Pregnancy is a choreographed physiological phenomenon that involves maternal-fetal cross-talk. Elucidating the cellular and molecular mechanisms involved in this communication will allow us to develop therapies for gestational complications. Little is known about the role of natural killer (NK) cells in the uterine microenvironment. In a recent study, Fu et al. (2017) used state-of-art technologies and showed that NK cells stimulate fetal growth via growth-promoting factors.
Strikingly, transplantation of uterine NK cells from normal mice reversed the negative pregnancy outcome in NK cell-deficient transgenic mice, as well as in aged mice. This new knowledge provides insight into the role of NK cells in the uterine microenvironment during pregnancy.
Approximately 20% of pregnancies result in embryonic death losses in mammals.1 Pregnancy is a complex physiological process influenced by multiple genetic, epigenetic, and microenvironmental factors, such as hormonal balance, immune tolerance, and angiogenesis. Fetal growth during development demands the expansion of cells, commonly followed by cellular specialization and the generation of distinct organs. Information on the control of this growth remains very limited. Effective regulation of fetal development is essential for successful mammalian reproduction. Disturbances in this process may lead to several gestational complications including infertility, fetal growth restriction, spontaneous abortion, and premature delivery. Survival of the embryo in the uterus is dependent on multiple cellular and molecular events that occur in this microenvironment.2 Hormones, cytokines, homeotic proteins, growth factors, and morphogens produced by a variety of cell types may influence pregnancy outcomes. The specific cells and the underlying mechanisms that directly contribute to the outcome of a pregnancy remain completely unknown. The lack of detailed knowledge about the cellular contributors that mediate pregnancy failures restricts the design of effective treatments.
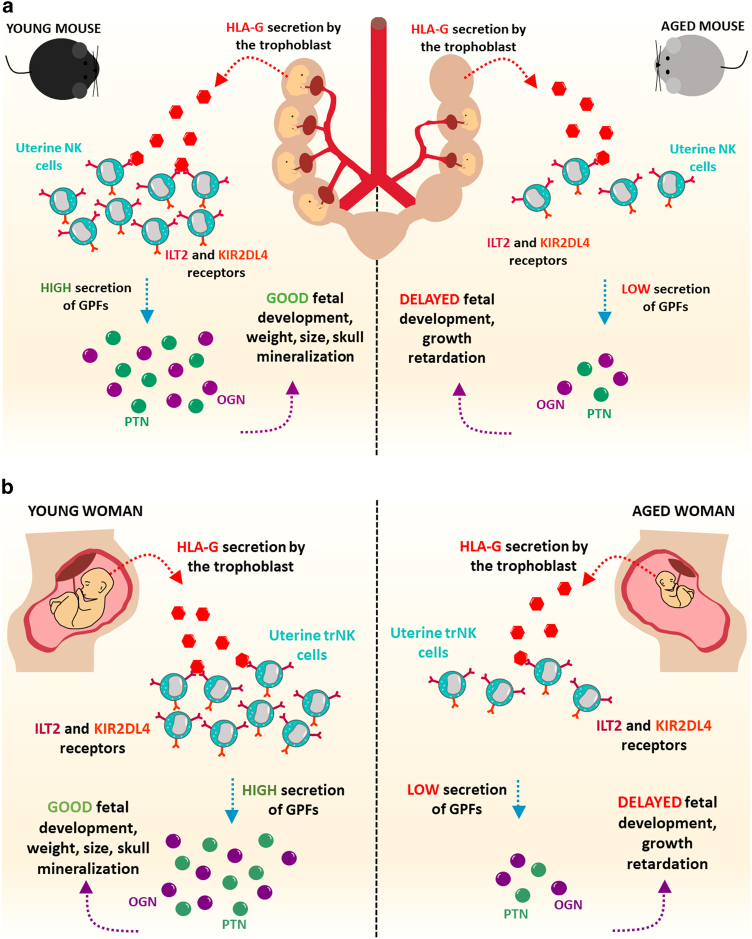
Understanding the regulation of fetal growth that occurs during pregnancy is a central question in reproduction research. The uterine microenvironment during pregnancy is highly complex and is replete with different cell populations, including activated immune cells. Most of the uterine immune cells identified during pregnancy are natural killer (NK) cells.3 These cells were classically named due to their capacity to eliminate target cells without previous priming in a “natural” manner. As an important component of innate lymphoid cells, NK cells are capable of perceiving and eradicating malignant cells and cells infected with intracellular pathogens, such as viruses, parasites, and bacteria.4 NK cells play essential roles in coordinating innate and adaptive immune responses by releasing a variety of molecules, including IFNγ, TGFβ, and IL10. The function of these cells in the uterus during normal pregnancy remains to be elucidated. In a recent article in Immunity, Fu et al.5 investigated the role of uterine NK cells during pregnancy. By using state-of-the-art techniques, the authors showed that NK cells induce fetal growth in the uterus. Fu and colleagues reported a NK cell subpopulation that expressed CD49a and Eomes produce growth-promoting factors such as pleiotrophin, osteoglycin, and osteopontin. The authors also showed using co-culture systems that a cross-talk between HLA-G on fetal trophoblasts and KIR2DL4 on uterine NK cells is needed to promote the secretion of growth-promoting factors by NK cells.5 Additionally, uterine NK cells from patients who experienced recurrent spontaneous abortion significantly decreased the expression of growth-promoting factors. To examine whether insufficient secretion of growth-promoting factors from NK cells in the uterus affects fetal growth, Fu and colleagues analyzed fetal growth in mice with genetically ablated NK cells. These experiments revealed that absence of maternal NK cells influenced the pregnancy outcome, leading to defective fetal development.5 Interestingly, aging was characterized by the decrease in the number of uterine CD49a-expressing NK cells and in the expression of growth-promoting factors (Fig. 1). Strikingly, a transfer of uterine NK cells from normal mice reversed the pregnancy outcome in NK cell-deficient transgenic mouse models, as well as in aged mice. Notably, NK cells from mice that do not express the pleiotrophin, osteoglycin, and osteopontin growth-promoting factors were not able to reverse the impairment in fetal development.5 Here, we discuss these findings and evaluate the recent advances in our understanding of the biology of NK cells in the uterus.
Fig. 1.
Uterine NK cells stimulate fetal growth via growth-promoting factors. NK cells are abundant in the uterus during pregnancy. The study by Fu et al.5 now suggests a novel role for NK cells in the uterine microenvironment. Uterine NK cells produce growth-promoting factors. During aging, the secretion of growth-promoting factors by NK cells is reduced and affects fetal development. Future studies will reveal in detail the cellular and molecular mechanisms involved in fetal growth in the uterine microenvironment
Perspectives/future directions
Pregnancy occurs in a complex cellular microenvironment in the uterus and contains a variety of cell types among hematopoietic and non-hematopoietic cells. In addition to NK cells, several other cell types have been reported to be abundant in the uterus, such as macrophages, dendritic cells, mast cells, T-regulatory cells, and others.6 Recent observations after genetic alterations in the uterus illustrate the relevance of specific cell types in the uterine microenvironment during pregnancy. Similar to what was done by Fu et al.5 for NK cells, the removal of other cell types significantly influenced the establishment of pregnancy as well.6 Interestingly, while the presence of some cells is important for a successful pregnancy, other cells may affect it negatively. For instance, genetically induced neutrophilia protects against pregnancy loss.7 Thus, a balanced microenvironment guarantees a successful pregnancy. Nevertheless, our understanding of the cross-talk between the different components of the uterine microenvironment during pregnancy is still limited. How the combination of molecules derived from various cell types affects fetal growth remains to be elucidated. Therefore, future studies should reveal the relationship between NK cells and other cell populations and how they influence fetal growth. Different cell populations may also have distinct functions at various time points during pregnancy and, by responding to changes in the microenvironment, each cell may secrete a different set of mediators.
An aberrance in the uterine vascular structure is associated with gestational pathologies, including intrauterine growth restriction, gestational diabetes, and preeclampsia. Pregnancy is characterized by a coordinated remodeling of the uterine vasculature, as well as the creation of new blood vessels (angiogenesis) in a coordinated way. These processes involve a number of cell populations, such as endothelial cells, pericytes, smooth muscle cells, fibroblasts, and others.8–12 How NK cells participate in uterine angiogenesis and communicate with these other cell types during pregnancy remains to be explored. Interestingly, uterine NK cells may produce pro-angiogenic factors such as vascular endothelial growth factor.13 The analysis of vascular beds after genetic disturbances in NK cells may address these questions.
Uterine NK cells release multiple bioactive molecules, including IL8, vascular endothelial growth factor, stromal cell-derived factor-1, interferon gamma-inducible protein-10, and others, which help in tissue building and remodeling.13 Thus, NK cells can induce a fetal growth-promoting milieu in the uterus. Fu et al.5 suggest that NK cells-derived growth-promoting factors contribute to fetal development. Nonetheless, several cell types may secrete growth-promoting factors. Transgenic mouse models have been widely used to study distinct cell populations within distinct tissue-microenvironments.14,15 The ability of not only eliminating cells but also deleting single genes in specific cellular populations in adult mice has allowed us to answer specific questions regarding the roles of molecules derived from different cell subsets in the regulation of several physiologic processes. The exact molecular mechanisms involved in fetal growth regulation by NK cells in vivo are yet not completely clear and will need to be revealed in future studies. Growth-promoting factors have not been conditionally deleted from uterine NK cells or from other possible sources, so there is no direct evidence that NK cells are the only/main functionally important source of these mediators for fetal growth. The generation of pleiotrophin-, osteoglycin-, and osteopontin-floxed mice crossed with NK cell-specific inducible CreER driver will allow us to specifically delete these growth-promoting factors in NK cells in vivo. In addition to studies in genetic mouse models, transcriptomic and single-NK cell analyses represent fundamental tools that will help us understand the role of NK cells in the uterine microenvironment during different stages of pregnancy. Moreover, are NK cells an important source for the production of other molecules for fetal development as well?
The findings from Fu et al.5 are based on the data obtained from Nfil3 knockout mice, a genetic model for NK cell deficiency, which was used to study the effect of NK cells on fetal growth during pregnancy. However, the basic leucine zipper transcription factor NFIL3, also known as E4BP4, has been implicated as an important regulator in other cells as well. Abnormalities in other cell populations were previously described in these mice that were not necessarily related to the absence of NK cells, such as the impairment in the development of CD8a dendritic cells, in cytokine production by CD4-expressing T cells, and in IgE class-switching in B cells.16 Interestingly, Nfil3 is also expressed in some nerve subtypes.17 Thus, Nfil3 knockout mice may have alterations in other cellular components of the uterine microenvironment that are important for fetal growth in addition to NK cells. This feature should be examined in future studies.
Importantly, how exactly the growth-promoting factors derived from NK cells affect fetal growth remains unclear. Which fetal cells are affected? Is this a direct effect, or do these mediators activate other maternal cells that promote fetal growth? Importantly, NK cells have been suggested to interact with stem cells and may alter their behavior.18 Do mediators derived from uterine NK cells affect the behavior of fetal stem cells? NK cell functional biology seems to be more complex than previously believed. Interestingly, although trophoblasts are known to control NK cell activation during pregnancy,19 what triggers NK cells to produce fetal growth-promoting factors remains questionable. Future in-depth work aiming to understand the behavior of NK cells in the uterine microenvironment will lead to additional insight about the roles of uterine NK cells in vivo.
Adult NK cells combat cancer, and major efforts are currently being done to exploit their properties in the clinic.11,20 Thus, tumoral NK cells likely do not promote the tissue growth that uterine NK cells induce during fetal development. When NK cells acquire and lose the capacity for producing growth-promoting factors remains to be elucidated. Are NK cells that promote tumor growth via growth-promoting factors present in the malignant cancer microenvironment?
In conclusion, the study by Fu et al.5 reveal a novel important regulatory role of uterine NK cells during fetal development. However, our understanding of the cross-talk between different cell types present in the uterine microenvironment remains limited, and the complexity of these interactions in distinct physiologic and pathologic conditions should be elucidated in future studies.
Acknowledgements
A.B. is supported by a grant from Instituto Serrapilheira/Serra-1708-15285, a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016), a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570-16)], and a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313-16)]. A.M. is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD).
Competing interests
The authors declare no competing interests.
References
- 1.Bazer FW, et al. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol. Hum. Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birbrair A. Stem cell microenvironments and beyond. Adv. Exp. Med. Biol. 2017;1041:1–3. doi: 10.1007/978-3-319-69194-7_1. [DOI] [PubMed] [Google Scholar]
- 3.Jabrane-Ferrat N, Siewiera J. The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology. 2014;141:490–497. doi: 10.1111/imm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padro Dietz C, Luong A. Innate lymphoid cells: the innate counterpart to T helper cells. Adv. Otorhinolaryngol. 2016;79:58–68. doi: 10.1159/000445130. [DOI] [PubMed] [Google Scholar]
- 5.Fu B, et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 2017;47:1100–1113. doi: 10.1016/j.immuni.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Teles A, et al. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation. Front. Immunol. 2013;4:158. doi: 10.3389/fimmu.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizugishi K, et al. Sphingolipid pathway regulates innate immune responses at the fetomaternal interface during pregnancy. J. Biol. Chem. 2015;290:2053–2068. doi: 10.1074/jbc.M114.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birbrair A, et al. Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias Moura Prazeres PH, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev. Biol. 2017;427:6–11. doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo, P. O. et al. Pericytes modulate myelination in the central nervous system. J. Cell. Physiol.10.1002/jcp.26348 (2017). [DOI] [PMC free article] [PubMed]
- 11.Paiva AE, et al. Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia. 2017;19:928–931. doi: 10.1016/j.neo.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa MA, et al. Pericytes constrict blood vessels after myocardial ischemia. J. Mol. Cell Cardiol. 2018;116:1–4. doi: 10.1016/j.yjmcc.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011;32:517–523. doi: 10.1016/j.it.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Andreotti JP, Lousado L, Magno LAV, Birbrair A. Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell. 2017;21:293–294. doi: 10.1016/j.stem.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra DAP, et al. Adipocytes role in the bone marrow niche. Cytom. A. 2017;93:167–171. doi: 10.1002/cyto.a.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motomura Y, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat. Immunol. 2011;12:450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacGillavry HD, et al. NFIL3 and cAMP response element-binding protein form a transcriptional feedforward loop that controls neuronal regeneration-associated gene expression. J. Neurosci. 2009;29:15542–15550. doi: 10.1523/JNEUROSCI.3938-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewett A, Man YG, Tseng HC. Dual functions of natural killer cells in selection and differentiation of stem cells; role in regulation of inflammation and regeneration of tissues. J. Cancer. 2013;4:12–24. doi: 10.7150/jca.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Human dNK cell function is differentially regulated by extrinsic cellular engagement and intrinsic activating receptors in first and second trimester pregnancy. Cell Mol. Immunol. 2017;14:203–213. doi: 10.1038/cmi.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sena, I. F. G. et al. Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med.10.1002/cam4.1375 (2018). [DOI] [PMC free article] [PubMed]