Dear Editor,
The progression of acute myeloid leukemia (AML)—the most severe blood/bone marrow cancer—is determined by the ability of malignant cells to escape host immune surveillance. However, the systemic regulatory mechanisms underlying this phenomenon remain largely unknown. In this study, we discovered a fundamental systemic biochemical strategy that allows AML cells to employ physiological systems within the body to survive and escape immune attack. We found that AML cells use a crucial human adrenal cortex hormone (cortisol) to induce the expression of neuronal receptor latrophilin 1 (LPHN1), which facilitates exocytosis. This receptor interacts with the blood plasma protein fibronectin leucine rich transmembrane protein 3 (FLRT3) to cause secretion of the immune suppressor galectin-9, which impairs the anticancer activities of cytotoxic lymphoid cells.
AML is a cancer of the blood and bone marrow that originates from self-renewing malignant immature myeloid cells and rapidly progresses into a systemic, and very often fatal, malignancy.1 AML cells employ physiological systems in the body to produce factors required for proliferation/disease progression.2,3 This includes the hijacking of stem cell factor (SCF), a major hematopoietic growth factor that controls AML progression and thus can become highly oncogenic.2,3 The expression and release of SCF can be triggered by AML cells via cytokines (e.g., interleukin-1β).2 Recent evidence clearly demonstrated that AML cells are also capable of impairing the activities of cytotoxic lymphoid cells (e.g., natural killer (NK) cells and cytotoxic T cells).4 One of the biochemical mechanisms underlying this phenomenon lies in the ability of AML cells to secrete the protein galectin-9. This tandem-type galectin binds the immune receptor Tim-3 and induces a variety of intracellular and cell-to-cell signaling events leading to the inactivation of NK cells, as well as the death of cytotoxic T cells.4,5 We recently reported that the process of galectin-9 secretion in AML cells is stimulated by the unique G protein-coupled receptor LPHN1, which normally functions in neurons to facilitate exocytosis.4,6 LPHN1 is also found in hematopoietic stem cells (HSCs), but its expression disappears at the early stages of their maturation.4,7 However, upon malignant transformation, AML cells preserve their abilities to express LPHN1 and to produce high levels of galectin-9 and Tim-3, in which the latter is involved in trafficking galectin-9 during the secretion process (HSCs express neither galectin-9 nor Tim-34).
It is currently unknown which molecular mechanisms trigger elevated levels of LPHN1 expression in primary human AML cells, and in general, the mechanisms of upregulation of LPHN1 expression at the genomic level remain unclear. It is also unknown whether FLRT3, a natural LPHN1 ligand,4,8 is present in human blood plasma and in other tissues associated with AML. Unraveling these mechanisms is crucial to understanding the pathways that control the ability of AML cells to protect themselves against cytotoxic lymphoid cells and, thus, was the aim of the present study.
Results and discussion
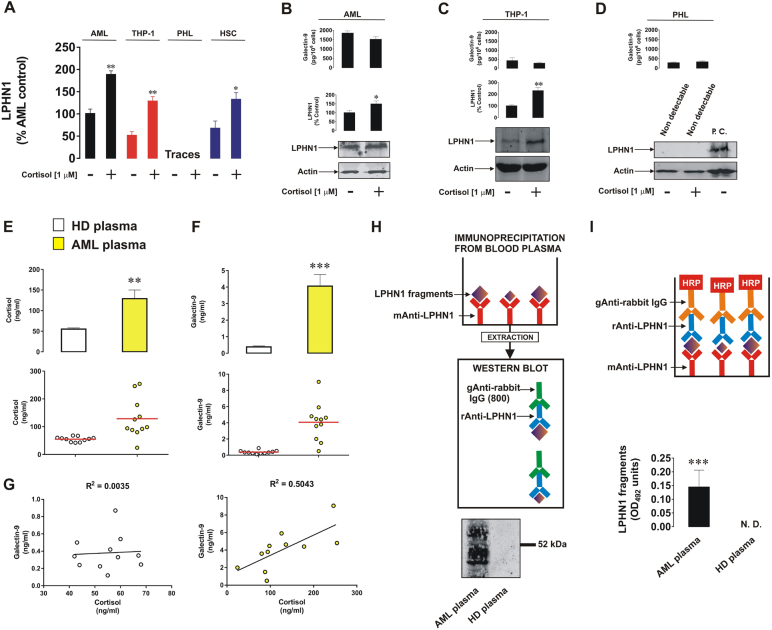
To investigate the effects of cortisol on LPHN1 transcription, we exposed primary and THP-1 human AML cells, primary human HSCs and primary healthy human leukocytes to 1 µM cortisol for 24 h and subjected to cells to quantitative real-time PCR to analyze LPHN1 mRNA levels. We found that all the tested cell types, except primary healthy leukocytes, transcribed detectable amounts of LPHN1 mRNA, and, in all these cases, the levels were significantly upregulated by treatment with cortisol (Fig. 1a). In both THP-1 and primary human AML cells, LPHN1 protein levels were also clearly upregulated (Fig. 1b, c). In contrast, primary human healthy leukocytes did not express detectable amounts of LPHN1 protein, and this was not altered by the effects of cortisol (Fig. 1d). Comparative analysis of LPHN1 protein expression in primary human AML cells, THP-1 cells, and primary human healthy leukocytes is shown in Supplementary figure 1.
Fig. 1.
Cortisol induces LPHN1 expression in human AML cells and in hematopoietic stem cells but not in primary healthy human leukocytes. Primary human AML cells, THP-1 cells, and hematopoietic stem cells, as well as primary healthy leukocytes were exposed to 1 µM cortisol for 24 h followed by analysis of LPHN1 gene transcription via quantitative real-time PCR (a) and Western blot analysis (b primary AML cells, (c) THP-1 cells, (d) PHL). For PHL, lysates from LPHN1-overexpressing NB2A cells were used as a positive control. ELISA was used to measure secreted galectin-9 levels. Blood plasma from 10 healthy donors and 10 AML patients was collected at the same time of the day to ensure comparability of the cortisol levels. Cortisol (e) and galectin-9 (f) levels were measured by ELISA, and the correlation between the levels of these two proteins was analyzed (g). Soluble LPHN1 fragments were immunoprecipitated and detected by Western blot (h) and ELISA (i), as outlined in the Materials and Methods section. Images are from one experiment but are representative of 4–6 replicates, all of which showed similar results. Data represent the mean values ± SEM of 6–10 independent experiments.; *p < 0.05; **p < 0.01; ***p < 0.01 vs. control
Cortisol treatments did not upregulate galectin-9 secretion in any of these cell types (Fig. 1b–d), suggesting that LPHN1 needs to be activated by a ligand to induce galectin-9 release.
Analysis of blood plasma levels of cortisol in AML patients vs. those in healthy donors (samples were collected at the same time of the day to avoid the influence of circadian dynamics) demonstrated that the cortisol levels were significantly higher in the blood plasma of AML patients than in healthy donors (Fig. 1e). Galectin-9 levels were also substantially higher in AML patients (Fig. 1f), which is in line with our previous observations.4 Furthermore, there was no correlation between cortisol and galectin-9 levels in the blood plasma of healthy donors, while in AML patients, there was a clear correlation (Fig. 1g), suggesting that galectin-9 secretion might be linked to LPHN1 expression in this circumstance.
If LPHN1 is expressed on the surface of blood cells, it can also be shed by proteolysis and therefore be present in the plasma. LPHN1 in blood plasma samples from AML patients was immunoprecipitated, extracted, and subjected to Western blot analysis using several LPHN1 antibodies. A clear fragment was detectable at ∼67–68 kDa, and smaller fragments were detectable as well, but only in plasma from AML patients, while in the blood plasma from healthy donors, there was no evidence of the presence of LPHN1 fragments (Fig. 1h). These fragments were also detectable by ELISA (Fig. 1i, see Materials and Methods for description of the ELISA format).
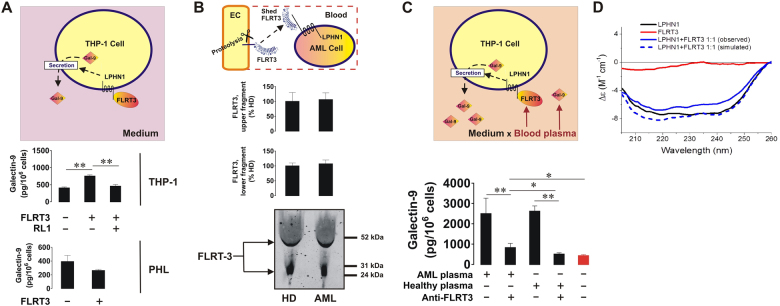
As reported before,4 we observed that exposure of THP-1 AML cells to 10 nM FLRT3 for 16 h resulted in a significant increase in galectin-9 secretion (Fig. 2a). This effect was not detectable in primary healthy human leukocytes (Fig. 2a). Importantly, 1 h pre-exposure of THP-1 cells to rabbit polyclonal antibody recognizing LPHN1 (clone name RL19) prior to the 16-h treatment with 10 nM FLRT3 attenuated FLRT3-induced galectin-9 release, confirming the involvement of LPHN1 in this process (Fig. 2a). The antibody employed specifically recognized target molecules on the surface of THP-1 cells (Supplementary figure 2). We used the mouse neuroblastoma cell line NB2A, which does not express LPHN1,10 as a negative control and measured the interaction of the antibody with the cell surface using a Li-Cor on-cell assay as described in the Materials and Methods (please see supplementary information). Exposure of THP-1 cells to 1 µg/ml RL1 for 16 h did not affect galectin-9 secretion levels (data not shown), suggesting that this antibody does not exert an LPHN1 agonistic effect.
Fig. 2.
FLRT3 induces galectin-9 secretion in AML cells in a LPHN1-dependent manner. THP-1 cells and PHL were exposed to 10 nM human recombinant FLRT3 for 16 h, followed by detection of secreted galectin-9 by ELISA. In THP-1 cells, the treatment was performed with or without 1 h pre-exposure to 1 µg/ml RL1 anti-LPHN1 polyclonal antibody (a). The levels of released FLRT3 fragments were analyzed in the blood plasma from healthy donors and AML patients using Western blot (b). THP-1 cells were exposed for 16 h to 10% blood plasma either from healthy donors or AML patients, with or without pretreatment with FLRT3-neutralizing antibody. The levels of secreted galectin-9 were analyzed using ELISA. (c) Secondary structure and conformational changes of LPHN1, FLRT3, and the complex of the two proteins were characterized using SRCD spectroscopy as outlined in the Materials and Methods (d). Images are shown from one representative experiment of four replicates, all of which showed similar results. Data are shown as the mean values ± SEM from four independent experiments; *p < 0.05; **p < 0.01 vs. control
Interestingly, we found that blood plasma from both healthy donors and AML patients contains approximately equal amounts of secreted FLRT3 (most likely by proteolytic shedding) with a molecular weight of approximately 55 kDa (which corresponds to the molecular weight of FLRT3 shed from the cell surface by proteinases11). Another specific band was observed at ∼27–28 kDa, which most likely corresponds to a smaller cleavage fragment of the FLRT3 extracellular domain (Fig. 2b). The amounts of this smaller fragment were also equal in blood plasma from healthy donors and AML patients (Fig. 2b). To explore which blood plasma-based ligands can induce galectin-9 secretion in AML cells, we cultured THP-1 cells in RPMI 1640 medium containing antibiotics (as outlined in Materials and Methods—see supplementary information) and replacing the 10% fetal bovine serum (FBS) with blood plasma from either healthy donors or AML patients. Cells were incubated for 16 h with or without a 30-min preincubation with anti-FLRT3 antibody to neutralize FLRT3 activity. Galectin-9 secretion levels were significantly higher in the presence of either sources of human blood plasma than in the presence of FBS (negative control). Anti-FLRT3 antibody attenuated galectin-9 secretion (Fig. 2c). The binding of LPHN1 and FLRT3 was further confirmed using SRCD spectroscopy. We found that the two proteins interact with each other with high affinity such that a conformational change is induced in both proteins, as seen from far UV synchrotron radiation circular dichroism (SRCD) spectra (Fig. 2d). This is further confirmation of the high-affinity interaction of LPHN1 and FLRT3 observed in previous studies8 using different techniques.
Taken together, our results demonstrate, for the first time, that cortisol upregulates LPHN1 expression at the transcriptional level, thus stimulating its translation in human AML cells. AML leads to decreased blood plasma glucose levels,5 which normally leads to upregulation of the secretion of corticotropin-releasing hormone (CTRH) from the hypothalamus.12 CTRH induces the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland.12 ACTH upregulates cortisol production in the adrenal cortex.12 Cortisol is then employed by AML cells. In healthy human leukocytes, cortisol is not capable of inducing LPHN1 transcription/translation, possibly because of gene repression. Interaction of AML cell-derived LPHN1 with released FLRT3 available in blood plasma facilitates the secretion of galectin-9. The latter protects AML cells against immune attack, which could otherwise be performed by NK cells or cytotoxic T cells (Supplementary figure 3). Importantly, LPHN1 fragments are present in the blood plasma from AML patients but not from healthy donors. These fragments were detectable by both Western blot analysis and ELISA, which indicates the possibility of detecting these fragments for a rapid AML diagnosis, although differential verification tests have yet to be performed. Our results suggest a fundamentally novel mechanism used by AML cells to progress the disease. They use a common endogenous human hormone (cortisol) to induce LPHN1 expression by employing a widely available ligand (FLRT3, which is always present in blood plasma) to escape host immune surveillance. Thus, AML cells engage crucial functional systems of the human body to support their survival and attenuate the anticancer activities of cytotoxic lymphoid cells. Our work indicates that galectin-9 and secreted FLRT3 are the most promising targets for anti-AML immune therapy.
Electronic supplementary material
Acknowledgements
This work was supported by a Daphne Jackson Trust postdoctoral fellowship (to I. M. Y.) and the University of Kent Faculty of Sciences Research Fund (to V. S.). We thank Diamond Light Source for access to B23 beamline (SM12578).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0053-8) contains supplementary material.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Wyszynski RW, Gibbs BF, Varani L, Iannotta D, Sumbayev VV. Interleukin-1 beta induces the expression and production of stem cell factor by epithelial cells: crucial involvement of the PI-3K/mTOR pathway and HIF-1 transcription complex. Cell. Mol. Immunol. 2016;13:47–56. doi: 10.1038/cmi.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasinska IM, et al. High mobility group box 1 (HMGB1) acts as an “alarmin” to promote acute myeloid leukaemia progression. OncoImmunology. 2018;7:e1438109. doi: 10.1080/2162402X.2018.1438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goncalves Silva I, et al. The tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves Silva I, et al. The immune receptor Tim-3 acts as a trafficker in a Tim-3/galectin-9 autocrine loop in human myeloid leukemia cells. OncoImmunology. 2016;5:e1195535. doi: 10.1080/2162402X.2016.1195535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumbayev VV, et al. Expression of functional neuronal receptor latrophilin 1 in human acute myeloid leukaemia cells. Oncotarget. 2016;7:45575–45583. doi: 10.18632/oncotarget.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiga A, et al. Transcriptome analysis of G protein-coupled receptors in distinct genetic subgroups of acute myeloid leukemia: identification of potential disease-specific targets. Blood Cancer J. 2016;6:e431. doi: 10.1038/bcj.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucard AA, Maxeiner S, Sudhof TC. Latrophilins function as heterophilic cell-adhesion molecules by binding to teneurins: regulation by alternative splicing. J. Biol. Chem. 2014;289:387–402. doi: 10.1074/jbc.M113.504779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volynski KE, et al. Latrophilin, neurexin, and their signaling-deficient mutants facilitate α-latrotoxin insertion into membranes but are not involved in pore formation. J. Biol. Chem. 2000;275:41175–41183. doi: 10.1074/jbc.M005857200. [DOI] [PubMed] [Google Scholar]
- 10.Silva JP, et al. Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl Acad. Sci. USA. 2011;108:12113–12118. doi: 10.1073/pnas.1019434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi S, et al. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011;30:2920–2933. doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabata I, Ogita F, Miyachi M, Shibayama H. Effect of low blood glucose on plasma CRF, ACTH, and cortisol during prolonged physical exercise. J. Appl. Physiol. 1991;71:1807–1812. doi: 10.1152/jappl.1991.71.5.1807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.