Abstract
Zika virus (ZIKV) has recently emerged in numerous tropical countries worldwide. In this study, we estimated ZIKV incubation period distribution using time-to-event models adapted to interval-censored data based on declared date of travels from 123 symptomatic travelers returning from areas with active ZIKV transmission. The median time and 95th percentile of ZIKV incubation period was estimated to 6.8 days (95% confidence interval [CI], 5.8–7.7 days) and 15.4 days (95% CI, 12.7–19.7 days), respectively. Determining the incubation period for ZIKV is beneficial to improve protection guidelines.
Keywords: arbovirus, incubation period, prevention guidelines, Zika
Zika virus (ZIKV) has recently emerged in numerous tropical countries all over the world, causing serious congenital disorders. Zika is a vector-borne disease, and viral infection is primarily acquired through infectious mosquitoes’ bite, with occasional sexual, maternofetal, and blood transfusion transmission [1]. Characterizing the ZIKV incubation period in humans is necessary to understand the temporal dynamics of the virus and thus to prevent outbreaks.
In this study, incubation period (IP) is defined as the time elapsed from virus acquisition to onset of symptoms in humans. Estimating the duration of IP in humans is not straightforward for vector-borne diseases because date of infection through the bite of an infectious vector cannot usually be determined. In the past, experimental exposure of human volunteers to infected mosquitoes allowed researchers to directly assess a 4–7 days IPs for dengue virus and yellow-fever virus [2, 3]. Those practices are now banned due to obvious ethical issues. In addition, existing animal models such as nonhuman primates and genetically modified mice are ethically sensitive, costly, and do not always depict human diseases. Exploitation of declared travel and symptom dates of travelers entering or leaving areas with ongoing virus transmission offers an alternative to indirectly assessing arbovirus IP distributions [4–6].
In this study, we estimated probabilities of ZIKV IP with interval-censored survival analyses based on declared traveling periods of symptomatic patients returning from areas with ongoing ZIKV transmission. Our estimates can help physicians exclude a Zika diagnosis in travelers with respect to other infectious diseases and provide valuable information for ZIKV outbreak simulation studies.
METHODS
A total of 126 travelers were initially included in our retrospective cohort study from April 2015 to September 2017. Inclusion criteria were as travelers who (1) presented Zika symptoms after their return from a transmission area, (2) tested positive for the presence of ZIKV ribonucleic acid (RNA) in body fluids by real-time reverse transcription-polymerase chain reaction (RT-PCR) (RealStar Zika Virus RT-PCR Kit; Altona Diagnostics, Hamburg, Germany), (3) returned from countries with a documented ongoing ZIKV transmission, (4) did not report to be pregnant, and (5) declared their symptoms onset date (denoted here as S) and travel dates (denoted here as A and D for arrival in and departure from the area with ZIKV transmission, respectively). Three patients were excluded from the analysis because they reported symptoms onset >30 days after their return from the transmission areas (DS period, ie, time from departure from the transmission area to symptom onset), which are periods that exceed the 3rd quartile of the DS period distribution by a factor of 6. Errors in travel declaration dates were suspected for these 3 patients. Zika virus RNA was detected postsymptom onset in plasma, serum, sperm, and urine in 22 (18.2%), 68 (56.2%), 2 (1.6%), and 29 (24%) patients, respectively, in a time not exceeding 8 days after symptom onset concerning plasma or serum, 27 days for urine, and 51 days concerning ZIKV RNA detection in the sperm. All data were obtained from the Centre National de Référence des Arbovirus (Marseille, France).
We assumed that exposure to ZIKV only occurred via the bite of an infectious mosquito in areas of active ZIKV transmission. The moment of infection by a virus via a mosquito bite is not directly observable, whereas the end event, the onset of symptoms, is always known for symptomatic patients. Thus, time of infection events were subject to single interval censoring. For travelers who experienced symptoms after returning from travel, the exposure interval was delineated by the arrival date and the departure date (AD period). For travelers who experienced illness during their travel in areas with ZIKV transmission, the exposure interval was delineated by the arrival date and the symptom onset date (AS period). We assumed that the IPs and the censoring times were independent (ie, independent censoring). The icfit function from the interval (version 1.1.0.1) [7] R package was used to calculate non-parametric maximum likelihood estimates (NPMLEs) of the IP distribution (generalization of the Kaplan-Meier estimate) with a modified bootstrap confidence interval (CI) method. Estimates were calculated over a set of time intervals called Turnbull intervals, which represent the innermost intervals over a group of individuals in which NPMLE can change [8]. The non-parametric log-rank test, as implemented in the ictest function with default parameters, was used to assess the statistical independence of the duration of IP with gender, age, geographic infection zone, presence of fever, retrobulbar headache, skin lesion, arthralgia, digestive signs, and meningeal signs. A Bayesian accelerated failure-time regression model (AFT) with a log-logistic error distribution was fitted to the data using the ic_bayes function from the icenReg R package [9] using a prior on the baseline scale parameter, 4 Markov chain Monte Carlo (MCMC) chains to run, 10000 samples, and 1000 samples discarded for burn in. Conclusions from 2 other independent studies [5, 6] revealed that once exposed to infectious mosquitoes, half of the exposed humans would develop ZIKV symptoms within 4–8 days. Therefore, we set the mean incubation prior to 6 days with a standard deviation of 2 days. Flat priors were implemented on the baseline shape and regression parameters. The log-logistic distribution was chosen over other distributions based on visual comparison between several parametric baseline distributions and the semi-parametric distribution, as implemented in the diag_baseline function [9]. In addition to the package specified above, the survival (version 2.41.3) [10] and flexsurv (version 1.1) [11] R packages were used to analyze the data in the R statistical environment.
RESULTS
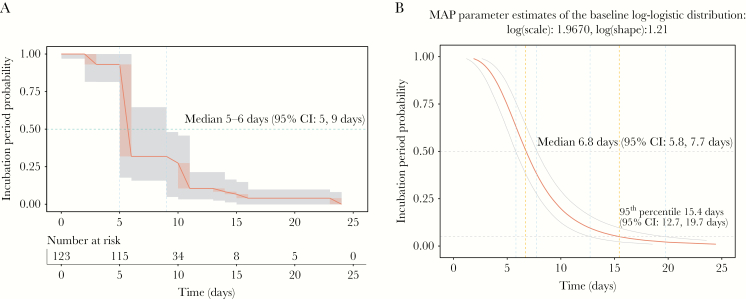
A total of 123 persons were included in our cohort, which comprises 58 females (47%) and 65 males (53%) with a median age of 38 years (mininimum, 1 year old; 1st quartile, 29 years old; 3rd quartile, 54 years old; maximum, 73 years old). Thirty persons (24.4%) experienced symptoms during their travel in areas with ZIKV transmission. The minimum ZIKV IP duration can only be directly observed among travelers who experienced symptoms during their journey in area with ZIKV transmission (the AS period), and this time frame was 3 days for a 58-year-old symptomatic male traveling in Martinique (French West Indies). On the other hand, the maximal ZIKV IP duration can only be directly observed among travelers who experienced symptoms after returning from areas with active ZIKV transmission (DS period), and this time frame was 23 days for a 25-year-old female returning from Martinique (Supplementary Figure 1). Because the date of ZIKV infection through an infectious mosquito bite cannot be directly observed, time-to-event models adapted to single interval-censored data were applied to estimate the IP distribution. A median time of 5–6 days (95% CI, 5–9 days) from infection to symptoms was estimated using a non-parametric method (Figure 1A). This median time was estimated as 6.8 days (95% CI, 5.8–7.7 days) when using Bayesian AFT regression (Figure 1B). The IP could not exceed 15.4 days (95% CI, 12.7–19.7 days) in 95% of cases, according to Bayesian AFT regression (Figure 1B). The effect of several factors on the duration of IP was tested. Age, geographic infection zone, presence of fever, skin lesions, retrobulbar headache, arthralgia, digestive, and meningeal signs were not shown to significantly impact the duration of ZIKV incubation. However, duration on ZIKV IP was significantly associated with gender (P = .032, log-rank test without adjustment for multiple testing). A deceleration factor of 0.74 was estimated (maximum a posteriori probability) using Bayesian AFT regression, which indicates a 26% shortening of the IP in male patients compared with female patients. The median time estimates of ZIKV IP was 8 days (95% CI, 6.4–9.6 days) for the female gender and 5.8 days (95% CI, 4.7–7.1 days) for the male gender.
Figure 1.
Probabilities of Zika virus (ZIKV) incubation period (IP) calculated by interval-censored survival analyses using declared traveling periods of symptomatic patients returning from areas with ZIKV transmission. Non-parametric maximum likelihood estimates (A) and Bayesian accelerated failure-time regression models (AFT) estimates (B) of ZIKV IP are represented. The table displaying number of individuals at risk to develop symptoms after ZIKV exposure via an infectious mosquito bite is provided in A. An AFT with a log-logistic error distribution was fit with a Bayesian regression model to provide Bayesian estimates. Non-parametric estimates are represented on B below the Bayesian regression curve. Medians are indicated for both analyses (A and B), and the 95th percentile is provided for the Bayesian analysis only (B). Light gray shading denotes 95% confidence intervals (CI).
DISCUSSION
To our knowledge, only 2 studies examined the duration of ZIKV IP so far. Both studies exploited declared travel and symptom dates of travelers visiting areas with ongoing virus transmission [5, 6]. Based on a new and completely independent data set, we confirm ZIKV IP distributions estimates found in these previous studies. We determined a median IP estimate of 6.8 days (95% CI, 5.8–7.7 days; n = 123 patients), whereas median estimates of 6.2 (95% CI, 5.7–6.6; n = 197 patients) days and 5.9 (95% CI, 4.4–7.6, n = 25 patients) days were determined by Krow-Lucal et al [6] and Lessler et al [5], respectively. The 95th percentile of the IP distribution was also concordant across all 3 studies with a value ranging from 11 to 16 days. These concordant results across studies support that exploitation of symptomatic travelers’ traveling dates with time-to-event models adapted to interval-censored data can be used as a reliable surrogate to estimate vector-borne diseases IPs in human.
One limitation to our study is that we could not assess the effect of the transmission mode on ZIKV IP by assuming that all infections occurred through an infectious mosquito bite. Occurrence of sexual and transfusion transmission during travel could have introduced a bias in our estimates, provided that ZIKV IP differs between transmission modes. Three patients were discarded in our analysis because they reported DS periods that deviate from other values. We have chosen to treat these values as data entry errors. Inclusion of these 3 patients does not change the median IP estimates (5–6 days, NPMLE method) but extend the 95th percentile of the IP distribution to 35–36 days instead of 15–16 days (NPMLE estimates).
It can be hypothesized that variability in the duration of ZIKV IP in humans can be explained by a combination of diverse environmental, human, and virus factors. In this study, we report that the male gender is significantly associated with a 26% shortening of the ZIKV IP. This association is not significant when considering P value correction for multiple testing. Inclusion of more patients might help to confirm this association with gender. In addition, the inclusion of a more exhaustive list of factors, such as virus genotypes, human body weight, and history of exposure to arboviruses, can further explain the variability of ZIKV IP. Because asymptomatic ZIKV infections are suspected to be involved in vector-borne transmission (as a far as we know, this was only documented for sexual transmission [12]), it would be of interest for epidemiological simulation modelers to assess whether the intrinsic IP distribution of asymptomatic infections differ from symptomatic ones. As far as we are aware, this could only be achieved by detecting asymptomatic Zika patients using cluster investigation around symptomatic index cases in travelers and by subsequently exposing them to noninfected mosquitoes [13].
CONCLUSIONS
In this study, we report that Zika IP distribution overlaps with the IP distribution of many arboviruses [14]. Characterization of ZIKV IP can help physicians to exclude a Zika fever diagnosis in travelers with respect to other infectious diseases. Furthermore, the IP, ie, the time between infectious bite and onset of symptoms, can be considered as a proxy for the intrinsinc IP, ie, the time between infectious bite and human-to-mosquito infection. Thus, the estimation of the time-related probability distribution of virus transmission after an infectious bite is also valuable to set up computer-based simulation outbreak scenarios, under the assumption that onset of symptoms concurs with the moment when a mosquito has the highest probability of infection during blood meal.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Sebastian Lequime for thoroughly reading this manuscript and insightful comments. We also thank the personnel at the French Centre National de Reference des Arbovirus who processed all Zika virus samples. We are particularly grateful to Olivier Merle, Laurent C. Bosio, and Thomas P. Canivez for performing polymerase chain reaction and recovering data.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was funded by the Direction Générale de l’Armement (DGA) (Grant No. PDH-2-NRBC-2-B-2113).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Sharma A, Lal SK. Zika virus: transmission, detection, control, and prevention. Front Microbiol 2017; 8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siler JF, Hall MW, Hitchens AP. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, immunity, and prevention. Philipp J Sci 1926; 29:1–304. [Google Scholar]
- 3. Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE. Incubation periods of Yellow fever virus. Am J Trop Med Hyg 2010; 83:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan M, Johansson MA. The incubation periods of Dengue viruses. PLoS One 2012; 7:e50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lessler JT, Ott CT, Carcelen AC, et al. Times to key events in the course of Zika infection and their implications: a systematic review and pooled analysis. Bull World Health Organ 2016; doi: 10.2471/BLT.16.174540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated incubation period for Zika virus disease. Emerg Infect Dis 2017; 23:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fay MP, Shaw PA. Exact and asymptotic weighted logrank tests for interval censored data: the interval R package. J Stat Softw 2010; 36:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gentleman R, Vandal AC. Computational algorithms for censored-data problems using intersection graphs. J Comput Graph Stat 2001; 10:403–21. [Google Scholar]
- 9. Anderson C. icenReg: regression models for interval censored data. JStat Softw 2017; 81 https://www.jstatsoft.org/article/view/v081i12. Accessed 5 March 2018. [Google Scholar]
- 10. Therneau T. A Package for Survival Analysis in S. version 2.38. 2016. https://CRAN.R-project.org/package=survival. Accessed 2 November 2017. [Google Scholar]
- 11. Jackson CH. flexsurv: a platform for parametric survival modeling in R. J Stat Softw 2016; 70:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect 2017; 23:296–305. [DOI] [PubMed] [Google Scholar]
- 13. Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A 2015; 112:14688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudolph KE, Lessler J, Moloney RM, et al. Incubation periods of mosquito-borne viral infections: a systematic review. Am J Trop Med Hyg 2014; 90:882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.