Abstract
Macrophages play a crucial role in maintaining homeostasis in the intestine, but the underlying mechanisms have not yet been elucidated fully. Here, we show for the first time that mature intestinal macrophages in mouse intestine express high levels of αvβ5 integrin, which acts as a receptor for the uptake of apoptotic cells and can activate molecules involved in several aspects of tissue homeostasis such as angiogenesis and remodeling of the ECM. αvβ5 is not expressed by other immune cells in the intestine, is already present on intestinal macrophages soon after birth, and its expression is not dependent on the microbiota. In adults, αvβ5 is induced during the differentiation of monocytes in response to the local environment and it confers intestinal macrophages with the ability to promote engulfment of apoptotic cells via engagement of the bridging molecule milk fat globule EGF-like molecule 8. In the absence of αvβ5, there are fewer monocytes in the mucosa and mature intestinal macrophages have decreased expression of metalloproteases and IL 10. Mice lacking αvβ5 on haematopoietic cells show increased susceptibility to chemical colitis and we conclude that αvβ5 contributes to the tissue repair by regulating the homeostatic properties of intestinal macrophages.
Keywords: Homeostasis, Intestine, Macrophage, Phagocytosis, αvβ5 integrin
Introduction
Macrophages (mϕ) are essential for both homeostasis and active immunity in the intestine, as well as playing pathogenic roles in inflammatory disorders such as Crohn’s disease (CD) and ulcerative colitis (UC) [1, 2]. Unlike many other tissue mϕ, those in the intestine require constant replenishment by circulating blood monocytes that then differentiate locally under control of factors present in their environment [3]. This process normally generates mϕ with high phagocytic activity and expression of scavenger receptors associated with clearance of apoptotic cells, but they lose the ability to produce proinflammatory mediators in response to conventional stimuli. Rather, they produce the anti-inflammatory cytokine IL-10, as well as trophic factors that maintain epithelial integrity and metalloproteinases (MMPs) involved in tissue remodeling [4, 5]. Thus resident intestinal mϕ may contribute to intestinal homeostasis by clearing effete tissue cells and repairing the resulting damage. However, the roles of individual scavenger molecules in these processes are unknown.
Here, we demonstrate that one of the most significantly upregulated proteins during intestinal mϕ development is αvβ5 integrin, an adhesion molecule crucial for clearance of effete rod and cone photoreceptor outer segment tips in the retina [6]. As mice lacking αvβ5 integrin had dysregulated populations of the monocyte-mϕ lineage in the intestine and were more susceptible to chemical colitis, αvβ5 may play a crucial role in the regulation of intestinal homeostasis by mϕ.
Results and discussion
αvβ5 Integrin on mature intestinal macrophages enables phagocytic uptake of apoptotic cells
Mature mϕ isolated from normal colon contained inclusion bodies that stained for cytokeratin (Fig. 1A and B), suggesting constitutive uptake of apoptotic epithelial cells in situ. Consistent with this, as colonic mϕ from CX3CR1gfp/+ mice differentiated into mature CX3CR1hi cells (P4) from Ly6ChiMHCII−CX3CR1int monocytes (P1) via Ly6ChiMHCII+CX3CR1int (P2) and Ly6ChiMHCII+CX3CR1int (P3) intermediaries (Supporting Information Fig. S1), they showed progressive upregulation of mRNA for receptors associated with uptake of apoptotic cells (Fig. 1C, Supporting Information Fig. S2 and [5]).
Figure 1.
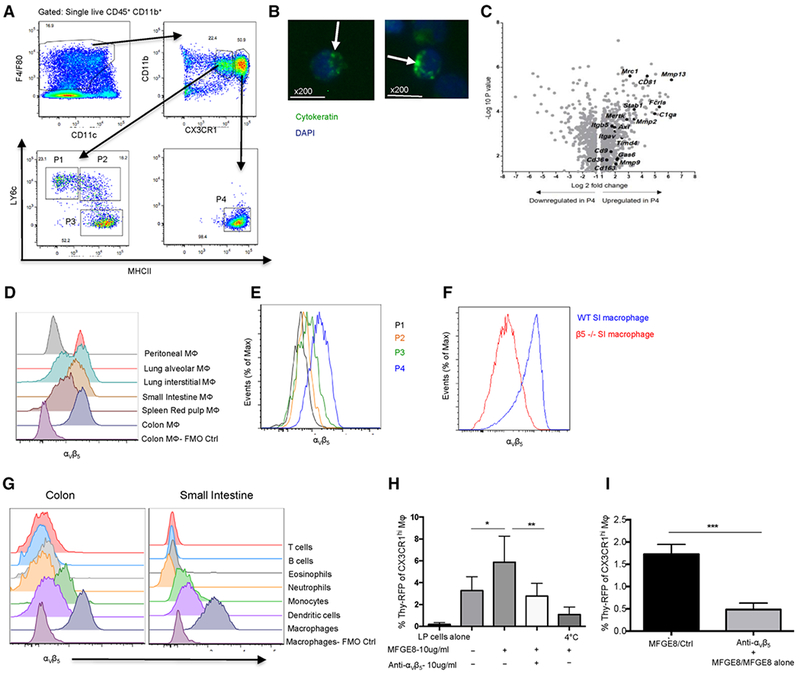
Expression of αvβ5 integrin by mature intestinal macrophages enables phagocytic uptake of apoptotic cells. (A) Gating strategy showing the differentiation of CX3CR1hiMHCIIhi colonic macrophages (P4) from CX3XR1intLy6ChiMHCII− monocytes (P1) via CX3CR1int intermediaries (P2 & P3) amongst live CD45+CD11b+F4/80+CD11c+/− cells in CX3CR1gfp/+ mice. (B) Cytokeratin+ inclusions (green – arrowed) in sorted CX3CR1hi macrophages (Final Magnification ×200). Image is representative of two independent experiments. (C) Volcano plot showing genes up- or downregulated in CX3XR1hiLy6C−iMHCII+ colonic macrophages compared with P1 monocytes. Data are from one microarray experiment using three biological replicates containing cells pooled from 4–5 mice [5] (GEO GSE84764) (D) Expression of integrin αvβ5 on CD11b+ F4/80hi peritoneal macrophages, alveolar macrophages (CD64hiCD11chiSiglecFhiMHCIIloCD11b−), lung interstitial macrophages (CD64loCD11bhiMHCII+SiglecFloCD11clo/+), splenic red pulp macrophages (F4/80hiMHCII+CD11blo/−CD11c−), and CX3CR1hi macrophages (P4) from small intestine and colon of Cx3cr1+/gfp mice. (E) Expression of αvβ5 on monocyte/macrophage subsets in the small intestine of Cx3cr1+/gfp mice. (F) αvβ5 staining of mature macrophages from the small intestine of WT→WT and Itgb5−/−→WT BM chimeric mice. (G) αvβ5 expression on CD3+ T lymphocytes, CD19+ B cells, SiglecF+CD11b+ eosinophils, Ly6G+CD11b+ neutrophils, Ly6ChiMHCII− monocytes, F4/80−CD11c+MHCII+ dendritic cells, and F4/80hiCX3CR1hiMHC+ macrophages from colon and small intestine of Cx3cr1+/gfp mice, together with the FMO for colon/SI macrophages. Histograms in D–G are representative of three independent experiments with 1–2 mice/experiment. (H, I) Frequencies of colon macrophages taking up apoptotic DS-Red thymocytes in presence of MFG-E8 and anti-αvβ5 antibody. *p < 0.05 vs MFG-E8 ligand alone; **p < 0.01 vs MFG-E8 + anti-αvβ5. ***p < 0.001 vs MFG-E8 ligand + anti-αvβ5 vs MFG-E8 alone, Student’s t-test + one-way ANOVA. Data in H, I are means + 1SD for eight mice/group pooled from three independent experiments.
As well as confirming the significant upregulation of genes encoding proteins involved in phagocytosis (C1qa, Fcrls, Cd36, Cd163, and Itgav), Q-PCR showed this also involved tissue remodeling metalloproteinase enzymes (Mmp2, Mmp9, and Mmp13) (Supporting Information Fig. S3). Of the partners for αv integrin, only the mRNA for Itgb5 (coding for integrin β5) was upregulated in colon mϕ, whereas that for β1, β3, β6, and β8 integrins did not alter (Supporting Information Fig. S3). Mature colonic macrophages expressed high levels of αvβ5 surface protein (Fig. 1D), as did small intestinal lamina propria (SILP) mϕ, interstitial and alveolar mϕ in the lung and red pulp mϕ in the spleen, but not F4/80hi peritoneal mϕ (Fig. 1D–F). This staining was specific, as it was absent from SILP mϕ in Itgb5−/− mice (Fig. 1F). αvβ5 expression in the colon and SILP was much higher on mϕ compared with T cells, B cells, eosinophils, neutrophils, and dendritic cells (Fig. 1G and data not shown). The expression of αvβ5 was not dependent on the microbiota, as it was already expressed by virtually all colonic CX3CR1hiMHCII+ mϕ and CX3CR1hiMHCII− mϕ 48 hours after birth and throughout the neonatal period, as well as in adult mice treated with broad spectrum antibiotics (Supporting Information Fig. S4A and B and data not shown). αvβ5 integrin recognizes phosphatidylserine on apoptotic cells in cooperation with CD81 and MFG-E8 as a bridging opsonin [7, 8]. αvβ5 integrin appears to have a similar role for intestinal mϕ, as their intrinsic ability to phagocytose apoptotic thymocytes in vitro was significantly enhanced in the presence of MFG-E8 and this was significantly reduced by anti-αvβ5 antibody (Fig. 1H and I, Supporting Information Fig. S4C).
Thus αvβ5 mediated phagocytosis of apoptotic cells may be one mechanism by which mϕ can contribute to intestinal homeostasis.
Role of integrin αvβ5 in the development of intestinal immune cells
To determine the role of αvβ5 in the development and function of intestinal mϕ, we generated chimeric mice in which irradiated CD45.1+CD45.2+ WT recipients received BM cells from CD45.2+ Itgb5−/− mice (Fig. 2A). Control chimeras received CD45.2+ WT BM and in both chimeras, >95% monocytes and mϕ in the colon were of donor origin, as were CD11b+ monocytes in blood (Supporting Information Fig. S5A and B). Itgb5−/−→WT chimeric mice developed normally and had no evidence of clinical disease, but they had significantly reduced proportions and numbers of MHCII− monocytes (P1) in the colon compared with WT→WT chimeric mice (Fig. 2B). There were no differences in the proportions or numbers of MHCII+ monocytes (P2) or mature mϕ (P3-4), or in the proportion of Ly6Chi monocytes in peripheral blood (Fig. 2B). Total dendritic cells and their subsets based on expression of CD103 and CD11b were also unaltered in Itgb5−/−→WT chimeric mice (Supporting Information Fig. S5C, data not shown), but the proportions and numbers of eosinophils were significantly higher in the colon of Itgb5−/−→WT chimeric mice (Supporting Information Fig. S5D). An identical pattern of myeloid cells was found in the SI LP of Itgb5−/−→WT chimeric mice (Supporting Information Fig. S6A and B). This reflected a lack of p5 integrin on the monocyte-mϕ lineage itself, as the effects were replicated in mixed BM chimeras in which CD45.1+CD45.2+ WT recipients were reconstituted with BM cells from CD45.1+ Ccr2−/− mice together with BM from CD45.2+ Itgb5−/− mice. As intestinal mϕ require constant, CCR2-dependent replenishment (Fig. 2C and [3]), they all lack αvβ5 integrin in such chimeras (Fig. 2D). Although Itgb5−/− BM was more efficient than WT BM in reconstituting colonic monocytes and mϕ in the colon under competitive conditions (Fig. 2E), the selective reduction in Itgb5−/−-derived Ly6ChiMHCII− monocytes (P1) seen in Itgb5−/− mice was replicated in the mixed chimeric system (Supporting Information Fig. S6C). There were no differences in the proportions of Ly6ChiMHCII+ monocytes (P2) or mature mϕ (P3-4) that were derived from Itgb5−/− or WT BM (Supporting Information Fig. S6C).
Figure 2.

Role of integrin αvβ5 in the development of immune cells. (A) Generation of Itgb5−/−→WT BM chimeras. (B) Frequency among CD45.2+ leukocytes (top panels) and absolute numbers (lower panels) of colonic macrophage subsets, together with monocytes in peripheral blood of Itgb5−/−→WT and WT→WT chimeric mice, determined by flow cytometry. Horizontal bars represent the medians and each symbol represents an individual mouse. Data pooled from three independent experiments with 4–5 mice/experiment. *p < 0.05, ***p < 0.001, two-tailed Mann–Whitney test. (C) Generation of CD45.1+Ccr2−/−:CD45.2+WT and CD45.1+Ccr2−/−:CD45.2+Itgb5−/− mixed BM chimeras. (D) Representative FACS staining of colonic macrophage subsets showing chimerism 8 weeks after reconstitution. (E) Frequencies of donor-derived CD45+ leukocytes amongst colonic monocyte and macrophage subsets in CD45.1+Ccr2−/−:CD45.2+WT and CD45.1+Ccr2−/−:CD45.2+Itgb5−/− mixed BM chimeras. Horizontal bars represent the medians and each symbol represents an individual mouse. Data pooled from two independent experiments with 3–4 mice/experiment. *p < 0.05, **p < 0.01, two-tailed Mann–Whitney test.
As the numbers of Ly6Chi monocytes were normal in the bloodstream of Itgb5−/− chimeric mice, the defect in intestinal P1 cells may reflect reduced recruitment. Alternatively, the fact that the mature colonic mϕ population remains unaffected in the absence of αvβ5 could suggest more rapid differentiation of monocytes after their arrival.
Role of integrin αvβ5 in homeostatic function and colon inflammation
As the numbers of mature mϕ were not affected by the absence of integrin αvβ5, we went on to examine whether any of their characteristic properties were altered.
As in intact mice, mϕ sorted from the colon of WT→WT BM chimeras showed marked upregulation of mRNA for Mmp2, Mmp9, and Mmp13 as they matured from monocytes (Fig. 3A). This did not occurwith Itgb5−/−→WT mϕ and although the mechanisms responsible for this are unclear, the results indicate that αvβ5 can influence MMP activity at the level of gene transcription; it can also regulate their enzymatic activity, as has been shown with microglia [9]. There was also a significant defect in the upregulation of Il10 mRNA in Itgb5−/−→WT mϕ (Fig. 3A). In contrast, transcripts for the scavenger molecules C1qA and CD163 showed normal upregulation in Itgb5−/−→WT mϕ, while the expression of mRNA for the chemokine CCL2 was enhanced (Supporting Information Fig. S7A), perhaps reflecting an attempt by these cells to respond to the reduced recruitment of monocytes. Dysregulated production of other chemokines might also help explain the increase in eosinophils that we observed in mice lacking αvβ5. Although αvβ5 integrin is thought to activate TGFβ [10, 11], colonic from Itgb5−→WT chimeric mice showed normal upregulation of genes involved in TGFβ mediated signaling (Tgfbr1) or whose expression is dependent on TGFβ (Il22) [5] (Supporting Information Fig. S7B). Interestingly, Itgb5−/−→WT mice showed a marked increase in the proportion of mature mϕ expressing TIM4, another phagocytic receptor that is upregulated during local differentiation from monocytes (Fig. 3B). As Tim4 expression correlates with long-term residence of mϕ in tissues [12, 13], our findings could indicate that mϕ differentiating in the absence of αvβ5 may have a longer lifespan than normal, thereby accounting the normal numbers of mature mϕ we observed. The lifespan, proliferative capacity, and differentiation kinetics of macrophages in the absence of αvβ5 warrant further investigation.
Figure 3.

Functional consequences of loss of integrin αvβ5 on macrophages. Q-PCR analysis of mRNA for (A) Mmp2, Mmp9, Mmp13, and Il10 by FACS sorted colonic monocytes (P1 and P2) and macrophages (P3/4) from Itgb5−/−→WT and WT→WT chimeric mice. Results show the mean of triplicate assays for each gene relative to cyclophillin A (CPA) calculated by the 2−dΔC(t) method. ND: not detected. (B) Proportion of each colonic macrophage subset in Itgb5−/−→WT and WT→WT chimeric mice expressing TIM4. Horizontal bars in A, B represent the medians and each symbol represents an individual mouse, with data pooled from two independent experiments with 3–5 mice/experiment. *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Mann–Whitney test. (C) Weight loss and (D) clinical disease activity during acute DSS colitis in Itgb5−/−→WT and WT→WT chimeric mice. Data represent means +1SD for 9–11 mice/group pooled from two independent experiments with 4–6 mice/experiment. *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test. (E) Frequency of colonic monocytes (P1 and P2), macrophages (P3/4), and eosinophils in chimeric mice on day 6 of DSS colitis and in controls. Horizontal bars represent the medians and each symbol represents an individual mouse. Data pooled from 5–6 mice/group from two independent experiments with 2–3 mice/experiment. *p < 0.05, **p < 0.01 two-tailed Mann–Whitney test.
We examined whether the dysregulated differentiation of colonic mϕ in the absence of αvβ5 had functional consequences in vivo. Oral administration of DSS led to clinical colitis and weight loss in all WT→WT and Itgb5−/→WT chimeric mice, but both parameters progressed more rapidly and showed increased severity in the Itgb5−/→WT chimeric mice (Fig. 3C and D). Colitis was associated with increased infiltration by P1 and P2 monocytes in both groups, together with a trend toward reduced numbers of mature mϕ (Fig. 3E). The expansion of P2 cells was significantly higher in Itgb5−/→WT mice, indicating that the chemical insult and/or resulting influx of microbes can overcome the intrinsic defect in monocyte accumulation (Supporting Information Fig. S7C). The already expanded population of eosinophils in steady-state Itgb5−/→WT mice did not increase further after administration of DSS as it did in colitic WT→WT mice (Fig. 3E). These findings that the absence of αvβ5 integrin leads to increased susceptibility to clinical disease and local inflammation in DSS colitis extend previous work showing that MFG-E8 can inhibit colitis and promote mucosal wound healing [14, 15]. Nevertheless, the loss of αvβ5 had only partial effects on colitis development and did not lead to intestinal inflammation under steady-state conditions in our conventional animal facility. Thus the requirement for αvβ5 may only become critical with advancing age, as is found for the blindness that occurs in Itgb5−/− mice [16]. Alternatively, its functions may be compensated for by other receptors for apoptotic cells, such as TAM receptors, GAS6, TREM-2, and CD300f. Although we were not able to examine such molecules in detail, TIM4 was expressed on a significantly increased proportion of mature colonic mϕ in Itgb5−/− mice. Redundancy of this kind amongst apoptotic receptors is found with the TAM receptor family, where the absence of more than one of Axl, Mer, or Tyro3 is needed to reveal autoimmune disease [17].
Concluding remarks
Our study reveals for the first time that αvβ5 integrin is highly expressed by mature intestinal mϕ as they differentiate from monocytes in situ in an age and microbiota independent manner. Mice lacking αvβ5 are more susceptible to intestinal inflammation, perhaps reflecting its roles in the clearance of damaged cells and tissue remodeling. However as the absence of αvβ5 had several other effects on the composition and properties of the intestinal environment, the exact mechanisms by which it regulates local homeostasis remain to be explored directly.
Materials and methods
In vivo procedures
WTC57Bl/6 (B6) (CD45.2+),B6.Ly5.1 (CD45.1+.CD45.2+), CD2-DsRed (a kind gift of Dr Robert Benson, Institute of Infection, Immunity and Inflammation), Cx3cr1gfp/+, and Ccr2−/− mice were bred and maintained under specific pathogen free conditions at the Central Research Facility at the University of Glasgow. Itgb5−/− mice [18] were bred and maintained in the Animal Research Facility at the Department of Biological Sciences, Fordham University, USA. All experiments were approved by the UK Home Office, or by the Institutional Animal Care Committee at Fordham University. All mice were used at 6-12 weeks of age.
Bone marrow chimeras were generated by reconstitution of lethally irradiated mice with 2–4 × 106 BM cells as described [4] and analyzed 8 weeks later. Colitis was induced by oral administration of 2% dextran sodium sulphate (DSS) [3] and scored as described in Supporting Information Table S1. Mice treated with antibiotics received ampicillin (1g/L), metronidazole (1g/L), neomycin (1g/L), gentamicin (1g/L), and vancomycin (0.5g/L) in the drinking water for 7 days.
Preparation of single cell suspensions
Lamina propria mononuclear cells were isolated from colon and small intestine by enzymatic digestion as described [19]. Spleens were chopped and digested in prewarmed RPMI 1640 (Gibco, Thermo Fisher Scientific, Paisley, UK) containing 1 mg/mL collagenase D (Roche) for 30 minutes in a shaking incubator at 37°C. Lung leukocytes and peritoneal exudate cells were isolated as described [12].
Phenotypic and transcriptional analysis
Cells were blocked with anti-CD16/32 (BioLegend, London, UK), stained with the antibodies shown in Supporting Information Table S2 and expression compared with fluorescence minus one (FMO) stains as negative controls. Cells were acquired using a FACSAria I or III cytometer (BD Biosciences) and sorted to a purity of >95% and analyzed using FlowJo software (Tree Star, Ashland, OR). Cytospins were stained with anti-mouse pan-cytokeratin antibody (C-11; Sigma-Aldrich), followed by image analysis using the EVOS Cell Imaging System (Life Technologies).
Gene expression was assessed in sorted cells by quantitative real time PCR (Q-PCR) (Supporting Information Table S3) and by microarray analysis as described previously [5] (GEO GSE84764).
Phagocytic uptake of thymocytes
Thymocytes from CD2-DsRed mice were irradiated with 30Gy and then were cultured for 4 h in RPMI 1640 supplemented with 10% foetal calf serum (FCS), 100 μg/mL streptomycin/penicillin and 1.25 μg/mL Fungizone (Gibco) (‘complete medium’). This process yielded 40–45% Annexin V+ positive cells (data not shown). A total of 2 × 106 LPMCs were cocultured for 2 hours at 37°C with 4–5 × 106 apoptotic thymocytes in the presence of 10 μg/mL milk fat globule-EGF factor 8 (R&D Systems, UK), with or without 10 μg/mL anti-αvβ5 integrin antibody. After incubation, cells were washed and analyzed by flow cytometry.
Statistical analysis
Data were compared using an unpaired Student’s t-test or Mann–Whitney two-tailed nonparametrical test, while multiple groups were compared using Kruskal–Wallis one-way ANOVA (GraphPad Prism 6, La Jolla, CA).
Supplementary Material
Acknowledgments:
This work was supported by the MRC UK (A.M.M., E.A.M., A.S.), the Stiftelsen Olle Engkvist Byggmästare, Sweden (A.K.K.) (grant number 2014/27), and by grant R01-EY26215 from the National Institutes of Health (S.C.F., C.Y.). We thank the staff of the central research facility and Jim Reilly and Shauna Kerr for technical assistance, and the University of Glasgow Flow Cytometry Core Facility for assistance with cell sorting.
Abbreviations:
- DSS:
dextran sodium sulphate
- LP:
lamina propria
- m:
macrophage
- MF-G8:
milk fat globule EGF-like molecule 8
- MMP:
metalloproteinase
- SI:
small intestine
- SILP:
small intestinal lamina propria
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Conflict of interest: The authors declare no commercial or financial conflict of interest
References
- 1.Kuhl AA, Erben U, Kredel LI and Siegmund B, Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol 2015. 6: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mowat AM and Agace WW, Regional specialization within the intestinal immune system. Nat. Rev. Immunol 2014. 14: 667–685. [DOI] [PubMed] [Google Scholar]
- 3.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M et al. , Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal. Immunol 2013. 6: 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B et al. , Constant replenishment from circulating monocytesmaintains the macrophage pool in the intestine of adultmice. Nat. Immunol 2014. 15: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schridde A, Bain CC, Mayer JU, Montgomery J, Pollet E, Denecke B, Milling SWF et al. , Tissue-specific differentiation of colonic macrophages requires TGFbeta receptor-mediated signaling. Mucosal. Immunol 2017. 10: 1387–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnemann SC, Bonilha VL, Marmorstein AD and Rodriguez-Boulan E, Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA 1997. 94: 12932–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y and Finnemann SC, Tetraspanin CD81 is required for the alpha v beta5-integrin-dependent particle-binding step of RPE phagocytosis. J. Cell Sci 2007. 120: 3053–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D and Finnemann SC, Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007. 104: 12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milner R, Crocker SJ, Hung S, Wang X, Frausto RF and del Zoppo GJ, Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J. Immunol 2007. 178: 8158–8167. [DOI] [PubMed] [Google Scholar]
- 10.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y and Tamaki K, Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum 2005. 52: 2897–2905. [DOI] [PubMed] [Google Scholar]
- 11.Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ et al. , Integrin alphavbeta5-mediated TGF-beta activation by airway smooth muscle cells in asthma. J. Immunol 2011. 187: 6094–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M et al. , Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun 2016. 7: ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S et al. , Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun 2016. 7: 10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz MM, Ishihara S, Mishima Y, Oshima N, Moriyama I, Yuki T, Kadowaki Y et al. , MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J. Immunol 2009. 182: 7222–7232. [DOI] [PubMed] [Google Scholar]
- 15.Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS et al. , Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Invest 2007. 117: 3673–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D and Finnemann SC, Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med 2004. 200: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemke G and Rothlin CV, Immunobiology of the TAM receptors. Nat. Rev. Immunol 2008. 8: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Griffiths M, Wu J, Farese RV Jr. and Sheppard D, Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell Biol 2000. 20: 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bain CC and Mowat AM, CD200 receptor and macrophage function in the intestine. Immunobiology 2012. 217: 643–651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


