Abstract
Objective
To compare persistence and adherence to triple therapy with the nonbiologic disease‐modifying antirheumatic drugs (DMARDs) methotrexate (MTX), hydroxychloroquine, and sulfasalazine, versus a tumor necrosis factor inhibitor (TNFi) plus MTX in patients with rheumatoid arthritis (RA).
Methods
Administrative and laboratory data were analyzed for US Veterans with RA initiating triple therapy or TNFi + MTX between January 2006 and December 2012. Treatment persistence 365 days postindex was calculated using 3 definitions. Definition 1 required no gap in therapy of ≥90 days for any drug in the original combination. Definition 2 required no added or switched DMARD, no decrease to nonbiologic DMARD monotherapy, and no termination of all DMARD therapies. Definition 3 was similar to definition 2 but allowed a switch to another drug within the same class. Adherence used a proportion of days covered of ≥80%. Propensity‐weighted analysis with matched weights was used to balance covariates.
Results
The analysis included 4,364 RA patients (TNFi + MTX, n = 3,204; triple therapy, n = 1,160). In propensity‐weighted analysis, patients in the TNFi + MTX group were significantly more likely than patients in the triple therapy group to satisfy all persistence criteria in definition 1 (risk difference [RD] 13.1% [95% confidence interval (95% CI) 9.2–17.0]), definition 2 (RD 6.4% [95% CI 2.3–10.5]), and definition 3 (RD 9.5% [95% CI 5.5–13.6]). Patients in the TNFi + MTX group also exhibited higher adherence during the first year (RD 7.2% [95% CI 3.8–10.5]).
Conclusion
US Veterans with RA were significantly more likely to be persistent and adherent to combination therapy with TNFi + MTX than triple therapy with nonbiologic DMARDs.
INTRODUCTION
Research estimates that 1.3–1.5 million adults in the US have rheumatoid arthritis (RA) 1, 2. The American College of Rheumatology guidelines for use of disease‐modifying antirheumatic drugs (DMARDs) and biologic agents in the treatment of RA 3, 4 recommend nonbiologic DMARD therapy alone, combination nonbiologic DMARD therapy (double or triple therapy), or biologic agents, including tumor necrosis factor inhibitors (TNFi), with or without methotrexate (MTX) or other DMARDs. The recommendations include tailoring treatment based on disease duration and disease activity 3, 4, 5, 6.
Box 1. Significance & Innovations.
Veterans who initiated combination therapy with a tumor necrosis factor inhibitor and methotrexate (TNFi + MTX) showed a higher proportion of persistence at 1 year compared with Veterans who initiated triple therapy.
Veterans who initiated TNFi + MTX combination therapy showed a higher proportion of adherence at 1 year compared with Veterans who initiated triple therapy.
The lower adherence and persistence in the triple therapy group may be due to the increased regimen complexity of multiday dosing.
Methods to improve adherence and persistence are needed for both treatment groups.
Three prospective clinical trials compared triple therapy (MTX + hydroxychloroquine + sulfasalazine) to a TNFi + MTX combination in patients with RA 7, 8, 9. One reported that a TNFi + MTX combination was clinically superior to triple therapy at 1 year 7, while the others showed similar clinical efficacy between the treatment arms 8, 9. Patients in all 3 trials had good‐to‐excellent treatment adherence. The nonblinded trial reported that 82% of patients who added a TNFi to MTX and 68% of patients who received triple therapy continued using their assigned therapy at 12 months 7; at 24 months, adherence rates were 70% and 57%, respectively 10. The other trials reported nondifferential adherence between treatment arms in patients remaining on protocol: 1 reported patients had good or excellent adherence at 94% of visits through 2 years of followup 8, and the other reported 78–79% of patients were adherent through 48 weeks of followup 9. However, these rates did not include patients who withdrew from the trials.
By contrast, a recent analysis of US commercially insured beneficiaries with RA found that less than one‐third of patients were persistent or adherent to triple therapy or etanercept‐MTX combination therapy, and triple therapy users had significantly lower odds of being persistent or adherent than users of 2‐drug biologic combination therapy 11. Discrepancies between trials and real‐world analyses may signal unrealized treatment benefits from nonpersistence and nonadherence. This analysis assessed US Veterans to explore persistence and adherence in a noncommercial health care system. The objective of this study was to compare persistence and adherence during the first year after initiation of triple therapy versus initiation of TNFi + MTX combinations in US Veterans with RA.
PATIENTS AND METHODS
Study design
This open cohort study used administrative and clinical databases with national data from the Department of Veterans Affairs (VA), Veterans Health Administration (VHA), Corporate Data Warehouse, Pharmacy Benefits Management, and Decision Support Services 12, 13, 14. The research was conducted in compliance with the Helsinki Declaration, approved by the Institution Review Board of the University of Utah (IRB_00012917), and reviewed by the Salt Lake City VA Research Review Committee.
This study included VA patients who initiated TNFi + MTX combination therapy or triple therapy (sulfasalazine, hydroxychloroquine, and MTX) between January 2006 and December 2012. TNFi drugs included adalimumab, certolizumab, etanercept, golimumab, and infliximab. Patients were ages ≥18 years and had observations recorded ≥182 days before and ≥365 days after starting combination therapy, with ≥1 health care encounter within 365 days after initiating therapy, to ensure that they were still actively using the VHA system. They were also required to have a diagnosis of RA (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] 714.0) within 182 days preindex or 28 days postindex.
Patients were excluded if they had a diagnosis (ICD‐9‐CM) of juvenile idiopathic arthritis (714.3x), psoriasis (696.1x), psoriatic arthritis (696.0x), ankylosing spondylitis (720.0x), Crohn's disease (555.xx), or ulcerative colitis (556.xx) in the baseline period, as some nonbiologic and/or biologic DMARDs are indicated for the treatment of these conditions. Patients could not have J‐codes for intravenous administration of MTX (J8610, J9250, and J9260) during the postindex period, which would suggest treatment for cancer.
Candidates for the triple therapy group had dispensing for all 3 medications (hydroxychloroquine, sulfasalazine, and MTX) that overlapped by ≥1 day. Candidates for the combination TNFi + MTX group had a dispensing or administration for a TNFi and MTX that overlapped by ≥1 day. The index date (day 1) was the date the last drug (index drug) required to complete the combination was dispensed or administered. To ensure the index drug was intended to be part of combination therapy, all other drugs (foundation drugs) in the triple therapy combination or TNFi + MTX combination were required to have 1 additional prescription refill/administration within 90 days after initiation of the newest index drug. This method ensured that the provider and patient intended to use combination therapy and were not just switching between DMARDs with an overlap. Supplementary Figure 1 (available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22944/abstract) illustrates the approach taken to identify patients who received triple therapy or TNFi + MTX combination therapy.
Patients with prescriptions for all combination drugs on the index date (both drugs in the TNFi + MTX group and all 3 drugs in the triple therapy group) were excluded if they had received each drug in the combination at least once during the preindex period (182 days before the index date). Patients in the TNFi + MTX group could not have a nonfoundation or nonindex biologic DMARD (TNFi or other) during the preindex period. Patients in either treatment group could not receive any nonbiologic DMARDs other than index or foundation between 30 days before and day 28. No other DMARDs beyond combination DMARDs (triple therapy and TNFi + MTX) could be available on the index date.
Persistence
Determination of persistence and adherence was based on days supply from the pharmacy outpatient package. Previous algorithms were developed to validate and correct quantity and days supply 15, 16, 17. Corrected days supply was used for all outpatient‐dispensed medications. The days supply for infliximab was assumed to be 56 days unless there was evidence of an infliximab infusion before then.
Persistence was analyzed with 3 definitions. Definition 1 was the primary outcome measure and represented complete persistence in taking both TNFi combination or all 3 triple therapy combination therapy drugs; definition 2 allowed dropping 1 nonbiologic DMARD, and definition 3 allowed dropping 1 nonbiologic DMARD or changing medications within a class.
Persistence definition 1 required a patient to continue all drugs in the combination without a ≥90‐day gap in therapy. A patient was considered nonpersistent if there was a gap of ≥90 days for any drug in the combination beyond the last day of a prescribed dispensing period. Patients who added another DMARD were considered persistent in the primary analysis if other drugs were continued without a gap in therapy. The date of nonpersistence was defined as the day immediately before the first gap of ≥90 days started for any treatment in the combination at the end of days supply or usual infusion dosing interval.
For persistence definition 2, patients were considered nonpersistent 1) if a new DMARD (biologic or nonbiologic) was started, either because of a switch in therapy or because the drug was added to the combination, 2) if, after a ≥90‐day gap in therapy, the patient received only nonbiologic DMARD monotherapy (i.e., the patient stopped 2 of 3 drugs in the triple combination or stopped TNFi in the TNFi + MTX combination), or 3) if the entire combination therapy was terminated. The date of nonpersistence was the earliest date on which the treatment was switched, added, or reduced to nonbiologic monotherapy or no therapy.
For persistence definition 3, patients were considered nonpersistent in the same manner as definition 2, but they were allowed to switch drugs within a class. Specifically, patients were considered nonpersistent 1) if a DMARD of another class was started, 2) if, after a ≥90‐day gap in therapy, the patient received only nonbiologic DMARD monotherapy, or 3) if the entire combination therapy was terminated. The date of nonpersistence was the earliest date on which the treatment was switched to a DMARD of another class or was reduced to nonbiologic monotherapy or no therapy.
Adherence
The proportion of days covered (PDC) was used to measure adherence 18. Patients with PDC ≥80% for each medication in the combination were considered adherent. For both persistence and adherence measures, patients were assumed not to be doubling up on medication when early refill occurred. An overlapping dispensing period because of early refill was shifted to a later time period (right shift) as if patients were continuously using the drug, but it was not used to fill gaps between previously dispensed episodes before overlapping days (left shift). The maximum allowed right shift for early refill was 14 days.
Covariates
Clinically plausible confounders of the relationship between initiating triple therapy or TNFi + MTX therapy were extracted from VHA data and used as baseline covariates. The baseline period was 1 year preindex for combination therapy for all diagnoses, medications, and utilization variables. Proportions of patients with positive rheumatoid factor (RF) or cyclic citrullinated peptide (CCP) antibody were based on patients with evaluable data. The Healthcare Cost and Utilization Project Clinical Classification System (HCUP CCS) for ICD‐9‐CM conditions was used to identify specific medical conditions. The HCUP CCS was also used for an overall measure of comorbidity by counting the unique number of single‐level conditions each patient experienced during the baseline year. Additional aggregate measures included the distinct count of VA Drug Class codes and the Rheumatic Disease Comorbidity Index (RDCI) score 19. Aggregate measures of health care utilization in the baseline period included yearly counts of rheumatology visits (stop code 314), urgent care visits (stop code 131), emergency department visits (stop code 130), and inpatient visits (admission counts). VA Drug Class codes were used to identify opioid analgesics (CN101), nonopioid analgesics (CN103), salicylates, antirheumatics (MS101), nonsalicylate nonsteroidal antiinflammatory drugs (MS102), and prednisone (HS051). Proton pump inhibitors were identified by string search on generic ingredients.
Statistical analysis
Propensity score analysis used matching weights to adjust for baseline patient characteristics and balance the 2 combination therapy groups 20, 21. The matched‐weight approach is theoretically equivalent to the 1:1 exact‐matching propensity score, which focuses on the subset of patients who have common support, meaning there is an expectation about clinical equipoise between the 2 treatment choices. The matched‐weight approach has proven to be more efficient and has better statistical properties than the 1:1 matching approach 20. Thirty‐four pretreatment baseline covariates were identified as possible confounders based on literature review and the assumption that these variables might influence treatment decisions and persistence at 1 year of followup. Covariates were then used to generate a propensity score ( ) using potential confounders (X) to model treatment choice (Z) for each patient using a logistic regression model:
The matching weight for patient i (Wi) was a variant of inverse probability weight 22 with min(1−ei, ei) in the numerator:
The matching‐weight estimator of the treated effect was calculated as follows, which can be interpreted as the difference in weighted risks between treatment groups:
Relative differences on the ratio scale were also reported. Variance was computed using the sandwich variance estimator 20.
The ability to check covariate balance between treatment groups is an advantage of propensity score methods over direct regression. Lack of balance often suggests that treatment comparison may not be feasible in certain subgroups of patients without extrapolation or that there may be residual bias due to confounding by covariates 23. Standardized differences were used to determine differences in covariate balance before and after weighting:
This formula can be computed using weighted means and variances. In typical applications of pair‐matching methods, standardized differences in a good match are a few percentage points. As a threshold for claiming balance, 10% has been advocated 23. Standardized differences using matching‐weights methods can easily reach below 1%. Therefore, the matching‐weights method may lead to a substantially better covariate balance than the pair‐matching method 20. Matched weights–adjusted Kaplan‐Meier plots were created to illustrate persistence among treatment groups. Microsoft SQL server and SAS, version 9.4, with Enterprise Guide, version 6.1, were used to prepare data and conduct statistical analyses.
RESULTS
Patient characteristics
Of 14,272 patients who received either combination therapy regimen during the study period, 4,364 met all inclusion and exclusion criteria (Table 1), including 3,204 who initiated TNFi + MTX and 1,160 who initiated triple therapy. Compared with patients who initiated TNFi + MTX, patients who initiated triple therapy were older (mean age 62.2 versus 61.2 years), more likely to be men (90.8% versus 86.6%), and more likely to have concurrent illness based on concomitant medications, comorbid conditions, RDCI scores, CCP antibody and RF positivity, smoking history, and health care visits (Table 2). Propensity score analysis using matched weights was used to balance covariates (Figure 1).
Table 1.
Sample attritiona
| No. removed | No. remaining | |
|---|---|---|
| Received combination therapy between 2006 and 2012 | – | 14,272 |
| Refilled foundation drug(s) | 1,179 | 13,093 |
| ≥365 followup days and ≥182 preindex days | 789 | 12,304 |
| Age >18 years | 0 | 12,304 |
| RA diagnosisb | 3,402 | 8,902 |
| Exclude PsO, PsA, AS, UC, CD, JIA diagnosisb | 567 | 8,335 |
| Exclude MTX J‐code | 56 | 8,279 |
| Exclude index drug during preindex period | 1,038 | 7,241 |
| ≥1 drug had no prescription in preindex period | 1,075 | 6,166 |
| Exclude noneligible biologic DMARD in preindex period | 202 | 5,964 |
| Exclude noneligible nonbiologic DMARDc | 1,551 | 4,413 |
| Exclude noneligible DMARD on index date | 49 | 4,364 |
RA = rheumatoid arthritis; PsO = psoriasis; PsA = psoriatic arthritis; AS = ankylosing spondylitis; UC = ulcerative colitis; CD = Crohn's disease; JIA = juvenile idiopathic arthritis; MTX = methotrexate; DMARD = disease‐modifying antirheumatic drug.
Between 182 days before and day 28.
Between 30 days before and day 28.
Table 2.
Propensity score analysis for unadjusted and adjusted covariates measured in the year before the start of followupa
| Unadjusted | Matched weights adjusted | |||||
|---|---|---|---|---|---|---|
| Method | TNFi + MTX | Triple therapy | Standard difference | TNFi + MTX | Triple therapy | Standard difference |
| No. of patients | 3,204 | 1,160 | – | 1,143.2 | 1,136.1 | – |
| Demographics | ||||||
| Age, mean ± SD years | 61.2 ± 10.8 | 62.2 ± 9.8 | 0.098b | 62.2 ± 6.1 | 62.2 ± 9.7 | 0.001 |
| Body mass index, mean ± SD kg/m2 | 29.4 ± 6.3 | 29.7 ± 8.0 | 0.038 | 29.7 ± 3.7 | 29.7 ± 7.9 | 0.001 |
| Male | 2,775 (86.6) | 1,053 (90.8) | 0.132c | 1,035.9 (90.6) | 1,029.5 (90.6) | < 0.001 |
| VA drug class | ||||||
| Distinct classes, mean ± SD | 9.3 ± 4.9 | 11.2 ± 5.2 | 0.373c | 11.1 ± 3.3 | 11.0 ± 4.8 | 0.038 |
| Opioid analgesics | 1,269 (39.6) | 479 (41.3) | 0.034 | 468.5 (41.0) | 462.3 (40.7) | 0.006 |
| Nonopioid analgesics | 733 (22.9) | 364 (31.4) | 0.192c | 351.8 (30.8) | 345.7 (30.4) | 0.007 |
| Salicylates, antirheumatic | 62 (1.9) | 34 (2.9) | 0.065d | 31.4 (2.7) | 31.2 (2.7) | < 0.001 |
| Prescription NSAID | 1,220 (38.1) | 500 (43.1) | 0.103b | 495.9 (43.4) | 491.6 (43.3) | 0.002 |
| Proton pump inhibitor | 1,272 (39.7) | 559 (48.2) | 0.172c | 555.5 (48.6) | 542.3 (47.7) | 0.017 |
| Any prednisone | 1,559 (48.7) | 649 (56.0) | 0.146c | 642.8 (56.2) | 629.3 (55.4) | 0.017 |
| CCS comorbidity | ||||||
| Distinct count, mean ± SD | 9.6 ± 5.9 | 10.6 ± 6.9 | 0.157c | 10.3 ± 3.9 | 10.3 ± 6.5 | 0.012 |
| Tuberculosis | 22 (0.7) | 3 (0.3) | 0.062 | 3.0 (0.3) | 3.0 (0.3) | < 0.001 |
| CHF, nonhypertensive | 56 (1.7) | 55 (4.7) | 0.170c | 40.8 (3.6) | 41.2 (3.6) | 0.003 |
| Pneumonia | 31 (1.0) | 21 (1.8) | 0.072§ | 15.4 (1.3) | 15.3 (1.3) | < 0.001 |
| Acute bronchitis | 47 (1.5) | 18 (1.6) | 0.007 | 16.1 (1.4) | 16.4 (1.4) | 0.003 |
| Urinary tract infection | 56 (1.7) | 12 (1.0) | 0.061 | 11.7 (1.0) | 11.6 (1.0) | 0.001 |
| Skin infection | 81 (2.5) | 37 (3.2) | 0.040 | 36.4 (3.2) | 34.8 (3.1) | 0.007 |
| Septicemia | 3 (0.1) | 6 (0.5) | 0.077b | 2.5 (0.2) | 2.2 (0.2) | 0.006 |
| Shock | 1 (0.0) | 1 (0.1) | 0.023 | 1.0 (0.1) | 1.0 (0.1) | < 0.001 |
| HIV infection | 3 (0.1) | 1 (0.1) | 0.003 | 1.0 (0.1) | 1.0 (0.1) | 0.001 |
| Hepatitis | 39 (1.2) | 15 (1.3) | 0.007 | 15.7 (1.4) | 14.9 (1.3) | 0.006 |
| Alcohol‐related disorders | 89 (2.8) | 62 (5.3) | 0.13c | 54.4 (4.8) | 54.2 (4.8) | 0.001 |
| Substance‐related disorders | 61 (1.9) | 32 (2.8) | 0.057 | 29.5 (2.6) | 28.8 (2.5) | 0.003 |
| RDCI score, mean ± SD | 1.6 ± 1.5 | 1.8 ± 1.6 | 0.130c | 1.8 ± 0.9 | 1.8 ± 1.6 | 0.009 |
| CCP antibody test and result | ||||||
| Any CCP antibody test | 2,375 (74.1) | 940 (81.0) | 0.166c | 866.5 (75.8) | 920.8 (81.1) | 0.128 |
| CCP antibody positive | 1,774 (55.4) | 682 (58.8) | 0.069§ | 613.5 (70.8) | 670.6 (72.8) | 0.045 |
| Rheumatoid factor test and result | ||||||
| Any rheumatoid factor test | 2,908 (90.8) | 1,079 (93.0) | 0.083§ | 1,042.8 (91.2) | 1,057.5 (93.1) | 0.069 |
| Rheumatoid factor positive | 1,995 (62.3) | 772 (66.6) | 0.090b | 740.8 (71.0) | 755.2 (71.4) | 0.008 |
| Smoking | ||||||
| Smoking status reported | 2,933 (91.5) | 1,082 (93.3) | 0.066 | 1,056.9 (92.5) | 1,058.9 (93.2) | 0.029 |
| Ever smoked | 2,048 (63.9) | 793 (68.4) | 0.094b | 770.3 (72.9) | 776.0 (73.3) | 0.009 |
| No. of visits, mean ± SD | ||||||
| Emergency department | 0.0 ± 0.2 | 0.0 ± 0.3 | 0.050 | 0.0 ± 0.2 | 0.0 ± 0.3 | 0.004 |
| Rheumatology | 2.5 ± 1.8 | 2.6 ± 1.7 | 0.096b | 2.6 ± 1.1 | 2.6 ± 1.7 | 0.014 |
| Urgent care | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.044 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.008 |
| Inpatient | 0.1 ± 0.4 | 0.2 ± 0.6 | 0.164c | 0.1 ± 0.3 | 0.1 ± 0.5 | 0.009 |
Values are the number (%) unless indicated otherwise. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate; VA = Veterans Affairs; NSAID = nonsteroidal antiinflammatory drug; CCS = Clinical Classification System; CHF = congestive heart failure; HIV = human immunodeficiency virus; RDCI = rheumatic disease comorbidity index; CCP = cyclic citrullinated peptide.
P < 0.01.
P < 0.001.
P < 0.05.
Figure 1.

Propensity score analysis with standardized difference scores before and after application of matched weights. VA = Veterans Affairs; NSAID = nonsteroidal antiinflammatory drug; CCS = Clinical Classification System; HIV = human immunodeficiency virus; RDCI = rheumatic disease comorbidity index; CCP = cyclic citrullinated peptide; ED = emergency department.
Persistence results
Crude analyses and those using a matched weights–adjusted model for each definition of persistence are summarized in Table 3. In each propensity‐weighted analysis, persistence was significantly higher for patients who initiated TNFi + MTX versus patients who initiated triple therapy. The absolute risk difference (RD) of persistence was 13.1% (95% confidence interval [95% CI] 9.2–17.0) for definition 1 (Figure 2), 6.4% (95% CI 2.3–10.5) for definition 2 (Figure 3), and 9.5% (95% CI 5.5–13.6) for definition 3 (Figure 4). Kaplan‐Meier plots for the propensity‐matched population by persistence definition consistently showed that persistence with triple therapy was significantly lower than with combination TNFi therapy.
Table 3.
Persistence and adherence for crude and adjusted rates at 1 yeara
| Outcome | TNFi + MTX | Triple therapy | Relative risk (95% CI) | Risk difference, % (95% CI) |
|---|---|---|---|---|
| Crude modelb | ||||
| Persistence | ||||
| Definition 1 | 1,361 (42.5) | 339 (29.2) | 1.45 (1.32–1.60) | 13.3 (10.1–16.4) |
| Definition 2 | 1,765 (55.1) | 550 (47.4) | 1.16 (1.09–1.24) | 7.7 (4.3–11.0) |
| Definition 3 | 1,937 (60.5) | 577 (49.7) | 1.22 (1.14–1.30) | 10.7 (7.4–14.1) |
| Adherence | 775 (24.2) | 201 (17.3) | 1.40 (1.21–1.61) | 6.9 (4.2–9.5) |
| Adjusted modelc | ||||
| Persistence | ||||
| Definition 1 | 483.66 (42.3) | 331.86 (29.2) | 1.45 (1.29–1.62) | 13.1 (9.2–17.0) |
| Definition 2 | 613.54 (53.7) | 536.88 (47.3) | 1.14 (1.05–1.23) | 6.4 (2.3–10.5) |
| Definition 3 | 676.31 (59.2) | 563.88 (49.6) | 1.19 (1.10–1.29) | 9.5 (5.5–13.6) |
| Adherence | 279.96 (24.5) | 196.98 (17.3) | 1.41 (1.20–1.66) | 7.2 (3.8–10.5) |
Values are the number (%) unless indicated otherwise. Definition 1 was no gap in therapy of ≥90 days for any drug in the original combination. Definition 2 was no added or switched disease‐modifying antirheumatic drug (DMARD), no decrease to nonbiologic DMARD monotherapy, and no termination of all DMARD therapies. Definition 3 was similar to definition 2 but allowed a switch to another drug within the same class. Adherence was proportion of days covered of ≥80% for each medication in the combination. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate; 95% CI = 95% confidence interval.
TNFi + MTX: n = 3,204; triple therapy: n = 1,160.
TNFi + MTX: n = 1,143.2; triple therapy: n = 1,136.1.
Figure 2.

Kaplan‐Meier plot for persistence with matching weights, definition 1: no gap in therapy of ≥90 days for any drug in the original combination. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/acr.22944/abstract.
Figure 3.
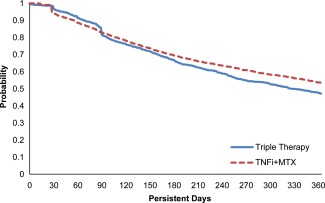
Kaplan‐Meier plot for persistence with matching weights, definition 2: no added or switched disease‐modifying antirheumatic drug (DMARD), no decrease to nonbiologic DMARD monotherapy, and no termination of all DMARD therapies. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/acr.22944/abstract.
Figure 4.

Kaplan‐Meier plot for persistence with matching weights, definition 3: similar to definition 2, but allowed a switch to another drug within the same class. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/acr.22944/abstract.
Adherence results
A higher proportion of patients in the TNFi + MTX group was considered adherent with all therapies at 1 year versus the triple therapy group (Table 3). The proportion of adherent patients (PDC ≥80%) was 24.2% for TNFi + MTX and 17.3% for triple therapy (P < 0.001) in the unadjusted model and 24.5% and 17.3%, respectively, in the adjusted model. The RD for adherence was 6.9% (95% CI 4.2–9.5) in the unadjusted model and 7.2% (95% CI 3.8–10.5) in the adjusted model.
Unadjusted PDCs for individual agents in the TNFi + MTX combination were 48.0% for TNFi and 40.4% for MTX (Table 4). Unadjusted PDCs for individual agents in the triple therapy combination were 29.8% for sulfasalazine, 48.6% for MTX, and 48.0% for hydroxychloroquine.
Table 4.
Unadjusted 1‐year adherence rate (≥80% proportion of days covered) by individual agenta
| Treatment group | Agent | Adherent patients | P |
|---|---|---|---|
| TNFi + MTX (n = 3,204) | Bothb | 775 (24.2) | – |
| TNFi | 1,538 (48.0) | – | |
| MTX | 1,295 (40.4) | – | |
| Triple therapy (n = 1,160) | Allb | 201 (17.3) | < 0.001c |
| Sulfasalazine | 346 (29.8) | – | |
| MTX | 564 (48.6) | – | |
| Hydroxychloroquine | 557 (48.0) | – |
Values are the number (%) unless indicated otherwise. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate.
Proportion of days covered in the first year was ≥80% for both/all drugs in the combination.
Between the TNFi + MTX and triple therapy groups.
DISCUSSION
Lower persistence and adherence represent deficient drug utilization and are often correlated with a greater extent of unrealized treatment benefit and poorer clinical outcomes in RA 24, 25, 26, 27. In this analysis of claims data for 4,364 US veteran patients with RA, initiation of the TNFi + MTX combination was associated with significantly greater treatment persistence in the first year compared with initiation of triple therapy with 3 nonbiologic DMARDs. An observational study of commercially insured patients also showed higher persistence with TNFi + MTX than with triple therapy 11. These findings are consistent with those in 1 open‐label clinical trial 7 but differ from those in 2 blinded clinical trials that showed very similar outcomes in persistence between patients taking TNFi + MTX and those using triple therapy 8, 9. Clinical trials typically try to identify treatment effects that represent the highest internal validity possible. Thus, it is often necessary to ensure that medication usage is tightly controlled and protocol based, leading to high, undifferentiated medication persistence and adherence in all treatment arms. As shown in this study, ideal medication usage environments in clinical trials may overestimate persistence and adherence in the real world where patients and/or physicians may alter therapy use on an as‐needed basis, and there is less patient selection (as in a trial) of patients more likely than not to adhere to the study protocol.
Three methods for defining persistence were used in this analysis to explore the sensitivity of various approaches that used different clinical assumptions. The primary analysis was a direct assessment of persistence without any clinical assumptions; this approach required a patient to continue all drugs in the combination for ≥365 days without any gap in therapy that was of ≥90 days. To explore the potential that patients who were doing well may have discontinued 1 nonbiologic DMARD of the combination, 2 alternative definitions of persistence were used. Persistence definition 2 incorporated the concept of clinical effectiveness into the analysis of persistence by allowing patients to reduce the use of nonbiologic DMARDs. In clinical practice, patients who respond to combination therapy and have low disease activity may interrupt or stop 1 or more nonbiologic DMARDs in the combination. Persistence definition 3 explored potential explanations for differences in persistence by allowing patients to be persistent during 1‐year followup, even if they switched drugs within a class, as long as they did not discontinue to nonbiologic DMARD monotherapy or to no DMARD therapy. The additional assumption was that drugs in the same class would have similar effectiveness, and thus, switching between them would be no different from persistence with the original drug. With these definitions of persistence, rates were higher in both treatment groups than for the primary method and continued to be statistically significantly higher for TNFi + MTX than for triple therapy, as seen with the primary method.
Adherence rates were also significantly higher for TNFi + MTX than for triple therapy. Adherence analysis of individual treatments found lower adherence to sulfasalazine (30%) compared with all other DMARDs (≥40%). One explanation that cannot be confirmed by this study is that once or twice daily therapy with hydroxychloroquine and sulfasalazine, which can require up to 6 tablets per day in divided doses, may be associated with lower persistence and adherence than drug schedules that require MTX injection once weekly and TNFi administration once weekly or less frequently. The observation that sulfasalazine adherence was much less than that for other agents supports this concept but is not conclusive. Previous reports show decreased adherence with multiple daily dosing of sulfasalazine for inflammatory bowel disease 28 and a general decrease in adherence with frequent medication dosing 29, 30. A lack of adherence may be associated with a lack of efficacy that could lead to termination of combination therapy. Provider and patient perceptions could also lead to differential adherence with different combination therapies.
The greatest 1‐day drops in persistence with the initiated combination were at 30 days and 90 days postindex. The clinical reason could not be fully determined but suggests that many patients did not refill their index drug after the first 30‐day or 90‐day prescription. In an additional analysis (not presented here), we evaluated persistence to therapy in each group among patients who were persistent for >100 days to determine whether observed differences were due to initial drops at 30 and 90 days. Persistence patterns were similar to those in the primary report, with a higher level of persistence in patients who initiated TNFi + MTX in comparison with triple therapy, suggesting that an early drop after a single index drug refill could not fully explain the difference in the observed persistence.
Overall, persistence and adherence were low, but these findings are consistent with other analyses conducted in the VA and commercial settings. In a previous study, we found overall adherence for TNFi during the index year to be 50% in RA patients 17. Adherence by TNFi agent ranged from 41% to 50%. Low persistence was also found in a commercial study comparing persistence between etanercept‐MTX and triple therapy initiators 11. At 1 year, only 28% of the etanercept‐MTX group remained persistent, while 18% of the triple therapy group remained persistent. In our study, persistence rates were dependent on the definition but reflected previously published expectations about persistence and adherence during the index year.
Nonadherence and nonpersistence could result in reduced clinical benefit with these therapies. We previously reported our experience using observational data in US Veterans to demonstrate that higher adherence with MTX is associated with improved clinical outcomes, as measured by mean disease activity scores over followup 27. We did not have clinical outcome measures to determine whether differences in adherence were associated with reduced clinical benefit; however, our prior report supports this expectation. Conversely, without clinical outcomes data, we could not determine whether differences in treatment effectiveness or tolerability led to observed differences in adherence and persistence in this analysis, but registry studies have reported that ineffectiveness and adverse events are the leading reasons for treatment discontinuation in RA 31, 32, 33, 34. Many other factors associated with medication adherence 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 were not addressed in this analysis, but there is no reason to suspect that these differences were unevenly distributed in this population between the TNFi + MTX and triple therapy groups. Strategies to improve adherence, particularly during initial treatment phases (e.g., at the first expected refill), possibly could lead to improved clinical outcomes that motivate patients to continue combination therapy.
This study has the strengths of a large population of Veterans across the US as well as a uniform system to capture pharmacy data, which allowed for comparison among these patients from different sites. This study also had several potential limitations. A diagnosis of RA was identified using ICD‐9‐CM diagnosis codes, which are subject to potential miscoding. We could only determine whether patients obtained DMARDs, not whether they actually took DMARDs as prescribed. As this study was a retrospective cohort study, results may not have indicated any causal relationships between exposure and outcomes 48. The study of US Veterans who are predominantly male, older, and with more comorbid conditions than a general RA population may not be generalizable to other patient populations. However, a separate study in a commercially insured population reported results similar to this study 11. The primary analysis provided the most direct assessment of whether a patient persisted with the original combination during the first year of followup, but it did not provide any information about reasons for nonpersistence. Although persistence definitions 2 and 3 were designed to include patients in persistence rates if their changes in therapy were consistent with effective treatment, these changes may also have reflected loss of efficacy, safety issues, drug costs, or patient or provider preferences that could not be assessed in this analysis. Other unmeasured confounders may have affected the association identified between treatment regimen and treatment patterns. Patients who initiated triple therapy had more comorbidities than patients who initiated TNFi + MTX based on baseline covariates, but these differences did not appear to confound the relationship between treatment groups and outcomes, because results in the weighted analysis were similar to crude results. The results of this study are intended to describe, not to influence, current clinical practice.
Given that both outcomes and exposure were defined using pharmacy dispensing and administration data, there was an inherent risk of information bias that could lead to classification error. While many Veterans use VA care exclusively, there are Veterans who receive care outside the VA system. Dual system use to treat RA possibly differed between treatment groups, but in this analysis, the risk for dual system use was assumed to be small. There was no risk of observer bias. This study was limited to adult patients with at least 6 months enrollment before initiating therapy and activity in the system for ≥1 year after the index event. Any correlation between length of enrollment and choice of index biologic agent was unlikely.
In summary, adult patients with RA in the VA were significantly more likely to be persistent and adherent in taking TNFi + MTX combination therapy than in using triple therapy with nonbiologic DMARDs, based on 3 methods used to analyze persistence and a measure of adherence. Additional research is needed to determine the extent to which safety, efficacy, or other factors contribute to differences in persistence and adherence with TNFi + MTX or triple therapy.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Sauer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sauer, Teng, Tang, Leng, Curtis, Mikuls, Harrison, Cannon.
Acquisition of data
Sauer, Teng, Leng, Curtis, Mikuls, Cannon.
Analysis and interpretation of data
Sauer, Teng, Tang, Leng, Curtis, Mikuls, Harrison, Cannon.
ROLE OF THE STUDY SPONSOR
This was an investigator initiated project funded by Amgen. Amgen had no role in the collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Amgen, but did require the review and approval of all authors.
Supporting information
Supplementary Figure 1. Criteria for identifying index into triple and TNFi+MTX combination treatments. A, Example with multiple foundation drugs and 1 index drug for combination therapy. B, Example with 1 foundation drug and 2 index drugs. C, Example where foundation drugs were not filled within 90 days, and patient was not indexed into combination therapy. MTX = methotrexate; No. = number; TNFi = tumor necrosis factor inhibitor.
ACKNOWLEDGMENTS
Jonathan Latham of PharmaScribe, LLC, and Dikran Toroser of Amgen Inc. assisted with the preparation and submission of the manuscript.
Supported by Amgen and by a Veterans Affairs Health Services Research and Development Award.
Dr. Sauer has received consulting fees from 3D Communications (less than $10,000).
Dr. Tang holds stock in Amgen.
Dr. Curtis has received honoraria and/or research grants from Roche/Genentech, UCB, Janssen, Corrona, Amgen (more than $10,000 each), and Pfizer, Bristol‐Myers Squibb, Crescendo, and AbbVie (less than $10,000 each).
Dr. Mikuls has received research support from Roche/Genentech (more than $10,000) and has received consulting fees from Pfizer (less/more than $10,000).
Dr. Harrison holds stock in Amgen.
Dr. Cannon has received research funding from Amgen (more than $10,000).
The copyright line for this article was changed on 23 October 2018 after original online publication.
REFERENCES
- 1. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 2. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken) 2010;62:460–4. [DOI] [PubMed] [Google Scholar]
- 3. Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 4. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux‐Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1‐year results of a randomised trial. Lancet 2009;374:459–66. [DOI] [PubMed] [Google Scholar]
- 8. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St.Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the Treatment of Early Aggressive Rheumatoid Arthritis trial. Arthritis Rheum 2012;64:2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307–18. [DOI] [PubMed] [Google Scholar]
- 10. Van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. Conventional combination treatment versus biological treatment in methotrexate‐refractory early rheumatoid arthritis: 2 year follow‐up of the randomised, non‐blinded, parallel‐group Swefot trial. Lancet 2012;379:1712–20. [DOI] [PubMed] [Google Scholar]
- 11. Bonafede M, Johnson BH, Tang DH, Shah N, Harrison DJ, Collier DH. Etanercept‐methotrexate combination therapy initiators have greater adherence and persistence than triple therapy initiators with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:1656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care 2004;27 Suppl 2:B22–6. [DOI] [PubMed] [Google Scholar]
- 13. Fihn SD, Francis J, Clancy C, Nielson C, Nelson K, Rumsfeld J, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood) 2014;33:1203–11. [DOI] [PubMed] [Google Scholar]
- 14. US Department of Veterans Affairs . VA informatics and computing infrastructure: corporate data warehouse (CDW). URL: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
- 15. Lu CC, Leng J, Cannon G, Zhou X, Harrison DJ, Shah N, et al. Accuracy of a natural language processing software designed to compute average weekly dose from narrative medication schedule [abstract]. Value Health 2014;17:A187–8. [Google Scholar]
- 16. Nelson SD, Lu CC, Teng CC, Leng J, Cannon GW, He T, et al. The use of natural language processing of infusion notes to identify outpatient infusions. Pharmacoepidemiol Drug Saf 2015;24:86–92. [DOI] [PubMed] [Google Scholar]
- 17. Sauer BC, Teng CC, He T, Leng J, Lu CC, Curtis JR, et al. Effectiveness and costs of biologics in veterans with rheumatoid arthritis. Am J Pharm Benefits 2015;7:280–9. [Google Scholar]
- 18. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40:1280–88. [DOI] [PubMed] [Google Scholar]
- 19. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken) 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat 2013;9:215–34. [DOI] [PubMed] [Google Scholar]
- 21. Li L, Greene TH, Sauer BC. Propensity score analysis with matching weights In: Pan W, Bai H, editors. Propensity score analysis: fundamentals and developments. New York: Guilford Press; 2015. p. 141–67. [Google Scholar]
- 22. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Contreras‐Yanez I, Cabiedes J, Villa AR, Rull‐Gabayet M, Pascual‐Ramos V. Persistence on therapy is a major determinant of patient‐, physician‐ and laboratory‐reported outcomes in recent‐onset rheumatoid arthritis patients. Clin Exp Rheumatol 2010;28:748–51. [PubMed] [Google Scholar]
- 25. Contreras‐Yanez I, Ponce De Leon S, Cabiedes J, Rull‐Gabayet M, Pascual‐Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease‐modifying antirheumatic drugs. Am J Med Sci 2010;340:282–90. [DOI] [PubMed] [Google Scholar]
- 26. Waimann CA, Marengo MF, de Achaval S, Cox VL, Garcia‐Gonzalez A, Reveille JD, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum 2013;65:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kane S. Does treatment schedule matter? Once daily versus divided doses of 5‐ASAs. Dig Dis 2010;28:478–82. [DOI] [PubMed] [Google Scholar]
- 29. Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med 1990;150:1881–4. [PubMed] [Google Scholar]
- 30. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 31. Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010;62:22–32. [DOI] [PubMed] [Google Scholar]
- 32. Markenson JA, Gibofsky A, Palmer WR, Keystone EC, Schiff MH, Feng J, et al. Persistence with anti‐tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol 2011;38:1273–81. [DOI] [PubMed] [Google Scholar]
- 33. Iannone F, Gremese E, Atzeni F, Biasi D, Botsios C, Cipriani P, et al. Longterm retention of tumor necrosis factor‐alpha inhibitor therapy in a large Italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol 2012;39:1179–84. [DOI] [PubMed] [Google Scholar]
- 34. Greenberg JD, Reed G, Decktor D, Harrold L, Furst D, Gibofsky A, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 2012;71:1134–42. [DOI] [PubMed] [Google Scholar]
- 35. Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr 2002;31 Suppl 3:S123–7. [DOI] [PubMed] [Google Scholar]
- 36. Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther 1998;20:764–71. [DOI] [PubMed] [Google Scholar]
- 37. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med 2008;149:795–804. [DOI] [PubMed] [Google Scholar]
- 38. Curtis JR, Delzell E, Chen L, Black D, Ensrud K, Judd S, et al. The relationship between bisphosphonate adherence and fracture: is it the behavior or the medication? Results from the placebo arm of the fracture intervention trial. J Bone Miner Res 2011;26:683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med 2004;19:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002;63:892–909. [DOI] [PubMed] [Google Scholar]
- 41. Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, et al. Adherence to antiretroviral medications and medical care in HIV‐infected adults diagnosed with mental and substance abuse disorders. AIDS Care 2009;21:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okuno J, Yanagi H, Tomura S. Is cognitive impairment a risk factor for poor compliance among Japanese elderly in the community? Eur J Clin Pharmacol 2001;57:589–94. [DOI] [PubMed] [Google Scholar]
- 43. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121–8. [DOI] [PubMed] [Google Scholar]
- 44. Sewitch MJ, Abrahamowicz M, Barkun A, Bitton A, Wild GE, Cohen A, et al. Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol 2003;98:1535–44. [DOI] [PubMed] [Google Scholar]
- 45. Stilley CS, Sereika S, Muldoon MF, Ryan CM, Dunbar‐Jacob J. Psychological and cognitive function: predictors of adherence with cholesterol lowering treatment. Ann Behav Med 2004;27:117–24. [DOI] [PubMed] [Google Scholar]
- 46. Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient‐provider race/ethnicity and language concordance matter? J Gen Intern Med 2010;25:1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Servellen G, Chang B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus‐infected men and women. AIDS Patient Care STDS 2002;16:269–81. [DOI] [PubMed] [Google Scholar]
- 48. Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report–part II. Value Health 2009;12:1053–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Criteria for identifying index into triple and TNFi+MTX combination treatments. A, Example with multiple foundation drugs and 1 index drug for combination therapy. B, Example with 1 foundation drug and 2 index drugs. C, Example where foundation drugs were not filled within 90 days, and patient was not indexed into combination therapy. MTX = methotrexate; No. = number; TNFi = tumor necrosis factor inhibitor.


