Abstract
Phenological and reproductive shifts of plants due to climate change may have important influences on population dynamics. Climate change may also affect invasive species by changing their phenology and reproduction, but few studies have explored this possibility. Here, we investigated the impact of climate change on the phenology, reproduction and invasion potential of two alien Solidago canadensis and Bidens frondosa and one native weed, Pterocypsela laciniata, all of which are in the Asteraceae family. The three species responded to simulated climate change by increasing reproductive investments and root/leaf ratio, prolonging flowering duration, and while the two alien species also displayed a mass-flowering pattern. Moreover, our experimental results indicated that the alien invasive species may have greater phenological plasticity in response to simulated warming than that of the native species (P. laciniata). As such, climate change may enhance the invasion and accelerate the invasive process of these alien plant species.
Introduction
Global mean temperature is predicted to increase 1.4–5.8 °C by 2100 as a result of growing greenhouse gases (GHG) concentrations in the atmosphere1–3. Climate change has already altered the population dynamics of species and their geographic ranges4 4, and along with biological invasions is a key driver affecting global biodiversity5–7. However, the effects of climate change and biological invasion on biodiversity are usually considered separately4,6. Moreover, the mechanisms by which climate change influence the specific influences of climate change on phenology and reproduction of invasive plants are poorly understood8.
Reproduction is a fundamental challenge for invaders when they are introduced into a new habitat. Thus, reproductive traits in general become important determinants of invasion9. Some reproductive traits, such as reproductive allocation and clonality, are positively associated with invasion10. However, the role of many other traits in invasion success, including seed dispersal patterns and flowering phenology, are less well known.
Most observations of climate-change responses have involved alterations of species’ phenologies11 which can have major influences on plant productivity12 and competition among species13–15. Evidence suggests that many plant species have advanced the timing of their phenological events in response to warming temperatures, and that the response modes of plant phenology to temperature are often nonlinear16. In southern Wisconsin, 18 of 55 species advanced spring events, whereas the others showed no change in timing in the 1980s and 1990s11,17. Earlier phenological activity increased the probability that developing fruit would reach full maturity15. Munson and Sher18 found that the flowering date of rare species in the Southern Rocky Mountains in Colorado has advanced 3.1 days every decade since the late 1800s, which indicated that the large shifts in plant phenology was related to climate. Moreover, flowering phenology directly influences pollination and the density of flowering individuals19, both of which directly impact plant reproductive success. Some successful invaders generally displayed earlier flowering or longer blooming periods, though several studies showed no significant differences in flowering phenology between native and non-native plants20. Other studies clearly showed that it is advantageous for an invasive species to flower for longer than a native plant21,22. However, the effects of climate change on the phenology of invasive plants is still unclear.
Lots of references showed that climate change has induced different responses of plant in flowering onset. Simulated warming leads to a significantly earlier onset of flowering in Silene acaulis23, Hibbertia hirsute24, and Gentiana formosa25. Temperature increase causes Arabidopsis halleri to advance flowering onset day and to shorten flowering duration, even leading to the loss of flowering opportunity26. On the other hand, flowering onset of Aster alpinus and Trollius farreri on the Tibetan plateau were significantly delayed under the warming climate27. Variable responses of native species in flowering phenology to climate change suggest that climate change may also affect the phenology of non-native species, which in turn would influence their invasion.
The Asteraceae are proportionally over-represented among invasive plant species worldwide28. Both Solidago canadensis and Bidens frondosa have become worldwide invasive herbs26 that have had serious ecological consequences in some countries27. Pterocypsela laciniata is a native weed growing in valleys, hillsides, forest margins, thickets, grasslands and wastelands in China. With the invasion of S. canadensis and B. frondosa in China habitat for P. laciniata, which is similar to that of the two alien species, is declining. To explore how simulated warming impacts the phenology, reproduction and invasion of Asteraceae plant species, S. canadensis, B. frondosa and P. laciniata were chosen as the subjects for the present study. Our main hypotheses are that climate change will enhance invasive ability of plant species by altering flowering phenology to promote reproduction and by increasing the number of offspring to increase reproductive allocation.
Materials and Methods
Plant species
Solidago canadensis L. is a perennial in the family Asteraceae widely distributed in the eastern US and Canada29, but is considered to be a serious invasive weed because of its strong range expansion and negative effects on native ecosystems in many countries in Europe, Asia and Oceania29–31.
Bidens frondosa L. is an annual weed native to North America that grows in wet, nutrient-rich mudsoils or muddy sand-soils on roadsides or the shores of rivers and lakes32, or in wastelands. This species has expanded its range throughout Europe and Asia33 and is considered one of the most widely distributed invasive species in China.
Pterocypsela laciniata (Houtt.) Shih is a perennial species in the family Asteraceae. It is native to China and mainly distributed in Shandong, Zhejiang and Jiangxi Provinces.
Experimental design
Experiments were conducted from March to December, 2013 at the biological experiment station of Jinggangshan University, China (27°06′31″–27°07′23″ N, 115°01′08″–115°02′05″ E). The climate of this area is subtropical monsoon climate. A simulated warming treatment and a control were designed to test the effects of climate change on the flowering phenology and reproductive allocation of the three tested species. Five replicates were set up for each temperature treatment in this experiment, and 6 plants were duplicated in each replicate. A total of 60 plants were planted in 60 flowerpots (21-cm d. × 21-cm h). All the plants used in the experiment were seedlings grown from seeds collected from the plants growing in the same region. Plants with similar growth potential were transplanted into the test pots for cultivation and observation.
Temperature was increased using conical OTCs that were designed to be 2.20 m in basal diameter, 0.80 m top diameter, and 1.30 m in height. The air temperature and relative humidity (at 30 cm above the soil surface) inside and outside of the OTCs were recorded at hourly intervals by an automatic temperature and humidity recorder (EM50, Decagon Devices Inc., Pullman, U.S.A) during the study period. The OTCs significantly increased the average monthly air temperature by a mean of 1.6 °C (t9 = 9.618, P < 0.001), but the observed decrease in relative humidity was not significant (mean difference 0.159%; t9 = 0.325, P = 0.581).
The flowering phenology of each plant was observed and recorded every 3 days until the last flower withered, including onset day (the day the first flower attained anthesis), endset day (the day the last flower attained anthesis), and the numbers of flowers per plant. The flowering duration, mean flowering amplitude (number of flowers per plant per day), and the relative flowering intensity and synchrony index were calculated for each species in each treatment. The number of flowers and fruits per plant was counted carefully. Seed weights of each treatment for each species were measured. The ramets of S. canadensis were also counted after harvesting.
The synchrony index (Si) of a population is used for detecting flowering synchrony and specific methods according to McIntosh34 and is given by:
| 1 |
Where ej ≠ i is the days of individual i and j overlap in their flowering, fi is the total flowering days of individual i, n is the number of individuals in the sample. Note that Si can range from 0–1, where 0 means there is no overlapping of flowering while 1 means there is complete overlap.
The relative flowering intensity of a plant was defined as the ratio of the number of flowers during the peak flowering stage to the largest number of flowers produced.
The plants were harvested after the height and numbers of leaves were recorded and all ripe fruits were collected. Harvested biomass was separated into roots, stems, leaves and fruits.
The biomass of each part of the plant was determined after oven drying at 80 °C for 48 hours, after which, the biomass allocation parameters were calculated as follows:
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
Statistical analysis
To examine the effects of warming on phenological parameters and reproduction potential for each species, T-tests were used to evaluate significant differences between treatments and controls (OTCs and CK) at α = 0.05. The analyses were performed with SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and OriginPro 8.0 (OriginLab, Northampton, MA, USA). The values reported in this paper are “Mean ± S.E.”.
Results
Effects on growth
Simulated warming significantly increased the height of each species (Fig. 1). The heights of S. canadensis, B. frondosa and P. laciniata were significantly greater in the OTC treatment (135.4 ± 2.9 cm, 106.2 ± 3.5 cm, 82.85 ± 2.0 cm, respectively) than that in the CK treatment (122.3 ± 3.6 cm, 94.6 ± 1.7 cm, 76.3 ± 1.4 cm, respectively; n = 5, p < 0.05).
Figure 1.
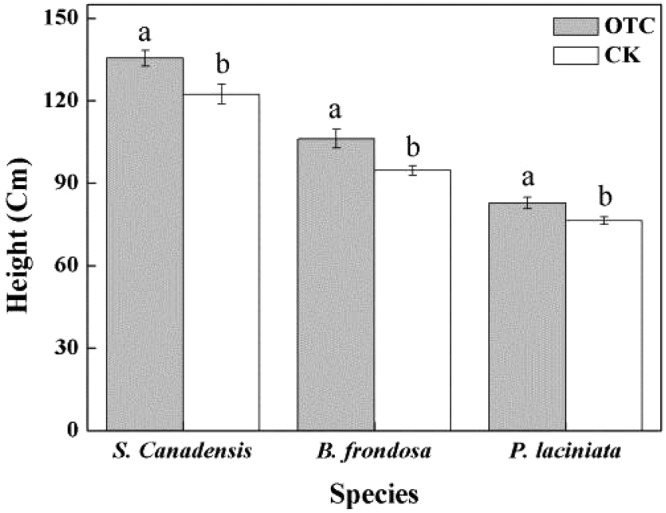
Effect of simulated warming on plant height of S. canadensis, B. frondosa and P. laciniata. Note: The values are “means ± S.E.”. Different letters indicate significant differences (P < 0.05).
Simulated warming decreased the leaf numbers of the two invasive plants, S. canadensis (decreased from 1291.7 ± 46.9 to 1004.2 ± 63.7) and B. frondosa (decreased from 2848.48 ± 164.4 to 2715.6 ± 105.6) (n = 5, p < 0.05), but had no significant effect on the leaf numbers of the native species (P. laciniata), which increased from 49.2 ± 3.2 to 54.3 ± 2.6 (Fig. 2).
Figure 2.
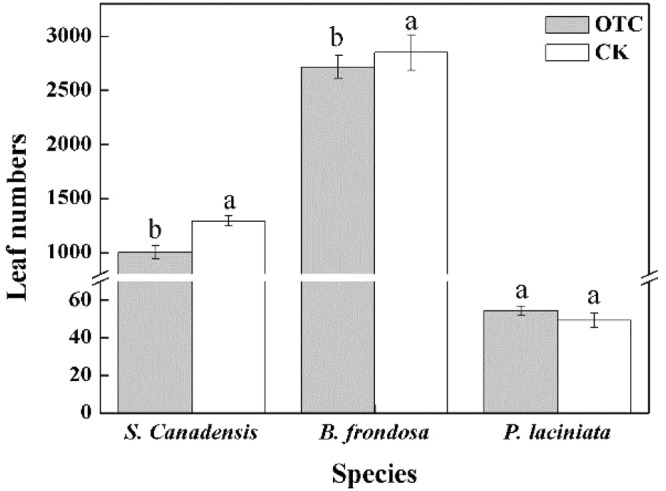
Effect of simulated warming on leaf number of S. canadensis, B. frondosa and P. laciniata.
Flowering phenology
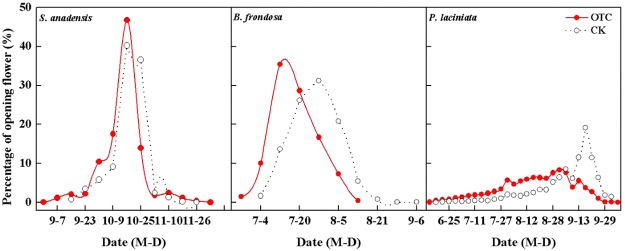
The peak flowering date and endset day of S. canadensis (49.0 ± 2.7 d and 77.0 ± 1.9 d), B. frondosa (65.0 ± 1.7 d and 94.0 ± 4.3 d) and P. laciniata (77.0 ± 1.9 d and 33.0 ± 2.5 d) were significantly advanced under simulated warming treatments. Simulated warming also significantly advanced the onset day (34.0 ± 2.6 d and 9.0 ± 0.9 d) and prolonged the flowering duration (44.0 ± 2.5 d and 85.0 ± 2.8 d) of S. canadensis and B. frondosa (n = 5, p < 0.05) (Table 1). It was also found to increase the flowering synchrony index of the invasive species, but decrease that of the native species. Warming didn’t significantly affect the relative flowering intensity for any of the species tested (Table 1, Fig. 3).
Table 1.
Flowering phenology index of S. canadensis, B. frondosa and P. laciniata.
| Items | Treatment | S. canadensis | B. frondosa | P. laciniata |
|---|---|---|---|---|
| Onset day (d) | OTC | 34 ± 2.645 | 9 ± 0.960 | 8 ± 1.483 |
| CK | 38 ± 2.379 | 41 ± 2.873 | 13 ± 1.715 | |
| P | 0.016 | 0.001 | 0.139 | |
| Peak flowering date (d) | OTC | 49 ± 2.684 | 65 ± 1.678 | 23 ± 1.944 |
| CK | 52 ± 2.44 | 82 ± 2.250 | 31 ± 1.181 | |
| P | 0.014 | 0.008 | 0.041 | |
| Endset day (d) | OTC | 77 ± 1.979 | 94 ± 4.326 | 33 ± 2.512 |
| CK | 73 ± 0.759 | 103 ± 1.331 | 56 ± 1.499 | |
| P | 0.042 | 0.100 | 0.004 | |
| Duration (d) | OTC | 44 ± 2.517 | 85 ± 2.824 | 26 ± 0.922 |
| CK | 36 ± 1.102 | 65 ± 1.575 | 35 ± 1.432 | |
| P | 0.047 | 0.005 | 0.017 | |
| Flower number | OTC | 5331 ± 557.78 | 401 ± 22.88 | 90 ± 4.590 |
| CK | 5725 ± 557.34 | 352 ± 13.98 | 100 ± 2.068 | |
| P | 0.429 | 0.040 | 0.024 | |
| Flowering synchrony index | OTC | 0.77 ± 0.036 | 0.85 ± 0.013 | 0.73 ± 0.022 |
| CK | 0.72 ± 0.043 | 0.67 ± 0.011 | 0.84 ± 0.019 | |
| P | 0.014 | 0.001 | 0.020 | |
| Relative flowering intensity | OTC | 0.54 ± 0.051 | 0.44 ± 0.064 | 0.53 ± 0.034 |
| CK | 0.47 ± 0.056 | 0.27 ± 0.044 | 0.53 ± 0.035 | |
| P | 0.227 | 0.111 | 0.986 |
Figure 3.
Effect of simulated warming on flowering phenology of S. canadensis, B. frondosa and P. laciniata.
Biomass allocation
The total biomass (64.9 ± 0.9 g · plant−1), leaf biomass (8.1 ± 0.1 g · plant−1) and shoot biomass (22.2 ± 0.6 g · plant−1) of S. canadensis and B. frondosa (56.8 ± 0.7, 7.4 ± 0.2 and 29.8 ± 0.5 g · plant−1) were significantly increased under simulated warming treatment; the root biomass of S. canadensis also increased, but not significantly. The shoot biomass of P. laciniata (0.4 ± 0.04 g · plant−1) was significantly increased in the warming treatment, but its root biomass decreased, and no significant difference in total biomass was displayed (Table 2).
Table 2.
Effect of simulated warming on biomass of S. canadensis, B. frondosa and P. laciniata.
| Items | Treatments | S. canadensis | B. frondosa | P. laciniata |
|---|---|---|---|---|
| Root biomass (g.plant−1) | OTC | 29.357 ± 0.520 | 10.966 ± 0.371 | 0.37 ± 0.04 |
| CK | 25.521 ± 0.544 | 10.108 ± 0.278 | 0.52 ± 0.04 | |
| P | 0.026 | 0.07 | 0.027 | |
| Shoot biomass (g.plant−1) | OTC | 22.192 ± 0.582 | 29.779 ± 0.499 | 3.22 ± 0.44 |
| CK | 20.435 ± 0.405 | 27.267 ± 0.463 | 1.92 ± 0.09 | |
| P | 0.014 | 0.008 | 0.042 | |
| Leaf biomass (g.plant−1) | OTC | 8.115 ± 0.143 | 7.408 ± 0.189 | 0.55 ± 0.08 |
| CK | 9.391 ± 0.307 | 8.425 ± 0.245 | 0.62 ± 0.03 | |
| P | 0.004 | 0.01 | 0.448 | |
| Flower biomass (g.plant−1) | OTC | 5.271 ± 0.276 | 8.613 ± 0.159 | 0.34 ± 0.08 |
| CK | 5.029 ± 0.223 | 7.776 ± 0.153 | 0.37 ± 0.04 | |
| P | 0.427 | 0.015 | 0.757 | |
| Total biomass (g.plant−1) | OTC | 64.935 ± 0.928 | 56.767 ± 0.741 | 4.48 ± 0.60 |
| CK | 61.376 ± 0.556 | 53.576 ± 0.888 | 3.44 ± 0.17 | |
| P | 0.04 | 0.034 | 0.158 |
Simulated warming significantly increased the ratio of root and shoot allocation (45.4% ± 0.4% and 34.0% ± 0.7%, n = 5, p < 0.05), but decreased the ratio of leaf allocation in S. canadensis (Table 3). The clonal ramets of S. canadensis increased significantly from 32.4 to 38.1 (p < 0.01), although its reproductive allocation didn’t increase after simulated warming. In B. frondosa, the ratio of reproductive allocation (14.8% ± 0.2%) and shoot allocation (52.7% ± 0.2%) increased significantly, while reproductive allocation of P. laciniata decreased significantly under simulated warming.
Table 3.
Biomass allocation of S. canadensis, B. frondosa and P. laciniata by simulated warming.
| Items | Treatments | S. canadensis | B. frondosa | P. laciniata |
|---|---|---|---|---|
| Root biomass ratio | OTC | 45.4% ± 0.4% | 19.5% ± 0.6% | 8.4% ± 0.8% |
| CK | 43.4% ± 0.7% | 19.2% ± 0.4% | 15.4% ± 0.9% | |
| P | 0.038 | 0.583 | 0.004 | |
| Shoot biomass ratio | OTC | 34.0% ± 0.7% | 52.7% ± 0.2% | 70.9% ± 2.9% |
| CK | 32.8% ± 0.7% | 51.0% ± 0.5% | 56.0% ± 1.9% | |
| P | 0.026 | 0.015 | 0.00.5 | |
| Leaf biomass ratio | OTC | 12.6% ± 0.3% | 13.0% ± 0.5% | 13.3% ± 2.1% |
| CK | 15.6% ± 0.5% | 15.9% ± 0.3% | 17.9% ± 0.7% | |
| P | 0.002 | 0.001 | 0.103 | |
| Reproductive allocation ratio | OTC | 8.0% ± 0.4% | 14.8% ± 0.2% | 7.4% ± 0.8% |
| CK | 8.3% ± 0.3% | 13.9% ± 0.2% | 10.8% ± 0.9% | |
| P | 0.187 | 0.037 | 0.028 | |
| Root/leaf ratio | OTC | 3.722 ± 0.129 | 1.650 ± 0.140 | 0.858 ± 0.105 |
| CK | 2.899 ± 0.113 | 1.231 ± 0.042 | 0.896 ± 0.067 | |
| P | 0.008 | 0.024 | 0.644 | |
| Root/shoot ratio | OTC | 0.493 ± 0.004 | 0.244 ± 0.010 | 0.093 ± 0.109 |
| CK | 0.473 ± 0.008 | 0.239 ± 0.007 | 0.173 ± 0.112 | |
| P | 0.058 | 0.600 | 0.004 |
Seed production
Simulated warming significantly increased the seed weight for both S. canadensis and B. frondosa (0.05 ± 0.0 g and 2.8 ± 0.04 g), the germination ratio for S. Canadensis (38.8 ± 2.4%), and the seed size of B. frondosa (2.7 ± 0.04 mm) (p < 0.05, Table 4).
Table 4.
Effect of simulated warming on seed of S. canadensis, B. frondosa and P. laciniata.
| Items | Treatments | S. canadensis | B. frondosa | P. laciniata |
|---|---|---|---|---|
| 1000-seed weight (g) | OTC | 0.0475 ± 0.000 | 2.783 ± 0.044 | 0.37 ± 0.006 |
| CK | 0.0465 ± 0.000 | 2.373 ± 0.063 | 0.35 ± 0.003 | |
| P | 0.021 | 0.005 | 0.053 | |
| Seed length (mm) | OTC | 1.558 ± 0.009 | 6.609 ± 0.069 | 3.268 ± 0.019 |
| CK | 1.557 ± 0.011 | 6.215 ± 0.057 | 3.260 ± 0.023 | |
| P | 0.959 | 0.001 | 0.778 | |
| Seed size (mm) | OTC | 0.559 ± 0.008 | 2.711 ± 0.041 | 1.230 ± 0.019 |
| CK | 0.548 ± 0.008 | 2.553 ± 0.030 | 1.200 ± 0.0166 | |
| P | 0.293 | <0.001 | 0.247 | |
| Germination ratio (%) | OTC | 38.8 ± 2.417 | — | 86.00 ± 2.608 |
| CK | 30.0 ± 1.414 | 91.20 ± 1.625 | ||
| P | 0.024 | 0.24 |
Note: The germination ratio of was not statistically analyzed, because there were only 3 seeds germinated in OTC but not seed germinated in CK.
Discussion
Temperature can directly or indirectly affect plant growth and biomass production. In the present study, the results showed that simulated warming increased the accumulation of root biomass, shoot biomass and total biomass in S. canadensis, and the flower biomass and total biomass in B. frondosa (Table 3), but decreased the root biomass in the native species P. laciniata (Table 1). These results suggested that warming significantly promotes the growth of our focal invasive species but not of the noninvasive species. S. canadensis can be cloned and propagated by subterranean stem sprouts. The rhizome can accommodate clonal growth by controlling the ratio of buds per node. Our results also showed that simulated warming significantly increased the ratio of root and shoot allocation, but decreased the ratio of leaf allocation in S. canadensis (Table 3), which indicated that simulated warming may promote the clonal reproduction of S. canadensis. These responses allow the invasive species to develop dominant populations in communities and become more competitive than noninvasive species.
Plants can change their biomass allocation pattern in response to a change of environmental conditions35. Invasive species have previously been shown to produce more biomass than native species36. In the present study, simulated climate change increased the shoot biomass of both the invasive species and native species, and promoted plant height growth, increasing the size of the plant stem. Plant stem tissue is large in these species, being a location for high energy storage, which could account for the observed increase in biomass. Increased investment in shoot biomass is conducive to plant growth and can improve competitive ability. Climate change also increased the root biomass allocation and decreased the leaf biomass allocation thereby significantly increasing root/leaf ratio in S. canadensis. This pattern is likely to increase water and nutrient utilization efficiency and increase the plant’s ability to adapt to varied environmental conditions and further enhance its competitive ability and invasion.
In contrast, a non-clonal species, B. frondosa responded to the simulated warming by increasing inflorescence biomass, total biomass and root/leaf ratio. This increase in biomass allocation to reproduction organs may increase population growth and colonization of new habitats. Hence, our results from simulated climate change are consistent with the hypothesis that warming may increase the invasion of non-native species37. The native species, P. laciniata significantly decreased its reproductive allocation, root mass, and root/shoot ratio under climate change. These results suggest that invasive species can improve their ability to adapt to the environment and enhance invasion by optimizing resource allocation to adapt to climate change.
Flowering phenology can affect pollinators’ behavior and their sexual reproductive fitness, and further affect plant reproductive success. Plants display a variety of responses to climate change38. Several studies have demonstrated that within a population, early-flowering plants produce more flowers and seeds than late-flowering plants39,40. All three focal species significantly advanced their onset day and peak flowering day, but this resulted in a prolonged flowering season for the two invasive species and a shortened season for the native species. At the same time, our results also indicated the invasive species increased their flowering synchrony, but the native species’ decreased after warming. Flowering pattern is an important factor affecting pollination success39; species with high flowering synchrony and a mass-flowering pattern can be more effective in attracting pollinators41–43.
Although climate change could provide new habitats for invasive species, they must overcome many unfavorable factors to invade the new habitat successfully44. For instance, they need to get enough nutrients to complete morphogenesis, and need enough pollinators to complete the process of sexual reproduction, a process that is impacted by flowering pattern, as discussed above. Flowering phenology and pattern can be an important factor for invaders in their successful colonization in new habitat.
It has been suggested that invasive species can respond better to climate change than native species by adjusting flowering times45. Our results demonstrated that the invasive species, S. canadensis and B. frondosa, displayed more plasticity than the native P. laciniata do in reproductive phenology in response to simulated climate change. The invasive species shifted and prolonged its flowering pattern. This result suggests that climate change might accelerate the process of biological invasion.
Acknowledgements
We thank Dr. Marc and Dr. Yonghong Xiao for comments on the manuscript. We are grateful to the National Natural Science Foundation of China (No. 41561012, 31360099, 31360138).
Author Contributions
Y.A.X. and W.H.H. designed the experiment, S.S.Z. and Y.S.C. performed the experiment, Y.A.X. did the statistical analysis and wrote the first draft of the manuscript, W.H.H. and Y.S.C. contributed substantially to the revisions.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pandey DN. GlobalClimate Change and Carbon Management in Multifunctional Forests. Current science. 2002;83:593–602. [Google Scholar]
- 2.Sathaye J, Shukla PR, Ravindranath NH. Climate change, sustainable development and India: Global and national concerns. Current science. 2006;90:314–325. [Google Scholar]
- 3.Hoegh-Guldberg O, et al. Coral reefs under rapidclimate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 4.Walther G-R, et al. Alien species in a warmer world: risks and opportunities. Trends in Ecology & Evolution. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Vitousek PM. Beyond Global Warming: Ecology and Global Change. Ecology. 1994;75:1861–1876. doi: 10.2307/1941591. [DOI] [Google Scholar]
- 6.Yang LH, Rudolf VHW. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecology letters. 2010;13:1–10. doi: 10.1111/j.1461-0248.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 7.Schroter D, et al. Ecosystem service supply and vulnerability to global change in Europe. Science. 2005;310:1333–1337. doi: 10.1126/science.1115233. [DOI] [PubMed] [Google Scholar]
- 8.Verlinden M, Nijs I. Alien plant species favoured over congeneric natives under experimental climate warming in temperate Belgian climate. Biological Invasions. 2010;12:2777–2787. doi: 10.1007/s10530-009-9683-1. [DOI] [Google Scholar]
- 9.Pyšek, P. & Richardson, D. M. In Biological Invasions 97–125 (Springer, 2008).
- 10.Williamson M, Fitter A. The varying success of invaders. Ecology. 1996;77:1661–1666. doi: 10.2307/2265769. [DOI] [Google Scholar]
- 11.Parmesan C. Ecological and Evolutionary Responses to Recent Climate Change. Annual Review of Ecology, Evolution, and Systematics. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 12.Hart R, Salick J, Ranjitkar S, Xu J. Herbarium specimens show contrasting phenological responses to Himalayan climate. Proceedings of the National Academy of Sciences. 2014;111:10615–10619. doi: 10.1073/pnas.1403376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badeck FW, et al. Responses of spring phenology to climate change. New Phytologist. 2004;162:295–309. doi: 10.1111/j.1469-8137.2004.01059.x. [DOI] [Google Scholar]
- 14.Fitchett JM, Grab SW, Thompson DI. Plant phenology and climate change: Progress in methodological approaches and application. Progress in Physical Geography. 2015;39:460–482. doi: 10.1177/0309133315578940. [DOI] [Google Scholar]
- 15.Kimball KD, Davis ML, Weihrauch DM, Murray GLD, Rancourt K. Limited alpine climatic warming and modeled phenology advancement for three alpine species in the Northeast United States. American journal of botany. 2014;101:1437–1446. doi: 10.3732/ajb.1400214. [DOI] [PubMed] [Google Scholar]
- 16.Iler AM, Høye TT, Inouye DW, Schmidt NM. Nonlinear flowering responses to climate: are species approaching their limits of phenological change? Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2013;368:0489. doi: 10.1098/rstb.2012.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley NL, Leopold AC, Ross J, Huffaker W. Phenological changes reflectclimate change in Wisconsin. Proceedings of the National Academy of Sciences. 1999;96:9701–9704. doi: 10.1073/pnas.96.17.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munson SM, Sher AA. Long-term shifts in the phenology of rare and endemic Rocky Mountain plants. American journal of botany. 2015;102:1268–1276. doi: 10.3732/ajb.1500156. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs EJ, Lobo JA, Quesada M. Effects of Forest Fragmentation and Flowering Phenology on the Reproductive Success and Mating Patterns of the Tropical Dry Forest Tree Pachira quinata. Conservation Biology. 2003;17:149–157. doi: 10.1046/j.1523-1739.2003.01140.x. [DOI] [Google Scholar]
- 20.Godoy O, Richardson DM, Valladares F, Castro-Díez P. Flowering phenology of invasive alien plant species compared with native species in three Mediterranean-type ecosystems. Annals of botany. 2009;103:485–494. doi: 10.1093/aob/mcn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadotte MW, Jon L-D. Ecological and taxonomic differences between native and introduced plants of southwestern Ontario. Ecoscience. 2001;8:230–238. doi: 10.2307/42901316. [DOI] [Google Scholar]
- 22.Lake JC, Leishman MR. Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biological Conservation. 2004;117:215–226. doi: 10.1016/s0006-3207(03)00294-5. [DOI] [Google Scholar]
- 23.Alatalo JM, Totland Ø. Response to simulated climatic change in an alpine and subarctic pollen-risk strategist. Silene acaulis. Global Change Biology. 1997;3:74–79. doi: 10.1111/j.1365-2486.1997.gcb133.x. [DOI] [Google Scholar]
- 24.Hovenden MJ, Wills KE, Schoor JKV, Williams AL, Newton PCD. Flowering phenology in a species-rich temperate grassland is sensitive to warming but not elevated CO2. New Phytologist. 2008;178:815–822. doi: 10.1111/j.1469-8137.2008.02419.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Reich PB, Li G, Sun S. Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology. 2011;92:1201–1207. doi: 10.1890/10-2060.1. [DOI] [PubMed] [Google Scholar]
- 26.Satake A, et al. Forecasting flowering phenology under climate warming by modelling the regulatory dynamics of flowering-time genes. Nature communications. 2013;4:2303. doi: 10.1038/ncomms3303. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Mu J, Niklas KJ, Li G, Sun S. Global warming reduces plant reproductive output for temperate multi-inflorescence species on the Tibetan plateau. New Phytologist. 2012;195:427–436. doi: 10.1111/j.1469-8137.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- 28.Hao JH, Qiang S, Chrobock T, van Kleunen M, Liu QQ. A test of baker’s law: breeding systems of invasive species of Asteraceae in China. Biological Invasions. 2011;13:571–580. doi: 10.1007/s10530-010-9850-4. [DOI] [Google Scholar]
- 29.Dong MEI, Lu B-R, Zhang H-B, Chen J-K, Li BO. Role of sexual reproduction in the spread of an invasive clonal plant Solidago canadensis revealed using intersimple sequence repeat markers. Plant Species Biology. 2006;21:13–18. doi: 10.1111/j.1442-1984.2006.00146.x. [DOI] [Google Scholar]
- 30.Abhilasha D, Quintana N, Vivanco J, Joshi J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? Journal of Ecology. 2008;96:993–1001. doi: 10.1111/j.1365-2745.2008.01413.x. [DOI] [Google Scholar]
- 31.Nolf M, Pagitz K, Mayr S. Physiological acclimation to drought stress in Solidago canadensis. Physiologia plantarum. 2014;150:529–539. doi: 10.1111/ppl.12100. [DOI] [PubMed] [Google Scholar]
- 32.Rahman A, Bajpai VK, Dung NT, Kang SC. Antibacterial and antioxidant activities of the essential oil and methanol extracts of Bidens frondosa Linn. International Journal of Food Science & Technology. 2011;46:1238–1244. doi: 10.1111/j.1365-2621.2011.02615.x. [DOI] [Google Scholar]
- 33.Brändel M. Dormancy and germination of heteromorphic achenes of Bidens frondosa. Flora. 2004;199:228–233. doi: 10.1078/0367-2530-00150. [DOI] [Google Scholar]
- 34.McIntosh ME. Flowering phenology and reproductive output in two sister species of Ferocactus (Cactaceae) Plant Ecology. 2002;159:1–13. doi: 10.1023/A:1015589002987. [DOI] [Google Scholar]
- 35.Zobel M, Zobel K. Studying plant competition: from root biomass to general aims. Journal of Ecology. 2002;90:578–580. doi: 10.1046/j.1365-2745.2002.00693.x. [DOI] [Google Scholar]
- 36.Szymura M, Szymura TH. Growth, phenology, and biomass allocation of alien Solidago species in central Europe. Plant Species Biology. 2015;30:245–256. doi: 10.1111/1442-1984.12059. [DOI] [Google Scholar]
- 37.Daws MI, Hall J, Flynn S, Pritchard HW. Do invasive species have bigger seeds? Evidence from intra- and inter-specific comparisons. South African Journal of Botany. 2007;73:138–143. doi: 10.1016/j.sajb.2006.09.003. [DOI] [Google Scholar]
- 38.Totland Ø, Alatalo J. Effects of temperature and date of snowmelt on growth, reproduction, and flowering phenology in the arctic/alpine herb, Ranunculus glacialis. Oecologia. 2002;133:168–175. doi: 10.1007/s00442-002-1028-z. [DOI] [PubMed] [Google Scholar]
- 39.Kudo Gaku, Suzuki Shizuo. Relationships between Flowering Phenology and Fruit-set of Dwarf Shrubs in Alpine Fellfields in Northern Japan: A Comparison with a Subarctic Heathland in Northern Sweden. Arctic, Antarctic, and Alpine Research. 2002;34(2):185–190. doi: 10.1080/15230430.2002.12003483. [DOI] [Google Scholar]
- 40.Xiao Y-A, et al. Pollination biology of Disanthus cercidifolius var. longipes, an endemic and endangered plant in China. Biologia. 2009;64:731–736. doi: 10.2478/s11756-009-0122-7. [DOI] [Google Scholar]
- 41.Herrera J. Flowering and fruiting phenology in the coastal shrublands of Doñana, south Spain. Vegetatio. 1986;68:91–98. [Google Scholar]
- 42.Ishii HS, Sakai S. Temporal variation in floral display size and individual floral sex allocation in racemes of Narthecium asiaticum (Liliaceae) American Journal Botanty. 2002;89:441–446. doi: 10.3732/ajb.89.3.441. [DOI] [PubMed] [Google Scholar]
- 43.van der Meer S, Jacquemyn H. The effect of phenological variation in sex expression on female reproductive success in Saxifraga granulata. American journal of botany. 2015;102:2116–2123. doi: 10.3732/ajb.1500324. [DOI] [PubMed] [Google Scholar]
- 44.Simberloff D, Von Holle B. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions. 1999;1:21–32. doi: 10.1023/A:1010086329619. [DOI] [Google Scholar]
- 45.Willis CG, et al. FavorableClimate Change Response Explains Non-Native Species’ Success in Thoreau’s Woods. PloS one. 2010;5:e8878. doi: 10.1371/journal.pone.0008878. [DOI] [PMC free article] [PubMed] [Google Scholar]