key regulators in driving a dense fibrotic peritumoral stroma and BCC infiltration. We bring experimental evidence for the role of TGFβ in peritumoral fibronectin deposition and BCC infiltration in murine models of infiltrative BCC and identify integrin α5β1-mediated phosphorylation of FAK, upon fibronectin adhesion, as a mechanism regulating BCC cell motility. The clinical relevance of our experiments is validated by the observation of increased TGFβ, fibronectin as well as membranous phospho-FAK in human infiltrative BCC. Hence, this work extends our understanding of the biology of aggressive infiltrative BCC and provides an essential step toward better prevention and improved treatment of BCC.
MATERIALS AND METHODS
Cell lines and cell culture.
UW_BCC cell line was generously provided by Dr. V. Spiegelman (Madison, WI). UW_BCC_T2 were obtained as previously described (Bal et al., 2017). ASZ_001 cell line was generously provided by Dr. E. Epstein (Oakland, CA). For all experiments, cells were grown in M154CF (Invitrogen, Basel, Switzerland) supplemented with 10% FBS, 1% P/S and CaCl2 0.05mM. 293T cells were obtained from ATCC (Manassas, VA). Dermal mouse embryonic fibroblasts (MEF) were obtained from 13.5 days-old C57BL/6 embryos as described in (A, 2007). 293T and MEF were grown in DMEM (Invitrogen, basel, Switzerland) supplemented with 10% FBS and 1% P/S. Cell lines were assessed for mycoplasma using the MycoSeq Mycoplasma real-time PCR detection kit (ThermoFisher Scientific, Waltham, MA) and frozen stocks were used for a maximum of 2 months.
Mouse model and drug treatment.
Adult male (5–7 weeks of age) NOD-SCID mice were used as host animals for grafted tumors. Primary tumors were initiated by injection of UW_BCC_T2 (5×105 cells/mouse) or ASZ_001 (5×105 cells/mouse) tumor cells together with MEF (1.5×106 cells/mouse) subcutaneously in 100μl of PBS:Matrigel 1:1. For intratumoral injection, mouse recombinant TGFβ1 (R&D Systems, Minneapolis, MN) was dissolved in 0.1% BSA, 4mM HCl and diluted in PBS before injection. PF-562271 inhibitor (MedKoo Biosciences, Chapel Hill, NC) was dissolved in DMSO and diluted in 5% DMSO (H2O) for oral gavage. K34C inhibitor was dissolved in DMSO and diluted in 0.2% DMSO (PBS) for intratumoral injections. Tumor volume was assessed as previously described (Kuonen et al., 2012). Infiltration was assessed on HE stainings in every quarter of a Chalkley grid (0= nodular; 1= slightly infiltrative; 2= infiltrative) and summed. For every slide, an infiltrative score from 0 (highly nodular) to 8 (highly infiltrative) was obtained. Mice were housed under standard conditions, and animal care was in compliance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Stanford University.
Human BCC samples.
Study on human samples obtained in Switzerland was approved by the cantonal ethical authority (VD, Switzerland) and written informed consents were obtained from participants. Study on human samples obtained from Stanford University was approved by the Stanford University Institutional Review Board (Stanford, CA), with a waiver ofconsent from participants.
Statistical analyses.
Statistical comparisons were performed by a two-tailed Student’s t test or one-way ANOVA with Tukey’s post test for multiple comparisons. Statistical analyses were performed using GraphPad Prism (La Jolla, CA). The data were considered to be significantly different when p < 0.05.
A more complete and detailed description of the methods is included in Supplementary Materials and Methods.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a MD-PhD clinician fellowship from CHUV-FBM (to FK), a Swiss National Research Foundation fellowship (to FK), a Fondation René Touraine fellowship (to FK), the Swiss National Research Foundation grant 31003A_159824 (to CR) and the NIH R01ARO46786 grant (to AO). We acknowledge Hanson Zhen for his help with the isolation of dermal mouse embryonic fibroblasts.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors state no conflict of interest.
REFERENCES
- EM A. Isolation and propagation of mouse embryonic fibroblasts and preparation of mouse embryonic feeder layer cells. Curr Protoc Stem Cell Biol 2007;Chapter 1:Unit1C 3. [DOI] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 2003;301(5629):94–6. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003;116(Pt 2):217–24. [DOI] [PubMed] [Google Scholar]
- Bal E, Park HS, Belaid-Choucair Z, Kayserili H, Naville M, Madrange M, et al. Mutations in ACTRT1 and its enhancer RNA elements lead to aberrant activation of Hedgehog signaling in inherited and sporadic basal cell carcinomas. Nat Med 2017. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer 2013; 13(7):511–8. [DOI] [PubMed] [Google Scholar]
- Barsky SH, Grossman DA, Bhuta S. Desmoplastic basal cell carcinomas possess unique basement membrane-degrading properties. J Invest Dermatol 1987;88(3):324–9. [DOI] [PubMed] [Google Scholar]
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol 1990;123(3):339–46. [DOI] [PubMed] [Google Scholar]
- Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet 2016;48(4):398–406. [DOI] [PubMed] [Google Scholar]
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol 1991;17(7):574–8. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009;9(2):108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccialanza M, Piccinno R, Cuka E, Alberti Violetti S, Rozza M. Radiotherapy of morphea-type basal cell carcinoma: results in 127 cases. J Eur Acad Dermatol Venereol 2014;28(12):1751–5. [DOI] [PubMed] [Google Scholar]
- Chu CY, Cha ST, Lin WC, Lu PH, Tan CT, Chang CC, et al. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12)-enhanced angiogenesis of human basal cell carcinoma cells involves ERK1/2-NF-kappaB/interleukin-6 pathway. Carcinogenesis 2009;30(2):205–13. [DOI] [PubMed] [Google Scholar]
- Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, et al. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res 2008;314(1):143–52. [DOI] [PubMed] [Google Scholar]
- Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 2011;1(4):482–97. [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 2011;178(3): 1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol 2006;19 Suppl 2:S127–47. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010;10(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley A, Shi-Wen X, Aden N, Tranah T, Desai N, Denton CP, et al. Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology (Oxford) 2010;49(11):2024–36. [DOI] [PubMed] [Google Scholar]
- Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008;8(10):743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev 2003;22(4):359–74. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 2003;19:173–206. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed) 2014;19:687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5(10):816–26. [DOI] [PubMed] [Google Scholar]
- Handa Y, Miwa S, Yamada M, Ono H, Suzuki T, Tomita Y. Multiple pigmented basal cell carcinomas arising in the normal-appearing skin after radiotherapy for carcinoma of the cervix. Dermatol Surg 2003;29(12):1233–5. [DOI] [PubMed] [Google Scholar]
- Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 2003;162(5):933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol 2012;30(13):1527–33. [DOI] [PubMed] [Google Scholar]
- Kallioinen M, Autio-Harmainen H, Dammert K, Risteli J, Risteli L. Discontinuity of the basement membrane in fibrosing basocellular carcinomas and basosquamous carcinomas of the skin: an immunohistochemical study with human laminin and type IV collagen antibodies. J Invest Dermatol 1984;82(3):248–51. [DOI] [PubMed] [Google Scholar]
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16(9):582–98. [DOI] [PubMed] [Google Scholar]
- Kasper M, Jaks V, Hohl D, Toftgard R. Basal cell carcinoma - molecular biology and potential new therapies. J Clin Invest 2012;122(2):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RM, McKeown-Longo PJ, Clark RA. EDA Fibronectin in Keloids Create a Vicious Cycle of Fibrotic Tumor Formation. J Invest Dermatol 2015; 135(7): 1714–8. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A 2010;107(46):20009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal-Vern A, Criswell BK. Burn scar neoplasms: a literature review and statistical analysis. Burns 2005;31(4):403–13. [DOI] [PubMed] [Google Scholar]
- Kuonen F, Laurent J, Secondini C, Lorusso G, Stehle JC, Rausch T, et al. Inhibition of the Kit ligand/c-Kit axis attenuates metastasis in a mouse model mimicking local breast cancer relapse after radiotherapy. Clin Cancer Res 2012;18(16):4365–74. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139(5):891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25(1):9–18. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2(2):329–33. [DOI] [PubMed] [Google Scholar]
- Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130(6):1091–103. [DOI] [PubMed] [Google Scholar]
- Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ. alpha vbeta 6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res 2008;68(9):3295–303. [DOI] [PubMed] [Google Scholar]
- Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 2005;24(12):2032–41. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell 2008;134(2):215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12(6):325–38. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol 2002;4(4):E83–90. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 2011;3(11):a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou S, Bravou V, Gyftopoulos K, Nakas D, Repanti M, Papadaki H. ILK expression in human basal cell carcinoma correlates with epithelial-mesenchymal transition markers and tumour invasion. Histopathology 2010;56(6):799–809. [DOI] [PubMed] [Google Scholar]
- Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene 2014;33(13):1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinno R, Benardon S, Gaiani FM, Rozza M, Caccialanza M. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat 2017:1–5. [DOI] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014;15(12):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci 2013;70(8):1335–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AM, Schaffner F, Janouskova H, Noulet F, Rognan D, Lelong-Rebel I, et al. Single cell tracking assay reveals an opposite effect of selective small non-peptidic alpha5beta1 or alphavbeta3/beta5 integrin antagonists in U87MG glioma cells. Biochim Biophys Acta 2014;1840(9):2978–87. [DOI] [PubMed] [Google Scholar]
- Roberts CJ, Birkenmeier TM, McQuillan JJ, Akiyama SK, Yamada SS, Chen WT, et al. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem 1988;263(10):4586–92. [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 2006;5(12):1026–33. [DOI] [PubMed] [Google Scholar]
- Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics 2014;46(7):223–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F, Ray AM, Dontenwill M. Integrin alpha5beta1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers (Basel) 2013;5(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Ortega S, Fernandez-Angel I, Dulanto-Campos E, Rodriguez-Archilla A, Linares-Solano J. Basal cell carcinoma arising in professional radiodermatitis of the nail. Br J Dermatol 2002;147(3):628–9. [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem 2007;282(20): 14845–52. [DOI] [PubMed] [Google Scholar]
- Telfer NR, Colver GB, Morton CA, British Association of D. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159(1):35–48. [DOI] [PubMed] [Google Scholar]
- Tien YW, Wu YM, Lin WC, Lee HS, Lee PH. Pancreatic carcinoma cells stimulate proliferation and matrix synthesis of hepatic stellate cells. J Hepatol 2009;51(2):307–14. [DOI] [PubMed] [Google Scholar]
- Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol 2008;18(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol 2009;129(4):1016–25. [DOI] [PubMed] [Google Scholar]
- Ueha S, Shand FH, Matsushima K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol 2012;3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res 2001;3(3):143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 1996;87(4):733–43. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 1998;94(5):625–34. [DOI] [PubMed] [Google Scholar]
- White ES, Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life 2011;63(7):538–46. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.