Abstract
Background
Fixed combination calcipotriol 50 μg/g (Cal) plus betamethasone 0.5 mg/g (BD) foam has been developed as a new treatment option for patients with psoriasis.
Methods
The randomized, parallel‐group, investigator‐blinded Phase III, 12‐week PSO‐ABLE study compared the efficacy and safety of Cal/BD foam with Cal/BD gel. Patients aged ≥18 years with mild‐to‐severe psoriasis were randomized 4:4:1:1 to once‐daily Cal/BD foam, Cal/BD gel, foam vehicle or gel vehicle (NCT02132936). The primary efficacy endpoint was the proportion of patients who were clear/almost clear with a ≥ 2 grade improvement according to the physician's global assessment of disease severity (i.e. treatment success) at week 4 for Cal/BD foam vs. week 8 for Cal/BD gel. Secondary efficacy endpoints included: proportion of patients achieving at least a 75% reduction in modified psoriasis area and severity index (mPASI75), and time to treatment success (TTTS). Safety was monitored throughout.
Results
A total of 463 patients were randomized: Cal/BD foam (n = 185), Cal/BD gel (n = 188), foam vehicle (n = 47), gel vehicle (n = 43); overall completion rate was 90%. Cal/BD foam achieved higher treatment success rates (38% vs. 22%; P < 0.001) and mPASI75 (52% vs. 35%; P < 0.001) by week 4 than Cal/BD gel by week 8. Median TTTS with Cal/BD foam was 6 weeks; this could not be determined for Cal/BD gel as 50% treatment success was not achieved (P < 0.001). Adverse drug reactions were reported in 14 (7.6%) Cal/BD aerosol foam patients and 7 (3.7%) Cal/BD gel patients; all were single events except for itch with Cal/BD aerosol foam (n = 5; 2.7%) and worsening psoriasis with Cal/BD gel (n = 3; 1.6%).
Conclusion
Cal/BD aerosol foam showed significantly greater efficacy after 4 weeks, than 8 weeks of treatment with Cal/BD gel, with similar tolerability.
Introduction
Psoriasis, a chronic, recurrent, immune‐mediated inflammatory disorder,1, 2 impairs quality of life (QoL)3, 4 to a similar extent to that experienced by patients with other chronic diseases such as diabetes or cancer.5 Although many patients with mild‐to‐moderate psoriasis are treated with topical therapy alone,6, 7, 8 adherence remains a significant issue as the daily treatment regimen can be cumbersome and time consuming.9 Studies have shown that the type of topical vehicle used can impact on adherence;10, 11 patients prefer a vehicle that is simple and fast to apply, quickly absorbed and non‐greasy.12
Topical formulations containing corticosteroids and/or vitamin D3 analogues are recommended for treating psoriasis.7, 8, 13, 14, 15 The efficacy and safety of the fixed combination of calcipotriol 50 μg/g (Cal) and betamethasone 0.5 mg/g as dipropionate (BD) has been confirmed in long‐term trials.16, 17, 18, 19 The ointment and gel formulations of this fixed combination are established first‐line treatments.20 An aerosol foam formulation of the fixed Cal/BD combination has been developed to enhance adherence and increase therapeutic options available. Previous Cal/BD aerosol foam studies have shown greater in vitro skin penetration compared with other formulations,21 and a significantly greater antipsoriasis effect over 4 weeks of treatment than Cal/BD ointment,22 vehicle23 and individual active ingredients,24 with a comparable tolerability profile.24, 25 The primary hypothesis of the PSO‐ABLE study was that 4 weeks of Cal/BD aerosol foam has superior efficacy compared with 8 weeks of Cal/BD gel. The PSO‐ABLE study also further investigated the fixed combination aerosol foam and gel, by assessing the efficacy and safety of up to 12 weeks of continued treatment.
Methods
Patients
Eligible patients were aged ≥18 years with mild‐to‐severe psoriasis vulgaris (according to the 5‐point physician's global assessment of disease severity [PGA]), and were amenable to topical therapy. Patients had between 2 and 30% of their body (i.e. trunk and/or limbs) surface area affected by psoriasis, with a modified (excluding the head, which was not treated) Psoriasis Area and Severity Index (mPASI) of ≥2. Patients were excluded if they had received biologic, systemic or phototherapy within 4–16 weeks (dependent on type of therapy) before randomization. Other exclusion criteria were as follows: planned excessive exposure of the treated area to sunlight; planned initiation or change to concomitant medication that could affect psoriasis; current diagnosis of guttate, erythrodermic, exfoliative or pustular psoriasis, or other inflammatory skin disorders; any skin infections; disorders of calcium metabolism associated with hypercalcaemia or hypersensitivity to any component of the investigational products. All patients provided written informed consent.
Study design
PSO‐ABLE was a prospective, multicentre, Phase III, parallel‐group, investigator‐blinded study (ClinicalTrials.gov NCT02132936) conducted in France, UK and the USA (Fig. 1). Patients were randomized 4:4:1:1 to once‐daily Cal/BD aerosol foam, Cal/BD gel, foam vehicle or gel vehicle for up to 12 weeks. Treatment was assigned via a central Interactive Web Response System (IWRS) in accordance with a computer‐generated randomization schedule; randomization was stratified by trial site and baseline disease severity (two strata: mild; at least moderate). Once patient eligibility was confirmed, site personnel entered subject number and PGA score into the IWRS, which then randomized the patient to one of the treatment arms and allocated a kit number. The institutional review board or independent ethics committee of each investigational site approved the protocol. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice.
Figure 1.
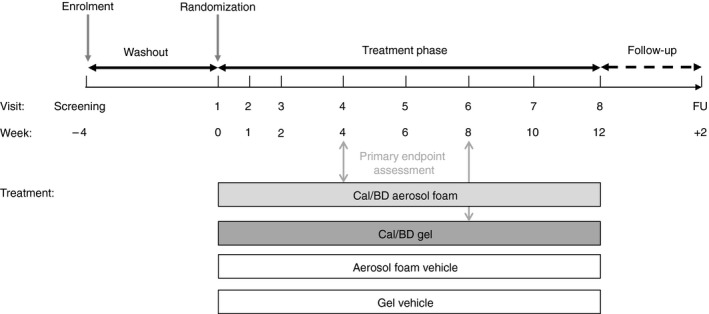
PSO‐ABLE study design. FU, follow‐up.
Objectives and assessments
The primary objective was to compare the efficacy of Cal/BD aerosol foam at week 4 to that of Cal/BD gel at week 8. The primary efficacy endpoint was the proportion of patients who were ‘clear’ or ‘almost clear’ with a ≥ 2 grade improvement in PGA‐assessed disease severity – which was defined as ‘treatment success’ – at week 4 for Cal/BD aerosol foam vs. week 8 for Cal/BD gel.
The secondary objective of the study was to compare the efficacy and safety of Cal/BD aerosol foam to Cal/BD gel for up to 12 weeks of treatment. Additional efficacy endpoints included: changes from baseline in mPASI score; proportion of patients achieving at least a 75% reduction in mPASI (mPASI75); proportion of patients achieving at least a 90% reduction in mPASI (mPASI90) and time to achieving treatment success (TTTS). Time to achieving mPASI75 was also assessed post hoc. Patient preferences and treatment satisfaction were assessed based on the Patient Preference Questionnaire (PPQ; at week 4) and the Topical Therapy Questionnaire (TTaQ; at weeks 4, 8 and 12).26 Both questionnaires were developed based on a systematic literature review, qualitative patient focus interviews and input from expert focus groups, with the aim of facilitating the early identification of specific non‐adherence factors in patients under topical treatment.26 For the TTaQ, a difference of ≥15% between the lowest proportion in one group and the highest proportion in the other group who agreed/strongly agreed with each statement was set as an arbitrary threshold to understand which treatment aspects led to markedly different responses. A number of additional patient‐reported outcomes were also assessed, but will be reported separately. Safety was assessed throughout the 12‐week treatment period by evaluating adverse events (AEs) and adverse drug reactions (ADRs; described as causally related to treatment), and by monitoring laboratory parameters. All AEs and ADRs were assessed according to the Medical Dictionary for Regulatory Activities (MedDRA) Version 15.1 (McLean, Virginia, USA).
Statistical analysis
For the sample size calculation, the proportion of patients achieving treatment success was assumed (based on data from previous studies) to be 52% for Cal/BD aerosol foam at week 4 and 34% for Cal/BD gel at week 8. The power analysis indicated that with a sample size of 168 patients in each group, Fisher's exact test would have 90% power to reject the null hypothesis of no difference in treatment success. As patients were randomized to the respective vehicle group in a 4:1 ratio, 42 patients were required for each vehicle group. Allowing for a withdrawal rate of approximately 8%, the aim was to enrol 460 patients in the following ratio: 184:184:46:46 (Cal/BD aerosol foam, Cal/BD gel, aerosol foam vehicle and gel vehicle).
Categorical outcomes were compared between treatment groups using the Cochran‐Mantel‐Haenszel method, adjusting for pooled centre (if a centre randomized <20 patients, it was pooled with a geographically neighbouring centre to form a pooled centre of ≥20 patients) and baseline PGA. For continuous outcomes, ANCOVA was used, adjusting for pooled centre, baseline PGA and baseline covariate. Missing values for efficacy endpoints were handled by applying multiple imputation (MI), except for TTTS, as no imputation was necessary as it was accounted for by the censoring of data in the model. The efficacy analysis set comprised all randomized patients. The safety analysis set comprised all patients who received at least one dose of study medication and for whom postbaseline safety data were available.
Results
Patients
In total, 504 patients from 41 centres (UK, n = 15; USA, n = 15; France, n = 11) were enrolled; the study was conducted between June 2014 and March 2015. Of these, 463 patients were randomized to Cal/BD aerosol foam (n = 185), Cal/BD gel (n = 188), foam vehicle (n = 47) or gel vehicle (n = 43) (Table 1). The overall completion rate was 89.8% (n = 416) (Fig. 2). Ten patients (5.4%) withdrew from Cal/BD aerosol foam and fourteen patients (7.4%) from Cal/BD gel. Most patients had moderate disease and the overall mean mPASI score was around 7.
Table 1.
Patient demographics and baseline characteristics
| Cal/BD aerosol foam (n = 185) | Cal/BD gel (n = 188) | Aerosol foam vehicle (n = 47) | Gel vehicle (n = 43) | |
|---|---|---|---|---|
| Mean age ± SD, years | 54.0 ± 14.5 | 54.5 ± 14.9 | 54.6 ± 14.2 | 51.9 ± 15.5 |
| Male:Female | 126:59 | 114:74 | 29:18 | 26:17 |
| Mean BMI ± SD, kg/m2 | 29.7 ± 6.1 | 30.3 ± 6.6 | 28.2 ± 4.8 | 28.8 ± 5.3 |
|
Baseline PGA Mild Moderate Severe |
54 (29.2) 109 (58.9) 22 (11.9) |
45 (23.9) 124 (66.0) 19 (10.1) |
14 (29.8) 30 (63.8) 3 (6.4) |
9 (20.9) 32 (74.4) 2 (4.7) |
| Mean duration of psoriasis vulgaris ± SD, years | 19.3 ± 14.1 | 19.0 ± 14.2 | 18.4 ± 13.3 | 20.8 ± 14.4 |
| Mean BSA ± SD, % | 7.1 ± 5.7 | 7.0 ± 5.5 | 7.9 ± 6.4 | 8.2 ± 6.3 |
| Mean mPASI ± SD | 7.1 ± 4.5 | 6.6 ± 3.6 | 7.2 ± 4.1 | 7.4 ± 5.0 |
BD, betamethasone 0.5 mg/g; BMI, body mass index; Cal, calcipotriol 50 μg/g; mPASI, modified psoriasis area and severity index; PGA, physician's global assessment.
Figure 2.
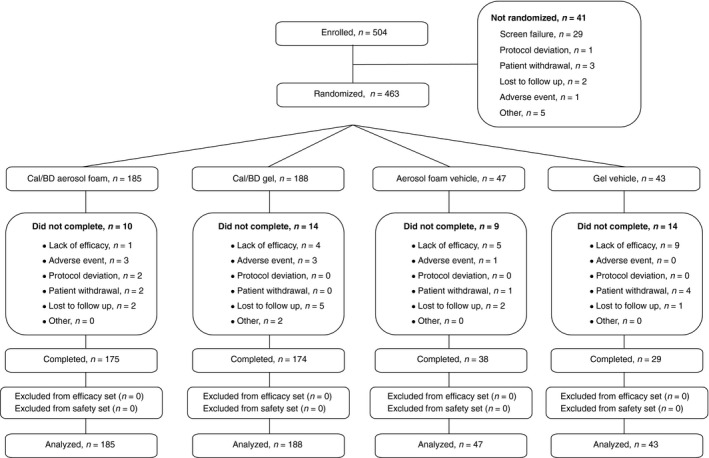
CONSORT diagram.
Efficacy
Treatment success
A significantly larger proportion of Cal/BD aerosol foam‐treated patients achieved treatment success at week 4, compared with Cal/BD gel‐treated patients at week 8 (38.3% vs. 22.5%; odds ratio [OR] 2.55, 95% CI: 1.46, 4.46; P < 0.001) (Fig. 3a). In the Cal/BD aerosol foam group, treatment success rates at week 4 were 18.9% (n = 10/53) in patients with mild disease, 44.8% (n = 47/105) in those with moderate disease and 50.0% (n = 11/22) in those with severe disease at baseline. Equivalent treatment success rates at week 8 in the Cal/BD gel‐treated patients were 0% (n = 0/42), 31.6% (n = 37/117) and 15.8% (n = 3/19), respectively.
Figure 3.
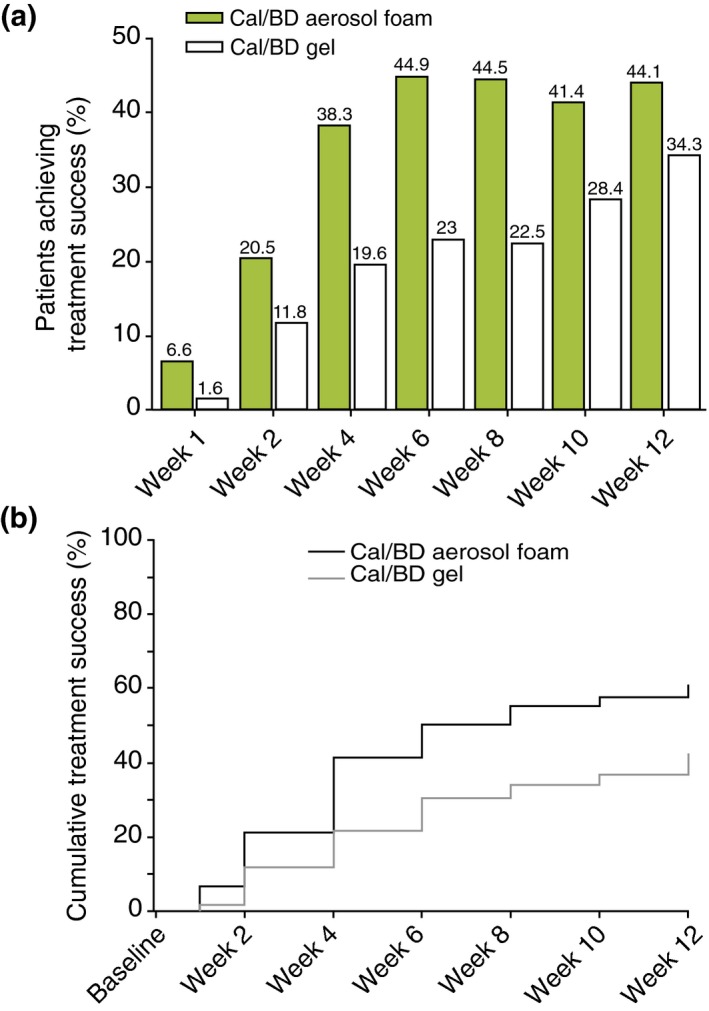
(a) Treatment success rates by visit (MI). (b) Time to treatment success, according to PGA (observed cases), in Cal/BD aerosol foam and gel groups. MI, multiple imputation.
Median TTTS with Cal/BD aerosol foam was 6 weeks; TTTS could not be determined for Cal/BD gel, as 50% treatment success was not achieved by 12 weeks (hazard ratio [HR] 1.97, 95% CI: 1.46, 2.65; P < 0.001) (Fig. 3b).
mPASI
Mean baseline mPASI was 7.1 in the Cal/BD aerosol foam group and 6.6 in the Cal/BD gel group. After baseline, mean mPASI was lower in the Cal/BD aerosol foam group than in the Cal/BD gel group at each time point up to week 12. Adjusted mean mPASI was significantly lower with Cal/BD aerosol foam than Cal/BD gel (4.50 vs. 5.20; adjusted difference −0.70; 95% CI: −1.05 to −0.35; P < 0.001) at week 1. The significant difference was maintained at week 4 for Cal/BD aerosol foam vs. week 8 for Cal/BD gel (2.18 vs. 2.77; adjusted difference −0.59; 95% CI: −1.11, −0.06; P = 0.028).
The proportion of patients achieving mPASI75 was greater with Cal/BD aerosol foam than Cal/BD gel throughout the study. mPASI75 was achieved by significantly more Cal/BD aerosol foam‐treated patients (52.1%) at week 4 than Cal/BD gel‐treated patients (34.6%) at week 8 (OR: 2.18, 95% CI: 1.37, 3.47; P < 0.001) (Fig. 4a). In the Cal/BD aerosol foam group, 54.7% (n = 29/53), 47.6% (n = 50/105) and 63.6% (n = 14/22) of patients with mild, moderate and severe baseline disease, respectively, achieved mPASI75 at week 4; equivalent proportions in the Cal/BD gel‐treated patients at week 8 were as follows: 40.5% (n = 17/42), 35.9% (n = 42/117) and 21.1% (n = 4/19), respectively. mPASI90 results with Cal/BD aerosol foam at week 4 vs. Cal/BD gel at week 8 were 22.2% vs. 10.7% (OR: 2.43, 95% CI: 1.22, 4.82; P = 0.009); at weeks 8 and 12 in the Cal/BD aerosol foam group, mPASI90 scores were 29.0% and 22.9%, respectively.
Figure 4.
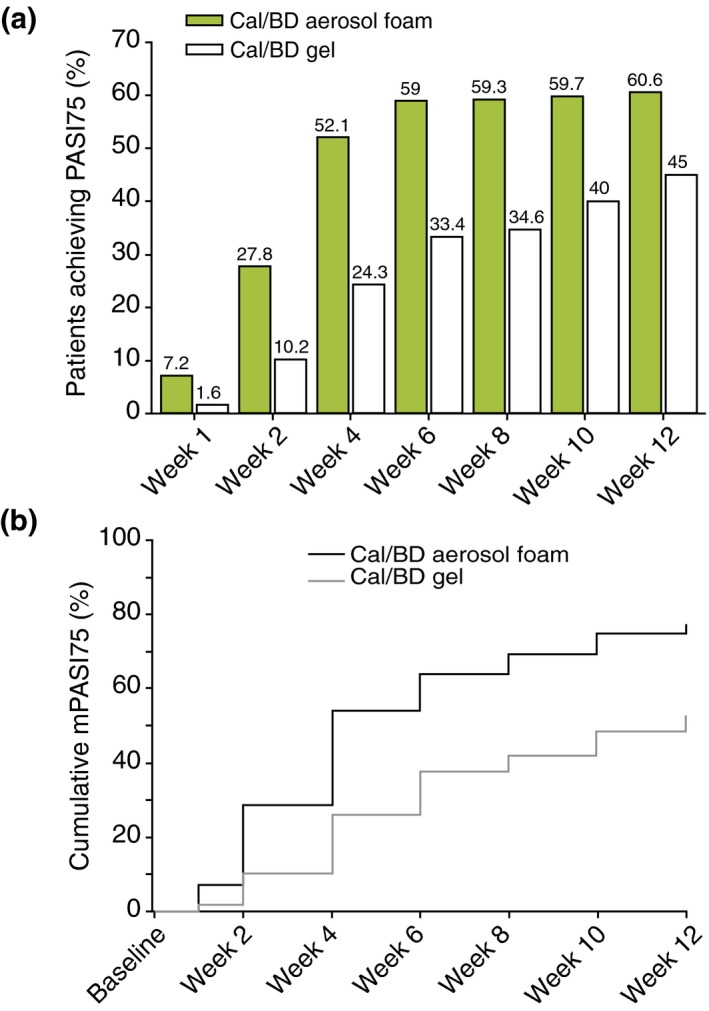
(a) mPASI75 by visit in each treatment group (MI). (b) Time to mPASI75, according to PGA (observed cases). MI, multiple imputation.
Median time to mPASI75 was 4 weeks with Cal/BD aerosol foam and 12 weeks with Cal/BD gel (Fig. 4b).
Drug consumption
The mean total amount of Cal/BD aerosol foam used from baseline to week 4 was 98.6 g, whereas the mean total amount of Cal/BD gel used from baseline to week 8 was 164.3 g. Over 12 weeks, the amount of Cal/BD aerosol foam used was 236.4 g, whereas the total amount of Cal/BD gel used was 193.1 g.
Safety
Adverse events were reported in a similar proportion of Cal/BD aerosol foam (n = 77, 41.6%) and the Cal/BD gel (n = 85, 45.2%) patients over the 12‐week treatment period. The most common AEs overall were upper respiratory tract infection, nasopharyngitis and vitamin D deficiency (Table 2); baseline blood levels of 25‐hydroxy vitamin D were classified as ‘low’ in 281 patients (60.7%). In the five Cal/BD foam patients with pruritus, it was lesional/perilesional in 3/5 and distant from lesions in 2/5 patients; all three patients in the Cal/BD gel and foam vehicle groups reported lesional/perilesional pruritus. Itch was reported as an AE by five patients receiving Cal/BD aerosol foam (2.7%) and two receiving Cal/BD gel (1.1%). Most AEs were mild or moderate; only 12 patients experienced a severe AE (n = 6 in the Cal/BD aerosol foam group, n = 5 in the Cal/BD gel group and n = 1 in the foam vehicle group).
Table 2.
Most common AEs during 12 weeks of treatment, by MedDRA primary system organ class and preferred term (>2 patients in the Cal/BD aerosol foam group)
| Cal/BD aerosol foam (n = 185) | Cal/BD gel (n = 188) | Aerosol foam vehicle (n = 47) | Gel vehicle (n = 43) | |
|---|---|---|---|---|
| Nasopharyngitis | 7 (3.8) | 4 (2.1) | 0 | 2 (4.7) |
| Vitamin D deficiency | 6 (3.2) | 5 (2.7) | 0 | 2 (4.7) |
| Upper respiratory tract infection | 5 (2.7) | 9 (4.8) | 1 (2.1) | 2 (4.7) |
| Pruritus | 5 (2.7) | 2 (1.1) | 1 (2.1) | 0 |
| Back pain | 5 (2.7) | 3 (1.6) | 1 (2.1) | 2 (4.7) |
| Diarrhoea | 4 (2.2) | 2 (1.1) | 0 | 2 (4.7) |
| Psoriasis | 4 (2.2) | 7 (3.7) | 1 (2.1) | 0 |
| Lower respiratory tract infection | 4 (2.2) | 1 (0.5) | 0 | 1 (2.3) |
| Gastro‐oesophageal reflux disease | 3 (1.6) | 1 (0.5) | 1 (2.1) | 0 |
| Influenza‐like illness | 3 (1.6) | 2 (1.1) | 0 | 1 (2.3) |
AE, Adverse event; BD, betamethasone 0.5 mg/g; Cal, calcipotriol 50 μg/g; MedDRA, Medical Dictionary for Regulatory Activities.
Serious AEs were reported in four patients (2.2%) receiving Cal/BD aerosol foam (congestive heart failure, gastro‐oesophageal reflux, prostate cancer, exacerbation of psoriasis) and three (1.6%) receiving Cal/BD gel (postprocedural haemorrhage, type 2 diabetes mellitus, ischaemic stroke). One serious AE was considered related to Cal/BD aerosol foam treatment (exacerbation of psoriasis after 69 days of treatment). There were no deaths. ADRs were reported in 14 patients (7.6%) in the Cal/BD aerosol foam group, and 7 (3.7%) in the Cal/BD gel group. All were single events except for itch with Cal/BD aerosol foam (n = 5; 2.7%) and worsening psoriasis with Cal/BD gel (n = 3; 1.6%). No clinically significant changes in mean albumin‐corrected serum calcium or spot urinary calcium:creatine ratio were seen in any treatment groups.
Patient preferences
Patient preference questionnaire
Both Cal/BD aerosol foam and gel scored higher than previous topical (Table 3) and systemic therapies (Table S1) on all preference parameters. However, a higher proportion of patients receiving Cal/BD aerosol foam than Cal/BD gel favoured their study treatment over previous treatments (i.e. ‘strongly agreed’ or ‘agreed’ with each statement). PPQ data were also evaluated based on previous topical treatment. In general, a similar proportion of patients receiving Cal/BD aerosol foam ‘strongly agreed’ or ‘agreed’ with each statement, irrespective of previous treatment (Fig. 5; Fig. S1 a–d).
Table 3.
Patient preferences at week 4 compared with previous topical therapies
| Cal/BD aerosol foam | Cal/BD gel | Aerosol foam vehicle | Gel vehicle | |
|---|---|---|---|---|
|
‘More effective’ (responders)
Strongly agree/agree Strongly disagree/disagree Does not apply to me |
(179) 158 (88.3) 16 (8.9) 5 (2.8) |
(181) 122 (67.4) 54 (29.8) 5 (2.8) |
(44) 21 (47.7) 23 (52.3) 0 |
(34) 11 (32.4) 23 (67.6) 0 |
|
‘Easier to apply’ (responders)
Strongly agree/agree Strongly disagree/disagree Does not apply to me |
(179) 137 (76.5) 39 (21.8) 3 (1.7) |
(179) 116 (64.8) 58 (32.4) 5 (2.8) |
(44) 28 (63.6) 15 (34.1) 1 (2.3) |
(34) 16 (47.1) 16 (47.1) 2 (5.9) |
|
‘Fewer side effects’ (responders)
Strongly agree/agree Strongly disagree/disagree Does not apply to me |
(178) 109 (61.2) 34 (19.1) 35 (19.7) |
(178) 92 (51.7) 49 (27.5) 37 (20.8) |
(44) 27 (61.4) 10 (22.7) 7 (15.9) |
(34) 12 (35.3) 14 (41.2) 8 (23.5) |
|
‘More tolerable’ (responders)
Strongly agree/agree Strongly disagree/disagree Does not apply to me |
(179) 145 (81.0) 23 (12.8) 11 (6.1) |
(179) 118 (65.9) 44 (24.6) 17 (9.5) |
(44) 23 (52.3) 18 (40.9) 3 (6.8) |
(34) 14 (41.2) 17 (50.0) 3 (8.8) |
|
‘Prefer current therapy’ (responders)
Strongly agree/agree Strongly disagree/disagree Does not apply to me |
(178) 149 (83.7) 25 (14.0) 4 (2.2) |
(179) 124 (69.3) 49 (27.4) 6 (3.4) |
(43) 21 (48.8) 21 (48.8) 1 (2.3) |
(33) 16 (48.5) 15 (45.5) 2 (6.1) |
BD, betamethasone 0.5 mg/g; Cal, calcipotriol 50 μg/g.
Figure 5.
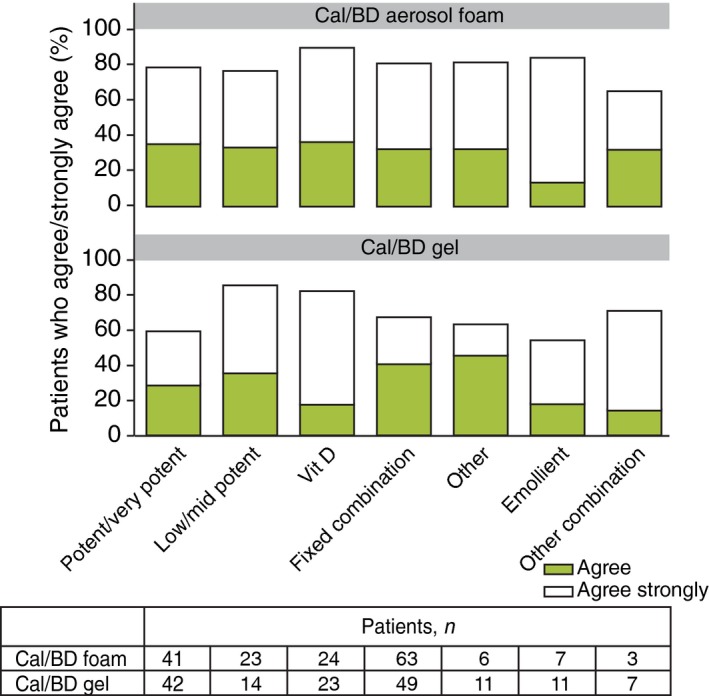
‘Prefer current therapy’: Patient preferences at week 4 compared with previous topical therapies, grouped by previous topical therapy.
Topical therapy questionnaire
Overall, a high proportion of patients in both the Cal/BD aerosol foam and gel groups agreed/strongly agreed with the TTaQ statements regarding satisfaction with effectiveness of therapy and how quickly therapy took effect, time spent on treatment and would repeat/continue on therapy (Table S2); other statements of interest are summarized in Table S2.
Discussion
In the PSO‐ABLE study, 4 weeks of Cal/BD aerosol foam was significantly more effective than 8 weeks of Cal/BD gel in patients with psoriasis. This superiority was obtained by week 1 and maintained throughout the 12‐week treatment period. Of note, Cal/BD aerosol foam was effective irrespective of baseline disease severity. Cal/BD aerosol foam was well tolerated, maintaining the safety profile of established fixed‐combination Cal/BD formulations.
As highlighted by the cumulative TTTS and time to mPASI75 (Figs. 3b and 4b), patients continued to achieve benefit from Cal/BD aerosol foam and gel throughout the study. This suggests that there can be a potential added benefit of continuing treatment for up to 12 weeks if a patient is not clear/almost clear of psoriasis after the initial recommended treatment period. The treatment success rate with Cal/BD aerosol foam at week 4 was slightly lower in PSO‐ABLE than in previous studies; the same was true for Cal/BD gel. One possible explanation for this could be that a greater proportion of patients with mild disease were enrolled (30% compared with 16% in PSO‐FAST,23 9% in one Phase II study24 and 16% in another Phase II study25). Whereas moderate and severe patients are considered to achieve treatment success if they are ‘clear’ or ‘almost clear’, mild patients need to be completely clear of psoriasis to be considered as such; this makes it more difficult for mild patients to achieve treatment success according to the predefined treatment success rule using PGA. Achieving complete clearance of psoriasis is a more stringent criterion than being ‘clear’ or ‘almost clear’, even for mild patients. This may also explain why the treatment success rates at week 4 were lower in patients with mild disease compared to those with moderate and severe disease (18.9% vs. 44.8% and 50.0%, respectively). Importantly, the proportion of patients achieving mPASI75 at week 4 in PSO‐ABLE (52%) was comparable to that observed in previous studies (53% in PSO‐FAST,23 49% in one Phase II study24 and 50% in another Phase II study25). Based on the study protocol, patients who achieved treatment success were allowed to discontinue treatment, but were required to attend all scheduled visits until study end.
The safety profile of Cal/BD aerosol foam was similar to previous studies23, 24, 25 and was consistent with the fixed combination gel and ointment formulations.27, 28, 29 The rates of AEs and ADRs with Cal/BD aerosol foam in PSO‐ABLE cannot be compared to previous studies due to the longer observation period (12 vs. 4 weeks). Nevertheless, the incidence of AEs and ADRs was low and similar to that observed in the Cal/BD gel and foam vehicle groups. Overall, this 12‐week study confirms that Cal/BD aerosol foam is well tolerated and may have a favourable benefit–risk profile in a chronic disease such as psoriasis.
Poor patient perception of effectiveness and concerns around the topical treatment formulation are drivers of inadequate adherence.30 Preference data indicate that a greater proportion of patients receiving Cal/BD aerosol foam than Cal/BD gel thought it was more effective, easier to apply and generally preferred it compared with previous topical and systemic therapies. These observations may be important in clinical practice, given that adherence to topical therapy remains a significant issue.9 Previous studies have shown that if patients think a treatment is effective and easy to use they are more likely to be adherent,9, 31 which should lead to better treatment outcomes. Although further studies are needed, these patient preference data suggest that Cal/BD aerosol foam may address some of the current unmet needs of the psoriasis patient population.
One possible criticism of the study design is that differing treatment periods were used for the primary endpoint. However, PSO‐ABLE was specifically designed to compare Cal/BD aerosol foam and Cal/BD gel based on the recommended treatment periods in the approved Food and Drug Administration labels, i.e. 4 weeks for Cal/BD aerosol foam vs. 8 weeks for Cal/BD gel. As such, the study design was deemed acceptable as it reflects the recommended use of each formulation in clinical practice.
In conclusion, this study shows that 4 weeks of Cal/BD aerosol foam treatment is significantly more effective than 8 weeks of treatment with Cal/BD gel (with lower drug consumption). This superior efficacy, combined with a similar safety profile and with the reported patient preference for Cal/BD aerosol foam over previous topical and systemic therapies, should lead to increased patient adherence and improved QoL and real‐world treatment outcomes. Patients who are not clear/almost clear of psoriasis after 4 or 8 weeks may benefit from continuing treatment for up to 12 weeks, with a favourable safety profile over this extended period.
Supporting information
Fig. S1. Patient preferences at week 4 compared with previous topical therapies, grouped by previous topical therapy (a) ‘more effective’; (b) ‘easier to apply’; (c) ‘fewer side effects’ and (d) ‘more tolerable’.
Table S1. Patient preferences at week 4 compared with previous systemic therapies.
Table S2. TTaQ statements: Proportion of patients who agreed/strongly agreed.
Acknowledgements
This study was sponsored by LEO Pharma. Medical writing support was provided by Andrew Jones PhD, from Mudskipper Business Ltd, funded by LEO Pharma.
Conflicts of interest
CP has been an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, LEO Pharma, Lilly, Novartis and Pfizer. LSG has been an investigator and advisor for LEO Pharma. FC has been an investigator and speaker for LEO Pharma. REK has been an investigator for LEO Pharma. DL and BB are employees of LEO Pharma. CEMG is a National Institute for Health Research Senior Investigator and has received honoraria, lecture fees or research grants from AbbVie, Amgen, BMS, Galderma, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Sandoz, Stiefela GSK company and UCB Pharma.
Funding sources
This study was sponsored by LEO Pharma.
References
- 1. Menter A, Gottlieb A, Feldman SR et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826–850. [DOI] [PubMed] [Google Scholar]
- 2. Schön MP, Boehncke W‐H. Psoriasis. N Engl J Med 2005; 352: 1899–1912. [DOI] [PubMed] [Google Scholar]
- 3. Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 2004; 51: 704–708. [DOI] [PubMed] [Google Scholar]
- 4. Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient‐membership survey. Arch Dermatol 2001; 137: 280–284. [PubMed] [Google Scholar]
- 5. Møller AH, Erntoft S, Vinding GR, Jemec GB. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ‐5D‐derived utility values. Patient Relat Outcome Meas 2015; 6: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebwohl M, Ting PT, Koo JY. Psoriasis treatment: traditional therapy. Ann Rheum Dis 2005; 64(Suppl 2): ii83–ii86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menter A, Korman NJ, Elmets CA et al Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60: 643–659. [DOI] [PubMed] [Google Scholar]
- 8. Nast A, Boehncke WH, Mrowietz U et al German S3‐guidelines on the treatment of psoriasis vulgaris (short version). Arch Dermatol Res 2012; 304: 87–113. [DOI] [PubMed] [Google Scholar]
- 9. Devaux S, Castela A, Archier E et al Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 61–67. [DOI] [PubMed] [Google Scholar]
- 10. Puig L, Carrascosa JM, Belinchon I et al Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr 2013; 104: 488–496. [DOI] [PubMed] [Google Scholar]
- 11. Tan X, Feldman SR, Chang J, Balkrishnan R. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv 2012; 9: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 12. Eastman WJ, Malahias S, Delconte J, DiBenedetti D. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis 2014; 94: 46–53. [PubMed] [Google Scholar]
- 13. Samarasekera E, Sawyer L, Parnham J, Smith CH. Assessment and management of psoriasis: summary of NICE guidance. BMJ 2012; 345: e6712. [DOI] [PubMed] [Google Scholar]
- 14. Paul C, Gallini A, Archier E et al Evidence‐based recommendations on topical treatment and phototherapy of psoriasis: systematic review and expert opinion of a panel of dermatologists. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 1–10. [DOI] [PubMed] [Google Scholar]
- 15. Devaux S, Castela A, Archier E et al Topical vitamin D analogues alone or in association with topical steroids for psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 52–60. [DOI] [PubMed] [Google Scholar]
- 16. Kragballe K, Austad J, Barnes L et al Efficacy results of a 52‐week, randomised, double‐blind, safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Daivobet®/Dovobet®/Taclonex®) in the treatment of psoriasis vulgaris. Dermatology 2006; 213: 319–326. [DOI] [PubMed] [Google Scholar]
- 17. Kragballe K, Austad J, Barnes L et al A 52‐week randomized safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris. Br J Dermatol 2006; 154: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 18. Lambert J, Hol CW, Vink J. Real‐life effectiveness of once‐daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: final analysis of the 52‐week PRO‐long study. J Eur Acad Dermatol Venereol 2015; 29: 2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luger TA, Cambazard F, Larsen FG et al A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long‐term management of scalp psoriasis. Dermatology 2008; 217: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother 2010; 11: 1999–2009. [DOI] [PubMed] [Google Scholar]
- 21. Hollesen Basse L, Olesen M, Lacour JP, Queille‐Roussel C. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol 2014; 134: S33: abst 192. [Google Scholar]
- 22. Queille‐Roussel C, Olesen M, Villumsen J, Lacour JP. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig 2015; 35: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leonardi C, Bagel J, Yamauchi P et al Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris ‐ a randomized Phase III study (PSO‐FAST). J Drugs Dermatol 2015; 14: 1468–1477. [PubMed] [Google Scholar]
- 24. Lebwohl M, Tyring S, Bukhalo M et al Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double‐blind, multicenter, three‐arm, Phase II study. J Clin Aesthet Dermatol 2016; 9: 34–41. [PMC free article] [PubMed] [Google Scholar]
- 25. Koo J, Tyring S, Werschler WP et al Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris ‐ A randomized phase II study. J Dermatolog Treat 2016; 27: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zschocke I, Mrowietz U, Lotzin A, Karakasili E, Reich K. Assessing adherence factors in patients under topical treatment: development of the Topical Therapy Adherence Questionnaire (TTAQ). Arch Dermatol Res 2014; 306: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menter A, Stein Gold L, Bukhalo M et al Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double‐blind, vehicle‐controlled trial. J Drugs Dermatol 2013; 12: 92–98. [PubMed] [Google Scholar]
- 28. Guenther L, van de Kerkhof PC, Snellman E et al Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double‐blind, vehicle‐controlled clinical trial. Br J Dermatol 2002; 147: 316–323. [DOI] [PubMed] [Google Scholar]
- 29. Douglas WS, Poulin Y, Decroix J et al A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol 2002; 82: 131–135. [DOI] [PubMed] [Google Scholar]
- 30. Zschocke I, Mrowietz U, Karakasili E, Reich K. Non‐adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol 2014; 28(Suppl 2): 4–9. [DOI] [PubMed] [Google Scholar]
- 31. Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol 2011; 25(Suppl 4): 9–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Patient preferences at week 4 compared with previous topical therapies, grouped by previous topical therapy (a) ‘more effective’; (b) ‘easier to apply’; (c) ‘fewer side effects’ and (d) ‘more tolerable’.
Table S1. Patient preferences at week 4 compared with previous systemic therapies.
Table S2. TTaQ statements: Proportion of patients who agreed/strongly agreed.


