Abstract
Many proteins provided with disulfide bridges in the native state undergo amorphous irreversible aggregation when these bonds are not formed. Here we show that egg lysozyme displays a clever strategy to prevent this deleterious aggregation during the nascent phase when disulfides are still absent. In fact, when the reduced protein assembles into a molten globule state, its cysteines acquire strong hyper-reactivity towards natural disulfides. The most reactive residue, Cys94, reacts with oxidized glutathione (GSSG) 3000 times faster than an unperturbed protein cysteine. A low pKa of its sulfhydryl group (6.6/7.1) and a productive complex with GSSG (KD = 0.3 mM), causes a fast glutathionylation of this residue (t1/2 = 3 s) and a complete inhibition of the protein aggregation. Other six cysteines display 70 times higher reactivity toward GSSG. The discovery of extreme hyper-reactivity in cysteines only devoted to structural roles opens new research fields for Alzheimer’s and Parkinson diseases.
Introduction
The aggregation of misfolded proteins, often generating amyloid fibrils or amorphous aggregates, is a phenomenon associated with over 20 diseases including Parkinson’s and Alzheimer diseases and type 2 diabetes mellitus1–5. Interestingly, disulfide bonds are present in more than 50% of proteins involved in amyloidosis6 and their reduction are often the origin of this phenomenon7–9. Lysozyme (Lyz), one of the most studied protein about its folding mechanism10, is an enzyme which in vitro forms similar aggregates when its four disulfides are broken by reduction7, so it is not clear why aggregation does not occur in vivo during the nascent phase when these disulfides are still to be formed. Motivated by the above argument, we explore here the possibility that some unknown expedient have been developed during evolution to avoid the protein aggregation and favor the correct folding. It is well known that the oxidative folding of lysozyme as well as of other proteins occurs in the endoplasmic reticulum promoted by an unusual high concentration of GSSG (about 0.4 mM) and the possible assistance of the protein disulfide isomerase (PDI) involved in the rearrangement of incorrect disulfides11,12. This process, without PDI and only in the presence of glutathione/oxidized glutathione (GSH/GSSG) (in a ratio similar to that found in the endoplasmic reticulum) is really fast and a number of pioneering studies reported that the early first disulfide appears in less than one min and three disulfides within a few minutes13–16. All these investigations were mainly finalized to characterize the temporal sequence of the disulfide formation and their identification but, in our opinion, some very important details have not been adequately enlightened. What is the true incipit of the oxidative folding, i.e. the identity of the cysteine that first interacts with GSSG? Is this residue characterized by a normal or unusual reactivity toward GSSG compared to a free cysteine? Does the early glutathionylation of this residue influence the aggregation process? Does exist an increased kinetic propensity of the other protein cysteines to form the native disulfides exhibiting a particular hyper-reactivity towards GSSG? As a matter of fact, structural cysteines only devoted to form disulfides have been always considered as “neutral” actors in the oxidative folding of many proteins. We demonstrate here that some of these residues may play a crucial and active role in the nascent phase of lysozyme to prevent deleterious protein aggregation exhibiting extraordinary reactivity toward GSSG. The exceptionality of this phenomenon is that this hyper-reactivity appears when the reduced lysozyme acquires the molten globule state i.e. when only about 30% of secondary and tertiary structures is formed, revealing, for the first time, that this rudimental build may accomplish sophisticated functions. A similar scenario has been recently found in the molten globule state of reduced albumin where Cys75 becomes more than 300 times more reactive towards GSSG17.
Results and Discussion
Interaction of GSSG with the reduced lysozyme
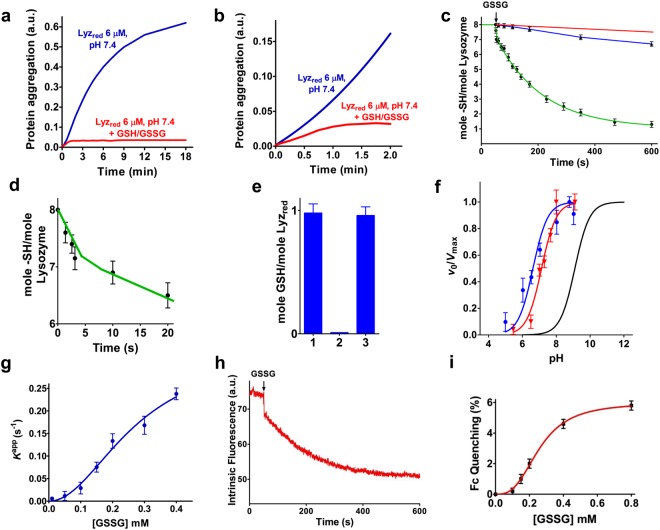
While the native oxidized lysozyme (Lyzox) is a protein which displays high solubility and good stability in solution over time, its reduced form (Lyzred) is highly unstable and when incubated at pH 7.4, a relevant aggregation occurs within a few minutes (Fig. 1a). Although the role of –SH groups in this phenomenon has previously been investigated7,18–20, the effect due to the addition of GSH and GSSG, at the concentrations similar to those found in the endoplasmic reticulum (2 mM and 0.4 mM, respectively)21 was not deeply examined in the past. Interestingly, a relevant decrease (about 60%) of the rate of aggregation occurs within a few seconds from the addition of the GSH/GSSG solution and a complete inhibition is achieved within one min (Fig. 1a,b). This evidence is quite surprising; a free cysteine would require a much longer time to react quantitatively with GSSG. Indeed, the second order kinetic constant for this reaction is 0.7 M−1 s−1 (pH 7.4 at 25 °C)17,22 (Fig. 2a) and assuming that at least 50% of one or a few protein cysteines must to be oxidized to avoid aggregation, an evident effect could not be observed before 40 min. An unperturbed protein cysteine would require even much longer time (about 140 min) as its pKa is 9.123, so the estimated k is about 0.2 M−1 s−1 (Fig. 2a). So the very fast inhibition of the lysozyme aggregation shown in Fig. 1 (panels a and b) suggests that at least one or more cysteines in lysozyme could have an unknown and particular hyper-reactivity toward GSSG. A fast interaction of a few cysteines of Lyzred with GSSG has been previously reported by several authors13,16. However, an in depth kinetic analysis, a comparison of the observed kinetic constants with that of a free cysteine with GSSG, and a correlation with the inhibition of the protein aggregation have not been made. Thus, we first studied the kinetics and stoichiometry of this reaction by following the disappearance of the protein sulfhydryl groups after the addition of GSSG. A very fast reaction (within 8–10 seconds) involving only one protein cysteine was initially observed. The reaction was then followed by a second slower near monophasic trend involving six remaining cysteines (Fig. 1c,d). The first event can be unequivocally identified as a rapid glutathionylation of a single cysteine. In fact, after only ten seconds of reaction between Lyzred and 0.4 mM GSSG, one equivalent of reduced GSH per mole of enzyme was released in solution (Fig. 1e).
Figure 1.
The effect of GSH/GSSG on Lyzred aggregation, pKa of Lyzred cysteines and evidence for a transient Lyzred-GSSG complex. (a) Blue line: Lyzred (6 µM) incubated in 0.2 M urea (37 °C). Red line: Same Lyzred solution after immediate addition of GSH/GSSG (2 mM/0.4 mM). (b) Expanded kinetics of the experiment shown in (a). (c) Green line: Disappearance of Lyzred sulfhydryl groups after addition of 0.4 mM GSSG to a 1.25 µM Lyzred solution (10 µM –SH groups) at pH 7.4 and 0.2 M urea (25 °C). Blue line: Disappearance of the sulfhydryl groups of free cysteine (10 µM) incubated with 0.4 mM GSSG as in the experiment with Lyzred (pH 7.4, 25 °C). Red line: Theoretical sulfhydryl groups disappearance of an unperturbed protein cysteine (10 µM) during the reaction with 0.4 mM GSSG. (d) Expanded kinetics of the experiment shown in c. (e) Reduced GSH produced after 10 s incubation of Lyzred with 0.4 mM GSSG at pH 7.4. Titration of GSH using NBD-Cl and glutathione transferase P1-1 (GSTP1-1) (see Methods) (column 1). Same experiment in the absence of GSTP1-1 (column 2). Same experiment in which the produced GSH was titrated with DTNB after filtration of the mixture on Amicon Ultra 10 kDa cut-off filter to remove the protein (column 3). (f) Average pKa of the seven reactive cysteines in Lyzred as calculated using DTNB (pKa = 6.6) (blue line); average pKa of the four reactive cysteines using NBD-Cl (pKa = 7.1) (red line). Black line is the theoretical curve for unperturbed cysteine (pKa = 9.1). v0/Vmax are the initial velocities normalized to those at full deprotonation (see Methods). (g) Apparent first order kinetic constants for the reaction of the most hyper-reactive cysteine with variable GSSG concentrations at pH 7.4 and 25 °C. (h) Quenching of the intrinsic fluorescence at 340 nm (λex = 295 nm) of Lyzred (1.25 µM, pH 7.4) after addition of GSSG 0.4 mM (25 °C). See (c) for comparison. The very fast fluorescence perturbation after the addition of GSSG occurs within one second. (i) The dependence of the fast fluorescence perturbation (occurring within 1 s) on the GSSG concentration. The error bars represent the S.D. from three independent experiments.
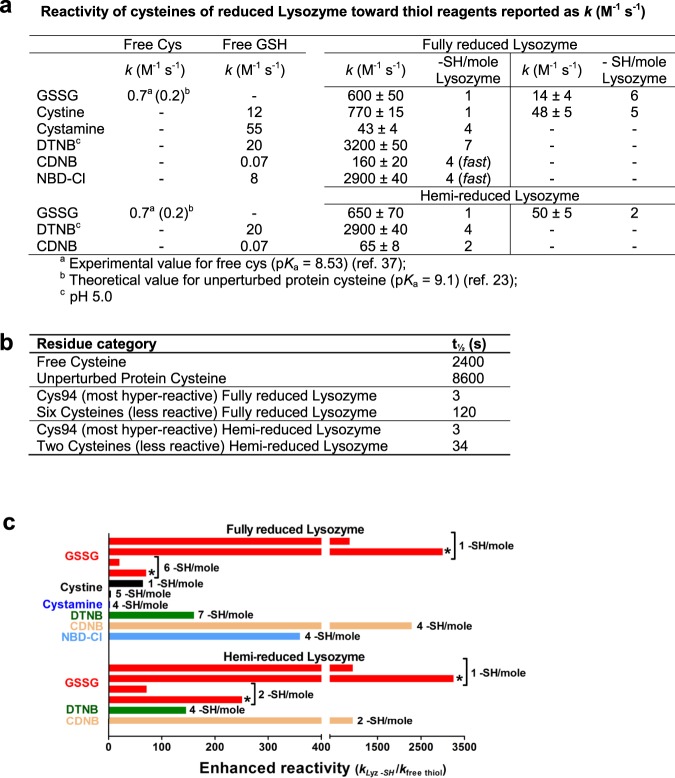
Figure 2.
Reactivity of Lyzred toward different disulfides and thiol reagents. (a) Second order kinetic constants k (M−1 s−1) for the reaction of the cysteines of Lyzred and hemi-reduced Lyz, free Cys and free GSH with natural disulfides and other thiol reagents calculated at pH 7.4 and 25 °C (DTNB at pH 5.0). Errors are reported as S.D. from five independent experiments. (b) t1/2 for the reaction of cysteines of Lyzred and hemi-reduced Lyz with GSSG at pH 7.4 and 25 °C. (c) Second order kinetic constants of Lyzred and hemi-reduced Lyz toward GSSG normalized to the corresponding constants found for free Cys (0.7 M−1 s−1) or () calculated for unperturbed protein Cys (0.2 M−1 s−1) (see Methods section). All other bars represent the second order kinetic constants of Lyzred and hemi-reduced Lyz in its reactions with other disulfides or thiol reagents normalized to the corresponding constants calculated for GSH. Note that electrostatic factors may have a critical role in determining the different kinetic constants for the reaction of free GSH (negatively charged) with cystine (neutral) and cystamine (positively charged).
The second order kinetic constant for the first oxidative event (600 ± 50 M−1 s−1) is about 850 fold higher than that of a free cysteine toward GSSG (0.7 M−1 s−1). The average second kinetic constant for the subsequent reaction of six remaining cysteines (14 ± 4 M−1 s−1) (Fig. 2a) is also more than one order of magnitude higher than that of a free cysteine (Fig. 2b). If the comparison is made considering the theoretical reactivity of an unperturbed protein cysteine toward GSSG (pKa = 9.1, k = 0.2 M−1 s−1)23, these incremental factors becomes 3000 and 70, respectively (Fig. 2c).
Lowered pKa and reversible complex Lyzred-GSSG cause hyper-reactivity
What is the cause of this hyper-reactivity? Given that only the deprotonated form of a thiol group is active in the thiol-disulfide exchange reactions (Fig. 3a), one possibility is that the pKa of these protein cysteines is lower than that of a free cysteine.
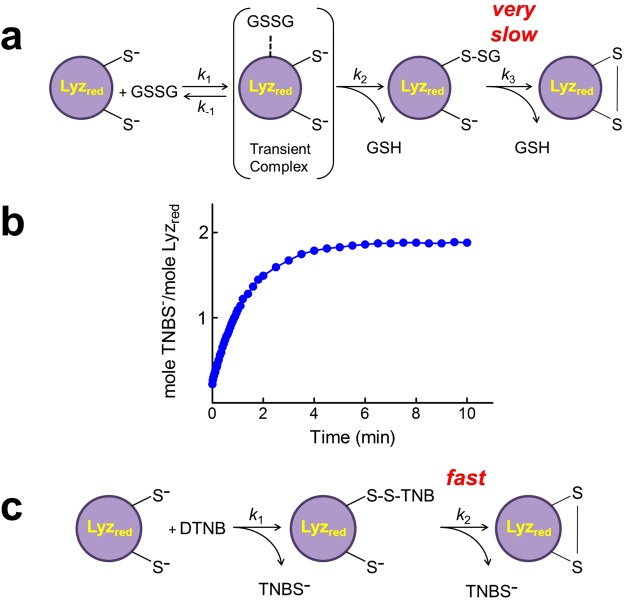
Figure 3.
Reaction of Lyzred with stoichiometric amounts of GSSG or DTNB. (a) Representative reaction scheme of Lyzred with stoichiometric GSSG. Note that only the deprotonated sulphydryl groups are the reactive species. (b) Time course of release of TNBS− when 1.25 µM DTNB reacts with 1.25 µM Lyzred at pH 5.0. (c) Representative reaction scheme of Lyzred with stoichiometric DTNB.
The measured average pKa value of the seven reactive cysteines with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (Fig. 1f) is 6.6, about 2.5 units lower than that of a “unperturbed” protein cysteine (9.1), while it results 7.1 for the four reactive cysteines with 4-chloro-7-nitrobenzofurazane (NBD-Cl) as thiol reagent (Fig. 1f). Both these values confirm a widespread increased acidity for most of the reduced cysteines. We note, however, that even assuming the lowest average pKa, i.e. 6.6, this would only cause, at pH 7.4, about 40 fold increased reactivity toward GSSG, a value approaching that observed experimentally for 6 cysteines in Lyzred but far from the thousands times enhanced reactivity found in a single residue (more details for this conclusion are reported in the Supplemental Discussion). We thus argue that an additional kinetic improvement must be present to trigger the astonishing enhanced reactivity toward GSSG found in one single cysteine. One possibility is the occurrence of a productive reversible complex Lyzred-GSSG which would speed the glutathionylation in a way resembling what occurs in the active site of an enzyme when two substrates interact productively. A convincing indication for this hypothesis was given by evaluating the rates of disappearance of the single hyper-reactive cysteine at different GSSG concentrations (Fig. 1g). In fact, the saturation behavior suggests a cooperative reversible binding of GSSG with S0.5 of 2.7 × 10−4 M. A very similar trend was observed by using a fluorescence approach. As shown in Fig. 1h the addition of 0.4 mM of GSSG to Lyzred causes a very fast intrinsic fluorescence perturbation at 340 nm, which occurs within 1 second (an event faster compared to the glutathionylation event), compatible with the formation of a reversible Lyzred-GSSG complex. The dependence of this very fast fluorescence perturbation on the different GSSG concentrations confirms a saturation trend and also a cooperative behavior with S0.5 of 3.1 × 10−4 M (Fig. 1i). This first event is followed by a second slower and more prominent fluorescence perturbation which is likely due to multiple glutathionylation events and intramolecular disulfide bond formation (Fig. 3a). In fact, quenching of fluorescence parallels the oxidation of the protein cysteines (see Fig. 1c,h).
Mass spectrometry analysis identifies the hyper-reactive cysteine
Mass spectrometry analysis confirmed the existence of the surprising hyper-reactive residue and allowed its identification. Lyzred was reacted with 0.4 mM GSSG at pH 7.4 for only ten seconds. Then 0.25 mM bromopyruvic acid was added to alkylate within 1–2 seconds the residual protein cysteines. A few drops of formic acid were added to lower the pH to 2.5. After a desalting step, the enzyme was subjected to limited proteolysis with pepsin followed by LC-MS/MS analysis. This procedure allowed to identify the glutathionylated residue as Cys94 (Supplemental Table 1). Interestingly, if the reaction with GSSG was prolonged for ten minutes, only the mixed disulfide Cys94SSG was quantitatively recovered while no other cysteines were found to be linked with GSH. This indicates that the natural Cys94-Cys76 disulfide is the last bridge to be formed in accordance with previous observations11,14,24,25, and that all other glutathionylated cysteines rapidly forms disulfides; among them, only a few being correct, as shown by the partial recovery of activity in the absence of GSH (Supplemental Fig. 1).
The interaction of Lyzred with GSSG displays specificity
The remarkable enhanced reactivity of Cys94 toward GSSG (about 3000 times) appears specific for this natural disulfide. In fact, while the absolute reactivity of a single residue of Lyzred for cystine is similar and even higher compared to the one for GSSG (k = 770 and 600 M−1 s−1, respectively) the enhanced reactivity toward cystine is only about 60 times and null toward cystamine (Fig. 2c). Checking these two disulfides, GSH was chosen as reference free thiol as its pKa (9.2) and its structure resembles a protein cysteine. We underline that the different kinetic properties of cystine and cystamine in their interaction with GSH are mainly due to electrostatic factors. A further interesting behavior of Lyzred was observed with organic hydrophobic compounds like DTNB, a well-known thiol reagent. The reaction is very fast at pH 7.4, so we preferred to perform all experiments at pH 5.0 and at low DTNB concentrations. Under these conditions DTNB reacts with an apparent near-monophasic behavior involving most of the protein cysteines. The average second order kinetic constant was 3200 M−1 s−1 i.e. 160 times higher than the one of GSH at the same pH value (Fig. 2a,c). This reagent gave other important informations. In fact, the reaction with sub-stoichiometric amounts of DTNB (1 mole DTNB with 1 mole of Lyzred, containing 8 reduced cysteines) releases 2 moles of TNBS− (Fig. 3b) demonstrating that, unlike Lyz-S-SG, the mixed disulfide Lyz-S-S-TNB rapidly evolves giving the intramolecular disulfide (Fig. 3c).
Hyper-reactivity was also found by reacting Lyzred with 1-chloro-2,4-dinitrobenzene (CDNB) and NBD-Cl, two well-known alkylating agents (Fig. 2a) involving four cysteines of Lyzred with enhanced reactivities spanning from 2200 to 360, respectively (Fig. 2a,c). Electrostatic factors (i.e., at pH 7.4 lysozyme is positively charged, NBD-Cl and CDNB neutral and negatively charged, respectively) are the probable cause of the different increased reactivities of Lyzred toward these two organic reagents.
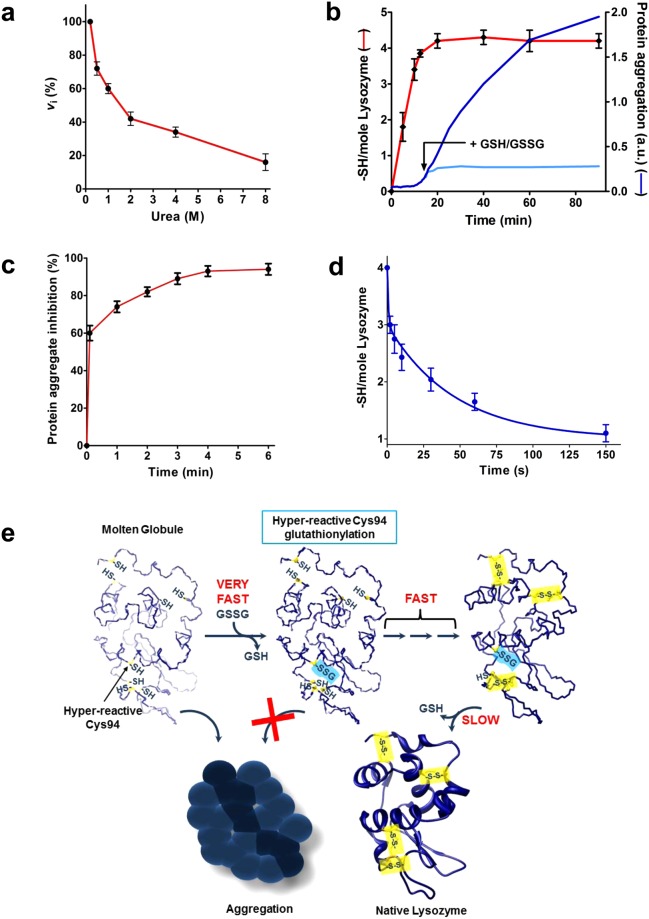
All these peculiar and never described kinetic properties of cysteines in Lyzred are surprisingly enabled by an only partially folded structure which is visible in the CD spectrum (Supplemental Fig. 2) and possibly identified as the so-called molten-globule conformation. Only about 30% of the native secondary structures are present in this primordial build. However, a residual secondary structure is still present in 8 M urea (Supplemental Fig. 2), in agreement with the presence a residual hyper-reactivity toward DTNB (Fig. 4a).
Figure 4.
The effect of urea on the hyper-reactivity of cysteines in Lyzred and the inhibition of the aggregation of the hemi-reduced lysozyme by GSH/GSSG. (a) Inhibition of the hyper-reactivity of cysteines in Lyzred toward DTNB by variable urea concentrations (1.25 µM Lyzred was reacted with 20 mM DTNB at pH 5.0). (b) Red line: Hemi-reduction of native Lyz (6 µM) incubated with 60 µM DTT in 0.01 M borate buffer pH 8.5 without urea. No aggregation occurs during the reduction at this pH. Blue line: Aggregation of the hemi-reduced Lyz when the pH was lowered to 7.4 with phosphate buffer 0.1 M. Light blue line: Inhibition of aggregation by addition of GSH/GSSG (2 mM/0.4 mM). (c) Expanded kinetics of inhibition of the protein aggregation reported in b after addition of GSH/GSSG (2 mM/0.4 mM). (d) Disappearance of three hyper-reactive sulfhydryls in the hemi-reduced Lyz (1.25 µM) after reaction with 0.4 mM GSSG at pH 7.4. Note that the first sulfhydryl reacts within 7 seconds. Errors are reported as S.D. from three independent experiments in panels (a–d). (e) Scheme of the protection mechanism of Lyzred to avoid the protein aggregation. Note that the natural Cys76-Cys94 is the last bridge to be formed11,14,24,25.
Cysteines of partially reduced lysozyme display also hyper-reactivity
Partial reduction of lysozyme, which could occur in vivo, for example caused by an excess of antioxidant drugs26,27, also triggers protein aggregation. After incubation of the enzyme with low dithiotreitol (DTT) concentration i.e. lysozyme:DTT (1:2) without urea, only one disulfide was reduced. The corresponding two sulfhydryls do not display any relevant hyper-reactivity and no protein aggregation occurs within ten min. On the basis of previous studies, the first disulfide that undergoes reduction may be the one involving Cys6 and Cys12728,29. Conversely, the subsequent reduction of a second disulfide, obtained using an higher DTT concentration (1:10), discloses one cysteine with strong hyper-reactivity toward DTNB and other reagents (Fig. 2a). The appearance of this residue is also accompanied by a massive protein aggregation (Fig. 4b). As above observed for the fully reduced lysozyme, the addition of GSH/GSSG mixture in a few seconds lowers to about 40% the velocity of the protein aggregation and a complete inhibition is achieved within 3–4 min (Fig. 4b,c). The parallel disappearance of one hyper-reactive sulfhydryl in a few seconds (Fig. 4d) demonstrates that this phenomenon is due to the glutathionylation of a single cysteine, as observed for Lyzred. An estimation of the reactivity of the hyper-reactive cysteine toward GSSG displayed similar kinetic properties found for Cys94 in the fully reduced enzyme (Fig. 2a) and thus a reasonable identification of this residue with Cys94. Even in this case, the enhanced reactivity of only a single cysteine toward GSSG is a critical property which opposes the deleterious event of the protein aggregation. The kinetic properties of this and of other interacting cysteines with different reagents are reported in Fig. 2a.
Conclusions
In conclusion, we have described a completely new property inherent the reduced and hemi-reduced forms of lysozyme which seems to be acquired during evolution to prevent deleterious protein aggregation both in the nascent phase or, once in the native state, after accidental reductive stress (see the representative scheme in Fig. 4e). The extraordinary enhanced reactivity of Cys94 toward GSSG indicates that the glutathionylation of this residue may represent in vivo the primordial event which carries out this protection. Although the reactivity toward cystine is similar, GSSG is the prime target in vivo due to its higher concentration in the endoplasmic reticulum (0.4 mM), compared to the cystine level (about 0.01 mM). Following endoplasmic reticulum redox perturbations, blocking the free thiol group of Cys94 with GSSG can prevent lysozyme aggregation during the folding process. The glutathionylated Cys94 will then form its native disulfide with Cys76 as a last event during the oxidative folding according to what reported in literature11,14,24,25. Interestingly, the analog mixed disulfide Cys95SSG in the human protein has been proposed as a protective group to prevent the formation of an incorrect disulfide bond during the protein folding30 and this additional role can be also extended to the corresponding disulfide of the chicken egg lysozyme. Moreover, it is well known that the folded lysozyme released from the cell might undergo occasional reductive stress within the extracellular matrix leading to protein aggregation and disease7. Glutathionylation of Cys94 by the GSSG present in the extracellular matrix might slow down the aggregation rate leading either to the reformation of the native molecule or to proteolytic degradation of the partially reduced lysozyme. Both a lowered pKa and a transient Lyzred-GSSG complex are the main determinants of the enhanced reactivity toward GSSG found for Cys94 while a lower average pKa value alone justifies the moderate increased reactivity of the remaining protein cysteines toward GSSG. An additional favorable hydrophobic interaction between Lyzred and other lipophilic reagents like DTNB, NBD-Cl and CDNB is the probable cause of the widespread relevant hyper-reactivity toward these reagents. A particular interest has been developed in the last years to identify hyper-reactive cysteines and an elegant proteomic assay has been published to profile quantitatively such cysteines mainly involved in catalytic mechanisms31. Thus, the discovery of these particular kinetic properties of cysteines only devoted to assume a structural role as disulfides in the native protein, as also recently found for Cys75 in human albumin17, is a completely new finding that opens interesting perspectives for the knowledge of molecular mechanisms underlying the correct protein folding during transit in the Golgi, as well as the molecular extracellular events responsible for misfolding diseases5 with a particular emphasis to lysozyme amyloidosis, rare non-neuropathic forms of hereditary amyloidosis32–35.
Methods
Materials
Lysozyme (Lyz) from chicken egg white (about 100,000 U/mg), L-cysteine (Cys), L-cystine, L-glutathione (GSH), oxidized glutathione (GSSG), cysteamine, cystamine, 1-chloro-2,4-dinitrobenzene (CDNB), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), 4-chloro-7-nitrobenzofurazane (NBD-Cl), dithiotreitol (DTT), bromopyruvic acid, lysozyme activity kit, pepsin A and all other reagents were from SIGMA-Aldrich (St. Louis, Mo, USA).
Lysozyme reduction
Lysozyme concentration was evaluated on the basis of an extinction coefficient of 37,970 M−1 cm−1 at 280 nm. Reduction of lysozyme was performed by dissolving the protein (0.05 mM final concentration) in 0.01 M sodium borate buffer, pH 8.5 with or without 8 M urea and incubating it with variable DTT concentrations (lysozyme:DTT, 1:2, 1:10) at 40 °C. The pH was always adjusted to 8.5 with 0.1 M NaOH. At fixed times, the content of reduced cysteines was evaluated on the basis of the residual DTT calculated using DTNB as –SH reagent (εM TNBS− = 14,100 M−1 cm−1 at pH 8.0) after filtration of the mixture on an Amicon Ultra filter with a cut-off of 10,000 Da. Alternatively, the reduced cysteines in Lyz were evaluated by reacting the reduced Lyz (1.25 µM) with 20 µM DTNB at pH 5.0 (εM TNBS− = 11,800 M−1 cm−1 at pH 5.0) taking advantage by the hyper-reactivity of all 8 protein cysteines with this reagent (Supplemental Fig. 3). Throughout the paper, the term “reduced lysozyme” (Lyzred) refers to that obtained after 40 min of reduction with DTT (lysozyme:DTT, 1:10) at 40 °C with 8 M urea. The reduced protein in 8 M urea shows 7.7 ± 0.3 reduced cysteines and very scarce enzyme activity (about 4%). On the other hand the reduction performed without urea with only two equivalents of DTT (Lyz 1.25 µM, 0.01 M sodium borate buffer pH 8.5, 30 min, 37 °C) reduces only one disulfide while two disulfides are reduced using a ten times molar excess in the same condition. This enzyme is defined in the text as “hemi-reduced” Lyz. DTT stock solutions were titrated with DTNB prior to use.
Inhibition of Lyzred aggregation by GSH/GSSG mixture
Lyzred used to follow the aggregation event was obtained by a reduction step similar to that above reported but using higher Lyz concentration i.e. 0.25 mM in 8 M urea and 2.5 mM DTT in 0.01 M sodium borate buffer, pH 8.5. The reduction progress and the final number of reduced cysteines were similar to those observed using more diluted conditions. Aggregation of Lyzred was observed by incubating the protein (6 µM) with a 0.2 M final concentration of urea in 0.01 M potassium phosphate pH 7.4. The turbidity due to protein aggregation was followed at 600 nm. The effect of GSH/GSSG (2 mM/0.4 mM) was observed by adding these reagents after 5 seconds after the beginning of the aggregation.
Reactivity of Lyzred cysteines toward GSSG
The interaction of Lyzred with GSSG at the level found in the endoplasmic reticulum was measured by incubating 1.25 µM of Lyzred in 0.2 M urea with 0.4 mM GSSG in 0.1 M potassium phosphate buffer, pH 7.4, 25 °C. At various times the loss of hyper-reactive cysteines due to reaction with GSSG was evaluated using DTNB as –SH titrant. Reactivity of free cysteine with GSSG was evaluated by following the disappearance of cysteine using bromopyrivuc acid17.
Titration of GSH with NBD-Cl and glutathione transferase isoform P1-1 (GSTP1-1)
GSH released after reaction of Lyzred (1.25 µM) with 0.4 mM GSSG at pH 7.4 for 10 seconds, was measured as follows; 0.5 ml of the incubation mixture was acidified to pH 5.0 with a few drops of formic acid to stop any redox reaction. The solution was passed through an Amicon Ultra (10 K Membrane) (Millipore, Cork, IRL). The filtrate was incubated with 0.02 mg GSTP1-1 and 0.2 mM NBD-Cl.
Alternative titration of GSH coming from the interaction of Lyzred with GSSG
GSH released after reaction of Lyzred (1.25 µM) with 0.4 mM GSSG at pH 7.4 after fixed incubation times (25 °C) was measured as follows: after reduction of 0.1 mM Lyz with 1 mM DTT in 0.01 mM borate buffer, pH 8.5 (50 min at 50 °C) containing 8 M urea, the solution was passed through a Sephadex G-25 column (1 × 20 cm) equilibrated with 20 mM sodium phosphate buffer, pH 7.4 containing urea 2 M and 1 mM EDTA. The collected protein (40 µM) is now without DTT. The protein was then diluted up to 4 µM with 20 mM potassium phosphate buffer, pH 7.4 containing 0.2 M urea and 0.1 mM EDTA. GSSG was then added (0.4 mM final concentration). At fixed times aliquots were acidified to pH 5 with acetate buffer (0.1 M final concentration) and 0.5 ml centrifuged at 15000 × g on an Amicon Ultra (10 K Membrane) (Millipore, Cork, IRL). The filtrate was brought to pH 8 with phosphate buffer and GSH titrated with DTNB.
pKa determination
The average pKa of cysteines in Lyzred was calculated on the basis of the different reactivity of these residues (1 µM Lyzred) with DTNB (20 µM) in 0.02 M Britton-Robinson buffers at different pH values (from 4.0 to 8.0). Below pH 7.0, appropriate TNBS− extinction coefficients at 412 nm were considered. A similar experimental approach was performed using NBD-Cl (20 µM with 1 µM of Lyzred) as thiol reagent at different pH values. The cysteine-NBD adduct absorbs at 419 nm (εM = 13,000 M−1 cm−1)36. v0 values are the initial velocity of reaction of Lyzred with DTNB or NBD-Cl as calculated spectrophotometrically in continuous at 412 nm and 419 nm, respectively. Observed initial velocities (v0), normalized to that calculated at full deprotonation (Vmax), were plotted against pH values. pKa values were calculated using a curve fitting analysis.
Circular dichroism and fluorescence analysis
CD spectra of native Lyz, Lyzred, and hemireduced Lyz were measured at 1.25 μM concentration in 10 mM potassium phosphate buffer pH 7.4, 25 °C. The setting panel of the spectropolarimeter Jasco J-600 was: slit 2 nm, sensibility 50 mdeg, range 245-200 nm, resolution 0.2 nm; using a quartz cuvette of 0.1-cm light path. The fluorescence measurements were performed in continuous on a Fluoromax-4 Horiba spectrofluorometer with slits 5 nm, excitation wavelength 295 nm, emission wavelength at 340, temperature 25 °C, with a quartz cuvette of 1-cm light path. The apparatus was provided with a rapid mixing device.
Reactivity of Lyzred toward several disulfides and thiol reagents
The reactivity of the sulfhydryl groups of Lyzred toward DTNB was evaluated spectrophotometrically in continuous at 412 nm where TNBS− absorbs. The experiments were performed both in the presence of residual DTT used to reduce the native Lyz (with minor corrections) or without DTT after G-25 Sephadex column, obtaining coincident results. In fact protein cysteines are 850 times (one cysteine) and 20 times (six cysteines) more reactive than DTT. In a typical experiment 1.25 µM Lyzred was reacted with 20 µM DTNB in acetate buffer pH 5.0. The kinetics follows an near-hyperbolic behavior so second order kinetic constants were evaluated on the basis of initial rate or of t1/2 at different DTNB concentrations. The reactivity toward cystine and cystamine was determined by reacting Lyzred (1.25 µM) with 0.4 mM of both disulfides in 0.1 M potassium phosphate buffer pH 7.4. At fixed times aliquots were placed in 0.1 M acetate buffer, pH 5.0 and the disappearance of the hyper-reactive cysteines of Lyzred was determined using DTNB as titrant. The reactivity toward CDNB was evaluated spectrophotometrically in continuous at 340 nm where the Cys-DNB adduct absorbs (εM = 9,600 M−1 cm−1)17. In a typical experiment Lyzred (1.25 μM) was reacted with variable CDNB concentrations (from 0.5 mM to 2 mM) in 0.1 M potassium phosphate buffer, pH 7.4. A slight turbidity due to the CDNB modified enzyme was subtracted by each determination. The reactivity of Lyzred toward NBD-Cl was determined as above described for CDNB using NBD-Cl (from 10 µM to 60 µM). The reaction was followed spectrophotometrically at 419 nm were the Cys-NBD adduct absorbs (εM = 13,000 M−1 cm−1)36. Second order kinetic constant for the reaction of free Cys toward GSSG (0.7 M−1 s−1) was determined previously17 (see Fig. 2a). The theoretical kinetic constant for an unperturbed protein cysteine was calculated by considering the pKa value of its sulfhydryl group = 9.1 instead of 8.53 of the free amino acid23,37. Second order kinetic constants for the reaction of free GSH toward all other reagents were calculated on the basis of initial velocity of the reaction of 10 µM of GSH (1 mM with CDNB) with each reagent in the same condition used for the assay with Lyzred. The velocity of the reaction of GSH with cystamine and cystine was evaluated by determining at fixed times the amount of cysteine or cysteamine released as a consequence of the reaction. Cysteamine and cysteine were determined on aliquots after reaction with 1 mM bromopyruvate. The reaction is almost instantaneous and the observed product is a cyclic sulfur compound (lanthionine ketimine and aminoethylcysteine ketimine) absorbing at 296 nm (εM = 6,200 M−1 cm−1)38.
Effect of urea concentration on the hyper-reactivity
The effect of urea on hyper-reactivity of Lyzred was assayed using DTNB as thiol reagent. In a typical experiment, Lyzred (1.25 µM) was incubated with 0.01 M potassium phosphate buffer, pH 7.4, in the presence of variable concentrations of urea (from 0.2 M to 8 M). After five min incubation the rate of reaction with DTNB (20 µM) was measured spectrophotometrically at 412 nm in 0.1 M acetate buffer pH 5.0 (25 °C).
Aggregation of the hemi-reduced Lyz
Concentrated hemi-reduced Lyz (6 µM) was produced by incubating Lyz with ten molar excess of DTT at pH 8.5. At the end of the reduction only two disulfides are reduced and a relevant aggregation occurs at pH 7.4 (see Fig. 4b).
Lysozyme activity
Activity of lysozyme was assayed by the lysozyme detection kit (Sigma-Aldrich, St. Louis, MO, USA) which uses Micrococcus lysodeikticus cell suspension as substrate39.
Mass spectrometry identification of hyper-reactive cysteine
Lyzred (1.25 μM) was incubated with GSSG (0.4 mM) in 0.01 M potassium phosphate buffer, pH 7.4. The reaction was stopped after 10 seconds or 10 min by adding 0.25 mM bromopyruvate which alkylated residual protein cysteines within 1–2 sec. Then the samples were lyophilized. A Lyzred solution (1.25 μM) was immediately alkylated with bromopyruvate and used as control. Samples were resuspended in 0.2% TFA and desalted by reversed-phase HPLC on a Phenomenex Jupiter C4 column (250 mm × 2.0 mm, 300 Å pore size) with a linear gradient from 10% to 95% of solvent B (0.07% TFA in 95% acetonitrile) in 30 min, at a flow rate of 200 μL/min using an Agilent Technologies 1100 HPLC (Agilent Technologies, USA). Protein fractions were collected and lyophilized. Controlled pepsin hydrolysis was carried out by dissolving the samples in 5% formic acid, pH 2.5 and adding pepsin at an enzyme to substrate ratio of 1:300 w/w at 37 °C for 2 hours. Sample was then lyophilized, resuspended in 0.2% formic acid and directly analyzed by nanoLC/MS-MS on an LTQ-XL Orbitrap mass spectrometer equipped with a nanoHPLC (ThermoFisher, USA). Peptides containing modified cysteine residues were selected using the ion extraction chromatograms of the corresponding doubly and triply charged ions and the assignments were confirmed by manual inspection of their fragmentation spectra.
Statistical and graphical analysis
Data are represented as means ± standard deviation (S.D.). Statistical analysis was performed using computer software packaged MedCalc (Mariakerke, Belgium). The graphic and results visualization were obtained using GraphPad Prism software (La Jolla, CA, USA).
Electronic supplementary material
Acknowledgements
Authors would like to thank Prof. Lorenzo Stella and Prof. Jens Z. Pedersen for helpful discussion. This research was supported by FFABR Grant from Italian Ministry of Education & Research to A.B. and by Consolidate The Foundation Grant to G.R.
Author Contributions
A.B., conducted and designed the spectroscopic and kinetic experiments, and analyzed the results. G.C. conducted the kinetic experiments and analyzed the results. C.M., F.C., M.C. and P.P. prepared sample for mass spectrometry and performed mass spectrometry experiments and evaluated the results. G.R. wrote the paper and coordinated the entire study. All authors contributed to writing and critical reading the manuscript and finally suggesting refinement alongside the final version of the present paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34439-y.
References
- 1.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 2.Dobson CM. Protein aggregation and its consequences for human disease. Prot. Pept. Lett. 2006;13:219–227. doi: 10.2174/092986606775338362. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Ann. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 4.Rambaran RN, Serpell LC. Amyloid fibrils: abnormal protein assembly. Prion. 2008;2:112–117. doi: 10.4161/pri.2.3.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossuto MF. Disulfide bonding in neurodegenerative misfolding diseases. Int. J. Cell Biol. 2013;2013:318–319. doi: 10.1155/2013/318319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossuto MF, et al. Disulfide bonds reduce the toxicity of the amyloid fibrils formed by an extracellular protein. Angew Chem Int Ed Engl. 2011;50:7048–7051. doi: 10.1002/anie.201100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M, Dutta C, Tiwari A. Disulfide-bond scrambling promotes amorphous aggregates in lysozyme and bovine serum albumin. J. Phys. Chem. B. 2015;119:3969–3981. doi: 10.1021/acs.jpcb.5b00144. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan R, Ravi VK, Kumar S, Kumar MV, Chandra N. Lysozyme: a model protein for amyloid research. Adv. Prot. Chem. Struct. Biol. 2011;84:63–111. doi: 10.1016/B978-0-12-386483-3.00003-3. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Yan J, Zhang X, Huang K. Disulfide bonds in amyloidogenesis diseases related proteins. Proteins. 2013;81:1862–1873. doi: 10.1002/prot.24338. [DOI] [PubMed] [Google Scholar]
- 10.Dobson CM, Evans PA, Radford SE. Understanding how proteins fold: the lysozyme story so far. Trends Biochem. Sci. 1994;19:31–37. doi: 10.1016/0968-0004(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg B, Chung EW, Robinson CV, Mateo PL, Dobson CM. The oxidative refolding of hen lysozyme and its catalysis by protein disulfide isomerase. EMBO J. 1999;18:4794–4803. doi: 10.1093/emboj/18.17.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg ME, Rudolph R, Jaenicke R. A kinetic study of the competition between renaturation and aggregation during the refolding of denatured-reduced egg white lysozyme. Biochemistry. 1991;30:2790–2797. doi: 10.1021/bi00225a008. [DOI] [PubMed] [Google Scholar]
- 13.Saxena VP, Wetlaufer DB. Formation of the three dimensional structure in Proteins. Rapid nonenzymatic reactivation of reduced lysozyme. Biochemistry. 1970;9:5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- 14.Anderson WL, Wetlaufer DB. The folding pathway of reduced lysozyme. J. Biol. Chem. 1976;251:3147–3153. [PubMed] [Google Scholar]
- 15.Roux P, Ruoppolo M, Chaffotte A-F, Goldberg ME. Comparison of the kinetics of S-S bond, secondary structure, and active site formation during refolding of reduced denatured hen egg white lysozyme. Protein Sci. 1999;8:2751–2760. doi: 10.1110/ps.8.12.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois T, Guillard R, Prieels J-P, Perraudin J-P. Comparison between the folding of reduced hen egg white lysozyme and that of reduced human milk lysozyme. Biochemistry. 1982;21:6516–6523. doi: 10.1021/bi00268a030. [DOI] [PubMed] [Google Scholar]
- 17.Bocedi A, et al. The extreme hyper-reactivity of selected cysteines drives hierarchical disulfide bond formation in serum albumin. FEBS J. 2016;283:4113–4127. doi: 10.1111/febs.13909. [DOI] [PubMed] [Google Scholar]
- 18.Cao A, Hu D, Lai L. Formation of amyloid fibrils from fully reduced hen egg white lysozyme. Protein Sci. 2004;13:319–324. doi: 10.1110/ps.03183404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi VK, Goel M, Kotamarthi HC, Ainavarapu SRK, Swaminathan R. Preventing disulfide bond formation weakens non-covalent forces among lysozyme aggregates. PLoS One. 2014;9:e87012. doi: 10.1371/journal.pone.0087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Ravi VK, Swaminathan R. How do surfactants and DTT affect the size, dynamics, activity and growth of soluble lysozyme aggregates? Biochem. J. 2008;415:275–288. doi: 10.1042/BJ20071499. [DOI] [PubMed] [Google Scholar]
- 21.Dixon BM, Heath SH, Kim R, Suh JH, Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid. Redox Signal. 2008;10:963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal. 2013;18:1623–1641. doi: 10.1089/ars.2012.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris TK, Turner GJ. Structural basis of perturbed pKa values of catalytic groups in enzyme active sites. IUBMB Life. 2002;53:85–98. doi: 10.1080/15216540211468. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg B, Chung EW, Robinson CV, Dobson CM. Characterization of the dominant oxidative folding intermediate of hen lysozyme. J. Mol. Biol. 1999;290:781–796. doi: 10.1006/jmbi.1999.2915. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi M, Taniyama Y, Kanaya S, Takao T, Shimonishi Y. Occurrence of S-(1,2-dicarboxyethyl)-cysteine at position 77 in mutant human lysozyme secreted by Saccharomyces cerevisiae. Eur. J. Biochem. 1990;187:315–320. doi: 10.1111/j.1432-1033.1990.tb15307.x. [DOI] [PubMed] [Google Scholar]
- 26.Michailidis Y, et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013;98:233–245. doi: 10.3945/ajcn.112.049163. [DOI] [PubMed] [Google Scholar]
- 27.Mentor S, Fisher D. Aggressive Antioxidant Reductive Stress Impairs Brain Endothelial Cell Angiogenesis and Blood Brain Barrier Function. Curr. Neurovasc. Res. 2017;14:71–81. doi: 10.2174/1567202613666161129113950. [DOI] [PubMed] [Google Scholar]
- 28.Radford SE, Woolfson DN, Martin SR, Lowe G, Dobson CM. A three-disulphide derivative of hen lysozyme. Structure, dynamics and stability. Biochem. J. 1991;273:211–217. doi: 10.1042/bj2730211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acharya AS, Taniuchi H. Preparation of a two-disulfide bonded enzymatically active derivative from hen egg lysozyme. Int. J. Pept. Protein Res. 1980;15:503–509. doi: 10.1111/j.1399-3011.1980.tb02928.x. [DOI] [PubMed] [Google Scholar]
- 30.Inaka K, Miki K, Kikuchi M, Taniyama Y, Matsushima M. Structure of a glutathionylated human lysozyme: a folding intermediate mimic in the formation of a disulfide bond. Acta Crystallogr. D Biol. Crystallogr. 1995;51:619–625. doi: 10.1107/S0907444994013478. [DOI] [PubMed] [Google Scholar]
- 31.Weerapana E, et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepys MB, et al. Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature. 1993;362:553–557. doi: 10.1038/362553a0. [DOI] [PubMed] [Google Scholar]
- 33.Granel B, et al. Lysozyme amyloidosis: report of 4 cases and a review of the literature. Medicine (Baltimore) 2006;85:66–73. doi: 10.1097/01.md.0000200467.51816.6d. [DOI] [PubMed] [Google Scholar]
- 34.Jean E, et al. A new family with hereditary lysozyme amyloidosis with gastritis and inflammatory bowel disease as prevailing symptoms. BMC Gastroenterol. 2014;14:159. doi: 10.1186/1471-230X-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pleyer C, Flesche J, Saeed F. Lysozyme amyloidosis, a case report and review of the literature. Clin. Nephrol. Case Stud. 2015;8:42–45. doi: 10.5414/CNCS108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkett DJ, Price NC, Radda GK, Salmon AG. The reactivity of SH groups with a fluorogenic reagent. FEBS Lett. 1970;6:346–348. doi: 10.1016/0014-5793(70)80095-3. [DOI] [PubMed] [Google Scholar]
- 37.Torchinskii, Y. M. & Dixon, H. B. F. Sulfhydryl and Disulfide Groups of Proteins p. 10 (Springer, Berlin, DR, 2013).
- 38.Cavallini, D., Ricci, G. & Federici, G. The ketimine derivatives of thiaLyzine, lanthionine, cystathionine, cystine: preparation and properties. In Sulfur Amino Acids: Biochemical and Clinical Aspects pp. 355–363 (Alan R, Liss, Inc NY, 1983). [PubMed]
- 39.Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta. 1952;8:302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.