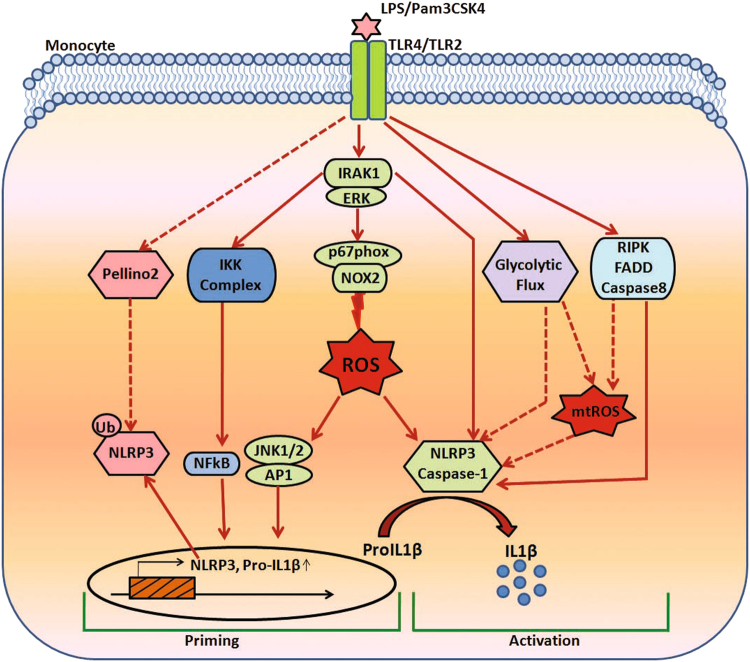
In a study published in Cellular and Molecular Immunology, Singh et al. demonstrated a new mechanism of monocyte IL-1β production.1 It was observed that TLR-4- and TLR-2-induced IRAK-ERK pathway cross-talk with p67phox-Nox-2 for reactive oxygen species (ROS) generation, thus regulating IL-1β transcription and processing in monocytes. This study not only establishes a direct link between IRAK and ERK, but also demonstrates the regulation of p67phox-Nox-2 by ERK for ROS and IL-1β production (Fig. 1). This study shows that the IRAK-ERK axis can regulate both IL-β transcription and processing through ROS.
Fig. 1.
IL-1β production in monocytes. Diagram depicting the probable mechanisms of IL-1β production in monocytes. The transcription and processing of IL-1β involve steps that promote inflammasome priming and activation, respectively. Dotted arrows indicate probable signaling pathways operating in monocytes. mtROS mitochondrial reactive oxygen species (mtROS)
The monocyte activation by TLR ligands leads to the production of inflammatory cytokines such as IL-1β and TNF-α, which exert diverse roles.2 Due to its high potency and diverse effects, IL-1β can modulate the progression of various pathological disorders such as rheumatoid arthritis, aneurysm, type I diabetes, atherosclerosis, and airway inflammation. Therefore, understanding the mechanism of IL-1β production may help in designing therapeutic strategies for various inflammatory disorders. Secreted IL-1β binds to its receptor IL-1βR and induces a cascade of signaling events, including the activation of IRAK, MAPK, and NF-κB, leading to the transcription of various inflammatory cytokines and growth factors. IL-1β affects both the adaptive and innate immune responses. It affects T cell maturation and the proliferation of B cells. Further, IL-1β promotes the expression of several inflammatory molecules such as nitric oxide, phospholipase A2, cyclooxygenase-2, and prostaglandin E2.3
Classically, it is known that IL-1β release involves two steps. In the first step, IL-1β is transcribed as an inactive precursor called pro-IL-1β, and priming of the inflammasome occurs. In the second step, processing of IL-1β takes place, which is dependent on the NLRP3 inflammasome and caspase-1, and leads to the formation of mature IL-1β that is secreted outside the cell. The TLR-dependent priming of the inflammasome involves the NF-κB-dependent enhanced transcription of pro-IL-1β and the increased expression of NLRP3.2 A study suggests the deubiquitination of NLRP34 as an important step in inflammasome priming. In this study, it was shown that the pharmacological inhibition of deubiquitination completely blocked NLRP3 activation both in mouse and human cells.4 However, these experiments were carried out in NLRP3-overexpressing cells, a condition that may mimic inflammasome priming to some extent. However, in murine macrophages, TLR-4-induced endogenous inflammasome priming was dependent on Pellino-2-induced NLRP3 and IRAK-1 ubiquitination.5 The absence of Pellino-2-induced IRAK-1-NLRP3 association and the downregulation of NLRP3 ubiquitination and priming.5 Thus, it seems that ubiquitination and deubiquitination both play important roles in inflammasome activation. In macrophages, IRAK-1 bypasses priming and directly links TLR activation with inflammasome activation and IL-1β production.6 IRAK-1SiRNA and the IRAK1/4 inhibitor significantly reduced TLR-2, TLR-4 induced caspase-1 activation, and IL-1β production in monocytes.1 However, because the IRAK-ERK axis was also involved in the generation of ROS and possible inflammasome activation, a direct interaction of IRAK with NLRP3 cannot be ruled out and needs to be ascertained.
Unlike murine monocytes, human monocytic cells possess an alternative pathway of inflammasome activation, where the first and second signals are both dependent on the TLR-4.7 In this study, TLR-4 induces IL-1β transcription and the TLR4-TRIF-RIPK1-FADD-CASP8 pathway leads to alternative inflammasome activation (Fig. 1). IL-1β is regulated not only transcriptionally by NF-κB and posttranslationally by the inflammasome but also posttranscriptionally by p38α-MK2.8
The TLR-2- and TLR-4-induced IRAK-ERK interaction and ERK-p67phox-Nox-2 association observed by Singh et al. indicates a transcription-independent regulation of ROS by the MAPK.1 Since ROS regulates IL-1β transcription through the JNK-AP-1 axis and induces caspase-1 activation,1 it can be speculated that ROS can be a master regulator of IL-1β transcription and processing. This study shows the importance of Nox-2 in ROS generation; however, whether other Nox isoforms, such as Nox-1 and Nox-4, also operate in a similar manner requires further investigation. It is known that ROS promotes the oxidation of TRX and the binding of relieved TXNIP to NLRP3, leading to NLRP3 activation and IL-β processing.9 Since the role of macrophage mitochondrial ROS in TLR-induced classical inflammasome activation is known,10 its role in monocyte alternative inflammasome activation needs to be ascertained.7 Whether TLR-2- and TLR-4-induced inflammasome activation and IL-β production also involve mitochondrial ROS in monocytes needs to be determined. As discussed earlier,11 regulated ROS production may lead to the controlled activation of inflammasomes and IL-1β production, but a sustained and elevated ROS may ultimately induce a high amount of IL-1β and pathological insult. Since mitochondrial or NOX-derived ROS may regulate monocyte inflammasome activation, IL-1β release and pyroptosis, it will be interesting to determine whether the source and extent of ROS generation regulate the different aspects of IL-1β production.
Though there is a general notion that upon microbial challenge, a shift from oxidative phosphorylation to glycolysis underlies the activation of all immune cells, a recent study sheds new light. In this study, transcriptome and metabolome analysis of monocytes stimulated with PAM3CSK4 Pam3CSK4(TLR-2) or LPS (TLR-4) showed significant differences in the tricarboxylic acid cycle, oxidative phosphorylation, and lipid metabolism pathways.12 Therefore, although Singh et al. demonstrated a significant role of Nox-2-derived ROS in TLR-2- and TLR-4-induced IL-1β production, it is quite possible that mitochondrial ROS and specific metabolic pathways may play significant roles in IL-1β production (Fig. 1). Therefore, although TLR-induced IL-1β release may be relatively similar, the underlying metabolic routes may vary significantly.12 In primary macrophages and human monocytes, increasing concentrations of lactate reduced TLR-4 mediated inflammasome priming, activation, and IL-1β release.13 As such, lactate delayed the monocyte inflammatory response.14
Translating the understanding of murine monocytic IL-1β production to humans must be done with caution, since there will be different mechanisms of IL-1β transcription, processing, and modification operating in the two species. At the same time, ROS can be a master regulator of IL-1β production since it may regulate the priming and processing of the inflammasome. Controlled ROS production may yield regulated IL-1β production; however, high ROS levels can induce pathological levels of IL-1β. The source and location of ROS may also define the aspect of IL-1β production that is regulated (Fig. 1). Understanding the role of TLR-specific metabolic pathways in ROS generation and IL-1β production will help in understanding the fine-tuning of the machinery involved in IL-1β production. In addition to this molecular mechanism, ROS-induced IL-1β transcription and processing require further studies. For secretory IL-1β production, although ROS can regulate both IL-1β transcription and processing, they may also require support from other pathways. This assumption comes from the fact that some inducers such as TNF, which do generate ROS, do not affect inflammasome and IL-1β production.15
Competing interests
The authors declare no competing interests. This is CDRI communication number 9695.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh A, et al. The IRAK-ERK-p67phox-Nox-2 axis mediates TLR4, 2-induced ROS production for IL-1beta transcription and processing in monocytes. Cell Mol. Immunol. 2016;13:745–763. doi: 10.1038/cmi.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 4.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphries F, et al. The E3 ubiquitin ligase Pellino2 mediates priming of the NLRP3 inflammasome. Nat. Commun. 2018;9:1560. doi: 10.1038/s41467-018-03669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin KM, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaidt MM, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, et al. Selective inhibition of the p38alpha MAPK-MK2 axis inhibits inflammatory cues including inflammasome priming signals. J. Exp. Med. 2018;215:1315–1325. doi: 10.1084/jem.20172063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 10.Heid ME, et al. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachmandas E, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat. Microbiol. 2016;2:16246. doi: 10.1038/nmicrobiol.2016.246. [DOI] [PubMed] [Google Scholar]
- 13.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015;457:412–418. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]