Abstract
Axitinib has emerged as a promising antineoplastic agent for the treatment of advanced renal cell carcinoma. Although the administration of axitinib was well-tolerated in clinical trials, the real-world safety and tolerability remain unverified. We herein report a patient with metastatic renal cell carcinoma who suddenly developed life-threatening hyperkalemia following the initiation of axitinib treatment. Although hyperkalemia has been reported with an incidence of <10%, acute severe hyperkalemia may be a considerably critical adverse event of axitinib therapy, especially in patients with risk factors for hyperkalemia. An abundance of caution for unusual and unpredictable toxicities is warranted when using axitinib.
Keywords: adverse event, axitinib, hyperkalemia, renal cell carcinoma
Introduction
Over the past few decades, novel forms of molecular-targeted agents have become available in cancer treatments, including the second-generation tyrosine kinase inhibitor axitinib (InlytaⓇ Tablets; Pfizer, New York, USA) (1,2). Since 2012, axitinib has been used worldwide as an antineoplastic agent for the treatment of metastatic or unresectable renal cell carcinoma after failure of a prior first-line therapy (1-4). Although hypertension, cardiac failure, and hemorrhaging have been reported as the most common dose-limiting toxicities of axitinib, the majority of cases have been suggested to be manageable with dose modification and standard supportive care (3,4). However, the need to be alert for acute severe hyperkalemia has never been emphasized. Given that targeted agents like axitinib have emerged as remarkable anticancer therapies in the current era, the awareness of un-emphasized critical adverse effects provides important clinical implications, particularly with regard to oncologic emergency.
We herein report a patient with metastatic renal cell carcinoma who developed acute severe hyperkalemia following the initiation of axitinib treatment.
Case Report
An 85-year-old woman presented with profound bradycardia due to acute hyperkalemia. She had a history of chronic kidney disease secondary to a left nephrectomy for renal clear cell carcinoma (pT3N0M0) at 64 years of age and was diagnosed with multiple pancreatic metastases at 83 years of age. She also had hypertension, which was treated with olmesartan (10 mg/day) and nifedipine (20 mg/day), and was prescribed lansoprazole (15 mg/day), estazolam (2 mg/day) and etizolam (0.5 mg/day) as well.
Three weeks before her presentation, she had been hospitalized for the initiation of axitinib (6 mg/day) treatment following progressive disease with sunitinib that had been administered as the first-line therapy for her metastatic renal cell carcinoma (5,6) (Fig. 1). The starting dose of axitinib was reduced from 5 mg twice daily (standard dose) to 3 mg twice daily due to concerns about her advanced age, concomitant hypertension, and a slight increase in plasma brain natriuretic peptide at baseline (Table). During the two-week hospitalization, she showed no adverse events related to the administration of axitinib, with serum levels of potassium within the normal range (4.5-4.8 mEq/L) and a trough plasma level of axitinib of 5.1 ng/mL, which was only marginally higher than the trough concentration to achieve a tumor response (>5.0 ng/mL) (7). After discharge, she remained in her usual state of health and had a performance status 0, consumed a regular diet without high potassium-content foods, and was not taking any natural or manufactured CYP3A4 inhibitors (e.g., macrolides, tetracyclines, azole antifungals, protease inhibitors, or grapefruit juice) that might affect the metabolism of axitinib. Three days after her discharge (17 days after the initiation of axitinib treatment), she complained of the sudden onset of general fatigue, nausea, and dizziness and was transferred to our emergency department.
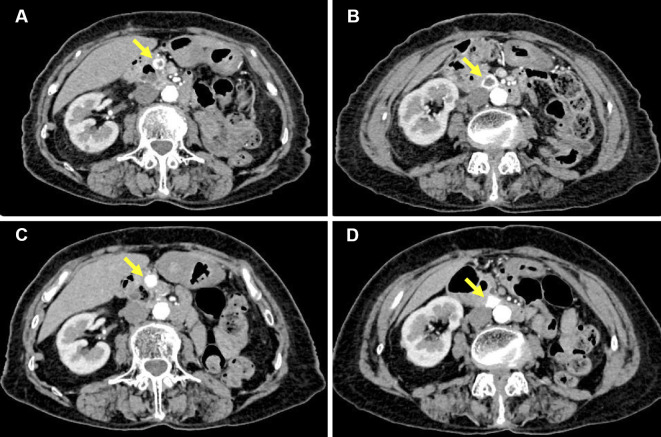
Figure 1.
Contrast-enhanced computed tomography of the abdomen. (A, B) The contrast-enhanced computed tomography images before axitinib treatment (one month before presentation) showed multiple metastatic lesions of renal cell carcinoma in the pancreas. They showed increased peripheral enhancement with central attenuation in the pancreatic nodules, indicating a progressive condition following first-line therapy with sunitinib for the metastatic renal cell carcinoma (5, 6). (C, D) The contrast-enhanced computed tomography images two months after presentation did not show any remarkable interval changes in the tumor size but did show marked enhancement of the metastatic lesions, indicating cancer progression after the cessation of axitinib (5, 6). Each arrow indicates the metastatic lesions of renal cell carcinoma in the pancreas.
Table.
Laboratory Data.
| Reference | Baseline† | On admission | On discharge | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood gas analysis | |||||||||
| Fraction of inspired oxygen | 0.21 | 0.21 | |||||||
| pH | NA | 7.328# | 7.445$ | ||||||
| pO2 (mmHg) | NA | 36.5# | 83.4$ | ||||||
| pCO2 (mmHg) | NA | 37.6# | 32.7$ | ||||||
| HCO3− (mmol/L) | NA | 19.2# | 22.1$ | ||||||
| Base excess (mmol/L) | NA | -1.1# | -1.4$ | ||||||
| Anion gap (mmol/L) | NA | 10.8# | +8.8$ | ||||||
| Lactate (mmol/L) | 0.37-1.65 | NA | 3.2# | 0.5$ | |||||
| Others | |||||||||
| BUN (mg/dL) | 8.0-20.0 | 23 | 50 | 18 | |||||
| Cre (mg/dL) | 0.32-0.84 | 0.99 | 1.96 | 0.95 | |||||
| eGFR (mL/min/1.73 m2) | >60 | 41 | 19 | 42 | |||||
| Na (mEq/L) | 136-145 | 141 | 140 | 145 | |||||
| Cl (mEq/L) | 98-107 | 107 | 108 | 109 | |||||
| K (mEq/L) | 3.5-5.1 | 4.5 | 7.7 | 4.2 | |||||
| P (mg/dL) | 2.2-4.1 | NA | 7.6 | 2.6 | |||||
| Ca (mg/dL) | 8.6-10.1 | 9.3 | 9.1 | 8.8 | |||||
| UA (mg/dL) | 3.0-5.5 | 6.3 | 10.5 | 5.6 | |||||
| T-bil (mg/dL) | 0.2-1.0 | 0.6 | 0.6 | 0.5 | |||||
| AST (IU/L) | 8-38 | 20 | 897 | 58 | |||||
| ALT (IU/L) | 4-43 | 13 | 648 | 206 | |||||
| Plasma glucose (mg/dL) | 68.0-109 | NA | 108 | 97 | |||||
| BNP (pg/mL) | <18.4 | 149.5 | 158.3 | 74.2 |
†Two days before the initiation of axitinib treatment;#venous;$arterial and on discharge from ICU.
ALT: alanine-aminotransferase, AST: aspartate-aminotransferase, BNP: brain natriuretic peptide, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, NA: not available, pCO2: partial pressure of carbon dioxide, pO2: partial pressure of oxygen, T-bil: total bilirubin, UA: uric acid
On presentation, she showed a decreased level of consciousness (Glasgow Coma Scale score of E3V4M6) with the following vital signs: heart rate, 25 beats/min; blood pressure, 98/58 mmHg; body temperature, 35.4℃; respiratory rate, 18 breaths/min, and oxygen saturation 100% in ambient air. Her height, weight, and body mass index were 141 cm, 45.0 kg, and 22.6 kg/m2, respectively. The electrocardiogram showed sinus arrest with a slow junctional escape rhythm (25 beats/min) (Fig. 2). Acute coronary syndrome was ruled out based on the normal findings of echocardiography and cardiac biomarkers, and her volume status evaluated by echocardiography was normal. Notably, laboratory data showed profound hyperkalemia of 7.7 mEq/L associated with hyperphosphatemia, hyperuricemia, a deteriorated renal function with modest metabolic acidosis, and liver dysfunction, none of which had been noted on the day of the discharge 3 days before the presentation (Table). Her hyperkalemia was acute and critical enough to cause sinus arrest and could not be attributed to metabolic acidosis (Table).
Figure 2.
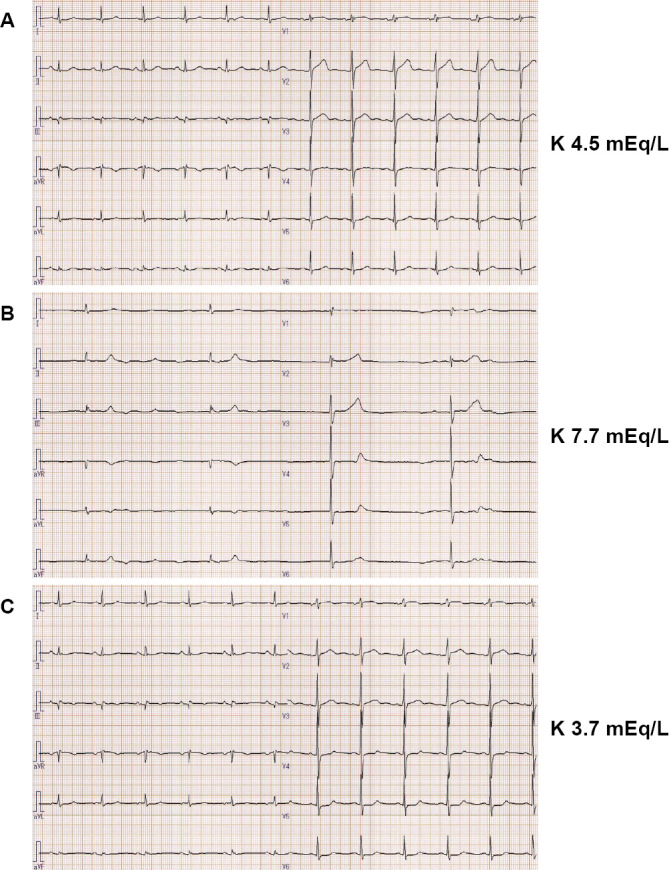
Electrocardiograms. (A) The electrocardiogram before the initiation of axitinib treatment showed a normal sinus rhythm (71 beats/min). (B) The electrocardiogram on her presentation to our department showed sinus arrest with a slow junctional rhythm (25 beats/min). (C) The electrocardiogram after the improvement of hyperkalemia showed a normal sinus rhythm (69 beats/min). The values on the right side correspond to the serum levels of potassium at each time point.
We initiated prompt therapies for the severe hyperkalemia with resultant sinus arrest via the intravenous administration of calcium gluconate, sodium bicarbonate, and insulin with glucose, in addition to intermittent furosemide infusions with saline hydration in order to facilitate urinary potassium excretion. She returned to a normal sinus rhythm immediately after the correction of hyperkalemia (Fig. 2). Her liver function normalized as well within a few days, a compatible course in the setting of shock liver. She did not show recurrence of hyperkalemia or bradyarrhythmia after the cessation of axitinib and olmesartan (Fig. 3), and she was discharged home in her premorbid state seven days after presentation.
Figure 3.
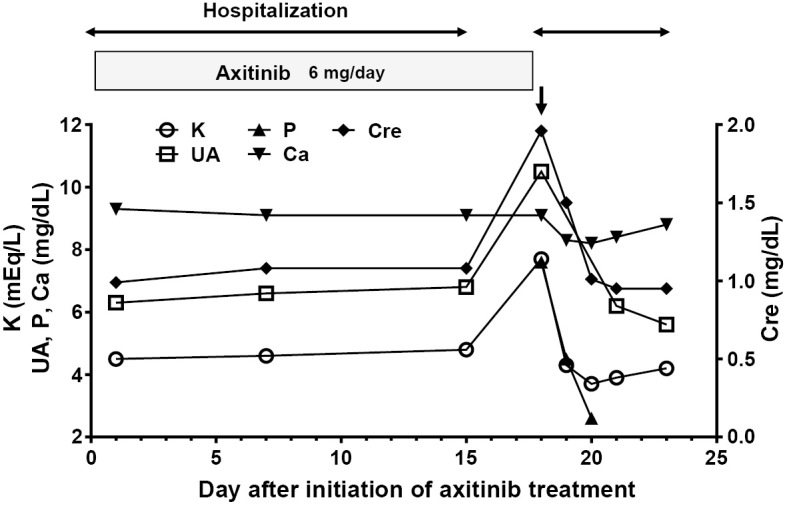
Clinical course of axitinib treatment and laboratory data. The vertical arrow indicates a hyperkalemic emergency, and the horizontal arrows indicate hospitalization. Cre: creatinine, P: phosphorus, UA: uric acid
Discussion
The present case highlighted two important clinical issues. First, serious hyperkalemia unexpectedly developed following the administration of axitinib in an elderly patient with moderate chronic kidney disease and hypertension who was being treated with an angiotensin II receptor antagonist. Second, physicians should be alert for unusual and unpredictable treatment-related hyperkalemia, especially in patients susceptible to develop hyperkalemia such as those with an advanced age, chronic kidney disease, and the concomitant use of drugs that may increase the serum potassium levels. To our knowledge, this is the first case report describing acute life-threatening hyperkalemia associated with the initiation of axitinib treatment.
Clinicians should consider the possibility of axitinib-induced hyperkalemia, especially in patients with predisposing factors for this electrolyte disturbance, such as those with an advanced age, preexisting renal insufficiency, and the concomitant administration of drugs that inhibit the renin-angiotensin-aldosterone system. Axitinib is metabolized predominantly in the liver through CYP3A4, and the renal function does not affect its clearance (1). The label instructions of axitinib describe the frequency of potassium disturbances of both hyper- and hypokalemia to be <10%. However, in phase I, II, and III trials, common adverse events related to axitinib included hypertension, fatigue, hand-foot syndrome, and gastrointestinal symptoms (8-13), and no unexpected adverse events, including severe hyperkalemia, occurred. Consistent with these clinical trials, recent real-world data have described no adverse events of hyperkalemia associated with the use of axitinib (4). Our patient was treated with axitinib at a reduced dose (3 mg twice daily) due to concerns associated with her advanced age, concomitant hypertension, and a slight increase in the plasma brain natriuretic peptide level at baseline (Table). This treatment was well-tolerated without adverse events during the two-week hospitalization with repeated monitoring of the liver and renal function, electrolytes, and blood pressure. Nonetheless, she developed an abrupt hyperkalemic emergency only three days after the discharge.
The mechanism by which axitinib causes hyperkalemia is largely unknown. Potassium homeostasis is finely maintained through multiple mechanisms in healthy and disease states (14). Iatrogenic hyperkalemia is a common complication of renin-angiotensin-aldosterone inhibitors, especially when elderly patients with preexisting chronic kidney disease are exposed to additional risk factors (15,16). However, apart from the initiation of axitinib treatment, our patient did not have such additional risk factors for the development of hyperkalemia, including the excessive intake of high potassium-content foods (17), use of non-steroidal anti-inflammatory drugs, volume depletion, systemic hypotension, and infections (15). An alternate explanation for the sudden onset of hyperkalemia in this patient may be tumor lysis syndrome, which is characterized by acute and severe hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia in response to anticancer therapy, leading to cardiac arrhythmia, acute kidney injury, and seizure (18). This syndrome most commonly occurs in patients with hematologic cancers but can also occur in those with solid tumors if they have a large tumor burden, a high proliferative rate, or a high chemosensitivity (18-20). By definition, these electrolyte and metabolic abnormalities need to be present simultaneously within three days before or up to seven days after the initiation of therapy (18). Our patient developed hyperkalemia 17 days after the initiation of axitinib treatment and thus did not meet these criteria. Furthermore, computed tomography images obtained before and after the initiation of axitinib treatment showed no significant interval changes in the size of the metastatic lesions.
Given that vascular endothelial growth factor receptors 1, 2, and 3, the selective targets of axitinib (1), are highly expressed in the kidneys (21,22), the possibility that drug-induced tubular dysfunction, or hyperkalemic type 4 renal tubular acidosis in particular, may be involved in the development of hyperkalemia should be considered (23,24). This mechanism is potentially supported by two clinical findings in this patient; first, the laboratory data on admission showed modest metabolic acidosis with a normal anion gap, and second, the data on the second day showed a low transtubular potassium gradient of 3.3 (less than 5) and low fractional excretion of potassium of 9.5% (less than 10%), which were indicative of reduced urinary excretion of potassium. Although these findings are inconclusive, axitinib may have, at least in part, directly contributed to or served as a second hit leading to the development of hyperkalemia in the present patient who was already susceptible to hyperkalemia due to her advanced age, chronic kidney disease, and the concomitant use of olmesartan.
Physicians should be alert for unusual and unpredictable treatment-related hyperkalemia, especially in patients using axitinib who have risk factors for developing hyperkalemia. Our patient had a number of risk factors for the development of hyperkalemia, including an advanced age, chronic kidney disease, and the concomitant use of a renin-angiotensin-aldosterone inhibitor. However, she had not had an episode of hyperkalemia until the initiation of axitinib treatment. A prospective study of metastatic renal cell carcinoma patients who were treated with axitinib in real-world practice showed that 86% of the patients had prior nephrectomy (4), indicating that a reduced renal function may be prevalent among patients who are eligible for axitinib treatment. Although no specific treatment of axitinib-induced hypertension is described in the literature, it has been reported that standard antihypertensive therapy is suitable for most patients with tyrosine kinase inhibitor-related hypertension (25). The current guidelines for the management of hypertension (26-28) recommend angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers as the drug of choice for the treatment of hypertension in patients with a variety of underlying renal and cardiovascular diseases, including chronic kidney disease, proteinuria, and heart failure. Indeed, angiotensin-receptor blockers are the second-most common antihypertensive drugs prescribed in Japan (27). Considering that hypertension is one of the most common adverse events in patients administered axitinib, the patients are likely to be prescribed renin-angiotensin-aldosterone inhibitors, which carry a risk of hyperkalemia, just as in our patient. In addition, proactive modifications aiming at the normalization of potassium homeostasis, including the dose reduction of renin-angiotensin-aldosterone inhibitors and dietary restriction of potassium, may be effective preventive strategies against axitinib-associated hyperkalemia in high-risk patients (29). The further accumulation of cases is needed to confirm whether or not axitinib directly causes hyperkalemia in patients without predisposing factors for this condition.
The advent of novel targeted therapies, including axitinib, has provided a promising therapeutic option for the treatment of advanced renal cell carcinoma, although these therapies may cause unanticipated adverse events of significance in the real-world clinical setting (2). The course of our patient provided two important clinical implications: first, serious hyperkalemia may unexpectedly occur following the initiation of axitinib treatment in patients susceptible to hyperkalemia, and second, physicians should be alert for unusual and unpredictable treatment-related hyperkalemia, especially in patients using axitinib who are at a high risk for hyperkalemia.
In conclusion, acute hyperkalemia may be a considerable adverse event associated with axitinib that clinicians should be aware of, especially in patients with risk factors for developing hyperkalemia, such as those with an advanced age, chronic kidney disease, and the concomitant use of drugs that inhibit the renin-angiotensin-aldosterone system.
Written informed consent was obtained from the patient for the publication of this case report.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D 11: 113-126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keefe DM, Bateman EH. Tumor control versus adverse events with targeted anticancer therapies. Nat Rev Clin Oncol 9: 98-109, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Borst DL, Arruda LS, MacLean E, Pithavala YK, Morgado JE. Common questions regarding clinical use of axitinib in advanced renal cell carcinoma. Am J Health Syst Pharm 71: 1092-1096, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Matias M, Le Teuff G, Albiges L, et al. Real world prospective experience of axitinib in metastatic renal cell carcinoma in a large comprehensive cancer centre. Eur J Cancer 79: 185-192, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Brufau BP, Cerqueda CS, Villalba LB, Izquierdo RS, Gonzalez BM, Molina CN. Metastatic renal cell carcinoma: radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. Radiographics 33: 1691-1716, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol 194: 157-165, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya N, Igarashi R, Suzuki-Honma N, et al. Association of pharmacokinetics of axitinib with treatment outcome and adverse events in advanced renal cell carcinoma patients. J Clin Oncol 33 (Suppl): 506, 2015. [Google Scholar]
- 8.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 23: 5474-5483, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 27: 4462-4468, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Eto M, Uemura H, Tomita Y, et al. Overall survival and final efficacy and safety results from a Japanese phase II study of axitinib in cytokine-refractory metastatic renal cell carcinoma. Cancer Sci 105: 1576-1583, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer 47: 2592-2602, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14: 552-562, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378: 1931-1939, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med 373: 60-72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet 382: 170-179, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Hoye A, Clark A. Iatrogenic hyperkalaemia. Lancet 361: 2124, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gennari FJ. Hypokalemia. N Engl J Med 339: 451-458, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 364: 1844-1854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholaou T, Wong R, Davis ID. Tumour lysis syndrome in a patient with renal-cell carcinoma treated with sunitinib malate. Lancet 369: 1923-1924, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Joshita S, Yoshizawa K, Sano K, et al. A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med 49: 991-994, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Witmer AN, Dai J, Weich HA, Vrensen GF, Schlingemann RO. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem 50: 767-777, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560-H576, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez Soriano J. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol 13: 2160-2170, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol 20: 251-254, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Leon-Mateos L, Mosquera J, Anton Aparicio L. Treatment of sunitinib-induced hypertension in solid tumor by nitric oxide donors. Redox Biol 6: 421-425, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34: 2159-2219, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 37: 253-390, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation 131: e435-e470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 10: 653-662, 2014. [DOI] [PubMed] [Google Scholar]