Abstract
A 58-year-old man with type 1 autoimmune pancreatitis was referred to nephrologists for severe proteinuria. Laboratory data revealed a high serum IgG4 level, hypoalbuminemia, and massive proteinuria, which were compatible with nephrotic syndrome. The renal pathological findings confirmed the diagnosis of secondary membranous nephropathy concurrent with IgG4-related tubulointerstitial nephritis. Despite the improvement of interstitial markers, the proteinuria was refractory to prednisolone, requiring cyclosporine to achieve complete remission. Membranous nephropathy is a rare manifestation of IgG4-related kidney disease. This case shows that the therapeutic response to prednisolone significantly differs between glomerular lesions and interstitial lesions of IgG4-related kidney disease.
Keywords: IgG4-related kidney disease, membranous nephropathy, tubulointerstitial nephritis, IgG-subclass, M-type phospholipase A2 receptor (PLA2R)
Introduction
IgG4-related disease (IgG4-RD) is a recently recognized clinical entity characterized by an elevated serum IgG4 concentration, a dense lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells, and a typical fibrotic lesion (“storiform” fibrosis) (1). Previous studies have reported the renal involvement of IgG4-RD, comprehensively termed IgG4-related kidney disease (IgG4-RKD) (2). Tubulointerstitial nephritis (TIN) with dominant IgG4-positive plasma cell infiltration and fibrosis is the most common histological finding and generally responds well to corticosteroid therapy (3,4). Glomerular lesions occasionally coincide with IgG4-related tubulointerstitial nephritis (IgG4-TIN), and a variety of glomerular diseases have been reported, including membranous nephropathy (MN), membranoproliferative glomerulonephritis, and minimal change disease (5,6). However, the underlying pathophysiology of glomerular lesions in the context of IgG4-RKD has yet to be elucidated.
We herein report a case of secondary MN concurrent with IgG4-TIN that required combination therapy of prednisolone (PSL) and cyclosporine (CyA).
Case Report
A 58-year-old man was admitted to our hospital with a 2-month history of persistent proteinuria. One year before admission, he had been diagnosed with type 1 autoimmune pancreatitis (AIP) and received PSL 35 mg/day. The AIP improved promptly, and the serum IgG4 level decreased from 473 mg/dL to 226 mg/dL. As PSL was tapered to 10 mg/day over the next 6 months, the AIP further improved, but the serum IgG4 level gradually increased. Two months before admission, proteinuria developed and rapidly became aggravated. He was referred to nephrologists for a further examination and treatment.
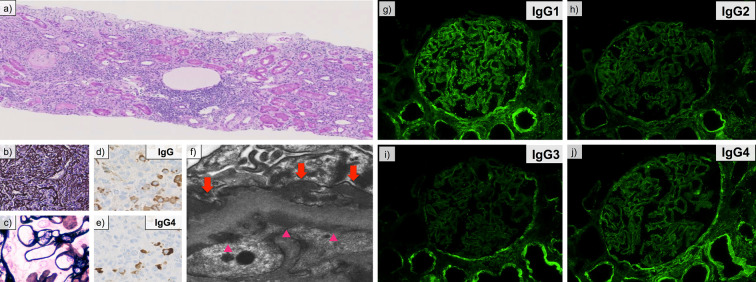
On admission, he was receiving PSL at 10 mg/day, and his AIP was in remission. He also had a history of diabetes mellitus due to AIP and PSL. His family history and social history were unremarkable. A physical examination revealed bilateral pitting edema on the lower extremities. Laboratory data at the referral revealed the following: serum albumin, 2.2 g/dL; serum total protein, 5.8 g/dL; serum creatinine, 0.80 mg/dL; serum IgG4 level, 495 mg/dL; serum IgE level, 1,934 IU/L; HbA1c, 8.5%; urinary protein excretion, 12.2 g/day; and urinary N-acetyl-β-D-glucosaminidase (NAG) 30.4 U/L. Based on these findings, he was diagnosed with nephrotic syndrome. Anti-nuclear antibody, hepatitis-B surface antigen, hepatitis C antibody, and cryoglobulin were negative. Serum IgG, IgA, IgM, and complement levels were within normal limits. Serum autoantibody against M-type phospholipase A2 receptor (anti-PLA2R antibody) was undetectable. Contrast-enhanced computed tomography (CT) revealed mild swelling of the mediastinal lymph nodes and pancreatic body. No renal involvement was noted, including low-density areas or mass lesions in the renal parenchyma. A percutaneous kidney biopsy was performed, and the specimen revealed interstitial infiltration of plasma cells (Fig. 1a) and storiform fibrosis (Fig. 1b). The glomerular basement structure was well-preserved on PAM staining (Fig. 1c). On immunohistochemistry, the ratio of IgG4-positive plasma cells to IgG-positive plasma cells was 44.8% (Fig. 1d and e). Electron microscopy revealed electron dense deposits in subepithelial and subendothelial regions along the glomerular basement membrane (Fig. 1f). Immunofluorescence staining revealed the presence of granular deposits of IgG, C3, and C1q along the glomerular basement membrane. Staining of IgM, IgA, and PLA2R was negative. On IgG-subclass staining, dominant IgG1 deposition was observed along the glomerular basement membrane, while staining for IgG2, IgG3, and IgG4 was comparatively weak (Fig. 1g-j). Other underlying etiologies, including infection, autoimmune disease, and malignancy, were excluded by systemic work-up. Based on these findings, we confirmed the diagnosis of secondary MN concurrent with IgG4-TIN.
Figure 1.
Renal pathological findings. (a) Interstitial infiltration of plasma cells (Hematoxylin and Eosin staining). Original magnification, ×100. (b) Characteristic “storiform” fibrosis (periodic acid-methenamine-silver; PAM stain). Original magnification, ×200. (c) Well-preserved glomerular structure (PAM stain). Original magnification, ×400. (d, e) An immunohistochemical study for IgG (d) and IgG4 (e). The IgG4/IgG-positive plasma cell ratio was 44.8%. (f) Subepithelial and subendothelial deposits (arrows and arrow heads, respectively.) on electron microscopy. Original magnification, ×20,000. (g-j) Immunofluorescence staining for IgG1 (g), IgG2 (h), IgG3 (i) and IgG4 (j). The deposition of IgG1 was dominant among IgG subclasses on the glomerular basement membrane.
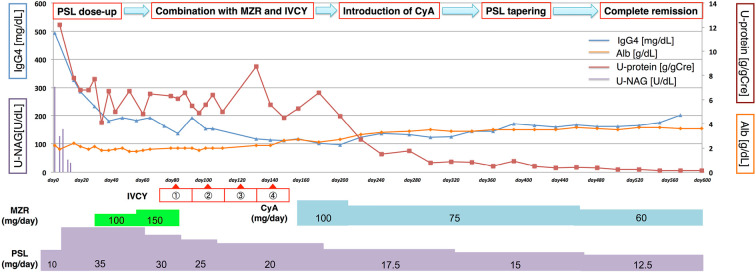
After the diagnosis, we increased the PSL dose to 35 mg/day (Fig. 2). Although urinary NAG decreased promptly to normal levels after increasing the PSL dosage, severe proteinuria persisted. The proteinuria was refractory to mizoribine (MZR) and intravenous cyclophosphamide (IVCY), so we administered CyA in addition to PSL. His urinary protein excretion had gradually decreased, and complete remission was achieved at 21 months' follow-up with a PSL dose of 12.5 mg/day. Although the PSL tapering had increased the serum IgG4 level again, the AIP and nephrotic syndrome were in remission under a maintenance PSL dose. The laboratory data at remission were as follows: serum IgG4 level, 203 mg/dL; serum albumin, 3.6 g/dL; and urinary protein 0.13 g/gCre.
Figure 2.
The clinical course after the diagnosis of secondary membranous nephropathy concurrent with IgG4-related tubulointerstitial nephritis. Although urinary NAG decreased promptly with the increase in the PSL dose, the proteinuria persisted even after combination treatment with mizoribine and IVCY. After the coadministration of CyA with PSL, the urinary protein excretion gradually decreased, and complete remission was achieved at 21 months of follow-up. Although the serum IgG4 level had been re-elevated by PSL tapering, the proteinuria was sufficiently suppressed. NAG: N-acetyl-β-D-glucosaminidase, PSL: prednisolone, MZR: mizoribine, IVCY: intravenous cyclophosphamide, CyA: cyclosporine
Discussion
Our patient presented with nephrotic syndrome during maintenance PSL therapy for AIP, and the pathological findings confirmed the diagnosis of secondary MN concurrent with IgG4-TIN, which was refractory to PSL therapy combined with mizoribine and IVCY. Glomerular lesions are rare manifestations of IgG4-RKD, and a previous report revealed that MN coincides with 7% of IgG4-RKD (7). The mechanism underlying the onset of MN in the context of IgG4-RKD remains unclear.
The characteristic pathological findings of IgG4-RKD include tubulointerstitial nephritis and storiform fibrosis (3). In MN concurrent with IgG4-TIN, the renal pathological findings are generally compatible with the features of secondary MN. Therefore, the exclusion of other underlying conditions is an important diagnostic rationale. In our case, the renal pathological findings revealed the dominant granular deposition of IgG1 along the glomerular basement membrane and the presence of electron-dense deposits on subendothelial and subepithelial lesions. On immunofluorescence staining, idiopathic MN generally shows IgG4-dominant deposits, while IgG2 or G3 is the dominant IgG subclass in secondary MN (8). Regarding secondary MN concurrent with IgG4-RD, previous reports have revealed several patterns of dominant deposition of IgG-subclasses on glomeruli (Table). Five cases showed the dominant deposition of IgG4 on glomeruli, while the other three showed the deposition of various IgG-subclasses, including IgG1, IgG2, and IgG3 (7,9-12). These findings indicate that the dominant deposition of IgG-subclass is not totally diagnostic for secondary MN concurrent with IgG4-RD (Table).
Table.
Fluorescence Staining Pattern of IgG-subclass and Treatment Response in Secondary Membranous Nephropathy Concurrent with IgG4-RD.
| Age and sex |
Staining pattern of IgG-subclass |
TIN | Tx | Proteinuria response |
U-pro (g/day) Pre Tx |
U-pro (g/day) Post Tx |
Length of f/u (months) |
Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67F | IgG2>IgG4>IgG1≒IgG3 | (+) | PSL | CR | 4.0 | 0.2 | 7 | (7) | ||||||||
| 67M | IgG4>IgG1≒IgG2>IgG3 | (+) | PSL+CyA | CR | 4.5 | 0.7 | 7 | (7) | ||||||||
| 53M | IgG4>IgG2≒IgG3>IgG1 | (−) | PSL+MMF | PR | 16 | 3.1 | 46 | (7) | ||||||||
| 34M | IgG4>IgG3>IgG1≒IgG2 | (−) | np | np | np | np | np | (7) | ||||||||
| 58M | IgG4>IgG1≒IgG2>IgG3 | (+) | PSL+Tac | CR | 15.7 | np | 18 | (9) | ||||||||
| 29F | IgG4>IgG1≒IgG2≒IgG3 | (+) | PSL+CyA | CR | 5.6 | np | 5 | (10) | ||||||||
| 54M | IgG3>IgG4>IgG1>IgG2 | (−) | PSL+Rit | NR | 3.9 | 5.6 | 17 | (11) | ||||||||
| 83M | IgG1≒IgG4>IgG2>IgG3 | (+) | PSL | NR | 2.3 | 1.6 | 4 | (12) | ||||||||
| 58M | IgG1>IgG2≒IgG4>IgG3 | (+) | PSL+CyA | CR | 12.2 | 0.1 | 21 | This case |
TIN: tubulointerstitial nephritis, PSL: prednisolone, CyA: cyclosporine, MMF: mycophenolate mofetil, Tac: tacrolimus, Rit: rituximab, CR: complete response, PR: partial response, NR: no response, np: not provided, Tx: treatment, f/u: follow up
M-type phospholipase A2 receptor (PLA2R) is a recently identified target antigen for idiopathic MN (13). The sensitivity and specificity of serum anti-PLA2R antibodies for idiopathic MN were reported to be >75% and 100%, respectively (14). In contrast, anti-PLA2R antibodies do not play a principal role in the pathogenesis of IgG4-RKD (15). Serum anti-PLA2R antibodies and the immunostaining for PLA2R on histology were negative in all reported cases (7,9-12), suggesting that negative results of serum anti-PLA2R antibodies and the negative immunostaining for PLA2R may contribute to the diagnosis of secondary MN concurrent with IgG4-RD. Although the prevalence of anti-PLA2R antibodies in Japanese patients is lower than in patients from other countries, no Japanese patients with secondary MN have been positive for anti-PLA2R antibodies (16). Therefore, the analysis of anti-PLA2R antibodies is still extremely valuable for differentiating secondary MN from idiopathic MN. In our case, undetected serum anti-PLA2R antibodies, characteristic renal pathological findings, and the exclusion of other underlying etiologies confirmed the diagnosis.
Generally, IgG4-RD and IgG4-TIN respond well to PSL therapy (4,17). A previous report demonstrated that 90% patients with IgG4-TIN showed a prompt response to corticosteroid therapy (18). In contrast, glomerular lesions of IgG4-RKD are occasionally refractory to PSL mono-therapy (Table). Of 9 previous cases, only 1 treated by PSL mono-therapy reached complete response of proteinuria, defined as <1 g/day proteinuria. Other cases required combination therapy with immunosuppressive agents to achieve a complete response or only achieved a partial response, defined as a >50% decrease in proteinuria but not reaching <1 g/day. The effectiveness of various immunosuppressive agents and B-cell depletion by rituximab therapy has been reported (19,20). In our case, while the urinary NAG level decreased promptly after the increase in the PSL dose, severe proteinuria persisted even under PSL combined with MZR and IVCY. Sustained combination therapy of CyA and PSL ultimately resulted in complete remission. These differences in the response to PSL between the glomerular lesion and the interstitial lesion were compatible with the findings of previous reports.
The serum IgG4 level is an important diagnostic marker for IgG4-RD (21). The serum IgG4 concentration is correlated with the number of involved organs and usually decreases immediately after PSL therapy (22,23). However, although more than 70% of cases of IgG4-RD exhibit a high serum IgG4 level (1), in the current consensus guideline statements on IgG4-RD, an elevated serum IgG4 concentration (≥135 mg/dL) is neither necessary nor sufficient for the diagnosis of IgG4-RD (3). Furthermore, the degree of serum IgG4 level elevation has only an imperfect correlation with the underlying disease activity (24). In previous reports, the serum IgG4 level was re-elevated without an apparent relapse in half of the patients with IgG4-RKD during maintenance PSL therapy (16). In our case, the serum IgG4 level was re-elevated by PSL tapering, but the proteinuria and AIP remained in remission, indicating that the serum IgG4 level does not directly reflect the activity of glomerular lesions. In order to effectively monitor the disease activity of IgG4-RKD, we should not rely solely on the serum IgG4 concentration, taking care to consider other clinical findings and laboratory data as well.
In conclusion, we described a refractory case of secondary MN concurrent with IgG4-TIN that presented with nephrotic syndrome during maintenance PSL therapy for AIP. The present case illustrates the important clinical and pathological features of secondary MN concurrent with IgG4-TIN. When a patient with IgG4-RD presents with proteinuria, the possibility of glomerular complications of IgG4-RKD should be considered. The renal pathology is the most important diagnostic clue, and the measurement of serum PLA2R antibodies also contributes to the diagnosis. In contrast to extra-renal lesions of IgG4-RD and IgG4-TIN, PSL therapy might be ineffective for treating the glomerular manifestation of IgG4-RKD, indicating that the underlying pathological mechanisms differ between interstitial and glomerular lesions of IgG4-RKD. Specific treatments for glomerular lesions of IgG4-RKD have yet to be established, indicating the need for further investigation.
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 366: 539-551, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Cortazar FB, Stone JH. IgG4-related disease and the kidney. Nat Rev Nephrol 11: 599, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25: 1181-1192, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 67: 1688-1699, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kawano M, Saeki T. IgG4-related kidney disease-an update. Curr Opin Nephrol Hypertens 24: 193-201, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano M, Mizushima I, Yamaguchi Y, et al. Immunohistochemical characteristics of IgG4-related tubulointerstitial nephritis: Detailed analysis of 20 Japanese cases. Int J Rheumatol 2012: 609795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander MP, Larsen CP, Gibson IW, et al. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 83: 455-462, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Kuroki A, Shibata T, Honda H, Totsuka D, Kobayashi K, Sugisaki T. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus, nephritis and idiopathic membranous nephropathy. Intern Med 41: 936-942, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Sueta S, Kondo M, Matsubara T, et al. Membranous nephropathy associated with type 1 autoimmune pancreatitis and dominant glomerular IgG4 deposit. CEN Case Rep 3: 18-23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazawa E, Nakamichi T, Nakayama K, et al. Case report; a case of IgG4-related tubulointerstitial nephritis complicated nephrotic syndrome. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 101: 759-762, 2012(in Japanese). [DOI] [PubMed] [Google Scholar]
- 11.Cravedi P, Abbate M, Gagliardini E, et al. Membranous nephropathy associated with IgG4-related disease. Am J Kidney Dis 58: 272-275, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Saeki T, Imai N, Ito T, Yamazaki H, Nishi S. Membranous nephropathy associated with IgG4-related systemic disease and without autoimmune pancreatitis. Clin Nephrol 71: 173-178, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11-21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck LH Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 77: 765-770, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Khosroshahi A, Ayalon R, Beck LH Jr, Salant DJ, Bloch DB, Stone JH. IgG4-related disease is not associated with antibody to the phospholipase A2 receptor. Int J Rheumatol 2012: 139409, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hihara K, Iyoda M, Tachibana S, et al. Anti-phospholipase A2 receptor (PLA2R) antibody and glomerular PLA2R expression in Japanese patients with membranous nephropathy. PLoS One 11: e0158154, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeki T, Kawano M, Mizushima I, et al. The clinical course of patients with IgG4-related kidney disease. Kidney Int 84: 826-833, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Saeki T, Nishi S, Imai N, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int 78: 1016-1023, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Khosroshahi A, Bloch DB, Deshpande V, Stone JH, et al. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 62: 1755-1762, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 91: 57-66, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 34: 1812-1819, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Cheuk W, Chan JK. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol 17: 303-332, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Okamoto A, Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas 31: 28-31, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 74: 14-18, 2015. [DOI] [PubMed] [Google Scholar]