Abstract
Camouflage has been a textbook example of natural selection and adaptation since the time of the earliest evolutionists. However, aside from correlational evidence and studies using artificial dummy prey, experiments directly showing that better camouflaged prey to predator vision are at reduced risk of attack are lacking. Here, we show that the level of camouflage achieved through colour adjustments towards the appearance of seaweed habitats is adaptive in reducing predation pressure in the prawn Hippolyte obliquimanus. Digital image analysis and visual modelling of a fish predator (seahorse) predicted that brown prawns would be imperfectly concealed against both brown and red seaweed respectively, whereas pink prawns should be well camouflaged only in red weed. Predation trials with captive seahorses (Hippocampus reidi), coupled with high-speed video analyses, closely matched model predictions: predation rates were similar for brown prawns between seaweed types, but pink individuals were attacked significantly less on red than brown weed. Our work provides some of the clearest direct evidence to date that colour polymorphism and colour change provides a clear adaptive advantage for camouflage, and also highlights how this can be asymmetric across morphs and habitats (i.e. dependent on the specific background-morph combination).
Introduction
The study of animal coloration has fascinated evolutionary biologists for centuries and provided important evidence of adaptation and natural selection1,2. Colour attributes may modulate individual fitness in many different ways, playing a crucial role in behavioural processes ranging from courtship and mate selection to predator deterrence through visual warning cues3. Furthermore, many animals spanning a wide array of taxonomic groups take advantage of their colour patterns for concealment against the surrounding environment3,4, mainly by adopting a camouflage strategy known as background matching4. In this type of camouflage, better concealed individuals are less frequently detected by visual predators and therefore their survival chances are higher5. This is a fundamental prediction of camouflage theory but, despite several emblematic cases consensually considered key examples of natural selection6–9, appropriate experimental evidence of the adaptive function of camouflage remains remarkably rare.
A substantial body of previous work has used artificial dummy prey10,11 or computer-generated stimuli12,13 to test the survival advantage of camouflage in the laboratory or in the field. Other studies, such as the classic example of camouflage and industrial melanism in the peppered moth (Biston betularia)6, have used correlational evidence, often based on morph-specific recapture rates, or artifical prey targets9 to support the hypothesis that better camouflaged individuals are less frequently attacked by predators. More recently, with a better understanding of the anatomy of predator eyes, spectral sensitivity and visual modelling, different studies have estimated how individuals are camouflaged based to the view of predators through the use of spectrometry14–18 or digital imagery19,20. However, while all these studies comprise important evidence that individuals are efficiently concealed against the substrate, no study has directly quantified how closely differently coloured individuals match their background to predator eyes, and then how matching effectively reduces predation rates in natural conditions. As such, the most basic, yet fundamental prediction of camouflage theory, is still poorly validated21.
Camouflage through colour change is commonplace in the animal kingdom and may be achieved over different time scales; from responses of less than a minute, when individuals are moving through a patchy background, to ontogenetic shifts over months or years, accompanying the transition between nursery and adult habitats21. In general terms, colour change is basically mediated by different endocrine and cellular processes, usually guided by vision, promoting modifications on the state and abundance of pigment-containing chromatophore cells21,22. Physiological colour changes refer to the dispersal or aggregation of pigments within chromatophores and determines patterns of fast colour change, within seconds or minutes, such as those observed in cephalopods23 or chameleons24. Slower morphological changes over days, weeks or months21,22 are more common and imply alterations in the quantity and proportion of chromatophore types and pigment content. Colour-changing species make ideal systems to investigate the adaptive value of camouflage21, because they allow testing the importance of colour adjustments of immigrant individuals upon contact with novel habitat, and also whether survival advantages of adjusted individuals are symmetrical between habitats. Despite its potential to unravel important ecological and evolutionary processes, suitable tests of the survival advantage of camouflage in colour-changing species are still rare. Some studies have used vision models to assess changes in concealment20,25, but they have not confirmed modelling outcomes with predation trials. Other studies include tethering or predator-exclusion experiments, but they have not modelled prey camouflage to the vision of predators26,27.
The marine prawn Hippolyte obliquimanus (Decapoda: Caridae) is a common seaweed-dwelling species found in shallow vegetated areas along the western Atlantic coast, from the Caribbean to Southern Brazil28. This species is polymorphic in colour, with individuals presenting homogeneous coloration that can be brown, yellow, green, red or pink, or comprising partially or fully transparent forms marked with stripes or spots29. Prawn polymorphism has been thought to function as protective coloration and to provide camouflage against the seaweed types where prawns live. Optimal concealment should be important in reducing both the detection and consumption of prawns by visual fish predators, especially those living in close association with seaweed, such as seahorses, gobies and blennies30,31. In Southeast Brazil, prawns exhibiting solid colour patterns on a range of brown to pink tones are commonly found associated with the brown seaweed Sargassum furcatum and the red weed Galaxaura marginata29 (Fig. 1). Both morphs are capable of changing their colour in the direction of their host substrates over a period of a few days32, but changes are more remarkable and prawns obtained better concealment when kept in the less intricate red seaweed Galaxaura32. Although based on colour reflectance alone, holding no relationship with any specific visual system33, those results are consistent with the hypothesis that camouflage through colour change is more important in the less structured habitat where shelter is limited (Galaxaura), compared to the physically more complex habitat (Sargassum) where refuges are more abundant and background matching probably less critical32. Although this species is widely distributed along the Central and South America28, there are no studies testing whether prawns from other regions and living on substrates of different coloration are also capable of changes to their colour and camouflage against variable backgrounds.
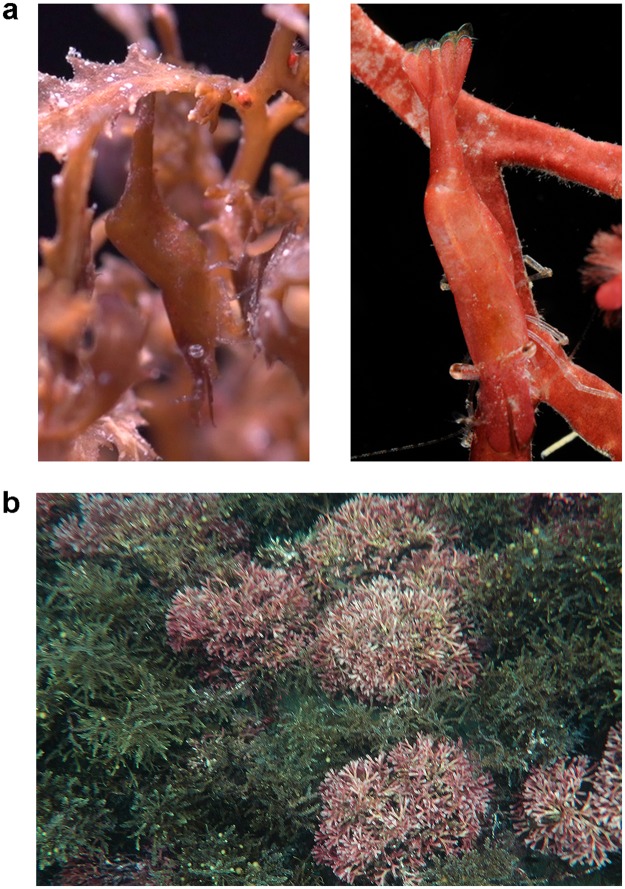
Figure 1.
Hippolyte obliquimanus colour morphs and seaweed habitats. (a) Brown (left) and pink (right) prawns resembling the colour of the brown seaweed Sargassum furcatum and the red-pink seaweed Galaxaura marginata, respectively. (b) Sargassum and Galaxaura canopies as commonly observed in shallow rocky reefs along the South-eastern Brazilian coast.
Here we tested the adaptive value of colour change and camouflage in H. obliquimanus prawns. First, we described colour variation within and between morphs to test whether ‘pink’ and ‘brown’ individuals can actually be viewed as distinct categories, and compare the colour of prawns and their host seaweed habitats to verify how closely they resemble their background. Based on their likelihood to remain unnoticed by a seahorse predator, which exhibits colour vision and detects prey primarily using visual cues31,34, we next quantified the camouflage of prawn morphs on both the host and the alternative seaweed habitat using image analyses and visual modelling. This translates in nature to the capacity of individuals to conceal in primary habitat, where they have remained long enough for colour adjustments to take place, and in secondary habitat shortly upon arrival. Finally, we tested model predictions in a manipulative experiment using real prey and predators. Considering previous results on habitat-specific prawn camouflage based on general colour reflectance32, we tested the hypothesis that the survival advantage of camouflage through colour change is dependent on the seaweed habitat, with much reduced detection and predation rates on individuals adjusting their coloration to the red seaweed Galaxaura compared to those shifting towards the brown seaweed Sargassum.
Results and Discussion
Our results validate the distinction of brown and pink prawns and their segregation between habitats, reinforcing the need to examine the adaptive value of camouflage separately in brown and red seaweed canopies. Principal component analyses applied on standardised seahorse cone catch values of prawns and seaweed indicate that ‘pink’ and ‘brown’ morphs of the prawn Hippolyte obliquimanus are clearly discrete colour entities to both the vision of humans and seahorses, and confirm that prawns tend to adjust their colour to the host seaweed since prawns categorized as pink and brown cohesively clustered with the seaweeds Galaxaura and Sargassum respectively (Fig. 2). Discriminant function analyses further validated the prawn classification, as all individuals were correctly reassigned to their morphs, and further supported the correspondence of prawn morphs to seaweed species, as 55 out of 60 prawns (92%) were correctly linked to their host weed. The few exceptions were invariably ‘brown’ prawns lying closer to the red Galaxaura than to the brown Sargassum pattern (crosses in Fig. 2). In fact, the wider spread of brown individuals in Fig. 2 indicates an overall less precise physiological response of prawns acclimating to Sargassum, and provides first evidence for less effective camouflage in these individuals compared to prawns established in Galaxaura.
Figure 2.
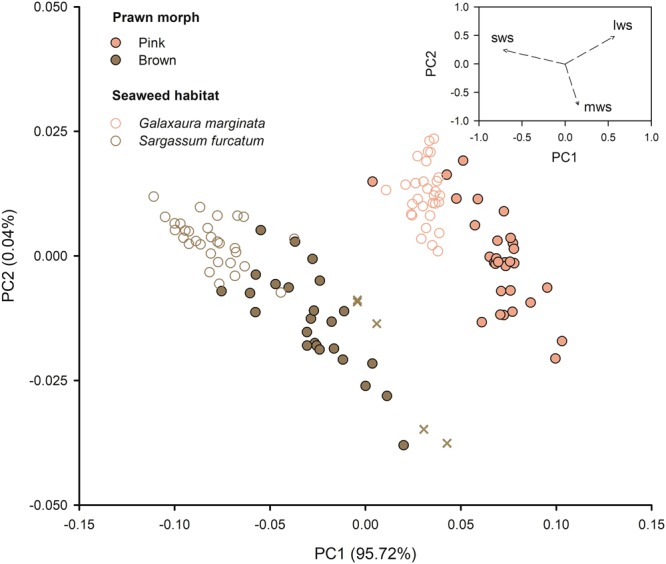
Background resemblance of prawn morphs against seaweeds. Principal Component Analysis applied to seahorse Hippocampus subelongatus cone catches showing colour variation of Hippolyte obliquimanus colour morphs (‘pink’ and ‘brown’ to the human vision) and seaweed habitats (red Galaxaura marginata and brown Sargassum furcatum). Percentage values correspond to the total variation explained by each component. The upper-right indent panel indicates that the shortwave colour channel (sws) is the main responsible for the segregation of groups. Brown crosses indicate the few (n = 5) cases in which prawn colour resemblance was closer to the alternative rather than to the host habitat colour (all ‘brown’ individuals which were actually closer to G. marginata). Sws, mws and lws stand for short, medium and long-wave sensitivity channels.
Predator discrimination of prawn morphs further suggests that any advantages of camouflage through colour change may be modest in Sargassum, but important in Galaxaura. Here, we used the discrimination model of Vorobyev and Osorio35 for colour and luminance and infer prey detectability based on “Just-Noticeable Differences” (JNDs) to seahorse vision. Briefly, prey are predicted to be discriminated from the background for JND values higher than 1, with detection chances increasing beyond that threshold level, even under unfavourable viewing conditions36. Contrasts of colour JNDs between prawns and background habitats are morph-specific, as indicated by the significant interaction term in Table 1. Namely, the colour discrimination of pink prawns in Galaxaura (mean JND ± SE; 1.99 ± 0.17) is much lower than in Sargassum (7.57 ± 0.28; Fig. 3a), while brown prawns were similarly discriminated in both algal habitats, above the colour detection threshold (3.24 ± 0.40; Fig. 3a). In other words, colour change should lead to superior camouflage and lower detection rates in Galaxaura but not in Sargassum (see how both prawn morphs and seaweed look like in the view of seahorses in the Supplementary Fig. S1). It is important to note that JND variation was lowest for pink prawns in Galaxaura and highest for brown morphs in Sargassum, further indicating that improved concealment to seaweed background relies on a precise physiological response leading to a standardised colour pattern. The markedly right-skewed distribution of JND values for brown prawns in Sargassum suggests that the poorer camouflage in this habitat is due to the relatively few individuals attaining exceedingly high JNDs (Fig. 3a). Results on luminance contrasts were less informative because they were consistently much higher than detection thresholds across level combinations of factors ‘prawn morph’ and ‘seaweed habitat’ (mean JND ± SE; 6.63 ± 0.62), and therefore are not likely to modulate any predator effects. The significant p-level of the interaction term (p = 0.046, Table 1) is attributed to morph-dependent habitat differences, with brown prawns showing lower JNDs in Sargassum (6.08 ± 1.20) than in Galaxaura (9.65 ± 1.41), and pink prawns showing similar JNDs between seaweed habitats (5.41 ± 0.74). Although being consistently lower for pink prawns on both habitats, all luminance contrasts were much higher than the putative threshold for detection, indicating that seahorses probably did not use this channel for detecting their prey and primarily base their hunting behaviour on colour cues34. However, we note that the achromatic version of the receptor noise model is much less tested than the chromatic model (the original model originally disregarded achromatic information altogether)35, and the mechanism of achromatic perception in fish is often poorly known and variable. Therefore, caution should be used with interpreting the overall magnitude of the luminance JND values. Additional behavioural experiments are necessary to understand the importance of both chromatic and achromatic signals in the visual repertoire of this predator37.
Table 1.
Summary results of prawn camouflage against seaweed backgrounds based on seahorse vision.
| Source of variation | df | Colour JNDs | Luminance JNDs | ||||
|---|---|---|---|---|---|---|---|
| MS | F | p | MS | F | p | ||
| Prawn morph – M | 1 | 35.7 | 21.50 | <0.001 | 92.0 | 4.42 | 0.040 |
| Seaweed habitat – S | 1 | 86.6 | 52.08 | <0.001 | 20.4 | 0.98 | 0.327 |
| M × S | 1 | 150.4 | 90.43 | <0.001 | 86.4 | 4.15 | 0.046 |
| Error | 56 | 1.7 | 20.8 | ||||
| Cochran’s C = 0.541; p < 0.01 | Cochran’s C = 0.356; p > 0.05 | ||||||
Results of two-way analyses of variance testing differences in “just-noticeable differences” (JNDs) for colour and luminance measurements, according to combinations of Hippolyte obliquimanus colour morphs (‘brown’, ‘pink’) and seaweed backgrounds (Galaxaura marginata, Sargassum furcatum). Cochran’s C: Cochran statistic testing variance heterogeneity.
Figure 3.
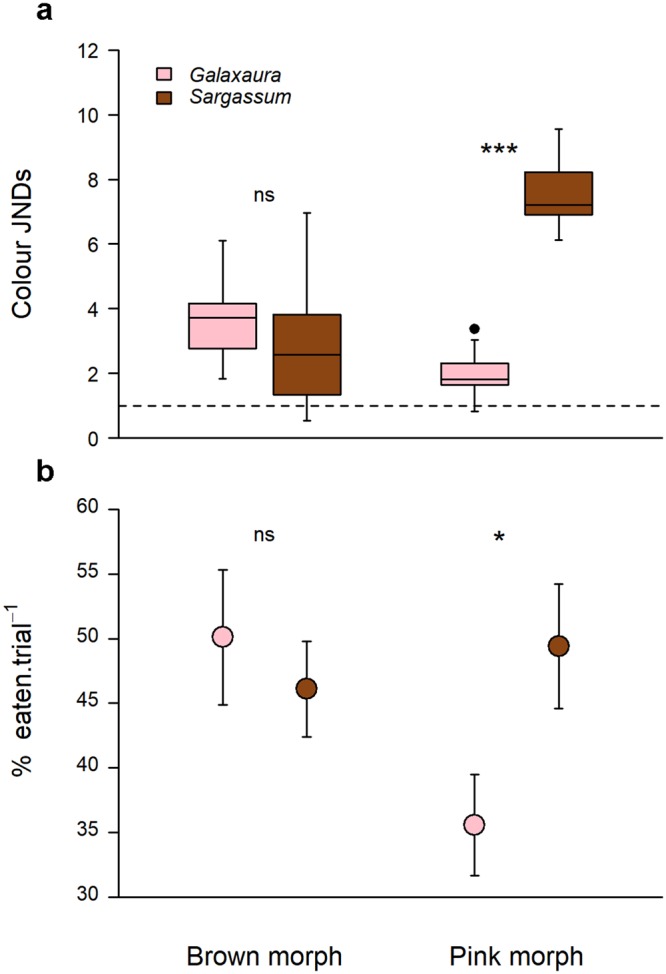
The adaptive value of camouflage in Hippolyte obliquimanus prawns. (a) Seahorse vision discrimination (as ‘just noticeable differences’; JNDs) of prawn morphs against seaweed habitats. Boxes display medians and inter-quartile ranges (IQRs), whiskers represent lowest and highest values within 1.5∗IQRs, and black filled circles represent outliers. The dashed line (JND = 1) indicates the threshold for visual discrimination of prawns by seahorses. ns: not significant; ***p < 0.001. (b) Seahorse predation rates, as the percentage of individuals consumed in 2 h trials (mean ± SE), on brown and pink prawn morphs when placed in Galaxaura and Sargassum habitats. ns: not significant; *p < 0.05.
Results of predation trials closely corresponded to colour JND modelling, thus supporting the adaptive value of camouflage through colour change as a mechanism to reduce predation rates on the prawn Hippolyte obliquimanus (Fig. 3). Habitat-dependent predation on prawn morphs is backed by the significance of the interaction term of the linear model examined (Table 2): seahorses Hippocampus reidi equally preyed on brown prawns held at the two seaweed habitats (mean ± SE; Sargassum: 46.1 ± 3.7%; Galaxaura: 50.1 ± 5.2%), but predation rates on pink individuals were reduced to almost 35% in Galaxaura compared to Sargassum (Sargassum: 49.4 ± 4.8%; Galaxaura: 35.6 ± 3.9%), indicating that colour change towards the background was efficient in the red but not in the brown seaweed environment (Fig. 3b). It is important to note that in spite of their much higher JNDs (Fig. 3a) pink prawns on Sargassum were eaten at similar rates than brown prawns on either habitat (Fig. 3b), suggesting that detection and predation rates would be high and fairly constant at JNDs over 3 or 4 (i.e. beyond the detection threshold). Interestingly, consumption rates were very consistent among seahorse individuals, as indicated by the non-significant random factor ‘seahorse ID’ nested in the morph*habitat interaction (Table 2). Positive effects of colour adjustments on prey survival may thus be pervasive, dampening any potential behavioural syndromes underlying individual-based differences among predators38,39. Consistent results among individual predators probably reflect specialized hunting techniques, involving a very specific pattern of prey spotting, approaching and striking common to all seahorse individuals (Fig. 4). High-speed video recordings (480 fps) taken during experimental trials confirmed that seahorses use primarily visual cues for prey detection, taking on average 4.28 ± 0.82 s (mean ± SD) to strike after first visual contact (Supplementary Video S1). Once detected, seahorses move slowly without losing eye contact until they reach a distance to prey that can be covered during a very fast strike (less than 0.063 s; Fig. 4). Still, our observations show that strikes involve an upward rotation of the head (frame 2 to 3), slightly increasing the path travelled by the mouth as revealed by models of seahorse feeding biomechanics40. According to these authors, an extended strike distance allows seahorses to probe a larger volume of water and hence locate prey more precisely, which could explain the very high percentage of successful attacks (90%) observed in our trials.
Table 2.
Summary results of seahorse predation on prawn colour morphs.
| Source of variation | Predation rate | |||
|---|---|---|---|---|
| df | MS | F | p | |
| Prawn morph – M | 1 | 0.031 | 3.39 | 0.103 |
| Seaweed habitat – S | 1 | 0.020 | 2.22 | 0.174 |
| Seahorse ID (M × S) | 8 | 0.009 | 0.43 | 0.888 |
| M × S | 1 | 0.076 | 8.38 | 0.020 |
| Error | 24 | 0.021 | ||
| Cochran’s C = 0.324; p > 0.05 | ||||
Results of mixed-model analysis of variance testing contrasts of seahorse Hippocampus reidi predation rates on prawn Hippolyte obliquimanus colour morphs maintained in different seaweed habitats (as percentage of individuals consumed by seahorses over 2 h trials). The factors ‘prawn morph’ and ‘seaweed habitat’ are fixed, while ‘seahorse ID’ is random and nested in the interaction of main factors. Cochran’s C: Cochran statistic testing variance heterogeneity.
Figure 4.
Sequence of still images from high-speed video footage (480 fps), over less than 1.5 s, showing a seahorse preying on a prawn camouflaged on brown seaweed Sargassum furcatum. The yellow arrow indicates the prawn position in the first frame. Note that the attack took shorter than 0.06 s (frame 2 to 3).
In this study we present novel evidence showing the adaptive value of camouflage through colour change. A wide range of recent studies have tested how types and levels of camouflage affect detection, but predominantly using artificial (human-made) ‘prey’ presented to human and other animal observers10,41. Furthermore, while iconic studies of the peppered moth quantified morph-specific survival advantage in different habitats6, and recent studies of wild birds have shown that camouflage level correlates with survival in the field19, no study has yet directly demonstrated that camouflage level, to predator vision, directly influences individuals’ survival chances. Here, the visual model we used closely predicted specific camouflage success for each H. obliquimanus colour morph on each seaweed background in terms of colour discrimination/detection to a seahorse predator. Therefore, our study is the first to quantitatively demonstrate that predation risk in an animal is directly related to predator-perceived levels of camouflage, and concurrently validates widely used but seldom tested models of visual discrimination. Although focusing in a specific seahorse predator, which exhibits colour vision34 and uses visual cues to detect prey31 (Fig. 4, Supplementary Video S1), our results should be generalizable to other fish potentially hunting H. obliquimanus, including gobies, blennies and pinfish species which are frequently found associated with Sargassum and Galaxaura canopies30,42. There is no specific information regarding the visual system or the existence of colour vision in these alternative predators, but studies on similar species have suggested that most of them use colour cues to detect prey37,43–46 and therefore would likely exhibit similar behaviour to seahorses and be potentially deceived by prawn camouflage.
In our study we found that the survival advantage of camouflage through colour change is asymmetric across different habitats. Colour concealment was shown to be adaptive for prawns shifting to pink in Galaxaura but not for prawns changing to shades of brown in Sargassum; a result consistent to our initial predictions. Adequate shelter and extensive foraging grounds provided by the more intricate architecture of Sargassum and accompanying epiphytic algae47,48 may be more important than concealing coloration to maintain high prawn densities in the brown weed habitat29. Differently, lower prey density and reduced shelter supply - two conditions known to increase per-capita predation pressure49,50 - make lower prey detection rates critical in the less complex Galaxaura canopy. In summertime, Sargassum blooms, becomes primary habitat and hosts very large prawn aggregations29, but by winter time the brown-weed have decayed51,52 and the perennial Galaxaura becomes a more important habitat. Fast colour change allowing camouflage in the red weed canopy32 may be therefore of paramount importance by increasing survival rates of overwintering individuals and hence ensuring population stability through time.
In conclusion, by integrating the more recent area of image analysis and visual modelling with classical behavioural experiments our study highlights an important future avenue of research in both sensory and behavioural ecology. The results we obtained represent a fundamental starting point for understanding the adaptive value of camouflage – one of the most common anti-predator strategies observed in nature – for many different species. In addition, colour change for camouflage is widespread in nature, being common in animals from both terrestrial and aquatic habitats21, which permits the generalization of our findings to different species living on heterogeneous habitats, such as many insects53, crabs54–56, fish25,57 and lizards58. It is important to appreciate, however, that both colour change and camouflage may differentially affect the survival of individuals in each of the different habitats where they live, since each background type will exhibit specific requirements that may change the close relationship between animal and substrate coloration.
Methods
Field sampling
Seaweeds Sargassum furcatum and Galaxaura marginata were collected by skin diving in the vicinities of the Centre for Marine Biology, São Sebastião, SP, Brazil (23°49′40′S; 45°25′22′W) during the spring of 2015 and summer of 2016. Prawns were sorted from seaweeds (as in29) and visually classified as brown or pink morphs, which proved to be a simple method for an accurate assignment32 (Fig. 2). Before being used in experiments, prawns and seaweeds were kept in indoor tanks (30 × 20 × 10 cm) at ambient temperature (~27 °C), supplied filtered running seawater and aeration. A random set of prawn and seaweed samples was separated for image analyses and visual modelling to measure the potential of prawn camouflage against algal habitats. Another set was used for predation experiments to test predictions of modelling results.
Prawn camouflage
Pieces of seaweed and living prawns (n = 30 for each seaweed species and prawn morph) were photographed in an acrylic chamber (5 × 5 cm) using a Nikon D80 digital camera, coupled with a Nikkor 60 mm lens and a UV-blocker filter (62 mm, Tiffen, USA). The camera was set up to capture only visible light (400 to 700 nm) because objects exhibited low overall UV reflection (as observed in images acquired with a UV-sensitive camera), and because fish preying on prawns are likely less sensitive to UV light43,59. Images were taken in RAW format, with manual white balancing and fixed aperture settings to avoid over-exposition60, and included black (7.5%) and white (91%) Spectralon reflectance targets (Labsphere, Congleton, UK) following the current standard procedure61. Illumination was provided by one human visible Colour Arc Lamp (70 W, 6500 K Iwasaki), coupled to a polytetrafluoroethylene diffuser cylinder around the photography chamber to ensure even lightning. Images were successfully linearized (R² ≥ 0.997 for all camera channels), using the curves modelled from eight Spectralon reflectance standards (from 2 to 99% reflectance) to correct for camera non-linear pixel responses to light intensity60,61. Photographs were equalized for changes in light conditions using 7.5% and 91% standards and saved as 32-bit multispectral images. All routines were performed using customized functions in the ImageJ software61,62.
Prawn and seaweed colour was analysed based on a seahorse vision model, since seahorses are abundant in seaweed beds63 and commonly prey on caridean prawns64, including H. obliquimanus31. Since there is no information on the visual system of the local seahorse predator Hippocampus reidi, the spectral sensitivities of the closely related species Hippocampus subelongatus59 were used for modelling. We assumed the visual capacity of the two seahorse species are similar as they both live in similar green-water vegetated habitats59,65. H. subelongatus has spectral peaks for single cones at 467 nm (shortwave sensitivity, SWS), and for double cones at 522 nm (mediumwave, MWS), 537 nm (medium-longwave, M-LWS), and 560 nm (longwave, LWS)59. A 50% light transmission cut-off at 425 nm was incorporated59, and a D65 standard irradiance spectrum was used as a measure of incident illumination66, compatible to the restricted shallow-water environment, of only a few cm, where predator-prey visual interactions take place (Fig. 4). We assumed that colour vision is encoded by independent spectral channels in double cones (DCs), as reported for the reef fish Rhinecanthus aculeatus45. Compared to other fish which have only one or two pigments in their DCs45,67, the seahorse H. subelongatus exhibits an unusual DC configuration, with three different cone types accounting for the medium-long wave sensitivity59. We thus assumed that H. subelongatus has a trichromatic visual system, but still modelled colour vision as encoded by SWS single cones plus LWS DCs, and either MWS DCs (‘Model 1’) or M-LWS DCs (‘Model 2’). We only report results for ‘Model 1’ because outputs of both models were very similar (Supplementary Table S1). Tetrachromatic vision was discarded because similar MWS and M-LWS cone types were present in DCs, strongly suggesting that one of them is used for luminance (i.e. achromatic) contrast59. Polynomial mapping was used to convert images from the camera colour space60,68 into values of seahorse cone catches, closely corresponding to spectrometry techniques19,20,25,61. Before building the model, we calculated the spectral sensitivity curves of our equipment20,69, and obtained the following sensitivity range and spectral peaks: SW; 400–550 nm (peak 472 nm), MW; 420–620 nm (peak 534 nm), LW; 550–700 nm (peak 596 nm).
Visual modelling resulted in multispectral images that were used to estimate photon catch values for each colour channel in the selected regions of interest (ROIs; prawn carapace and abdomen, from the region behind the eyes to the end of the third abdominal somite, avoiding the stomach region, and seaweed fronds). A principal component analysis (PCA) on the covariance matrix of the standardized cone data was used to visualise colour differences between morphs and backgrounds and to determine the channels responsible for clustering. Prawn and seaweed PC scores (PC1 and PC2) were used to create discriminate functions to, respectively, confirm morph classifications and validate the correspondence of morphs to seaweed species. The ‘lda’ function from the MASS library in R70 was used to run discriminant function analyses. A widely implemented log-linear form of colour discrimination model35, which assumes limitation by receptor noise, was used to predict chromatic and achromatic perception as “just noticeable differences” (JNDs). Since behavioural data backing visual discrimination is lacking for H. subelongatus, we used a conservative and frequently adopted Weber fraction value (0.05) for the most abundant cone type66, and set cone proportions to LWS = 0.44, MWS = 1.00, M-LWS = 0.89 and SWS = 0.5659. Colour detection by predators is expected at JNDs higher than 1.0036. We then calculated colour and luminance contrasts in single prawn-seaweed random pairings, resulting in 15 independent JNDs for each prawn morph/seaweed species combination. Colour and luminance JNDs were analysed separately using a 2-way ANOVA, in which factors ‘prawn morph’ (brown or pink) and ‘seaweed type’ (Sargassum or Galaxaura) were fixed and orthogonal. Variances remained heterogeneous for colour JNDs even after log transformation. Still, we proceed with the analysis using raw data because this was a balanced design with a large sample size (n = 15), which makes the test robust to variance heterogeneity71. The Student-Newman-Keuls (SNK) procedure was used for a posteriori comparisons.
Laboratory predation trials
There were different reasons to select seahorses as ideal model predators in this study. First, seahorses are specialised consumers of seaweed-dwelling invertebrates, curling their tail around weed thalli or holdfasts and ambushing prey upon visual detection63. Second, caridean prawns have been ranked first or second in seahorse diet72,73. Regarding our focal species, the prawn Hippolyte obliquimanus is heavily consumed by Hippocampus reidi, preferring this prey to amphipods and brine shrimp31. Third, H. obliquimanus and H. reidi are common species in our study region29,74 and therefore the predator-prey interaction addressed here should be quite frequent at the sampling area.
A set of ten cubic aquaria (25 × 25 × 25 cm), supplied a thin layer of natural sand covering the bottom and constant flow of 5-µm filtered seawater, was maintained in natural temperature (26.5 °C ± 1.1) and salinity (31.1 ± 0.7) conditions. Five of these aquaria were used to maintain stocks of freshly collected seaweeds, prawns and seahorses, and the other five were used for experimental trials. Prawn stocks were kept with their original plant hosts (‘brown’ prawns on Sargassum and ‘pink’ prawns on Galaxaura). Three non-reproductive H. reidi individuals (S1: female, height 11.4 cm; S2: female, height 10.6 cm; S3: male, height 11.4 cm) were collected by snorkelling from seaweed meadows in the same area (ICMBio-approved license #55633-1) and kept in individual tanks where they were fed ad libitum a variety of seaweed-dwelling invertebrates. Predation trials were carried out under natural daylight in aquaria provided with a clump of either Sargassum or Galaxaura (approx. 50 ml), devoid of any mobile epifauna after brief immersion in freshwater. In each tank, 20 individuals of either the brown or pink morph were included and left to acclimatize for 10 minutes before the addition of a single seahorse, initially caged in a 5 mm mesh-bag. After 20 minutes, when all prawns had settled on seaweed, the predator was released and left in tanks for 2 hours. Predation rate was calculated as the proportion of prawns that were consumed until the end of the trial. A maximum of two experimental aquaria were run at the same time and combinations among levels of factors ‘prawn morph’, ‘seaweed habitat’ and ‘seahorse ID’ were randomly replicated in time, three times, summing 36 trials over 1.5 months. The tank used in each trial was also randomly chosen to avoid potential artefacts due to uncontrolled spatial variation of any physical variables within laboratory space. We also certified that seahorses were left without food for at least 20 hours before their use in trials, ensuring that complete gastric evacuation has occurred31. In some trials (n = 10) we used a high-speed camera (Sony NX-FS700R, coupled with a Nikkor 60 mm lens, capturing images at 480 fps) to record seahorse hunting behaviour. All experimental procedures complied with Brazilian ethical standards.
Predation rate was analysed using a specific ANOVA model in which the factors ‘prawn morph’ (brown and pink) and ‘seaweed habitat’ (Sargassum and Galaxaura) were considered fixed and orthogonal. ‘Seahorse ID’ (S1, S2 and S3) was included as a random factor, nested in the interaction of main factors, allowing proper replication and a test for the generality of predation effects. As for JND comparisons, we used the SNK post hoc test to further examine significant sources of variation.
Ethics
Collection of seahorses and their maintenance in the laboratory together with their use in the experiments complied with Brazilian ethical standards and were licensed accordingly [‘Instituto Chico Mendes de Conservação da Biodiversidade’ (ICMBio), license number #55633-1].
Electronic supplementary material
Acknowledgements
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) which granted a PhD fellowship to RCD (#2012/17003-0, #2015/04484-8), and a visiting professor grant to MS (#2015/22258-5). We are grateful to Devi Stuart-Fox and two anonymous reviewers for their valuable comments on an earlier draft of this manuscript, to Jolyon Troscianko for his advice in image analyses and visual modelling, and to Alvaro Migotto for his help in recording high-speed videos. This is a contribution of the Research Centre for Marine Biodiversity of the University of São Paulo (NP‐Biomar/USP).
Author Contributions
R.C.D., M.S. and A.A.V.F. designed the study. R.C.D. obtained the digital images and conducted the predation experiment. R.C.D., M.S. and A.A.V.F. designed the analyses. R.C.D. conducted analyses and together with M.S. and A.A.V.F. wrote the first draft and revised the manuscript.
Data Availability
The data generated and analysed during the current study are available from the corresponding author on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34470-z.
References
- 1.Darwin, C. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, 10.4324/9780203509104 (1859).
- 2.Wallace A. Mimicry and other protective resemblances among animals. Westminster Rev. London Ed. 1867;1(July):1–43. [Google Scholar]
- 3.Caro T, Sherratt TN, Stevens M. The ecology of multiple colour defences. Evol. Ecol. 2016;30:797–809. doi: 10.1007/s10682-016-9854-3. [DOI] [Google Scholar]
- 4.Stevens M, Merilaita S. Animal camouflage: current issues and new perspectives. Philos. Trans. R. Soc. Ser. B, Biol. Sci. 2009;364:423–427. doi: 10.1098/rstb.2008.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. Biol. Sci. 2007;274:1457–64. doi: 10.1098/rspb.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kettlewell H. Selection experiments on industrial melanism in the Lepidoptera. Heredity (Edinb). 1955;9:323–342. doi: 10.1038/hdy.1955.36. [DOI] [Google Scholar]
- 7.Cain AJ, Sheppard PM. Natural Selection in Cepaea. Genetics. 1954;39:89–116. doi: 10.1093/genetics/39.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cott, H. Adaptive coloration in animals. (Methuen, 1940).
- 9.Walton OC, Stevens M. Avian vision models and field experiments determine the survival value of peppered moth camouflage. Commun. Biol. 2018;1:118. doi: 10.1038/s42003-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuthill IC, et al. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. [DOI] [PubMed] [Google Scholar]
- 11.Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF. Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 2003;66:541–550. doi: 10.1006/anbe.2003.2235. [DOI] [Google Scholar]
- 12.Karpestam E, Merilaita S, Forsman A. Reduced predation risk for melanistic pygmy grasshoppers in post-fire environments. Ecol. Evol. 2012;2:2204–2212. doi: 10.1002/ece3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond AB, Kamil AC. Visual predators select for crypticity and polymorphism in virtual prey. Nature. 2002;415:609–613. doi: 10.1038/415609a. [DOI] [PubMed] [Google Scholar]
- 14.Théry M, Casas J. Predator and prey views of spider camouflage. Nature. 2002;415:133. doi: 10.1038/415133a. [DOI] [PubMed] [Google Scholar]
- 15.Russell BJ, Dierssen HM. Use of hyperspectral imagery to assess cryptic color matching in Sargassum associated crabs. PLoS One. 2015;10:4–11. doi: 10.1371/journal.pone.0136260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cournoyer BL, Cohen JH. Cryptic coloration as a predator avoidance strategy in seagrass arrow shrimp colormorphs. J. Exp. Mar. Bio. Ecol. 2011;402:27–34. doi: 10.1016/j.jembe.2011.03.011. [DOI] [Google Scholar]
- 17.Hanlon RT, Chiao CC, Mäthger LM, Marshall NJ. A fish-eye view of cuttlefish camouflage using in situ spectrometry. Biol. J. Linn. Soc. 2013;109:535–551. doi: 10.1111/bij.12071. [DOI] [Google Scholar]
- 18.Chiao C, Wickiser JK, Allen JJ, Genter B, Hanlon RT. Hyperspectral imaging of cuttlefish camouflage indicates good color match in the eyes of fish predators. Proc. Natl. Acad. Sci. USA. 2009;108:9148–9153. doi: 10.1073/pnas.1019090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN. Camouflage predicts survival in ground-nesting birds. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens M, Lown AE, Wood LE. Color change and camouflage in juvenile shore crabs Carcinus maenas. Front. Ecol. Evol. 2014;2:1–14. doi: 10.3389/fevo.2014.00014. [DOI] [Google Scholar]
- 21.Duarte RC, Flores AAV, Stevens M. Camouflage through colour change: mechanisms, adaptive value and ecological significance. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160342. doi: 10.1098/rstb.2016.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagnara, J. T. & Hadley, M. E. Chromatophores and color change: a comparative physiology of animal pigmentation. (Prentice-Hall, 1973).
- 23.Hanlon R. Cephalopod dynamic camouflage. Curr. Biol. 2007;17:400–404. doi: 10.1016/j.cub.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Ligon RA, McCartney KL. Biochemical regulation of pigment motility in vertebrate chromatophores: a review of physiological color change mechanisms. Curr. Zool. 2016;62:237–252. doi: 10.1093/cz/zow051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens M, Lown AE, Denton AM. Rockpool gobies change colour for camouflage. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hultgren KM, Mittelstaedt H. Color change in a marine isopod is adaptive in reducing predation. Curr. Zool. 2015;61:739–748. doi: 10.1093/czoolo/61.4.739. [DOI] [Google Scholar]
- 27.Hultgren KM, Stachowicz JJ. Alternative camouflage strategies mediate predation risk among closely related co-occurring kelp crabs. Oecologia. 2008;155:519–528. doi: 10.1007/s00442-007-0926-5. [DOI] [PubMed] [Google Scholar]
- 28.Udekem d’Acoz C. Redescription of Hippolyte obliquimanus Dana, 1852, and comparison with Hippolyte williamsi Schmitt, 1924 (Decapoda, Caridea) Crustaceana. 1997;70:469–479. doi: 10.1163/156854097X00050. [DOI] [Google Scholar]
- 29.Duarte RC, Flores AAV. Morph-specific habitat and sex distribution in the caridean shrimp Hippolyte obliquimanus. J. Mar. Biol. Assoc. United Kingdom. 2017;97:235–242. [Google Scholar]
- 30.Dubiaski‐Silva J, Masunari S. Natural diet of fish and crabs associated with the phytal community of Sargassum cymosum C. Agardh, 1820 (Phaeophyta, Fucales) at Ponta das Garoupas, Bombinhas, Santa Catarina State, Brazil. J. Nat. Hist. 2008;42:1907–1922. doi: 10.1080/00222930802126896. [DOI] [Google Scholar]
- 31.Felício AKC, Rosa IL, Souto A, Freitas RHA. Feeding behavior of the longsnout seahorse Hippocampus reidi Ginsburg, 1933. J. Ethol. 2006;24:219–225. doi: 10.1007/s10164-005-0189-8. [DOI] [Google Scholar]
- 32.Duarte RC, Stevens M, Flores AAV. Shape, colour plasticity, and habitat use indicate morph-specific camouflage strategies in a marine shrimp. BMC Evol. Biol. 2016;16:218. doi: 10.1186/s12862-016-0796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens M. Avian vision and egg colouration: concepts and measurements. Avian Biol. Res. 2011;4:168–184. doi: 10.3184/175815511X13207790177958. [DOI] [Google Scholar]
- 34.Lee HR, Bumsted Obrien KM. Morphological and behavioral limit of visual resolution in temperate (Hippocampus abdominalis) and tropical (Hippocampus taeniopterus) seahorses. Vis. Neurosci. 2011;28:351–360. doi: 10.1017/S0952523811000149. [DOI] [PubMed] [Google Scholar]
- 35.Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. London Ser. B - Biol. Sci. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- 37.Kelber A, Vorobyev M, Osorio D. Animal colour vision - behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/S1464793102005985. [DOI] [PubMed] [Google Scholar]
- 38.Webster MM, Ward AJW, Hart PJB. Individual boldness affects interspecific interactions in sticklebacks. Behav. Ecol. Sociobiol. 2009;63:511–520. doi: 10.1007/s00265-008-0685-2. [DOI] [Google Scholar]
- 39.Sweeney K, et al. Predator and prey activity levels jointly influence the outcome of long-term foraging bouts. Behav. Ecol. 2013;24:1205–1210. doi: 10.1093/beheco/art052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Wassenbergh S, Roos G, Ferry L. An adaptive explanation for the horse-like shape of seahorses. Nat. Commun. 2011;2:162–165. doi: 10.1038/ncomms1159. [DOI] [PubMed] [Google Scholar]
- 41.Karpestam E, Merilaita S, Forsman A. Detection experiments with humans implicate visual predation as a driver of colour polymorphism dynamics in pygmy grasshoppers. BMC Ecol. 2013;13:17. doi: 10.1186/1472-6785-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ornellas AB, Coutinho R, Ornellas AB, Coutinho R, Coutinho R. Spatial and temporal patterns of distribution and abundance of a tropical fish assemblage in a seasonal Sargassum bed, Cabo Frio Island, Brazil. J. Fish Biol. 1998;53:198–208. [Google Scholar]
- 43.Utne-Palm AC, Bowmaker JK. Spectral sensitivity of the two-spotted goby Gobiusculus flavescens (Fabricius): a physiological and behavioural study. J. Exp. Bol. 2006;209:2034–41. doi: 10.1242/jeb.02171. [DOI] [PubMed] [Google Scholar]
- 44.Cheney KL, Skogh C, Hart NS, Marshall NJ. Mimicry, colour forms and spectral sensitivity of the bluestriped fangblenny, Plagiotremus rhinorhynchos. Proc. Biol. Sci. 2009;276:1565–1573. doi: 10.1098/rspb.2008.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pignatelli V, Champ C, Marshall J, Vorobyev M. Double cones are used for colour discrimination in the reef fish, Rhinecanthus aculeatus. Biol. Lett. 2010;6:537–539. doi: 10.1098/rsbl.2009.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guthrie, D. M. Role of vision in fish behaviour. In Behaviour of Teleost Fishes (ed. Picher, T. J.) 89–121 (Chapman & Hall, 1993).
- 47.Tanaka MO, Leite FPP. Spatial scaling in the distribution of macrofauna associated with Sargassum stenophyllum (Mertens) Martius: analyses of faunal groups, gammarid life habits, and assemblage structure. J. Exp. Mar. Bio. Ecol. 2003;293:1–22. doi: 10.1016/S0022-0981(03)00233-8. [DOI] [Google Scholar]
- 48.Duarte, R. C., Flores, A. A. V., Vinagre, C. & Leal, M. C. Habitat-dependent niche partitioning between colour morphs of the algal-dwelling shrimp Hippolyte obliquimanus. Mar. Biol. 164 (2017).
- 49.Anderson TW. Predator responses, prey refuges, and density-dependent mortality of a marine fish. Ecology. 2001;82:245–257. doi: 10.1890/0012-9658(2001)082[0245:PRPRAD]2.0.CO;2. [DOI] [Google Scholar]
- 50.White JW, Warner RR. Safety in numbers and the spatial scaling of density-dependent mortality in a coral reef fish. Ecology. 2007;88:3044–3054. doi: 10.1890/06-1949.1. [DOI] [PubMed] [Google Scholar]
- 51.Jacobucci GB, Tanaka MO, Leite FPP. Factors influencing temporal variation of a Sargassum filipendula (Phaeophyta: Fucales) bed in a subtropical shore. J. Mar. Biol. Assoc. United Kingdom. 2009;89:315. doi: 10.1017/S0025315409002306. [DOI] [Google Scholar]
- 52.Leite F, Turra A. Temporal variation in Sargassum biomass, Hypnea epiphytism and associated fauna. Brazilian Arch. Biol. Technol. 2003;46:665–671. doi: 10.1590/S1516-89132003000400021. [DOI] [Google Scholar]
- 53.Eacock A, Rowland HM, Edmonds N, Saccheri IJ. Colour change of twig-mimicking peppered moth larvae is a continuous reaction norm that increases camouflage against avian predators. PeerJ. 2017;5:e3999. doi: 10.7717/peerj.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caro T. The functional significance of coloration in crabs. Biol. J. Linn. Soc. 2018;124:1–10. doi: 10.1093/biolinnean/bly021. [DOI] [Google Scholar]
- 55.Nokelainen O, Stevens M, Caro T. Colour polymorphism in the coconut crab (Birgus latro) Evol. Ecol. 2018;32:75–88. doi: 10.1007/s10682-017-9924-1. [DOI] [Google Scholar]
- 56.Detto T, Hemmi JM, Backwell PRY. Colouration and colour changes of the fiddler crab, Uca capricornis: A descriptive study. PLoS One. 2008;3:1–10. doi: 10.1371/journal.pone.0001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortesi F, et al. From crypsis to mimicry: changes in colour and the configuration of the visual system during ontogenetic habitat transitions in a coral reef fish. J. Exp. Biol. 2016;219:2545–2558. doi: 10.1242/jeb.139501. [DOI] [PubMed] [Google Scholar]
- 58.Smith KR, et al. Color change for thermoregulation versus camouflage in free-ranging lizards. Am. Nat. 2016;188:668–678. doi: 10.1086/688765. [DOI] [PubMed] [Google Scholar]
- 59.Mosk V, et al. Spectral sensitivities of the seahorses Hippocampus subelongatus and Hippocampus barbouri and the pipefish Stigmatopora argus. Vis. Neurosci. 2007;24:345–354. doi: 10.1017/S0952523807070320. [DOI] [PubMed] [Google Scholar]
- 60.Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007;90:211–237. doi: 10.1111/j.1095-8312.2007.00725.x. [DOI] [Google Scholar]
- 61.Troscianko J, Stevens M. Image Calibration and Analysis Toolbox - a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 2015;6:1–32. doi: 10.1111/2041-210X.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasband, W. ImageJ [online] (1997).
- 63.Foster S, Vincent A. Life history and ecology of seahorses: implications for conservation and management. J. Fish Biol. 2004;65:1–61. doi: 10.1111/j.0022-1112.2004.00429.x. [DOI] [Google Scholar]
- 64.Kendrick AJ, Hyndes GA. Variations in the dietary compositions of morphologically diverse syngnathid fishes. Environ. Biol. Fishes. 2005;72:415–427. doi: 10.1007/s10641-004-2597-y. [DOI] [Google Scholar]
- 65.Rosa IL, et al. Population characteristics, space use and habitat associations of the seahorse Hippocampus reidi (Teleostei: Syngnathidae) Neotrop. Ichthyol. 2007;5:405–414. doi: 10.1590/S1679-62252007000300020. [DOI] [Google Scholar]
- 66.Wyszecki, G. & Stiles, W. Colour science: concepts and methods, quantitative data and formulae. (Wiley-Interscience, 2000).
- 67.Cortesi F, et al. Phenotypic plasticity confers multiple fitness benefits to a mimic. Curr. Biol. 2015;25:949–954. doi: 10.1016/j.cub.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Westland, S. & Ripamonti, C. Computational Color Science. (John Wiley & Sons Ltd., 2004).
- 69.Pike TW. Using digital cameras to investigate animal colouration: estimating sensor sensitivity functions. Behav. Ecol. Sociobiol. 2011;65:849–858. doi: 10.1007/s00265-010-1097-7. [DOI] [Google Scholar]
- 70.Venables, W. & Ripley, B. Modern Applied Statistics with S. (Springer, 2002).
- 71.Underwood, A. Experiments in ecology: their logical design and interpretation using analysis of variance. (Cambridge University Press, 1997).
- 72.Yip MY, Lim ACO, Chong VC, Lawson JM, Foster SJ. Food and feeding habits of the seahorses Hippocampus spinosissimus and Hippocampus trimaculatus (Malaysia) J. Mar. Biol. Assoc. United Kingdom. 2015;95:1033–1040. doi: 10.1017/S0025315414001660. [DOI] [Google Scholar]
- 73.Woods CMC. Natural diet of the seahorse Hippocampus abdominalis. New Zeal. J. Mar. Freshw. Res. 2002;36:655–660. doi: 10.1080/00288330.2002.9517121. [DOI] [Google Scholar]
- 74.Freret-Meurer NV, Andreata JV. Field studies of a Brazilian seahorse population, Hippocampus reidi Ginsburg, 1933. Brazilian Arch. Biol. Technol. 2008;51:743–751. doi: 10.1590/S1516-89132008000400012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analysed during the current study are available from the corresponding author on request.