Abstract
A 73-year-old woman with massive ascites associated with a giant hepatic mass accompanied by arterio-portal (AP) shunt was admitted to our hospital. Based on contrast-enhanced computed tomography (CT) and angiography findings, hepatic hemangioma with AP shunt and ascites due to portal hypertension was diagnosed. Transcatheter arterial embolization (TAE) by N-butyl-2-cyanoacrylate (NBCA) was performed without complications. The patient's ascites disappeared, and her liver function test results improved after the treatment. The patient has maintained a steady state for two years. This case indicates that TAE with NBCA is a safe and effective treatment for hepatic hemangioma accompanied by AP shunt.
Keywords: arterio-portal shunt, hepatic hemangioma, ascites, portal hypertension, transcatheter arterial embolization, N-butyl-2-cyanoacrylate
Introduction
Hepatic hemangioma is the most common benign neoplasm of the liver. The reported prevalence at autopsy ranges from 3% to 20% (1). In most cases, hemangiomas are asymptomatic and have no complications. A few cases in adults, however, have presented with spontaneous bleeding, rupture, obstructive jaundice, portal hypertension, and Kasabach-Merritt syndrome (2-6).
Arterio-portal (AP) shunt, a rare vascular disorder with various origins, represents an infrequent cause of portal hypertension (7). With the development of abdominal imaging procedures, AP shunt associated with hemangioma has been shown to be not uncommon (8). While many patients with hepatic hemangioma accompanied by AP shunt are asymptomatic, a few adult cases have been reported to require treatment for severe clinical symptoms (9-11).
We herein report a patient with ascites due to portal hypertension caused by AP shunt associated with hepatic hemangioma who was successfully treated with transcatheter arterial embolization (TAE) by N-butyl-2-cyanoacrylate (NBCA).
Case Report
A 73-year-old woman was referred and admitted to our hospital for the further evaluation of ascites and a giant liver mass suggestive of hepatic hemangioma with portal hypertension. For two months before referral, she had experienced abdominal distension, anorexia, and weight gain. Dynamic computed tomography (CT) in the arterial phase of contrast administration revealed a giant hepatic mass with early peripheral enhancement and progressive globular centripetal filling in the left lobe of the liver, suggesting hemangioma, and also showed AP shunt and massive ascites (Fig. 1). The patient's ascites had not improved despite diuretic therapy with spironolactone (50 mg/day) and furosemide (40 mg/day). Therefore, the patient was referred and admitted to our hospital for further investigation and management. She had no relevant medical history, such as liver dysfunction, abdominal trauma, epistaxis, or other congenital abnormalities. Her family history of hereditary hemorrhagic telangiectasia was negative.
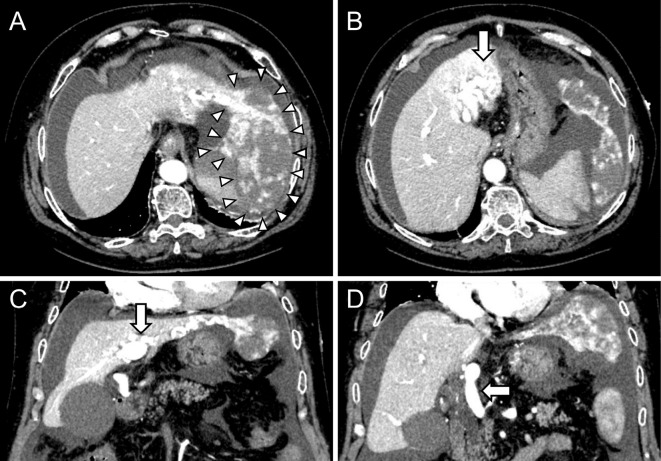
Figure 1.
Dynamic CT images showed a 10-cm pedunculated giant hepatic mass suggesting hemangioma in the left lobe of the liver (arrowheads) with massive ascites (A), an enlarged and tortuous hepatic artery (arrow) (B), an AP shunt (arrow) (C), and retrograde enhancement into the trunk of the portal vein (arrow) (D). AP: arterio-portal
On a physical examination, she had no remarkable findings except for abdominal distension, and telangiectasia was not detected in either the mucosa or skin. Laboratory data on admission showed mild hypoalbuminemia and a slight increase in the serum alanine aminotransferase (ALT) level (Table). The serum tumor marker levels were within the normal range, and positron emission-CT findings were negative. Esophagogastroduodenoscopy showed esophageal varices without a red color sign. Angiography indicated hemangioma in the left lobe in the liver, and retrograde filling of the trunk of the portal vein as hepatofugal flow from the middle hepatic artery and the inferior branch of the left hepatic artery, suggesting an AP communication (Fig. 2A and B). Selective hepatic artery angiogram demonstrated proximal branches of the left hepatic artery to the left portal vein with retrograde flow into the portal vein as hepatofugal flow (Fig. 2C). The right hepatic artery and portal vein showed no abnormal findings. Therefore, we diagnosed the lesion as hepatic hemangioma with AP shunt, which induced portal hypertension following ascites.
Table.
Laboratory Data on Admission.
| Value | Reference range | |||
|---|---|---|---|---|
| White blood cell count (/μL) | 3,920 | 3,500-8,500 | ||
| Hemoglobin (g/dL) | 12.9 | 11.5-15.0 | ||
| Platelet count (104/μL) | 11.6 | 15.0-35.0 | ||
| Total protein (g/dL) | 6.3 | 6.7-8.3 | ||
| Albumin (g/dL) | 3.8 | 4.0-5.5 | ||
| Blood urea nitrogen (mg/dL) | 10 | 8-22 | ||
| Creatinine (mg/dL) | 0.63 | 0.4-17 | ||
| Sodium (mmol/L) | 141 | 138-146 | ||
| Potassium (mmol/L) | 3.2 | 3.6-4.9 | ||
| Chloride (mmol/L) | 103 | 99-109 | ||
| Calcium (mg/dL) | 8.6 | 8.7-10.3 | ||
| Glucose (mg/dL) | 173 | 70-109 | ||
| HbA1c (%) | 6.2 | 4.6-6.2 | ||
| Aspartate aminotransferase (U/L) | 27 | 13-33 | ||
| Alanine aminotransferase (U/L) | 38 | 6-27 | ||
| Alkaline phosphatase (U/L) | 241 | 115-359 | ||
| Lactate dehydrogenase (U/L) | 258 | 119-229 | ||
| Total bilirubin (mg/dL) | 0.6 | 0.3-1.2 | ||
| Gamma-glutamyltranspeptidase (U/L) | 49 | 10-47 | ||
| Choline esterase (U/L) | 227 | 168-470 | ||
| Total cholesterol (mg/dL) | 179 | 128-219 | ||
| Triglyceride (mg/dL) | 87 | 40-234 | ||
| Creatine kinase (U/L) | 69 | 45-163 | ||
| Amylase (U/L) | 25 | 37-125 | ||
| C-reactive protein (mg/dL) | 0.210 | 0.000-0.300 | ||
| Prothrombin time (%) | 90 | 70-130 | ||
| Alpha-fetoprotein (ng/mL) | 2 | 0.0-7.0 | ||
| PIVKA-II (mAU/mL) | 35 | 0.00-40.00 | ||
| Carcinoembryonic antigen (ng/mL) | 2.6 | 0.0-5.0 | ||
| CA19-9 (U/mL) | 7 | 0-37 |
PIVKA-II: protein induced by vitamin K antagonist-II
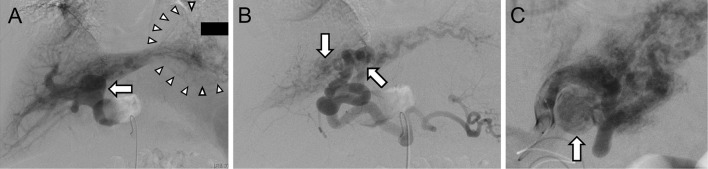
Figure 2.
Angiography showed retrograde filling of the trunk of the portal vein as hepatofugal flow (arrow), contrast-filled vascular space as hepatic hemangioma (arrowheads) (A), and an AP shunt between the left hepatic artery and the left portal vein (arrows) (B). Selective hepatic artery angiogram demonstrated proximal branches of the left hepatic artery to the left portal vein with retrograde flow into the portal vein as hepatofugal flow (arrow) (C). AP: arterio-portal
To reduce the portal hypertension, TAE was performed from branches of the left hepatic artery. As embolic agents for TAE, gelform particles were used out of concern for hepatic infarction or portal vein thrombosis. However, follow-up CT 6 days after TAE showed recanalization in the AP shunt. Therefore, TAE was performed a second time with 0.5 mL of 20% NBCA added to the AP shunt 12 days after the initial round of TAE (Fig. 3A and B). Although angiography after embolization with NBCA showed residual AP shunt, the arterial retrograde flow to the trunk of the portal vein with hepatofugal flow was decreased (Fig. 3C).
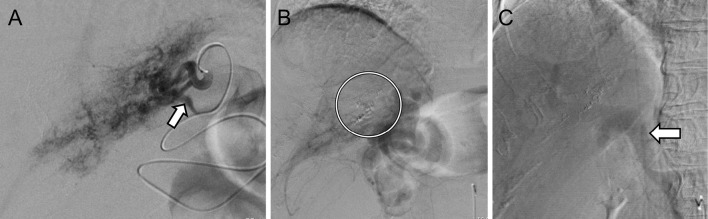
Figure 3.
Angiography showed re-canalization in the AP shunt (arrow) (A). TAE was performed with NBCA (circle) (B), and arterial flow to the trunk of the portal vein was decreased (arrow) (C). AP: arterio-portal, TAE: transcatheter arterial embolization, NBCA: N-butyl-2-cyanoacrylate
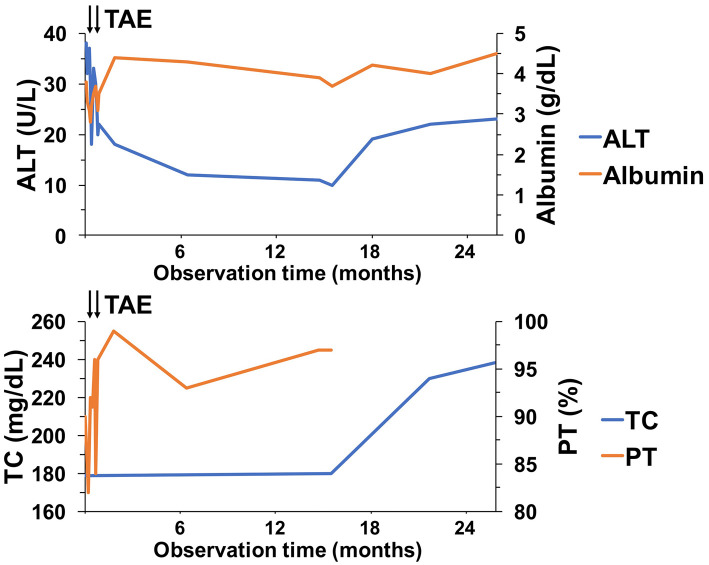
The patient had an uneventful clinical course following embolization, and her ascites was decreased. Subsequently, diuretics were tapered to 25 mg/day of spironolactone alone. The patient was discharged 8 days after the second round of TAE. Two months after the second round of TAE, a repeat CT scan confirmed the complete loss of ascites and the reduction of the AP shunt, indicating a decrease in the hepatofugal portal outflow (Fig. 4), although the hepatic hemangioma in the left lobe of the liver had not changed. Thus, the administration of spironolactone was discontinued. After two years of follow-up, the patient remains asymptomatic without ascites. CT has shown no exacerbation of the AP shunt, and the serum levels of ALT, albumin, total cholesterol, and prothrombin time have improved (Fig. 5).
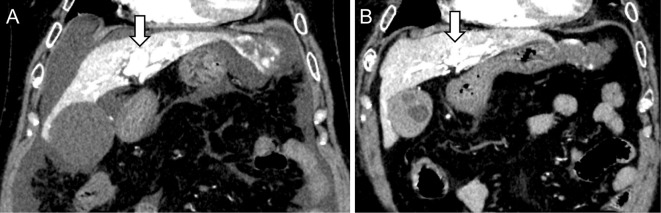
Figure 4.
A CT scan obtained 2 months after the second TAE procedure. Dynamic CT images showed the loss of ascites and the reduction of the AP shunt, indicating a decrease in the hepatofugal portal outflow (arrow). (A) Image obtained before TAE. (B) Image after the second TAE procedure. TAE: transcatheter arterial embolization, AP: arterio-portal
Figure 5.
Changes in the liver function test results in the clinical course. The serum levels of ALT, albumin, total cholesterol, and prothrombin time improved after TAE. TAE: transcatheter arterial embolization, ALT: alanine aminotransferase, TC: total cholesterol, PT: prothrombin time
Discussion
Hepatic hemangioma with AP shunt was first reported in 1977 (12), and it has been diagnosed increasingly frequently due to improvements in imaging techniques. The prevalence of AP shunts is estimated to be 26% in patients with hepatic hemangioma (8). Hepatic hemangioma with AP shunt is most often asymptomatic, and symptomatic cases of hemangioma with AP shunt that require treatment are very rare (9-11). The number of cases of symptomatic hepatic hemangioma with AP shunt might be low because cases of both hepatic hemangioma and AP shunt are generally asymptomatic (2,13). However, large hepatic hemangiomas are more likely to cause symptoms, such as abdominal pain or discomfort, nausea, and vomiting (2). In contrast, when AP shunt causes portal hypertension, the most common manifestations are gastrointestinal bleeding, ascites, and diarrhea (13). Therefore, we need to understand that patients with hepatic hemangioma with AP shunt may present with these symptoms in some cases.
In terms of diagnostic imaging findings, dynamic CT shows hemangiomas as peripheral enhancement with subsequent slow progressive central filling and AP shunts as an enlarged hepatic artery with a dilated intrahepatic portal vein (8,14). Furthermore, hepatic arteriography can be used to confirm AP shunts, which can be treated with TAE (7). More recently, it has been reported that color Doppler sonography is useful for diagnosing and monitoring hepatic hemangioma with an AP shunt (15). Unfortunately, we did not evaluate the AP shunt by color Doppler sonography in the present case. If color Doppler sonography had been performed, a more detailed blood flow evaluation might have been possible.
Since most cases of hepatic hemangioma with AP shunt are asymptomatic, intervention is rarely indicated, and observation is sufficient. However, in cases of symptomatic hepatic hemangioma with AP shunt, treatments such as surgical resection, TAE, hepatic artery ligation, and liver transplantation were considered in three previously reported cases (9-11). In those cases, the hepatic hemangiomas with AP shunts were not cured by TAE with metallic coils or gelform particles alone. In our patient, although TAE with NBCA was unable to occlude the AP shunt completely, the procedure decreased the blood flow from the hepatic artery to the portal vein, which resulted in the resolution of the ascites without any complications, such as distal organ ischemia due to embolization. However, the appearance of the hepatic hemangioma was not changed.
Metallic coils, gelform particles, and liquid agents are commonly used as embolic agents for TAE of AP shunts. In our case, gelform particles were used for embolization during the first TAE procedure due to concerns about complications, such as hepatic infarction or portal vein thrombosis. However, TAE with gelform particles was ineffective; we therefore used NBCA as the embolic agent for the second TAE procedure. Metallic coils were not used as the embolic agent in our case due to the presence of multiple AP shunts and the lack of segmentation in the hepatic arteries. Furthermore, not only ascites but also the findings of liver function tests improved after TAE in our patient. The effectiveness of TAE in this case may have been due to the suppression of portal hypertension as the size of the AP shunt decreased. However, regarding improvements in portal hypertension, the effect of TAE on esophageal varices was not evaluated in this patient because esophagogastroduodenoscopy was not performed after TAE treatment.
In summary, the patient presented here had an AP shunt that was successfully treated by TAE with subsequent improvement of the ascites and liver function without complications. Although a variety of embolic agents have been used over the years, TAE with NBCA is extremely useful for the treatment of symptomatic AP shunts with hepatic hemangioma.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol 39: 401-412, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SS, Charny CK, Fong Y, et al. . Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg 97: 392-402, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Zhao W, Guo X, Dong J. Spontaneous rupture of hepatic hemangioma: a case and literature review. Int J Clin Exp Pathol 8: 13426-13428, 2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Lasonoff JE, Millis JM. Liver hemangioma complicated by obstructive jaundice. Am J Surg 196: e3-e4, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Katoh H, Dohke M, Okushiba S. A giant hemangioma with secondary portal hypertension: a case report of successful surgical treatment. Hepatogastoenterology 44: 1212-1214, 1997. [PubMed] [Google Scholar]
- 6.Oak CY, Jun CH, Cho EA, et al. . Hepatic hemangioma with Kasabach-Merritt syndrome in adult patient. Korean J Gastroenterol 67: 220-223, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Guzman EA, McCahill LE, Rogers FB. Arterioportal fistula: introduction of a novel classification with therapeutic implications. J Gastrointest Sirg 10: 543-550, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kim KW, Kim TK, Han JK, Kim AY, Lee HJ, Choi BI. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology 219: 707-711, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Morimoto T, Yamamori T, Moriyasu F, Yamaoka Y. Atypical liver hemangioma with shunt: long-term follow-up. J Hepatobiliary Pancreat Surg 9: 750-754, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava DN, Sharma S, Yadav S, Nundy S, Berry M. Pedunculated hepatic hemangioma with arterioportal shunt: treated with a angio-embolization and surgery. Australas Radiol 42: 151-153, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Shimada M, Matsumata T, Ikeda Y, et al. . Multiple hepatic hemangiomas with significant arterioportal venous shunting. Cancer 73: 304-307, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Winograd J, Palubinskas AL. Arterio-portal venous shunting in cavernous hemangioma of the liver. Radiology 122: 331-332, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Vauthey JN, Tomczak RJ, Helmberger T, et al. . The arterioportal fistula syndrome: clinicopathologic features, diagnosis, and therapy. Gastroenterology 113: 1390-1401, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Kim T, Federle MP, Baron RL, Peterson MS, Kawamori Y. Discrimination of small hemangiomas from hypervascular malignant tumors smaller than 3 cm with three-phase helical CT. Radiology 219: 699-706, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Lim KJ, Kim KW, Jeong WK, et al. . Colour Doppler sonography of hepatic haemangiomas with arterioportal shunts. Br J Radiol 85: 142-146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]