Abstract
In many cases, hippocampal neurogenesis appears to be a hallmark of antidepressant treatments. One novel technique for inducing this type of neurogenesis is using focused ultrasound waves, in conjunction with circulating microbubbles, to open the blood-brain-barrier. The present experiment aimed to test whether this technique has antidepressant effects in a rodent model. Rats were subjected to 1, 2 or 3 weekly treatments of magnetic resonance-guided focused ultrasound in order to open the blood-brain-barrier in the hippocampal region. Before and after treatments, animals went through modified forced swim tests. 1 week after the final treatment, animals that received 2 weekly treatments showed antidepressant-like effects on behavioural measures in comparison to untreated controls. This was not the case for animals that received 1 or 3 weekly treatments. Effects had disappeared by 5 weeks following the first ultrasound treatment. These results suggest that focused ultrasound may be used for inducing short-term antidepressant effects.
Keywords: focused ultrasound, forced swim test, blood-brain barrier, antidepressants, neurogenesis
1. Introduction
Depression is one of the most prevalent mental disorders worldwide. The World Health Organization estimates that unipolar depressive disorders accounted for 4.5% total disability adjusted life years (DALYs) at the turn of the century, making depression the fourth leading cause of disease burden [1]. Perhaps more concerning is the fact that at least 12% of patients are resistant to the current means of treatment [2]. This point underscores the need for novel treatment strategies in combating this disease.
The association between neurogenesis and many of the common treatments for depression is of increasing interest to the mood disorder research field. This interest is borne of the fact that many of the treatments that have reasonable antidepressant effects in animal models are accompanied by hippocampal neurogenesis. The treatments that produce this cell proliferation include a number of pharmacological interventions, models of electroconvulsant therapy and even simple physical exercise [3–7]. More importantly, some of these antidepressant effects may be dependent on the neurogenesis that is produced. One of the first examples of this was the demonstration that the antidepressant-like behavioural effects of fluoxetine were abolished by blocking the drug’s ability to induce hippocampal neurogenesis [8, 9].
A technique that continues to show promise in its ability to noninvasively manipulate the central nervous system (CNS) is focused ultrasound (FUS). At various parameters, this technology is capable of producing a number of interesting or beneficial effects on the brain including neuromodulation [10, 11], increasing neurotrophic factors [10], and decreasing Alzheimer’s disease-like pathology [12]. Under the proper parameters and with the aid of circulating microspheres, FUS also has the ability to transiently and reversibly open the blood-brain-barrier (BBB) [13, 14]. This important effect allows for greater access to the CNS, particularly for molecules that the BBB normally renders impermissible. Interestingly, when directed towards opening the BBB in the hippocampal region, FUS is accompanied by increased hippocampal neurogenesis [12, 15–16].
Whether FUS-mediated BBB opening might have antidepressant effects is yet to be tested. While many antidepressant effects may be accompanied by or even rely upon hippocampal neurogenesis, this neurogenesis does not seem sufficient for producing the antidepressant effects in all cases. For example, Sahay et al. [17] demonstrated that increasing the survival of adult-born neurons via ablation of the proapoptotic gene, Bax, is not sufficient to produce antidepressant-like effects on a number of behavioural measures. The present study, therefore, aimed to test whether FUS-induced BBB opening in the area of the hippocampus might have antidepressant effects. We tested whether one or multiple FUS treatments affected behaviour in the modified forced swim test (FST) [18], a variation of the industry standard test developed by Porsolt et al. [19,20].
2. Methods
2.1 Animals
69 adult male Sprague-Dawley rats (299–567g) were used for this study. Animals were housed in the Sunnybrook Research Institute animal facility and had ad libitum access to food and water. Rats received FUS once (N=11), twice (N=10), or 3 times (N=10) with 1 week in between each FUS treatment. The age/weight of the animals and the time of testing can impact the performance on the FST. 10 age-matched control rats were, therefore, assigned to each of the experimental groups to account for the difference in testing time-point (total = 30 control rats). Finally, 8 rats were used as drug-treated positive controls for behavioural tests. All procedures were approved by the institutional Animal Care Committee (Sunnybrook Research institute, Toronto, Ontario, Canada) and were in accordance with guidelines provided by the Canadian Council on Animal Care and the Animals for Research Act.
2.2 FUS
FUS-mediated BBB opening was achieved with methods described by O’Reilly and Hynynen [14]. Briefly, animals were deeply anaesthetized with isoflurane (2% @ 1 L/min with medical air), the scalp was depilated, and each animal was fitted with a tail vein catheter. Each animal was then fitted, in a supine position, onto a sled that allowed coupling between the animal’s bare scalp and a degassed water bath. Using a 7-Telsa MRI scanner (BioSpin 7030; Bruker, Billerica, Massachusetts), T1- and T2-weighted scans of the animals’ heads were acquired in order to register locations of ultrasound focus. Animals were then administered a microbubble contrast agent (Definity; Lantheus Medical Imaging, North Billerica, Massachusetts) via the tail vein at a dose of 0.02 ml/kg. Immediately following this, animals were sonicated using a custom-built transducer with a 1.68 MHz center frequency. The transducer was 75 mm in diameter, with a 60 mm radius of curvature, and was fitted with a custom-built polyvinylidene difluoride hydrophone in the center. FUS was delivered to a region containing the hippocampus in 10 ms bursts at a 1 Hz burst repetition frequency for 120 s. Once the presence of ultraharmonic acoustic emissions, which are indicative of enhanced microbubble activity, were registered, the acoustic pressure was reduced by 50% for the remainder of the sonication [14]. After sonication, contrast-enhanced T1-weighted images were acquired. Contrast enhancement was achieved by administering the gadolinium-based contrast agent, Gadovist (Schering AG, Berlin, Germany), via the tail vein at a dose of 0.2 ml/kg prior to imaging.
2.3 Modified Forced Swim-Tests
1 week prior to the initial FUS treatment, 1 week after the final FUS treatment and 5 weeks after the initial FUS treatment, modified FSTs were carried out as described by Slattery and Cryan [21]. The swimming arena was a 45 cm high clear cast acrylic cylinder with a diameter of 20 cm. The cylinder was filled to 30 cm with 23–25°C water. Fresh water was used for each animal. At each testing time point, animals were given a 15 min swimming session on the day before the actual test. 24 h after this pre-test session, animals were placed back into the cylinder and allowed to swim for 5 min. These 5 min tests were captured on video with Logitech® c525 HD Webcams. After each swimming session animals were dried off, warmed with a heating lamp, and returned to their home cages.
2.4 Drug Injections
At the baseline testing time point, an additional 8 animals were tested under the influence of the antidepressant fluoxetine as a positive control for the ability of the test to model antidepressant effects. Immediately after the pre-test and 1 h before the test, these animals were given i.p. injections of fluoxetine hydrochloride (Sigma) at a dose of 20 mg/kg [Q].
2.5 Data Collection and Statistical Analysis
Videos were scored using BORIS [22], Version 2.72 (www.http:// http://www.boris.unito.it/) by an observer blinded to the identification and condition of the animals being scored. The behaviours of interest were swimming (horizontal movements throughout the swim cylinder), climbing (upward-directed movements), and immobility (floating without struggle, including movements necessary to keep head above water). The 300 s test was then divided into 5 s periods and the predominant behaviour in each period was scored. This produced a total of 60 scores for each animal at each testing time point. An independent t-test was conducted for each behaviour of interest between each FUS group and their testing time-matched controls.
3. Results
3.1 Baseline and Positive Control Tests
Paired t-tests revealed that animals receiving fluoxetine injections swam more (Figure 1; t(67)=2.269, p=0.040) and spent less time immobile (Figure 1; t(67)= −2.484, p=0.026) than 8 randomly selected animals at baseline (before any experimental animals had received FUS treatments).
Figure 1.
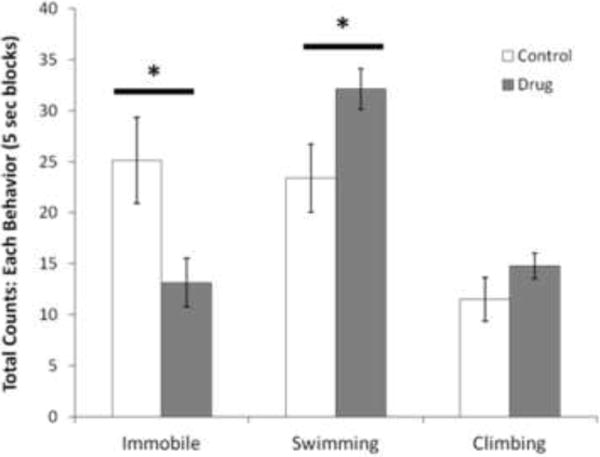
Pre-treatment with fluoxetine hydrochloride has anti-depressant effects on the modified forced swim test. Fluoxetine-treated animals are compared to saline-treated animals on the total counts of swimming behaviour, climbing behaviour and immobility. *p<0.05. n=8 rats per group. Results are mean ± SEM.
3.2 Post-Treatment Tests
For animals that received 1 FUS treatment, there was no difference in the time spent swimming or the time spent immobile in comparison to their matched controls, either 1 week after their treatment (Figure 2A–B; swimming: t(19)= 0.599, p=0.556; immobility: t(19)= − 0.256, p=0.800) or 5 weeks after their treatment (Figure 2A–B; swimming: t(19)= 0.250, p=0.805; immobility: t(19)= −1.170, p=0.256).
Figure 2.
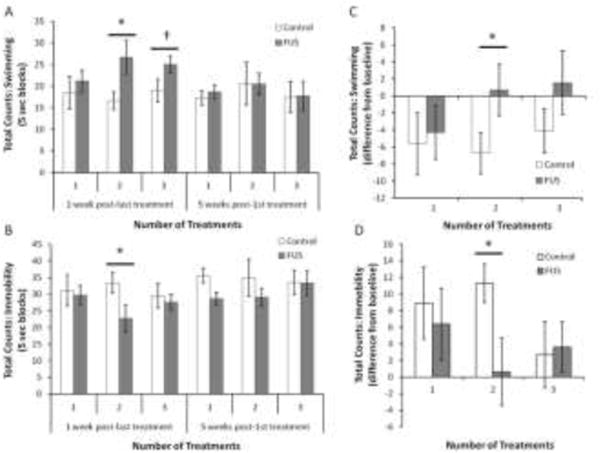
FUS has dose-response effect on anti-depressant effects on the modified forced swim test. The total counts of swimming activity (A) and immobility (B) of sonicated animals are compared to the matched controls at 1 amount of swimming (C) and the amount of immobility (D) between the baseline and the post-FUS tests (1 week) are also considered. *p<0.05, †p=0.085. n=10–11 rats per group. Results are mean ± SEM.
Animals that received 2 FUS treatments swam more (Figure 2A–B; t(18)= 2.288, p=0.034) and spent less time immobile (Figure 2A–B; t(18)= −2.107, p=0.049) in comparison to their matched controls. This effect had disappeared by 5 weeks after the initial treatment (Figure 2A–B; swimming: t(18)= −0.020, p=0.984; immobility: t(18)= −1.141, p=0.269).
For animals that received 3 FUS treatments, there was no difference in the amount of time spent immobile in comparison to matched controls 1 week after their final treatment (Figure 2B; t(18)= −0.442, p=0.664). They trended towards swimming more than their matched controls at this time point (Figure 2A; t(18)= 1.862, p=0.085) but this effect did not reach statistical significance. There was no effect of FUS on either swimming behaviour or immobility 5 weeks after the initial treatment (Figure 2A–B; swimming: t(18)= 0.090, p=0.929; immobility: t(18)= − 0.049, p=0.961).
3.3 FUS Difference from Baseline
In order to confirm that the effects seen in animals who received 2 weeks of treatment were in response to the treatment, we conducted further t-tests on the difference from baseline in swimming and immobile behaviours. On the first post-treatment testing session, control animals decreased their swimming behaviour more so than did FUS treated animals in comparison to the baseline tests (Figure 2C; t(18)= −2.195, p=0.041). In comparison to their baseline sessions, control animals also spent more time immobile than did FUS-treated animals (Figure 2D; t(18)= 2.273, p=0.036).
4. Discussion
Here, we show that 2 weekly treatments of FUS-induced BBB opening have antidepressant-like effects on behaviour in the modified FST. 1 treatment at the given exposure does not seem sufficient for inducing these effects. Furthermore, 3 weekly treatments, while trending in the right direction, were certainly not as potent as 2 treatments and the antidepressant effects did not reach statistical significance. We, therefore, show a dose-response curve in FUS treatment on antidepressant-like activity.
It is not yet clear why these treatments might display such a dose-response pattern. One possible explanation is that continual exposure to the treatment paradigm counteracts the beneficial effects of the treatment. Certain aspects, most notably continued exposure to anaesthetic, may require more recovery time than was granted in this study. However, repeated FUS may yet prove to be beneficial under the proper treatment regimen. Kobus et al. [23] demonstrated that repeated weekly treatments of FUS-mediated BBB opening resulted in little or no histological signs of damage in the brain parenchyma or vasculature above what is normally seen following a single treatment. Any negative effects of the treatment were, therefore, not likely due to a destructive impact of ultrasound on the brain tissue. In addition, 3 repeated exposures of FUS have been shown to have beneficial effects on physiology and behaviour in rodents. In a mouse model of Alzheimer’s Disease, 3 repeated weekly treatments reduced amyloid plaques and increased performance on a memory task [12].
Unfortunately, we cannot say whether these results are related to the amount of neurogenesis that FUS may induce. Newly divided cells can be labelled by the thymidine analog, 5′-bromo-2′-deoxyuridine (BrdU), which incorporates the DNA of dividing cells during the S-phase of the cell cycle. While we did inject each animal and their control with BrdU (i.p. 50mg/kg) 24 hours after each FUS treatment, FUS-treated animals did not appear to display increased neurogenesis. However, after conducting a follow-up study, we found that, in order to properly capture the period of FUS-related neurogenesis, multiple days of post-treatment BrdU injections are required (Figure 3). Future work, determining whether multiple FUS treatments influences either the amount of neurogenesis or the survival and integration of newly generated neurons may help answer questions pertaining to the apparent dose-response nature of our results.
Figure 3.
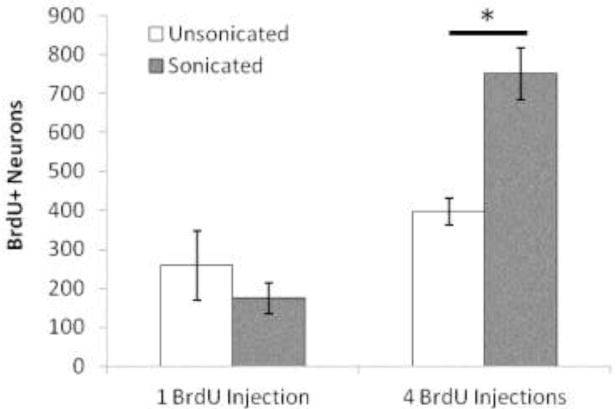
Capturing FUS-induced neurogenesis requires multiple injections of BrdU. Graph represents the number of NeuN and DCX labelled neurons that colabel with BrdU in the CA1 region of the hippocampus. The sonicated hemisphere is compared to the non-sonicated hemisphere when animals are injected with a single dose of BrdU at 50mg/kg 24hrs post-FUS (1 BrdU Injection) and when animals receive 4 daily injections of BrdU at a dose of 50mg/kg starting at 24hrs post-FUS (4 BrdU Injections). *p<0.05. n=3 rats per group.
Alternative means of producing the antidepressant effect may include long-term changes in neural excitability or potential. FUS-induced BBB opening causes changes in both BOLD signaling and somatosensory-evoked potentials in the short and longer-terms [24]. However, the parameters that cause longer-term (1-week) changes in evoked potentials are also those that cause red blood cell extravasations. The parameters used in this study are consistent with a safe-range for blood-brain barrier opening. Similar parameters cause neuromodulatory effects that disappear by 2 days following the application of FUS [24]. It is, therefore, unlikely that neuromodulatory effects lasted long enough to account for differences in performance on the FST. However, the effect of repeated BBB-opening on sustaining neuromodulatory effects for longer periods is currently unknown.
In addition to the neurogenic and neuromodulatory potential of FUS-mediated BBB opening, this technique also allows for greater access to the central nervous system for circulating drugs (see Hynynen [25]). This, coupled with the fact that loading microbubbles or microdroplets with drugs allows for local release of payload upon application of FUS [26] offers the potential for a dual-strategy approach for combating depression whereby FUS effects are supplemented with pharmaceutical effects at doses a fraction of the size that are currently found to be effective.
5. Conclusions
The present study demonstrates that FUS may be used for inducing some antidepressant effects in rats. Determining the dynamics of this effect may help develop this as a novel treatment for mood disorders. Among potential mechanisms, hippocampal neurogenesis, neuromodulation, and increases in growth/neurotrophic factors are a few candidates that are associated with FUS at various parameters with and without microbubbles [10–12,15–16, 27].
Highlights.
The blood-brain-barrier of rats is transiently opened with focused ultrasound
2 weekly treatments results in short-term antidepressant effects.
Longer-term effects are absent at 5 weeks post-treatment
Acknowledgments
The authors would like to extend their thanks to Shawna Rideout-Gros and Viva Chan for animal preparation and care.
Funding Sources
This work was supported by funding from The National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01 EB003268), the Canadian Institutes for Health Research (FRN 119312), and the Canada Research Chair Program (awarded to KH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
KH is the founder of FUS Instruments, from which he receives non-study related support. FUS Instruments developed the focused ultrasound system used in this project.
References
- 1.Üstün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996– 2013. Psychiatr Serv. 2014;65:977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- 3.Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Neurogenic effects of fluoxetine are attenuated in p11 (S100A10) knockout mice. Biol Psychiatry. 201;67:1048–1056. doi: 10.1016/j.biopsych.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 7.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Belzung C. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 9.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tyler WJ. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, Jolesz FA. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, Hynynen K. Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. 2014;273:736–745. doi: 10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits 1. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly MA, Hynynen K. K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooney SJ, Shah K, Yeung S, Burgess A, Aubert I, Hynynen K. Focused Ultrasound-Induced Neurogenesis Requires an Increase in Blood-Brain Barrier Permeability. PloS One. 2016;11:e0159892. doi: 10.1371/journal.pone.0159892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarcelli T, Jordão JF, O’Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7:304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahay A, Scobie KN, Hill AS, O’carroll CM, Kheirbek MA, Burghardt NS, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Porsolt RD, Le Pichon M, Jalfre ML. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 20.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 21.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant- like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 22.Friard O, Gamba M. BORIS: a free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods Ecol Evol. 2016;7:1325–1330. [Google Scholar]
- 23.Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety validation of repeated Blood–Brain barrier disruption using focused ultrasound, Ultrasound. Med Biol. 2016;42:481–492. doi: 10.1016/j.ultrasmedbio.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu PC, Liu LH, Lai HY, Lin CY, Tsai HC, Pei YC. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci Rep. 2015;5:15477. doi: 10.1038/srep15477. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine. 1999;11:416–423. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- 25.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60:1209–1217. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug Carriers. J Pharm Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]


