Interferon (IFN)-induced transmembrane protein 3 (IFITM3) is a potent antiviral factor capable of restricting numerous virus infections.1 Viruses inhibited by IFITM3 generally enter cells via endocytosis, and are trafficked to IFITM3-positive intracellular membrane compartments.1,2 IFITM3-positive compartments are acidic and stain positive for a variety of endosomal, lysosomal and autophagosomal markers.2,3 Although the membrane fusion of many endocytic viruses, such as influenza virus, is triggered by acidic pH, fusion is blocked in the presence of IFITM3, and virions are degraded2,4,5 (Figure 1). It has been proposed that IFITM3 alters membrane properties, including rigidity and curvature, to inhibit virus membrane fusion.5 The molecular mechanism underlying these IFITM3-induced membrane alterations is not well characterized, and whether IFITM3 has other cellular functions is also not known.
Figure 1.
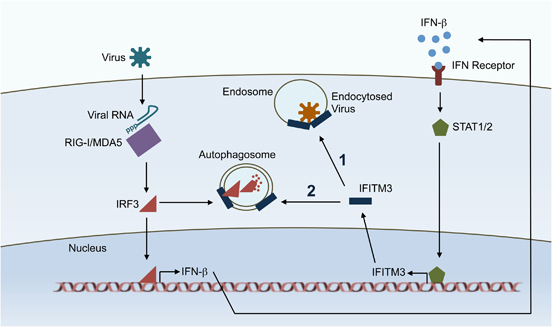
Dual functions of IFITM3 in the innate antiviral immune response. Cellular sensors, such as RIG-I and MDA5, detect virus infection. These proteins recognize viral products, including RNA bearing uncapped 5′-phosphates. Subsequently, the transcription factor IRF3 is activated and is translocated to the nucleus for induction of IFN-β. Secreted IFN-β signals through its receptor via STAT proteins and induces production of IFITM3 and other IFN-stimulated genes. IFITM3 blocks the membrane fusion of endocytosed viruses, such as influenza virus (function 1), and is newly shown to promote IRF3 degradation in autophagosomes, providing feedback inhibition of the IFN-β induction pathway (function 2).
IFITM3 is particularly well characterized as a restriction factor able to limit influenza virus infections, and is responsible for a significant portion of the antiviral action of type I IFNs against influenza virus in cells.6,7 As such, IFITM3 knockout mice succumb to sublethal doses of influenza virus and exhibit increased lung pathology compared to control mice.8,9 Further, homozygosity of a single nucleotide polymorphism in the human IFITM3 gene, rs-12252-C, has been associated with increased morbidity and mortality in individuals hospitalized from infections with the 2009 pandemic H1N1 influenza A virus or with an emergent H7N9 virus.8,10,11 This polymorphism is thought to result in an alternatively spliced IFITM3 transcript coding for a truncated and mislocalized protein, though evidence for a truncated variant of IFITM3 is lacking.8 Nonetheless, IFITM3 is well established as a critical component of the innate anti-influenza virus immune response in mice and humans.1
Although IFITM3 is appreciated primarily as an antiviral protein able to block virus membrane fusion, we, and others, have also noted the ability of IFITM3 to induce autophagosome formation in cells as indicated by LC3 lipidation and puncta formation.2,3 We previously demonstrated that antiviral activity of IFITM3 is independent of the canonical ATG5-dependent autophagy pathway, thus decoupling autophagy induction by IFITM3 from its antiviral activity.3 This suggested that, in addition to directly inhibiting viruses, IFITM3 may also play a role in regulating autophagy, though the biological relevance of such a role has been difficult to identify. New work by Jiang et al. 12 published in this edition of Cellular and Molecular Immunology, identified a novel function for IFITM3 in inhibiting IFN-β induction by promoting degradation of IFN regulatory factor 3 (IRF3), a critical transcription factor necessary for activating IFN-β production upon infection. The authors propose that this IRF3 degradation occurs within IFITM3-associated autophagosomes (Figure 1). This work describes the first reported immunoregulatory role for IFITM3 in the type I IFN pathway.
Jiang et al. 12 made the astute observation that IFITM3 expression reduces production of IFN-β during Sendai virus infection (an IFITM3-insensitive virus) or after PolyI:C treatment of cells. By artificially inducing IFN-β through overexpression of various components of the IFN-β induction pathway, they then determined that IFITM3 acts late in the pathway, possibly by affecting IRF3. They went on to show that indeed IRF3 levels inversely correlate with IFITM3 levels; when IFITM3 was overexpressed, IRF3 levels decreased, and when IFITM3 expression was knocked down, IRF3 levels increased. Interestingly, the decrease in IRF3 in the presence of IFITM3 was reversed by treatment of cells with the autophagy inhibitor 3-methyladenine, but not by treatment with the proteasome inhibitor MG132, indicating that IFITM3 promotes IRF3 degradation in autophagosomes. This was further supported by the finding that IFITM3 co-immunoprecipates with the autophagy proteins, Beclin1 and LC3. The authors also observed co-immunoprecipitation of IFITM3 and IRF3, suggesting that these proteins interact, and that IFITM3 may direct IRF3 to autophagosomes. Experiments using cells with genetic deficiencies in autophagosome components in the future will confirm the specific involvement of the autophagy pathway in IFITM3-mediated degradation of IRF3, and will allow the mapping of specific proteins involved in this dampening of the type I IFN response. Overall, this work identifying a feedback inhibition mechanism for the IFN-β induction pathway by IFITM3 highlights the importance and growing appreciation for negative regulators of the type I IFN response during infections.13
This new report has provided insights into a potential biological function for autophagy induction by IFITM3, and also introduces several new research directions that should be exciting areas of investigation in the future. The observed degradation of IRF3 upon expression of IFITM3 did not occur when IFITM1 or IFITM2 was overexpressed, thus identifying a major difference between these highly homologous proteins.12 IFITM2 and IFITM3 share roughly 90% amino-acid identity, and exhibit similar antiviral activities in vitro.6 Thus, it has remained enigmatic that IFITM2 is seemingly unable to compensate for defects in IFITM3 in limiting the severity of influenza virus infections in mice and humans.8,10,11 This new work may suggest that the immunoregulatory role of IFITM3 that distinguishes it from IFITM1 and IFITM2 is critically involved in limiting infection severity in vivo. It will be interesting to map specific amino acids within IFITM3 that mediate interaction with IRF3 and to determine whether these are conserved in IFITM1 and IFITM2. Further, since IFITM3 is regulated by several post-translational modifications, including palmitoylation, ubiquitination and phosphorylation, it will be informative to determine how these IFITM3 modifications impact the degradation of IRF3 and the induction of IFN-β.3,7,14 The discovery of an immunoregulatory role for IFITM3 may also indicate that IFITM3 could have broader effects on immune responses against pathogens beyond the viruses for which it directly blocks membrane fusion. Thus, a wider examination of the role of IFITM3 in immune responses and pathogen susceptibility may be warranted by this exciting new work.
Acknowledgements
TMM is supported by a Gilliam Graduate Fellowship from the Howard Hughes Medical Institute. JSY is supported by NIH Grant AI130110.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bailey CC, Zhong G, Huang IC, Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annu Rev Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen LM, et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7:e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yount JS, Karssemeijer RA, Hang HC. S-palmitoylation and ubiquitination differentially regulate interferon-induced transmembrane protein 3 (IFITM3)-mediated resistance to influenza virus. J Biol Chem. 2012;287:19631–19641. doi: 10.1074/jbc.M112.362095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai TM, Marin M, Chin CR, Savidis G, Brass AL, Melikyan GB. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014;10:e1004048. doi: 10.1371/journal.ppat.1004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li K, Markosyan RM, Zheng YM, Golfetto O, Bungart B, Li M, et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9:e1003124. doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yount JS, Moltedo B, Yang YY, Charron G, Moran TM, López CB, et al. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol. 2010;6:610–614. doi: 10.1038/nchembio.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey CC, Huang IC, Kam C, Farzan M. Ifitm3 limits the severity of acute influenza in mice. PLoS Pathog. 2012;8:e1002909. doi: 10.1371/journal.ppat.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Zhang A, Wan Y, Liu X, Qiu C, Xi X, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci USA. 2013;111:769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YH, Zhao Y, Li N, Peng YC, Giannoulatou E, Jin RH, et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is aFssociated with severe influenza in Chinese individuals. Nat Commun. 2013;4:1418. doi: 10.1038/ncomms2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang LQ, Tian X, Hu YH, Sun MS, Yan S, Lei CQ et al. IFITM3 inhibits virus-triggered induction of type I interferon by mediating autophagosome-dependent degradation of IRF3. Cell Mol Immunol 2017. In Press. [DOI] [PMC free article] [PubMed]
- 13.Porritt RA, Hertzog PJ. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 2015;36:150–160. doi: 10.1016/j.it.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Chesarino NM, McMichael TM, Hach JC, Yount JS. Phosphorylation of the antiviral protein interferon-inducible transmembrane protein 3 (IFITM3) dually regulates its endocytosis and ubiquitination. J Biol Chem. 2014;289:11986–11992. doi: 10.1074/jbc.M114.557694. [DOI] [PMC free article] [PubMed] [Google Scholar]


