Abstract
Objective
Low urine pH is associated with several metabolic diseases, such as dyslipidemia, diabetes, and metabolic syndrome. However, the association between low urine pH and non-alcoholic fatty liver disease (NAFLD) remains unknown. Therefore, we conducted a community-based cross-sectional study to investigate this association.
Methods
Between April 2013 and March 2014, the records of 4,945 Japanese subjects who had undergone annual health checkups were reviewed to identify subjects who met the diagnostic criteria for NAFLD.
Patients
Based on urine pH, the participants were classified into four groups; a low urine pH was defined as ≤5.5. Of the 3,411 subjects who qualified for enrollment, 1,028 met the diagnostic criteria for NAFLD.
Results
The prevalence of NAFLD was significantly increased with decreasing urine pH in both men and women (p<0.01 and p=0.02, respectively). A multivariate analysis, including adjustments for age, metabolic markers, and the renal function, showed a significant association between low urine pH and NAFLD in men and women (odds ratio, 1.37; 95% confidence interval, 1.01-1.85, p=0.04 and odds ratio, 1.73; 95% confidence interval, 1.15-2.62, p<0.01, respectively).
Conclusion
Our study indicates that NAFLD is associated with a low urine pH in both sexes, findings that might help clinicians identify patients at high risk for NAFLD.
Keywords: low urine pH, non-alcoholic fatty liver disease, metabolic disease, community-based study, health checkup
Introduction
Recent lifestyle changes have led to the development of various metabolic diseases (1,2) and problems, including the growth of medical spending and decreased life expectancy (3,4). Non-alcoholic fatty liver disease (NAFLD) is the manifestation of metabolic diseases in the liver (5,6), and untreated NAFLD can progress to non-alcoholic steatohepatitis, cirrhosis, and liver failure (5-9). Furthermore, NAFLD is a risk factor for diabetes (10), dyslipidemia (11), and other metabolic diseases (11,12), and it may complicate cardiovascular (13) and chronic kidney diseases (14). Therefore, the early diagnosis is important in patients with NAFLD.
Urine pH depends on both acid excretion into the urine and the production and excretion of ammonia, which is responsible for urinary buffering. Recently, several studies have shown the association between urine pH and metabolic diseases: the 24-h urine pH is inversely associated with the body mass index (BMI) (15,16). In addition, low urine pH is significantly associated with the waist circumference, homeostasis model assessment of insulin resistance (HOMA-IR), fasting plasma glucose, hemoglobin A1c, triglycerides, high-density lipoprotein cholesterol, and metabolic syndrome (17-19).
Although NAFLD is closely related to these diseases (10-14), its relationship with urine pH is unknown. Therefore, we conducted this community-based study to investigate this association. The results of this study might help clinicians identify patients at high risk for NAFLD.
Materials and Methods
This community-based cross-sectional study was conducted among 4,945 Japanese subjects (2,490 men and 2,455 women) who had undergone annual health checkups at the Ehime General Health Care Association between April 2013 and March 2014. Data from the health checkups included a review of each subject's medical history, prescription medications, a questionnaire to assess the frequencies and quantities of alcohol consumption, a physical examination, and routine biochemical assessment. Body weight and height were measured without shoes or heavy outer clothing for the calculation of the BMI (kg/m2). Blood pressure was measured using an automated sphygmomanometer while the subjects were in the seated position. Waist circumference and blood samples were collected in a ≥10 hours fasting condition, and an analysis of total cholesterol, triglycerides, fasting plasma glucose, and uric acid was performed. Other blood chemistry variables, including serum creatinine, alanine aminotransferase (ALT), hepatitis B surface antigen (HBs-Ag), and hepatitis C antibody (anti-HCV), were also measured. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese Society of Nephrology equation (20). Midstream urine samples were collected in the morning and measured using dipstick testing. Fatty liver was diagnosed using abdominal ultrasonography (Hitachi Avius, Tokyo, Japan) by trained technicians who were unaware of the subjects' individual data. Of the four known criteria for fatty liver identification using ultrasonography (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring) (21), only the evidence of hepatorenal contrast and liver brightness were required for this diagnosis.
The study protocol was approved by the Ehime University Hospital Research Ethics Board (Approval ID #110405, University Hospital Medical Information Network ID: UMIN000011953) according to the Declaration of Helsinki, as revised in 2013, and all study procedures were conducted in accordance with guidelines on good clinical practices, as well as local ethical and legal requirements.
After the assessment of the medical and medication histories and laboratory data at the checkup, 1,534 subjects who met one or more of the following exclusion criteria were excluded from the study: 1) urine pH of ≥7.5 (n=351), which was considered an abnormally high pH indicating a urinary infection (22); 2) eGFR of <60 mL/min/1.73 m2 (n=318); 3) positive serology for HBs-Ag (n=56) or anti-HCV (n=36); 4) current use of anti-diabetic (n=115), lipid-lowering (n=321), or anti-hypertensive (n=458) regimens; and/or 5) consumption of alcohol (≥30 g/day in men or ≥20 g/day in women; in accordance with a previous study) (23) (n=398). Ultimately, the data of 3,411 subjects (1,595 men and 1,816 women) were analyzed (Fig. 1). We defined a low urine pH as ≤5.5, with reference to previous reports (22,24).
Figure 1.
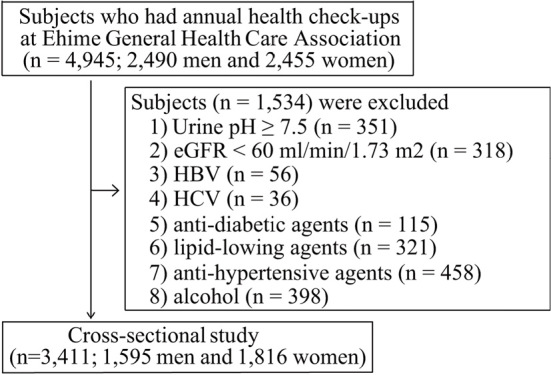
A schematic flow chart of subject selection. EGFR: estimated glomerular filtration rate, HBV: hepatitis B virus, HCV: hepatitis C virus
All of the subjects were assigned a numerical code to maintain anonymity, and all data were stored in a secure database. The JMP software program, version 11 (SAS Institute Japan, Tokyo, Japan), was used for all statistical analyses. The Mann-Whitney U test was used to analyze between-group differences in the baseline characteristics, such as the age, results of physical examinations, and routine biochemical variables; the chi-squared test was used to analyze the presence or absence of fatty liver; and the Cochran-Armitage test was used to assess trends in the incidence rates of NAFLD according to urine pH. To determine whether or not urine pH was independently associated with the incidence rate of NAFLD, a multivariate analysis was performed using a logistic regression model adjusted for the following variables, which are associated with metabolic disease and the renal function: the age and BMI (Model 1); the age, BMI, waist circumference, systolic blood pressure, total cholesterol, triglycerides, uric acid, creatinine, and fasting plasma glucose (Model 2); and the age, BMI, waist circumference, systolic blood pressure, total cholesterol, triglycerides, uric acid, creatinine, fasting plasma glucose, and ALT (Model 3). All data were expressed as the mean±standard deviation, all p values were 2-tailed, and p<0.05 was considered statistically significant.
Results
Characteristics of the subjects
The characteristics of subjects with low and normal urine pH (pH ≤5.5 and pH >5.5) are shown in Table 1. The low urine pH group had significantly higher uric acid, creatinine, and ALT levels in both sexes, higher levels of triglycerides in men, and lower systolic blood pressures in women than the normal urine pH group (p<0.05).
Table 1.
Baseline Characteristics.
| Men | Women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤pH 5.5 (n=780) |
>pH 5.5 (n=815) |
p value | ≤pH 5.5 (n=1,865) |
>pH 5.5 (n=151) |
p value | |||||||||
| Age | (years) | 45.6±7.9 | 45.5±7.9 | 0.86 | 45.6±8.1 | 44.9±8.1 | 0.02 | |||||||
| BMI | (kg/m2) | 23.7±3.3 | 23.4±3 | 0.26 | 21.4±3.5 | 21.5±3.4 | 0.44 | |||||||
| Waist circumference | (cm) | 83.3±8.9 | 82.7±8.1 | 0.3 | 76.1±9.3 | 76.1±8.8 | 0.94 | |||||||
| Systolic blood pressure | (mmHg) | 111±14.8 | 111.5±15 | 0.58 | 101.5±13.7 | 104.2±15.9 | <0.01 | |||||||
| Total cholesterol | (mg/dL) | 203±31.5 | 201.8±32.1 | 0.32 | 205±32.5 | 202.7±33.8 | 0.06 | |||||||
| Triglycerides | (mg/dL) | 129.1±100.3 | 114.7±74.9 | <0.01 | 76.9±43.9 | 73.1±37.1 | 0.24 | |||||||
| Uric acid | (mg/dL) | 6.3±1.2 | 6.1±1.2 | <0.01 | 4.4±1 | 4.3±0.9 | <0.01 | |||||||
| Creatinine | (mg/dL) | 0.86±0.1 | 0.85±0.1 | 0.02 | 0.64±0.07 | 0.63±0.08 | <0.01 | |||||||
| Fasting plasma glucose | (mg/dL) | 97.7±14.8 | 96.3±12.2 | 0.06 | 90.2±11.5 | 89.5±7.8 | 0.8 | |||||||
| ALT | (U/L) | 28.7±25.6 | 25.3±17 | <0.01 | 17.3±12.5 | 16.3±17.6 | <0.01 | |||||||
| NAFLD | (%) | 44.2 | 36.7 | <0.01 | 19.4 | 14.4 | <0.01 | |||||||
Values are expressed as mean±standard deviation.
For continuous values, differences among groups were assessed using the Mann-Whitney U test. The Chi-square test was employed for comparisons of prevalence.
BMI: body mass index, ALT: alanine aminotransferase, NAFLD: non-alcoholic fatty liver disease
The association between urine pH and NAFLD prevalence
The NAFLD prevalence was greater in the low urine pH group than in the normal pH group (44.2% vs. 36.7% in men, 19.4% vs. 14.4% in women; p<0.01; Table 1). To elucidate the relationship between urine pH and NAFLD, we examined the NAFLD prevalence according to urine pH. As the urine pH decreased, the NAFLD prevalence significantly increased in men (p<0.01; Fig. 2A) as well as in women (p=0.02; Fig. 2B).
Figure 2.
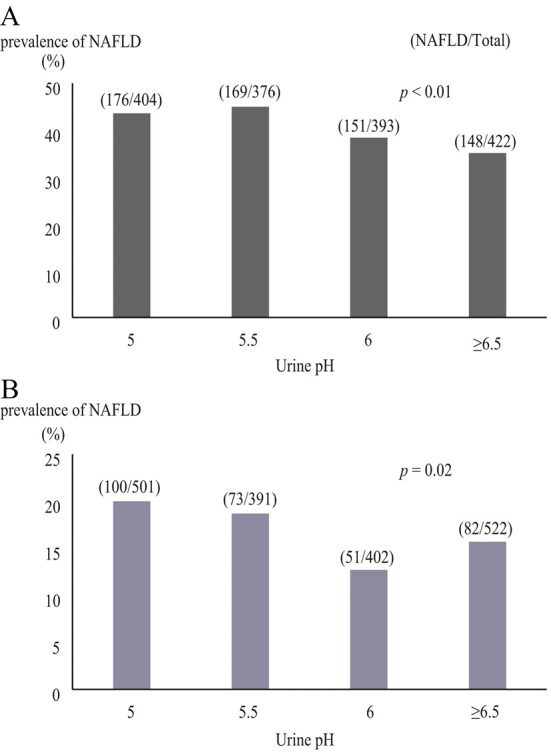
The association between urine pH and the non- alcoholic fatty liver disease prevalence in (A) men, and (B) women. The p value was determined by the Cochran-Armitage test. NAFLD: non-alcoholic fatty liver disease
Factors associated with NAFLD
A univariate analysis showed that low urine pH, age, ALT, and metabolic markers such as the BMI, waist circumference, systolic blood pressure, total cholesterol, triglycerides, uric acid, and fasting plasma glucose were significantly associated with NAFLD in men and women (p<0.01; Table 2).
Table 2.
Results of Univariate Analysis of Associated Factors for NAFLD.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||||||
| Age | (years) | 1.02 (1.01-1.03) | <0.01 | 1.04 (1.03-1.06) | <0.01 | |||||
| BMI | (kg/m2) | 2.27 (2.09-2.47) | <0.01 | 2.17 (2-2.37) | <0.01 | |||||
| Waist circumference | (cm) | 1.34 (1.31-1.38) | <0.01 | 1.34 (1.3-1.38) | <0.01 | |||||
| Systolic blood pressure | (mmHg) | 1.04 (1.03-1.05) | <0.01 | 1.05 (1.04-1.06) | <0.01 | |||||
| Total cholesterol | (mg/dL) | 1.01 (1.01-1.02) | <0.01 | 1.01 (1.009-1.016) | <0.01 | |||||
| Triglycerides | (mg/dL) | 1.01 (1.01-1.01) | <0.01 | 1.03 (1.023-1.031) | <0.01 | |||||
| Uric acid | (mg/dL) | 1.54 (1.41-1.69) | <0.01 | 2.72 (2.35-3.16) | <0.01 | |||||
| Creatinine | (mg/dL) | 0.41 (0.15-1.15) | 0.09 | 0.29 (0.06-1.37) | 0.12 | |||||
| Fasting plasma glucose | (mg/dL) | 1.067 (1.055-1.081) | <0.01 | 1.12 (1.1-1.14) | <0.01 | |||||
| ALT | (U/L) | 1.1 (1.09-1.12) | <0.01 | 1.07 (1.06-1.09) | <0.01 | |||||
| ≤pH 5.5 | (%) | 1.37 (1.12-1.67) | <0.01 | 1.43 (1.12-1.84) | <0.01 | |||||
NAFLD: non-alcoholic fatty liver disease, OR: odds ratio, CI: confidence interval, BMI: body mass index, ALT: alanine aminotransferase
The association of low urine pH with NAFLD
Model 1, adjusted for the age and BMI, showed that low urine pH was significantly associated with NAFLD in men and women (p=0.01 and p<0.01, respectively; Table 3). Model 2 included adjustments for age, metabolic markers, and the renal function and revealed a significant association between low urine pH and NAFLD in men and women (p=0.04 and p<0.01, respectively; Table 3). Model 3 included adjustments for age, metabolic markers, the renal function, and ALT and revealed a significant association between low urine pH and NAFLD in women (p<0.01, respectively; Table 3).
Table 3.
Associations between Low Urinary PH and NAFLD.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||||
| Model 1 | 1.44 (1.09-1.9) | 0.01 | 1.82 (1.27-2.64) | <0.01 | ||||
| Model 2 | 1.37 (1.01-1.85) | 0.04 | 1.73 (1.15-2.62) | <0.01 | ||||
| Model 3 | 1.28 (0.93-1.76) | 0.13 | 1.74 (1.16-2.65) | <0.01 | ||||
Model 1: adjusted for age and BMI.
Model 2: adjusted for age, BMI, waist circumference, systolic blood pressure, total cholesterol, triglycerides, uric acid, creatinine, and fasting plasma glucose.
Model 3: adjusted for age, BMI, waist circumference, systolic blood pressure, total cholesterol, triglycerides, uric acid, creatinine, fasting plasma glucose, and ALT.
NAFLD: non-alcoholic fatty liver disease, OR: odds ratio, CI: confidence interval, BMI: body mass index, ALT: alanine aminotransferase
Characteristics of NAFLD with low urine pH
The characteristics of NAFLD with low and normal urine pH (≤pH 5.5 and > pH 5.5) are shown in Table 4. NAFLD patients with low urine pH had significantly higher ALT levels in both sexes and significantly lower systolic blood pressures and higher fasting plasma glucose levels in women than those with normal urine pH (p<0.05).
Table 4.
Characteristics of NAFLD with Low Urine PH.
| Men | Women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤pH 5.5 (n=345) |
>pH 5.5 (n=299) |
p value | ≤pH 5.5 (n=173) |
>pH 5.5 (n=133) |
p value | |||||||||
| Age | (years) | 46.4±7.3 | 46.2±7.2 | 0.93 | 48.1±7.6 | 47.1±7.4 | 0.18 | |||||||
| BMI | (kg/m2) | 26±3.1 | 25.9±2.8 | 0.75 | 26.2±3.4 | 26.8±4.1 | 0.4 | |||||||
| Waist circumference | (cm) | 89.8±7.6 | 89.5±7.1 | 0.77 | 88.6±7.4 | 89±8.5 | 0.63 | |||||||
| Systolic blood pressure | (mmHg) | 116.1±16.1 | 116.7±15.3 | 0.48 | 111±16.3 | 116.2±18.7 | 0.02 | |||||||
| Total cholesterol | (mg/dL) | 209.9±31 | 210.3±31.2 | 0.74 | 217.6±33.1 | 212.4±33.4 | 0.16 | |||||||
| Triglycerides | (mg/dL) | 164.6±119.6 | 149.3±95 | 0.16 | 118±64.7 | 108±53.1 | 0.36 | |||||||
| Uric acid | (mg/dL) | 6.7±1.2 | 6.5±1.3 | 0.2 | 5.1±1 | 5±1.2 | 0.19 | |||||||
| Fasting plasma glucose | (mg/dL) | 102.3±19 | 100.6±15.7 | 0.36 | 99.5±19.1 | 95±8.7 | 0.03 | |||||||
| ALT | (U/L) | 38.8±33.9 | 35.2±22.9 | <0.01 | 26.5±19.9 | 22.6±13.2 | 0.03 | |||||||
Values are expressed as mean±standard deviation.
For continuous values, differences among groups were assessed using the Mann-Whitney U test.
NAFLD: non-alcoholic fatty liver disease, BMI: body mass index, ALT: alanine aminotransferase
Discussion
In this community-based cross-sectional study, we examined the relationship between low urine pH and NAFLD, with results showing that the NAFLD prevalence increased as urine pH decreased. In addition, urine pH was significantly associated with NAFLD in both sexes. This significant association remained after adjustment for potential metabolic confounders, although the significance in men was lost in a multivariate analysis adjusted for ALT. Furthermore, NAFLD patients with low urine pH had higher ALT levels in both sexes than those with normal urine pH. Our results are supported by those of previous studies that showed a relationship between low urine pH and metabolic disorders, such as a high BMI, abnormal glucose tolerance, and metabolic syndrome, which are associated with NAFLD (11,15-19,25,26).
A urinalysis is a convenient and cheap test routinely conducted at health checkups and in clinical practice on site. Although urine specific gravity, urine glucose, and ketonuria are useful in screening for dehydration, endocrinopathy, kidney disease, and diabetes, the importance of urine pH in metabolic disease has not been elucidated. Several recent studies have demonstrated the association between urine pH and metabolic diseases (11,15-19,25,26). We therefore believe that conducting a simple urinalysis, especially for urine pH, might increase the probability of diagnosing metabolic diseases, including NAFLD.
The association between urine pH and NAFLD might be related to insulin resistance. Abate et al. conducted a study involving 55 healthy volunteers and 13 patients with uric acid nephrolithiasis to examine whether or not insulin resistance is associated with low urine pH using the hyperinsulinemic euglycemic clamp technique, and they reported that insulin resistance contributed to low urine pH (27). Maalouf et al. evaluated the association between metabolic diseases and 24-h urine pH in 148 kidney stone-free adults and showed that low 24-h urine pH was associated with HOMA-IR as well as the BMI, fasting glucose, triglycerides, systolic blood pressure, and high-density lipoprotein cholesterol (17). In addition, experimental studies have shown that insulin increases the production and activity of the Na+/H+ exchanger in renal tubular cell lines (28). In contrast, fatty liver is thought to induce insulin resistance. Patients with fatty liver have elevated levels of hepatokines, such as fetuin-A and selenoprotein P (29,30). These hepatokines are associated with insulin resistance through the inhibition of insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1 (31) as well as the inactivation of adenosine monophosphate-activated protein kinase (32). Furthermore, fatty liver can impair the ability of insulin to activate glycogen synthase (33). Therefore, NAFLD may be associated with low urine pH through the induction of insulin resistance.
Our results showed that the strength of the association between urine pH and NAFLD was higher in women than in men. This association may be due to hormonal changes related to menopause. Kato et al. found that the urine pH of postmenopausal women was lower than that of premenopausal women (34). Furthermore, NAFLD is reported to be twice as common in postmenopausal women as in premenopausal women, and hormonal replacement therapy for postmenopausal women reduces the risk of NAFLD (35). Therefore, there may be a strong relationship between low urine pH and NAFLD in women due to estrogen.
The strengths of our study include the use of a large sample size and the analysis by sex. Furthermore, our research population reflects the Japanese regional general population, as our study used data from the annual checkup offered by the local government. In addition, the NAFLD prevalence obtained in this study was similar to that previously reported (36,37). However, our study had several limitations. First, we measured urine pH using spot urine samples instead of 24-h urine samples. However, previous reports have shown that the pH of a spot urine sample is correlated with that of a 24-h sample (38). Second, we used dipstick testing to measure urine pH. However, dipstick test strips are reported to be as reliable as an electrochemical pH meter (39). Third, we did not examine the presence of factors that affect urine pH, such as renal tubular acidosis, urinary tract infections, chronic diarrheal illness, primary hyperparathyroidism, chronic respiratory diseases, medications, and diet. Fourth, we did not completely exclude from the study patients who might have had liver diseases. Although we measured HBs-Ag and anti-HCV and asked the subjects about their history of liver disease, we did not completely exclude other etiologies, such as autoimmune hepatitis and primary biliary cirrhosis, of which the patients might have been unaware. Finally, the information regarding the alcohol intake was collected via self-report, so misreporting may have been a source of bias.
However, despite these limitations, our study had several important findings. NAFLD was associated with low urine pH in both sexes, and the NAFLD prevalence significantly increased as urine pH decreased. These results might help clinicians conveniently identify patients at high risk of NAFLD.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by a grant-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (JSPS KAKENHI 15K00874 to T.M., 15K09006 to Y.H.), and a research grant from Ehime University.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4-14, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274-285, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 49: 608-612, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 173: 309-314, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917-923, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50: 1844-1850, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 123: 1705-1725, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka Y, Hashimoto E, Yatsuji S, et al. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. J Gastroenterol 39: 1215-1218, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Hashimoto E, Taniai M, et al. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol 37: 154-160, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bae JC, Rhee EJ, Lee WY, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care 34: 727-729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake T, Kumagi T, Hirooka M, et al. Metabolic markers and ALT cutoff level for diagnosing nonalcoholic fatty liver disease: a community-based cross-sectional study. J Gastroenterol 47: 696-703, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Aneni EC, Oni ET, Martin SS, et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J Hypertens 33: 1207-1214, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 13: 1579-1584, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y, Ryu S, Sung E, et al. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism 57: 569-576, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis 48: 905-915, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol 17: 1422-1428, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2: 883-888, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Cho YH, Lee SY, Jeong DW, et al. The association between a low urine pH and the components of metabolic syndrome in the Korean population: Findings based on the 2010 Korea National health and nutrition examination survey. J Res Med Sci 19: 599-604, 2014. [PMC free article] [PubMed] [Google Scholar]
- 19.Otsuki M, Kitamura T, Goya K, et al. Association of urine acidification with visceral obesity and the metabolic syndrome. Endocr J 58: 363-367, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol 38: 954-961, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi N, Fukui M, Tanaka M, et al. Low urine pH is a predictor of chronic kidney disease. Kidney Blood Press Res 35: 77-81, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005-2023, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Hara S, Tsuji H, Ohmoto Y, et al. High serum uric acid level and low urine pH as predictors of metabolic syndrome: a retrospective cohort study in a Japanese urban population. Metabolism 61: 281-288, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Miyake T, Kumagi T, Hirooka M, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol 48: 413-422, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes mellitus. Diabetes Care 38: 1673-1679, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Abate N, Chandalia M, Cabo-Chan AV Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 65: 386-392, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Klisic J, Hu MC, Nief V, et al. Insulin activates Na(+)/H(+) exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol 283: 532-539, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Choi HY, Hwang SY, Lee CH, et al. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab J 37: 63-71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29: 853-857, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Haasemann M, Nawratil P, Müller-Esterl W. Rat tyrosine kinase inhibitor shows sequence similarity to human alpha 2-HS glycoprotein and bovine fetuin. Biochem J 274: 899-902, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misu H, Takamura T, Takayama H, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab 12: 483-495, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345-32353, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Kato Y, Yamaguchi S, Kakizaki H, Yachiku S. Influence of estrus status on urinary chemical parameters related to urolithiasis. Urol Res 33: 476-480, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 122: 1649-1657, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol 47: 586-595, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143: 722-728, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br J Nutr 99: 1335-1343, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Desai RA, Assimos DG. Accuracy of urinary dipstick testing for pH manipulation therapy. J Endourol 22: 1367-1370, 2008. [DOI] [PubMed] [Google Scholar]


