Abstract
Cardiovascular disease (CVD) and cancer are leading causes of mortality and morbidity worldwide. Strategies to improve their treatment and prevention are global priorities and major focus of World Health Organization’s joint prevention programs. Emerging evidence suggests that modifiable risk factors including diet, sedentary lifestyle, obesity and tobacco use are central to the pathogenesis of both diseases and are reflected in common genetic, cellular, and signaling mechanisms. Understanding this important biological overlap is critical and may help identify novel therapeutic and preventative strategies for both disorders. In this review, we will discuss the shared genetic and molecular factors central to CVD and cancer and how the strategies commonly used for the prevention of atherosclerotic vascular disease can be applied to cancer prevention.
1. Introduction
In 2012, two-thirds of global non-communicable disease deaths were attributable to cardiovascular disease (CVD) and cancer [1], both associated with significant morbidity and poor health-related quality of life [2,3]. The incidence of CVD and cancer is increasing in all socioeconomic classes worldwide [1,4]. According to the 2002 World Health Report [5] and 2011 United Nations High-Level Meeting on Non-communicable Diseases [6], the global reduction of four common modifiable risk factors including unhealthy diet, sedentary lifestyle, excess alcohol consumption and tobacco use can help prevent four prevalent diseases including CVD, cancer, type 2 diabetes (T2DM) and chronic obstructive pulmonary disease.
The importance of common modifiable risk factors for both CVD and cancer is reflected in our emerging understanding of the shared genetics and molecular mechanisms that are central to the pathogenesis of both diseases [7]. Mounting evidence supports the use of medications like aspirin, statins and inhibitors of the reninangiotensin-aldosterone system (RAAS) in the prevention of a variety of malignancies [8–11]. Understanding the significant biological overlap between CVD and cancer is crucial and may foster the development of novel therapeutic and preventive strategies for both diseases. In this review, we will discuss the shared genetics and cellular molecular pathways central to CVD and cancer to provide additional credence for the WHO’s recommendation that health promotion and prevention programs should be directed jointly at CVD and cancer [1,5,6].
2. Shared molecular and genetic pathways in CVD and cancer
2.1. Systemic inflammation & oxidative stress
Atherosclerosis is a chronic inflammatory disease of the arterial wall and is the principal pathology leading to myocardial infarction, stroke, and peripheral vascular disease [12]. The continuous accumulation of lipid and inflammatory cells in the arterial intima is central to atherogenesis. Monocyte/macrophages and other inflammatory cells elaborate diverse pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF- α), and interferon-γ (IFN-γ) which foster further cell recruitment and inflammation. The highly dynamic, lipid rich and chronically inflamed microenvironment of the plaque also contains an abundance of reactive oxygen species (ROS) and oxidized low-densitylipoprotein cholesterol (LDL), driving further inflammatory signaling and gene expression cascades including nuclear factor-kB (NF-kB) [13], hypoxia inducible factor-1α (HIF-1α) [14], and signal transducer and activator of transcription (STAT) [15] which drive cell recruitment, foam cell formation and angiogenesis.
Since Virchow’s 19th-century hypothesis of an inflammationecancer relationship [16], accumulating evidence has supported a complex interplay between inflammation and carcinogenesis [17]. Epidemiological data suggest that over 25% of all cancers are triggered by chronic inflammation [18]. In most solid tumors, malignant cells interact in a complex, chronically inflamed extracellular microenvironment, enriched with macrophages, inflammatory cytokines, growth factors, and reactive oxygen species. This complex interaction activates a wide array of intracellular signaling pathways including Janus-activated kinase (JAK), protein kinase B (Akt), and mitogen-activated protein kinase (MAPK). These cascades of events can in turn lead to transcriptional activation of pro-inflammatory, pro-survival and proteolytic programs via STATs, NF-kB, and HIF-1α [17,19–21]. Moreover, ROS and resultant reactive nitrogen species (RNS) can induce DNA damage and modulate the expression of oncogenes and tumor suppressor genes [17,20].
In summary, common intracellular signaling cascades lead to chronic inflammation, oxidative stress and the activation of cellular processes that underlie both diseases.
2.2. Adenosine 5′ monophosphate-activated protein kinase (AMPK)
AMPK is a key regulator of cellular metabolism in most cells. Emerging evidence supports a pleiotropic and overall protective effect for AMPK in CVD. AMPK activation reduces ROS formation [22] and inflammation [23] by inhibiting immune cell adhesion [24], foam cell formation [25] and vascular smooth muscle cell (VSMC) proliferation [26], all central events in the development of atherosclerosis. Furthermore, pharmacological activation of AMPK reduces atherosclerotic plaque size in apolipoprotein E-deficient mice [25]. AMPK also plays a critical role in maintaining glucose homeostasis and improves insulin sensitivity [27]. Interestingly, medications such as metformin, thiazolidinediones (TZDs) and statins, may in part exert their vascular protective effects through activation of AMPK [24].
AMPK activation has also been demonstrated to prevent malignant growth in a variety of tumors, including glial [28], breast [29], lung [30], liver [31], stomach [32], and prostate [33]. AMPK activation inhibits tumorigenesis by regulating signaling pathways such as PI3K, mTOR, and p53, which are involved in cellular proliferation, cell cycle progression and cellular survival [26,34]. Metformin, activates the AMPK indirectly through inhibiting complex I of the mitochondrial respiratory chain, leading to an increased AMP:ATP ratio [35]. Multiple retrospective studies and metaanalyses in patients with type II diabetes showed that use of metformin was associated with overall 30% lower risk of cancer compared with other antidiabetic medications and this protective effect was more prominent in hepatocellular and colorectal cancers [36–38]. This retrospective evidence is further supported by animal studies reporting a delayed onset of malignant tumor growth in tumor-prone mice due to heterozygous mutations in PTEN, which inhibits PI3K/mTOR pathway. Interestingly, phenformin, another type of biguanide class of antidiabetic drugs, had an even more pronounced effect due to its greater membrane permeability [35]. Treatment of the mice with oral phenformin from 3 weeks after tumor initiation prolonged survival of the mice and delayed tumor progression as well as increasing expression of markers of necrosis and apoptosis in the tumors [39].
2.3. Peroxisome proliferator-activated receptor-γ (PPAR-γ)
PPAR-γ is a widely expressed ligand activated transcription factor, which regulates the expression of multiple genes involved in lipid and glucose homeostasis. It is also a major receptor for the TZD class of insulin-sensitizing drugs [23]. In addition to its effect on glucose metabolism and insulin resistance, PPAR-γ activation can reduce atherosclerosis [40,41] by improving endothelial function and inhibiting the production of pro-inflammatory cytokines, foam cell formation, and VSMC proliferation [23,42,43]. PPAR-γ activation also lowers blood pressure by interfering with angiotensin-II mediated pathways [43,44]. PPAR-γ is also expressed in a number of solid tumors including colon, breast, bladder, lung, and gastric cancers [23] and acts as a tumor suppressor by reducing proliferation and angiogenesis and promoting differentiation. Moreover, TZD class of PPAR-γ agonists have been shown to inhibit the Wnt/β-catenin signaling pathway [45], which functions in the self-renewal capability of cancer stem cells including prostate [46], bladder [47], colon [45], and breast [48] cancer, and also lymphoma [49]. TZD analogs have also recently been identified as potent inhibitors of IGF-1 receptors [50], which are aberrantly activated in several cancers [51]. The inhibition of pro-survival IGF-1 receptor signaling by TZDs may partially explain the observed anti-cancer effects of TZDs. The understanding of anti-cancer effects of TZDs still remains elusive and a careful re-examination of its chemistry and pharmacological effect is needed. In addition to anti-cancer effects [45–47,49,52,53], pioglitazone class of TZDs have the potential to attenuate atherosclerosis and reduce the risk of cardiovascular events [42,43]. A recent multi-center clinical trial in non-diabetic insulin resistant patients with a previous history of transient ischemic attack or stroke demonstrated that risk of stroke and myocardial infarction was lower among patients who received pioglitazone than those who received placebo. A meta-analysis of six randomized clinical trials (4 on pioglitazone and 2 on rosiglitazone) in diabetic and non-diabetic patients after coronary stent implantation demonstrated that those who received TZDs in addition to standard therapy were less likely to undergo revascularization due to stent restenosis at 6-month follow-up [54]. It is noteworthy that despite proven beneficial effect of pioglitazone in reducing myocardial infarction, stroke, and cardiovascular mortality in patients with type II diabetes [55–58], there is conflicting data regarding possible increased risk of myocardial infarction, with no change in cardiovascular mortality, with use of rosiglitazone [59]. Hence, only pioglitazone might be considered as a potential drug for joint pharmacologic prevention of CVD and cancer.
2.4. Fatty acid synthase
Fatty acid synthase (FAS), one of the key enzymes involved in de novo fatty acid synthesis, is abundant in atherosclerotic plaques and plays an important role in atherogenesis [23,60]. In experimental models of atherosclerosis, foam cell formation is reduced in FAS deficient macrophages due to increased cholesterol efflux and reduced uptake of oxidized LDL [60]. Palmitate, an end product of FAS activity, plays an important role by triggering proinflammatory effects in macrophages and VSMCs [61]. FAS is overexpressed in a variety of solid tumors including breast, ovary, endometrium, prostate, colon, esophagus, stomach, pancreas, thyroid and melanoma [23,62]. Overexpression of FAS in malignant tumors is proposed to provide an abundant alternate energy supply in the relatively hypoxic tumor microenvironment [23]. FAS can also affect signaling through the Wnt/β-catenin pathway via palmitoylation of Wnt proteins and plays an important role in cancer stem cell renewal [63]. Moreover, FAS levels are inversely correlated with phosphatase and tensin homologue (PTEN), an important tumor suppressor [64] and its inhibition induces programmed cell death in human breast [65], prostate [64], and pancreas [66] cancer cells. Taken together, FAS is yet another example of the important biological overlap between CVD and cancer.
2.5. Plasminogen activator inhibitor-1
Plasminogen Activator Inhibitor-1 (PAI-1) is a multifunctional protein with the ability to regulate fibrinolysis and many other cellular processes [67]. Increased levels of circulating PAI-1 are associated with an elevated risk of atherogenesis and cardiovascular events, T2DM, and several cancers [67–69]. PAI-1 mRNA is increased in atherosclerotic plaques and may stabilize the fibrin matrix, providing a scaffold for migrating cells, stimulate VSMC proliferation and LDL uptake into the lesion [67]. The role of PAI-1 in malignant tumors has been primarily attributed to its proangiogenic function in the extracellular milieu that may contribute to tumor cell growth, invasion, and metastasis [70]. Moreover, it has been recently observed that PAI-1 protects tumor cells from Fas/Fas ligand-mediated apoptosis [70].
2.6. Obesity and adipokines
Epidemiological studies have demonstrated a robust link between CVD and obesity. Truncal obesity has been shown to be an independent modifiable risk factor for CAD as identified the INTERHEART Study [71]. Moreover, obese and overweight individuals may also be at increased risk of developing a variety of cancers including breast, uterus, esophagus, pancreas, colorectal, and kidney [72]. The causal link between obesity and both cancer and CVD is further strengthened by evidence of decreased incidence of MI, stroke and cancer in patients with weight loss following bariatric surgery [73]. Adipose tissue secretes numerous bioactive proteins including pro-inflammatory cytokines (e.g. TNF-α and IL-6), PAI-1, and adipokines (e.g. adiponectin and leptin) [74–77]. These in turn increase activities of PI3K, MAPK, and STAT3 pathways, which play important roles in atherosclerosis as well as angiogenesis and malignant growth [69]. Among the aforementioned adipokines, the role of leptin in connecting the obesity, CVD, and cancer is noteworthy. Except for in lipodystrophy, leptin levels are elevated in obese patients. Recent studies have shown that leptin dysregulation plays a critical role in the development and metastasis of different malignant tumors by modifying its microenvironment, promoting migration of endothelial cells, and recruitment of macrophages and monocytes, which secrete vascular endothelial growth factor and proinflammatory cytokines that will promote angiogenesis of tumors [78,79]. Leptin has been shown to promote endothelial dysfunction through decreasing the bioavailability of nitric-oxide, upregulating the endothelin-1 secretion, and activating JAK/STAT pathway and increasing ERK signaling [80]. The involvement of leptin in CVD is further supported by its induction of osteoblastic differentiation and calcification of vascular cells, promoting platelets aggregation and arterial thrombosis, and inducing the accumulation of cholesterol in macrophages in hyperglycemic conditions via activation of acylCoA: cholesterol O-acyltransferase (ACAT), and the release of monocyte colony-stimulating factor [80]. Although, PPAR-γ activators like TZDs are pro-adipogenic, they downregulate leptin gene expression [81] and therefore inhibit Akt-dependent migration of vascular smooth muscle cell through inhibition of eNOS [82]. These findings highlight the potential therapeutic role of TZDs in prevention of diabetes-associated vasculoproliferative disorders.
2.7. Wnt signaling pathway
Signaling by the Wnt family of secreted glycolipoproteins plays an important role in the regulation of cell proliferation, polarity, migration and cell fate determination [83]. In the ‘canonical’ Wnt/β-catenin signaling pathway, binding of Wnts to the transmembrane Frizzled and lipoprotein receptor-related proteins (LRP5 and LRP6), prevents the cytoplasmic degradation of b-catenin. Translocation of β-catenin nucleus allows for interaction with members of the T cell factor/lymphoid enhancer factor (TCF/LEF) family including TCF7L2 to promote expression of genes involved in proliferation, and survival [84,85]. Aberrant Wnt signaling plays an important role in the pathogenesis of atherosclerosis [85–87]. Wnt and downstream signaling targets can promote endothelial dysfunction and inflammation [88,89], monocyte adhesion [7], and vascular calcification [90]. Wnt/β-catenin pathway also plays a crucial role in proliferation of VSMCs [91]. Interestingly, impaired LRP6-TCF7L2 activity enhances VSMC plasticity, increasing neointimal thickening, in the absence of excessive lipid accumulation [85]. Aberrant Wnt signaling functions is also linked to a number of cancers including prostate [46], bladder [47], colon [45], breast [48] and lymphoma [49]. Using a drosophila model of insulin resistance Hirabayashi et al. [92] showed that the transcriptional upregulation of insulin receptor by Wnt/TCF7L2 in Ras/Src-transformed tumors may explain increased growth and metastatic capability of malignant tumors in patients with T2DM and/or obesity. Taking together, the Wnt signaling pathway is an emerging biological link between CVD, diabetes and cancer, and may be novel therapeutic target in the future.
2.8. LRP6 mutation
In 2007, a novel neomorphic missense mutation (R611C) in the LRP6 coding region on chromosome 12p was identified in an Iranian family with autosomal dominant premature CAD, metabolic syndrome (dyslipidemia, hypertension, and diabetes), and osteoporosis [87]. Further studies demonstrated that LRP6R611C plays an important role in insulin resistance, hypertriglyceridemia and LDL, non-alcoholic fatty liver disease, and accumulation of ectopic fat [93–101]. The LRP6R611C mutation promoted PDGF stimulated VSMC proliferation and [86], dedifferentiation and neointimal thickening via the non-canonical Wnt signaling pathway [85]. Recent studies have shown that overexpression of LRP6 enhances cell proliferation and induces tumorigenesis in fibrosarcoma [102], hepatocellular carcinoma [103], and breast [104,105] and colorectal [106] cancer. In conclusion, aberrant activation of LRP6 gene plays a major role in pathophysiology of both diseases and may be as one of the genetic links between CVD and cancer.
2.9. TCF7L2 polymorphism
The TCF7L2 gene located on chromosome 10q25.3 is a transcription cofactor involved in canonical Wnt signaling pathway [107]. Single nucleotide polymorphisms (SNPs) of TCF7L2 gene, in particular the T allele of rs7903146, have been associated with T2DM in diverse ethnic populations, making TCF7L2, one of the strongest diabetes susceptibility genes known to date [108–110]. Muendlein et al. [107] recently reported that rs7903146 and two other variants of TCF7L2 are linked with angiographically evident coronary atherosclerosis, particularly in diabetic patients. Interestingly, polymorphisms of TCF7L2 are also associated with increased risk of breast, endometrial, colorectal, and recurrent prostate cancer [111–116]. Altogether, TCF7L2 polymorphism is another example for genetic linkage of CVD and cancer, and their shared metabolic risk factors including T2DM and obesity.
2.10. DYRK1B mutations
DYRK1B is a member of the Dyrk family of proteins, a group of evolutionarily conserved protein kinases that are involved in cell differentiation, survival, and proliferation [117,118]. It is overexpressed in many types of human tumors, particularly ovarian, lung, and pancreatic cancers [119–121]. A gain of function mutation in DYRK1B gene (R102C) was recently identified through linkage analysis of three large families premature CAD, central obesity, hypertension, and T2DM [122]. Furthermore, R102C mutant cell lines demonstrate enhanced adipogenesis and glucose-6phosphatase expression in vitro [122]. DYRK1B may thus represent a novel biological overlap between cancer cell survival and premature coronary atherosclerosis.
2.11. Methylenetetrahydrofolate reductase (MTHFR) C677Tpolymorphism
MTHFR is a key enzyme involved in the synthesis of methionine from homocysteine and folate. Increased plasma homocysteine is an independent risk factor for CAD [123–125]. Homocysteine has multiple effects on atherosclerosis including enhanced lipid peroxidation, endothelial dysfunction and reduced nitric oxide, as well as stimulating VSMC proliferation [126]. Moreover, homocysteine can lead to platelet activation and promote thrombosis [123]. C677T is the most common genetic variant of the MTHFR gene producing a less active, thermolabile mutant and increased plasma homocysteine and reduced folate levels [123–125,127]. Several recent meta-analyses have revealed that the C677T polymorphism is associated with increased risk of CAD [123,124], MI [125], peripheral arterial disease [128], and ischemic and hemorrhagic stroke [129–132].
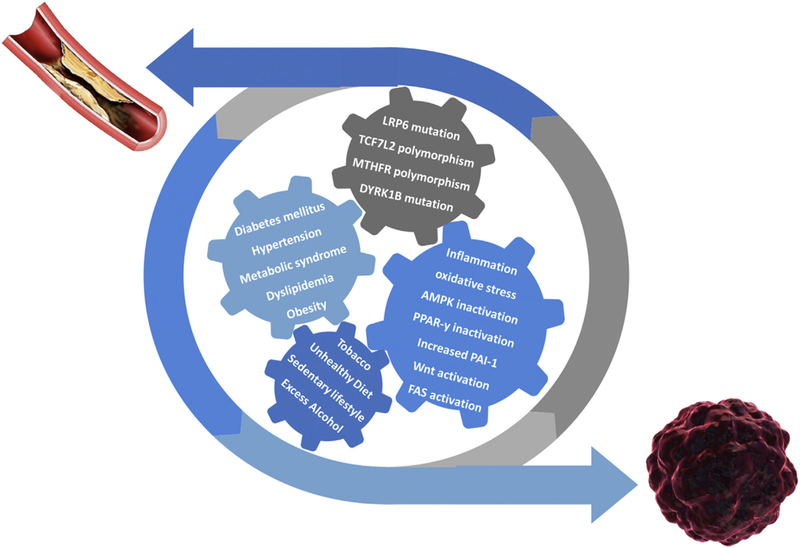
Mounting evidence has also supported an association of the MTHFR C677T polymorphism with a variety of cancers including breast, prostate, colorectal, cervical, oral, ovarian, esophageal, gastric, pancreatic, lung, bladder cancer, hepatocellular carcinoma and acute lymphoblastic leukemia [127]. The C677T mutation can result in reduced folate levels and may increase the incidence of cancer [127,133]. Folate plays a key role in epigenetic processes by regulating DNA methylation [133]. Altered DNA methylation can lead to increased expression of various oncogenes [127] see (Fig. 1).
Fig. 1.
Interplay of shared molecular pathways, genetic alterations, metabolic disorders, and environmental life-style related factors in the dynamic process of developing cardiovascular disease and cancer.
AMPK, adenosine 5ʹ monophosphate-activated protein kinase; PPAR-γ, peroxisome proliferator-activated receptor-γ; PAI-1, plasminogen activator inhibitor-1; FAS, fatty acid synthase.
3. Joint pharmacologic prevention for CVD and cancer
Effective prevention strategies include both pharmacologic and non-pharmacologic components. In line with the significant effect of prevention strategies on global disease incidence, the WHO has identified global targets for major environmental and lifestyle related risk factors [1]. In addition are cornerstones of the prevention and management of CVD. A growing body of evidence supports a role for statins, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, and aspirin in cancer prevention. Although currently no organization endorses widespread use of these drugs for primary prevention of cancer, they may be considered among strategies for joint prevention of CVD and cancer as evidence for risks and benefits is established see (Table 1).
Table 1.
Potential drugs for joint pharmacologic prevention of cardiovascular disease and cancer.
| Drug | Direct Target | Indirect Targets | Action on CVD | Action on Cancer |
|---|---|---|---|---|
| Statins | HMG-CoA-reductase inhibition | • AMPK activation | Improving endothelial function | Tumor-suppressor and anti-cancer role through: |
| • Inhibition of Cyclines & cycline-dependent kinases | Plaque stabilization | ↑ Apoptosis | ||
| ↓ Atherosclerosis progression | ↓ Proliferation | |||
| • Up-regulation of tumor-suppressors (p53, p27, p21) | ↓ Myocardial infarction and stroke | ↓ Invasion | ||
| ↓ Cardiovascular mortality | ↑ Radiosensitization | |||
| • Inhibition ofPI3K, serine—threonine kinases, NF—κB, and MAPKs signaling pathways | ↓ DNA damage | |||
| ASA | Inhibition of COX1 | • AMPK activation? | ↓ Myocardial infarction and stroke | ↓ Cancer incidence |
| ↓ Cardiovascular mortality | ↓ Cancer death | |||
| ACEIs/ARBs | ACE inhibition/Angiotensin II receptor antagonism | • ↓ VEGF expression | Improving endothelial function | ↓ Cancer incidence |
| • PPAR-γ activation | Plaque stabilization | Tumor-suppressor and anti-cancer role through: | ||
| ↓ Atherosclerosis progression | ↓ DNA damage | |||
| ↓ Myocardial infarction and stroke | ↑Apoptosis | |||
| ↓ Cardiovascular mortality | ↓ Differentiation ↓ Angiogenesis ↓ Cell growth |
|||
| Metformin | Unknown | • AMPK activation | ↓ Cancer incidence | |
| Tumor suppression by regulating cellular proliferation, cell cycle progression and cellular survival | ||||
| TZDs | PPAR-γ agonism | • AMPK activation | ↓ Coronary and carotid atherosclerosis | Tumor suppression through: |
| • Wnt/β-catenin signaling pathway inhibition | ↓ Angiogenesis | |||
| • IGF-1 inhibition | ↓ Thrombus formation and acute | ↑ Apoptosis | ||
| • Inhibition of leptin gene expression | myocardial infarction and stroke | ↓ Self-renewal of cancer cells | ||
| ↓ Blood pressure | ↑ Differentiation | |||
HMG-CoA-reductase, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; AMPK, Adenosine 5′ monophosphate -activated protein kinase; PI3K, phosphoinositide 3- kinase; NFekB, nuclear factor kappa-B; MAPK, mitogen-activated kinases; CVD, cardiovascular disease; COX1, cyclooxygenase 1; ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists; ACE, angiotensin-converting enzyme; VEGF, vascular endothelial growth factor; PPAR-γ, peroxisome proliferator-activated receptor-γ; TZDs, thiazolidinediones.
3.1. Statins
Lipid lowering therapy with statins is the cornerstone of medical therapy for primary and secondary prevention of CVD [134]. In addition to potent LDL lowering, statins can improve endothelial function, promote plaque stabilization, and exert antioxidant and anti-inflammatory effects [135]. Although not currently part of medical therapy for malignancy, a potential tumor-suppressor and anti-cancer role for statins thought to be independent of their lipidreducing properties is also emerging. Statin use is associated with reduced incidence of colorectal, liver, breast, skin, prostate, melanoma, head and neck, lung and pancreatic cancers [136–140]. Statins also inhibit production of the mevalonate pathway products involved in posttranslational modification of cell signaling proteins. This modulation of cellular signaling may have pro-apoptotic, antiproliferative, anti-invasive, and radiosensitizing effects [23,135,136]. Statins may also play a protective role against DNA damage which is a key factor in pathophysiology of both atherosclerosis and cancer [141]. Recent studies have shown that statins slightly increase the incidence of new-onset T2DM [142, 143] and the effect varies as per the dosage and type used [142]. However, most of the investigators are of the opinion that the risk of T2DM with statins can be outweighed by the long-term benefits in preventing complications [142,144]. Taken together, statins are interesting candidates for application in CVD and cancer prevention. In patients with high risk of T2DM, statins should be cautiously used and clinicians should vigilantly monitor for incident T2DM in patients on statins [142,144].
3.2. Angiotensin converting enzyme inhibitors (ACEIs)/angiotensinreceptor blockers (ARBs)
Evidence suggests that long-term exposure to ACEIs/ARBs is associated with decreased overall risk of cancer [9,145], with potentially stronger effects in colorectal, pancreatic, breast, prostate, and lung cancers [8,9,11,145–148]. The main effector peptide of the renin-angiotensin-aldosterone system, angiotensin II, induces angiogenesis [149,150], cell proliferation [151] and DNA synthesis [152]. Blocking angiotensin II decreases preneoplastic lesions, cell growth, angiogenesis, and VEGF levels in experimental models of cancer [8,146,153]. Moreover, it has been established that blockade of the RAAS exerts potent anti-atherosclerotic effects through anti-hypertensive effects and through anti-inflammatory, anti-proliferative and antioxidant properties [134]. Hence, the role of ACEIs/ARBSs in joint pharmacologic prevention of CVD and cancer may have to be examined in clinical studies.
3.3. Aspirin
The role of aspirin in the secondary prevention of CVD is well established [154,155]. It reduces the risk of recurrent major coronary events and stroke by 20% and 19%, respectively [156]. Although uncertainties exist regarding the net beneficial effect (decreased MI vs. increased bleeding) of aspirin for primary prevention of CVD in low risk populations [155], guidelines of the American College of Cardiology, American Heart Association, American Diabetes Association, as well as a position paper of the European Society of Cardiology Working Group on Thrombosis, recommend its use in individuals at high risk of CVD and not at increased risk of bleeding [155].
Aspirin may also reduce cancer-related mortality. The first and the most powerful preventive effect was observed in colorectal cancer and then extended to other malignancies, especially adenocarcinomas [157]. In long-term follow-up of participants in five randomized trials of cardiovascular prevention, Rothwell and colleagues [158] reported that daily aspirin at any dose reduced risk of colorectal cancer by 24% and reduced associated mortality by 35% after 8e10 years. Daily aspirin use was associated with a 21% reduced risk of cancer death during the trials, with benefit only apparent after 5 years, and reduced mortality due to several cancers during post-trial follow-up to 20 years [159]. These data were reinforced by a more recent meta-analysis of 11 RCTs, reporting that daily aspirin use significantly reduces risk of non-vascular death by 12% and of cancer death by 15%, with benefit seen within 3 years for high doses (≥300 mg/day) and after 5 years for low doses (<300 mg/day) [10]. Taken together, pending the results of several ongoing studies on efficacy of aspirin in primary prevention of CVD in high-risk patients [155], aspirin is considered a candidate drug for joint prevention of CVD and cancer in high-risk populations.
3.4. Polypill
The concept of combining multiple classes of cardiovascular medications (including aspirin, statins, ACEIs/ARBs) into a single pill, so-called “polypill”, has gained great interest in the past decade. There are several ongoing trials evaluating the short-term and long-term efficacy of a fixed-dose polypill strategy in primary and secondary prevention of CVD. Taking into account the promising effects of aspirin, statins, and ACEIs/ARBs on primary prevention of cancers, assessment of the efficacy of the polypill in the primary prevention of cancer in high-risk populations should be considered.
4. Conclusions
While diverse diseases, CVD and cancer have many similarities. These include common lifestyle-related risk factors, and shared cellular, signaling and genetic pathways. Moreover, the interaction between genetics and environment adds further complexity to both diseases and will be an important area for future research [160]. Clearly, multifaceted interventions to address these shared issues are needed and will include individual and population-level behavioral and policy strategies as well as novel pharmacologic approaches. Modifying common risk factors and targeting of common disease pathways may prove to be the most powerful strategy for prevention of both cancer and CVD.
Acknowledgments
Financial support
Arya Mani is supported by R35HL135767 grant from NHLBI and Yale Liver Center Pilot Grant from NIDDK.
Conflict of interest
Margot K. Davis receives support for her research by the Vancouver Coastal Health Institute Mentored Clinician Scientist Award. Authors declare that there are no actual or potential conflicts of interest concerning this paper.
Abbreviations:
- CVD
cardiovascular disease
- CAD
coronary artery disease
- T2DM
type II diabetes mellitus
- ACEIs/ARBs
angiotensin converting enzyme inhibitors/ angiotensin receptor blockers
- RAAS
renin-angiotensin-aldosterone system
- VSMC
vascular smooth muscle cell
- AMPK
adenosine 5′ monophosphate-activated protein kinase
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PAI-1
plasminogen activator inhibitor-1
- TZDs
thiazolidinediones
- WHO
World Health Organization.
References
- [1].In, Geneva, Global Status Report on Noncommunicable Diseases 2014, 2014.
- [2].Lipscomb J, Gotay CC, Snyder CF, Patient-reported outcomes in cancer: a review of recent research and policy initiatives, CA Cancer J. Clin 57 (2007) 278–300. [DOI] [PubMed] [Google Scholar]
- [3].Dickens C, Cherrington A, McGowan L, Depression and health-related quality of life in people with coronary heart disease: a systematic review, Eur. J. Cardiovasc. Nurs 11 (2012) 265–275. [DOI] [PubMed] [Google Scholar]
- [4].Daar AS, Singer PA, Persad DL, et al. , Grand challenges in chronic noncommunicable diseases, Nature 450 (2007) 494–496. [DOI] [PubMed] [Google Scholar]
- [5].In Geneva, The World Health Report 2002-Reducing Risks, Promoting Healthy Life, 2002. [DOI] [PubMed] [Google Scholar]
- [6].In, New York, Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases, 2011. [Google Scholar]
- [7].Lee DK, Nathan Grantham R, Trachte AL, et al. , Activation of the canonical Wnt/beta-catenin pathway enhances monocyte adhesion to endothelial cells, Biochem. Biophys. Res. Commun 347 (2006) 109–116. [DOI] [PubMed] [Google Scholar]
- [8].Deshayes F, Nahmias C, Angiotensin receptors: a new role in cancer? Trends Endocrinol. Metab. TEM 16 (2005) 293–299. [DOI] [PubMed] [Google Scholar]
- [9].Mc Menamin UC, Murray LJ, Cantwell MM, et al. , Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review, Cancer Causes Control CCC 23 (2012) 221–230. [DOI] [PubMed] [Google Scholar]
- [10].Rothwell PM, Price JF, Fowkes FG, et al. , Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials, Lancet (Lond. Engl.) 379 (2012) 1602–1612. [DOI] [PubMed] [Google Scholar]
- [11].Attoub S, Gaben AM, Al-Salam S, et al. , Captopril as a potential inhibitor of lung tumor growth and metastasis, Ann. N. Y. Acad. Sci 1138 (2008) 65–72. [DOI] [PubMed] [Google Scholar]
- [12].Olijhoek JK, van der Graaf Y, Banga JD, et al. , The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm, Eur. Heart J 25 (2004) 342–348. [DOI] [PubMed] [Google Scholar]
- [13].Pateras I, Giaginis C, Tsigris C, et al. , NF-kappaB signaling at the crossroads of inflammation and atherogenesis: searching for new therapeutic links, Expert Opin. Ther. Targets 18 (2014) 1089–1101. [DOI] [PubMed] [Google Scholar]
- [14].Vink A, Schoneveld AH, Lamers D, et al. , HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages, Atherosclerosis 195 (2007) e69–75. [DOI] [PubMed] [Google Scholar]
- [15].Agrawal S, Febbraio M, Podrez E, et al. , Signal transducer and activator of transcription 1 is required for optimal foam cell formation and atherosclerotic lesion development, Circulation 115 (2007) 2939–2947. [DOI] [PubMed] [Google Scholar]
- [16].Balkwill F, Mantovani A, Inflammation and cancer: back to Virchow? Lancet (Lond. Engl.) 357 (2001) 539–545. [DOI] [PubMed] [Google Scholar]
- [17].Kundu JK, Surh YJ, Emerging avenues linking inflammation and cancer, Free Radic. Biol. Med 52 (2012) 2013–2037. [DOI] [PubMed] [Google Scholar]
- [18].Hussain SP, Harris CC, Inflammation and cancer: an ancient link with novel potentials, Int. J. Cancer. J. Int. du Cancer 121 (2007) 2373–2380. [DOI] [PubMed] [Google Scholar]
- [19].Colotta F, Allavena P, Sica A, et al. , Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability, Carcinogenesis 30 (2009) 1073–1081. [DOI] [PubMed] [Google Scholar]
- [20].Wu Y, Antony S, Meitzler JL, et al. , Molecular mechanisms underlying chronic inflammation-associated cancers, Cancer Lett. 345 (2014) 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu H, Ouyang W, Huang C, Inflammation, a key event in cancer development, Mol. Cancer Res. MCR 4 (2006) 221–233. [DOI] [PubMed] [Google Scholar]
- [22].Morrow VA, Foufelle F, Connell JM, et al. , Direct activation of AMPactivated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells, J. Biol. Chem 278 (2003) 31629–31639. [DOI] [PubMed] [Google Scholar]
- [23].Cabarcas SM, Hurt EM, Farrar WL, Defining the molecular nexus of cancer, type 2 diabetes and cardiovascular disease, Curr. Mol. Med 10 (2010) 744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ewart MA, Kennedy S, AMPK and vasculoprotection, Pharmacol. Ther 131 (2011) 242–253. [DOI] [PubMed] [Google Scholar]
- [25].Chen B, Li J, Zhu H, AMP-activated protein kinase attenuates oxLDL uptake in macrophages through PP2A/NF-kappaB/LOX-1 pathway, Vasc. Pharmacol 85 (2015) 1–10. [DOI] [PubMed] [Google Scholar]
- [26].Motoshima H, Goldstein BJ, Igata M, et al. , AMPK and cell proliferationeAMPK as a therapeutic target for atherosclerosis and cancer, J. Physiol 574 (2006) 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Xu S, Mihaylova MM, et al. , AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice, Cell Metab. 13 (2011) 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Giri S, Nath N, Smith B, et al. , 5-aminoimidazole-4-carboxamide-1-beta-4ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase, J. Neurosci. Off. J. Soc. Neurosci 24 (2004) 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Swinnen JV, Beckers A, Brusselmans K, et al. , Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype, Cancer Res. 65 (2005) 2441–2448. [DOI] [PubMed] [Google Scholar]
- [30].Li J, Jiang P, Robinson M, et al. , AMPK-beta1 subunit is a p53-independent stress responsive protein that inhibits tumor cell growth upon forced expression, Carcinogenesis 24 (2003) 827–834. [DOI] [PubMed] [Google Scholar]
- [31].Meisse D, Van de Casteele M, Beauloye C, et al. , Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells, FEBS Lett. 526 (2002) 38–42. [DOI] [PubMed] [Google Scholar]
- [32].Saitoh M, Nagai K, Nakagawa K, et al. , Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase, Biochem. Pharmacol 67 (2004) 2005–2011. [DOI] [PubMed] [Google Scholar]
- [33].Xiang X, Saha AK, Wen R, et al. , AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms, Biochem. Biophys. Res. Commun 321 (2004) 161–167. [DOI] [PubMed] [Google Scholar]
- [34].Wang W, Guan KL, AMP-activated protein kinase and cancer, Acta Physiol. (Oxf. Engl.) 196 (2009) 55–63. [DOI] [PubMed] [Google Scholar]
- [35].Kim J, Yang G, Kim Y, et al. , AMPK activators: mechanisms of action and physiological activities, Exp. Mol. Med 48 (2016) e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Evans JMM, Donnelly LA, Emslie-Smith AM, et al. , Metformin and reduced risk of cancer in diabetic patients, BMJ (Clin. Res. Ed.) 330 (2005) 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Decensi A, Puntoni M, Goodwin P, et al. , Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis, Cancer Prev. Res. (Phila. Pa.) 3 (2010) 1451–1461. [DOI] [PubMed] [Google Scholar]
- [38].Singh S, Singh PP, Singh AG, et al. , Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis, Am. J. Gastroenterol 108 (2013) 881–891 quiz 892. [DOI] [PubMed] [Google Scholar]
- [39].Shackelford DB, Abt E, Gerken L, et al. , LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin, Cancer Cell 23 (2013) 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen Z, Ishibashi S, Perrey S, et al. , Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL, Arterioscler. Thromb. Vasc. Biol 21 (2001) 372–377. [DOI] [PubMed] [Google Scholar]
- [41].Nakaya H, Summers BD, Nicholson AC, et al. , Atherosclerosis in LDLRknockout mice is inhibited, but not reversed, by the PPARgamma ligand pioglitazone, Am. J. Pathol 174 (2009) 2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang N, Yin R, Liu Y, et al. , Role of peroxisome proliferator-activated receptor-gamma in atherosclerosis: an update, Circ. J. Off. J. Jpn. Circ. Soc 75 (2011) 528–535. [DOI] [PubMed] [Google Scholar]
- [43].Ivanova EA, Parolari A, Myasoedova V, et al. , Peroxisome proliferatoractivated receptor (PPAR) gamma in cardiovascular disorders and cardiovascular surgery, J. Cardiol 66 (2015) 271–278. [DOI] [PubMed] [Google Scholar]
- [44].Sugawara A, Takeuchi K, Uruno A, et al. , Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferatoractivated receptor-gamma in vascular smooth muscle cells, Endocrinology 142 (2001) 3125–3134. [DOI] [PubMed] [Google Scholar]
- [45].Sabatino L, Pancione M, Votino C, et al. , Emerging role of the beta-cateninPPARgamma axis in the pathogenesis of colorectal cancer, World J. Gastroenterol 20 (2014) 7137–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sikka S, Chen L, Sethi G, et al. , Targeting PPARgamma signaling cascade for the prevention and treatment of prostate cancer, PPAR Res. 2012 (2012) 968040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yan S, Yang X, Chen T, et al. , The PPARgamma agonist Troglitazone induces autophagy, apoptosis and necroptosis in bladder cancer cells, Cancer Gene Ther. 21 (2014) 188–193. [DOI] [PubMed] [Google Scholar]
- [48].Conzen SD, Minireview: nuclear receptors and breast cancer, Mol. Endocrinol. (Baltim. Md.) 22 (2008) 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu JJ, Dai XJ, Xu Y, et al. , Inhibition of lymphoma cell proliferation by peroxisomal proliferator-activated receptor-gamma ligands via Wnt signaling pathway, Cell Biochem. Biophys 62 (2012) 19–27. [DOI] [PubMed] [Google Scholar]
- [50].Pollak M, The insulin and insulin-like growth factor receptor family in neoplasia: an update, Nat. Rev. Cancer 12 (2012) 159–169. [DOI] [PubMed] [Google Scholar]
- [51].Mughal A, Kumar D, Vikram A, Effects of Thiazolidinediones on metabolism and cancer: relative influence of PPARgamma and IGF-1 signaling, Eur. J. Pharmacol 768 (2015) 217–225. [DOI] [PubMed] [Google Scholar]
- [52].Denning GM, Stoll LL, Peroxisome proliferator-activated receptors: potential therapeutic targets in lung disease? Pediatr. Pulmonol 41 (2006) 23–34. [DOI] [PubMed] [Google Scholar]
- [53].Monami M, Dicembrini I, Mannucci E, Thiazolidinediones and cancer: results of a meta-analysis of randomized clinical trials, Acta Diabetol. 51 (2014) 91–101. [DOI] [PubMed] [Google Scholar]
- [54].Geng D-f, Jin D-m, Wu W, et al. , Effect of thiazolidinediones on in-stent restenosis in patients after coronary stenting: a meta-analysis of randomized controlled trials, Atherosclerosis 202 (2009) 521–528. [DOI] [PubMed] [Google Scholar]
- [55].Dormandy JA, Charbonnel B, Eckland DJ, et al. , Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial, Lancet (Lond. Engl.) 366 (2005) 1279–1289. [DOI] [PubMed] [Google Scholar]
- [56].Erdmann E, Dormandy JA, Charbonnel B, et al. , The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study, J. Am. Coll. Cardiol 49 (2007) 1772–1780. [DOI] [PubMed] [Google Scholar]
- [57].Lincoff AM, Wolski K, Nicholls SJ, et al. , Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials, Jama 298 (2007) 1180–1188. [DOI] [PubMed] [Google Scholar]
- [58].Wilcox R, Bousser MG, Betteridge DJ, et al. , Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial in macroVascular Events 04), Stroke 38 (2007) 865–873. [DOI] [PubMed] [Google Scholar]
- [59].Paredes S, Matta-Coelho C, Monteiro AM, et al. , Cardiovascular safety of type 2 diabetes medications: review of existing literature and clinical implications, Horm. (Athens, Greece) 15 (2016) 170–185. [DOI] [PubMed] [Google Scholar]
- [60].Schneider JG, Yang Z, Chakravarthy MV, et al. , Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis, J. Biol. Chem 285 (2010) 23398–23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wu D, Liu J, Pang X, et al. , Palmitic acid exerts pro-inflammatory effects on vascular smooth muscle cells by inducing the expression of C-reactive protein, inducible nitric oxide synthase and tumor necrosis factor-alpha, Int. J. Mol. Med 34 (2014) 1706–1712. [DOI] [PubMed] [Google Scholar]
- [62].Mullen GE, Yet L, Progress in the development of fatty acid synthase inhibitors as anticancer targets, Bioorg. Med. Chem. Lett 25 (2015) 4363–4369. [DOI] [PubMed] [Google Scholar]
- [63].Fiorentino M, Zadra G, Palescandolo E, et al. , Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer, Lab. Investig. J. Tech. Methods Pathol 88 (2008) 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bandyopadhyay S, Pai SK, Watabe M, et al. , FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis, Oncogene 24 (2005) 5389–5395. [DOI] [PubMed] [Google Scholar]
- [65].Pizer ES, Jackisch C, Wood FD, et al. , Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells, Cancer Res. 56 (1996) 2745–2747. [PubMed] [Google Scholar]
- [66].Bian Y, Yu Y, Wang S, et al. , Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer, Biochem. Biophys. Res. Commun 463 (2015) 612–617. [DOI] [PubMed] [Google Scholar]
- [67].Ploplis VA, Effects of altered plasminogen activator inhibitor-1 expression on cardiovascular disease, Curr. Drug Targets 12 (2011) 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Iwaki T, Urano T, Umemura K, PAI-1, progress in understanding the clinical problem and its aetiology, Br. J. Haematol 157 (2012) 291–298. [DOI] [PubMed] [Google Scholar]
- [69].Hursting SD, Hursting MJ, Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer, Arterioscler. Thromb. Vasc. Biol 32 (2012) 1766–1770. [DOI] [PubMed] [Google Scholar]
- [70].Fang H, Placencio VR, DeClerck YA, Protumorigenic activity of plasminogen activator inhibitor-1 through an antiapoptotic function, J. Natl. Cancer Inst 104 (2012) 1470–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yusuf S, Hawken S, Ounpuu S, et al. , Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study, Lancet (Lond. Engl.) 364 (2004) 937–952. [DOI] [PubMed] [Google Scholar]
- [72].Renehan AG, Tyson M, Egger M, et al. , Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies, Lancet (Lond. Engl.) 371 (2008) 569–578. [DOI] [PubMed] [Google Scholar]
- [73].Sjostrom L, Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery, J. Intern. Med 273 (2013) 219–234. [DOI] [PubMed] [Google Scholar]
- [74].Golpaie A, Tajik N, Masoudkabir F, et al. , Short-term effect of weight loss through restrictive bariatric surgery on serum levels of vaspin in morbidly obese subjects, Eur. Cytokine Netw 22 (2011) 181–186. [DOI] [PubMed] [Google Scholar]
- [75].Karbaschian Z, Hosseinzadeh-Attar MJ, Giahi L, et al. , Portal and systemic levels of visfatin in morbidly obese subjects undergoing bariatric surgery, Endocrine 44 (2013) 114–118. [DOI] [PubMed] [Google Scholar]
- [76].Tajik N, Golpaie A, Keshavarz SA, et al. , Decreased plasma levels of ceruloplasmin after diet-induced weight loss in obese women, J. Endocrinol. Investig 35 (2012) 566–569. [DOI] [PubMed] [Google Scholar]
- [77].Tajik N, Keshavarz SA, Masoudkabir F, et al. , Effect of diet-induced weight loss on inflammatory cytokines in obese women, J. Endocrinol. Investig 36 (2013) 211–215. [DOI] [PubMed] [Google Scholar]
- [78].Ando S, Catalano S, The multifactorial role of leptin in driving the breast cancer microenvironment, Nature reviews, Endocrinology 8 (2011) 263–275. [DOI] [PubMed] [Google Scholar]
- [79].Garofalo C, Surmacz E, Leptin and cancer, J. Cell. Physiol 207 (2006) 12–22. [DOI] [PubMed] [Google Scholar]
- [80].Ntaios G, Gatselis NK, Makaritsis K, et al. , Adipokines as mediators of endothelial function and atherosclerosis, Atherosclerosis 227 (2013) 216–221. [DOI] [PubMed] [Google Scholar]
- [81].Kallen CB, Lazar MA, Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes, Proc. Natl. Acad. Sci. U. S. A 93 (1996) 5793–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Giaginis C, Tsantili-Kakoulidou A, Theocharis S, Peroxisome proliferatoractivated receptor-gamma ligands: potential pharmacological agents for targeting the angiogenesis signaling cascade in cancer, PPAR Res. 2008. (2008) 431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].MacDonald BT, Tamai K, He X, Wnt/beta-catenin signaling: components, mechanisms, and diseases, Dev. Cell 17 (2009) 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Garcia-Jimenez C, Garcia-Martinez JM, Chocarro-Calvo A, et al. , A new link between diabetes and cancer: enhanced WNT/beta-catenin signaling by high glucose, J. Mol. Endocrinol 52 (2014) R51–R66. [DOI] [PubMed] [Google Scholar]
- [85].Srivastava R, Zhang J, Go GW, et al. , Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease, Cell Rep. 13 (2015) 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Keramati AR, Singh R, Lin A, et al. , Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mani A, Radhakrishnan J, Wang H, et al. , LRP6 mutation in a family with early coronary disease and metabolic risk factors, Science 315 (2007) 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Christman MA 2nd, Goetz DJ, Dickerson E, et al. , Wnt5a is expressed in murine and human atherosclerotic lesions, Am. J. Physiol. Heart Circ. Physiol 294 (2008) H2864–H2870. [DOI] [PubMed] [Google Scholar]
- [89].Kim J, Kim J, Kim DW, et al. , Wnt5a induces endothelial inflammation via beta-catenin-independent signaling, J. Immunol. (Baltim. Md. 1950) 185 (2010) 1274–1282. [DOI] [PubMed] [Google Scholar]
- [90].Shao JS, Cheng SL, Pingsterhaus JM, et al. , Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals, J. Clin. Investig 115 (2005) 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Marinou K, Christodoulides C, Antoniades C, et al. , Wnt signaling in cardiovascular physiology, Trends Endocrinol. Metab. TEM 23 (2012) 628–636. [DOI] [PubMed] [Google Scholar]
- [92].Hirabayashi S, Baranski TJ, Cagan RL, Transformed Drosophila cells evade diet-mediated insulin resistance through wingless signaling, Cell 154 (2013) 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu W, Mani S, Davis NR, et al. , Mutation in EGFP domain of LDL receptorrelated protein 6 impairs cellular LDL clearance, Circ. Res 103 (2008) 1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Go GW, Mani A, Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis, Yale J. Biol. Med 85 (2012) 19–28. [PMC free article] [PubMed] [Google Scholar]
- [95].Liu W, Singh R, Choi CS, et al. , Low density lipoprotein (LDL) receptorrelated protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure, J. Biol. Chem 287 (2012) 7213–7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ye ZJ, Go GW, Singh R, et al. , LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake, J. Biol. Chem 287 (2012) 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Singh R, De Aguiar RB, Naik S, et al. , LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans, Cell Metab. 17 (2013) 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Singh R, Smith E, Fathzadeh M, et al. , Rare nonconservative LRP6 mutations are associated with metabolic syndrome, Hum. Mutat 34 (2013) 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Go GW, Srivastava R, Hernandez-Ono A, et al. , The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue, Cell Metab. 19 (2014) 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Song K, Wang S, Mani M, et al. , Wnt signaling, de novo lipogenesis, adipogenesis and ectopic fat, Oncotarget 5 (2014) 11000–11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang S, Song K, Srivastava R, et al. , Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 29 (2015) 3436–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li Y, Lu W, He X, et al. , LRP6 expression promotes cancer cell proliferation and tumorigenesis by altering beta-catenin subcellular distribution, Oncogene 23 (2004) 9129–9135. [DOI] [PubMed] [Google Scholar]
- [103].Tung EK, Wong BY, Yau TO, et al. , Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion, PLoS One 7 (2012) e36565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Liu CC, Prior J, Piwnica-Worms D, et al. , LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 5136–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang J, Li Y, Liu Q, et al. , Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis, Oncogene 29 (2010) 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lemieux E, Cagnol S, Beaudry K, et al. , Oncogenic KRAS signalling promotes the Wnt/beta-catenin pathway through LRP6 in colorectal cancer, Oncogene 34 (2015) 4914–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Muendlein A, Saely CH, Geller-Rhomberg S, et al. , Single nucleotide polymorphisms of TCF7L2 are linked to diabetic coronary atherosclerosis, PLoS One 6 (2011) e17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang J, Hu F, Feng T, et al. , Meta-analysis of associations between TCF7L2 polymorphisms and risk of type 2 diabetes mellitus in the Chinese population, BMC Med. Genet 14 (2013) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tong Y, Lin Y, Zhang Y, et al. , Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta-analysis, BMC Med. Genet 10 (2009) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cauchi S, El Achhab Y, Choquet H, et al. , TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis, J. Mol. Med. (Berl. Ger.) 85 (2007) 777–782. [DOI] [PubMed] [Google Scholar]
- [111].Wang F, Jiang L, Li J, et al. , Association between TCF7L2 polymorphisms and breast cancer susceptibility: a meta-analysis, Int. J. Clin. Exp. Med 8 (2015) 9355–9361. [PMC free article] [PubMed] [Google Scholar]
- [112].Folsom AR, Pankow JS, Peacock JM, et al. , Variation in TCF7L2 and increased risk of colon cancer: the Aherosclerosis Risk in Communities (ARIC) Study, Diabetes Care 31 (2008) 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Domenyuk VP, Litovkin KV, Verbitskaya TG, et al. , Identification of new DNA markers of endometrial cancer in patients from the Ukrainian population, Exp. Oncol 29 (2007) 152–155. [PubMed] [Google Scholar]
- [114].Connor AE, Baumgartner RN, Baumgartner KB, et al. , Associations between TCF7L2 polymorphisms and risk of breast cancer among Hispanic and non-Hispanic white women: the Breast Cancer Health Disparities Study, Breast Cancer Res. Treat 136 (2012) 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen CS, Huang CY, Huang SP, et al. , Genetic interaction analysis of TCF7L2 for biochemical recurrence after radical prostatectomy in localized prostate cancer, Int. J. Med. Sci 12 (2015) 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Burwinkel B, Shanmugam KS, Hemminki K, et al. , Transcription factor 7like 2 (TCF7L2) variant is associated with familial breast cancer risk: a case-control study, BMC Cancer 6 (2006) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tejedor F, Zhu XR, Kaltenbach E, et al. , minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila, Neuron 14 (1995) 287–301. [DOI] [PubMed] [Google Scholar]
- [118].Yang EJ, Ahn YS, Chung KC, Protein kinase Dyrk1 activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells, J. Biol. Chem 276 (2001) 39819–39824. [DOI] [PubMed] [Google Scholar]
- [119].Deng X, Friedman E, Mirk kinase inhibition blocks the in vivo growth of pancreatic cancer cells, Genes & Cancer 5 (2014) 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Friedman E, Mirk/dyrk1B kinase in ovarian cancer, Int. J. Mol. Sci 14 (2013) 5560–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gao J, Zheng Z, Rawal B, et al. , Mirk/Dyrk1B, a novel therapeutic target, mediates cell survival in non-small cell lung cancer cells, Cancer Biol. Ther 8 (2009) 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Keramati AR, Fathzadeh M, Go GW, et al. , A form of the metabolic syndrome associated with mutations in DYRK1B, N. Engl. J. Med 370 (2014) 1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li YY, Methylenetetrahydrofolate reductase C677T gene polymorphism and coronary artery disease in a Chinese Han population: a meta-analysis, Metab. Clin. Exp 61 (2012) 846–852. [DOI] [PubMed] [Google Scholar]
- [124].Hou X, Chen X, Shi J, Genetic polymorphism of MTHFR C677T and premature coronary artery disease susceptibility: a meta-analysis, Gene 565 (2015) 39–44. [DOI] [PubMed] [Google Scholar]
- [125].Xuan C, Bai XY, Gao G, et al. , Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls, Arch. Med. Res 42 (2011) 677–685. [DOI] [PubMed] [Google Scholar]
- [126].Thambyrajah J, Townend JN, Homocysteine and atherothrombosisemechanisms for injury, Eur. Heart J 21 (2000) 967–974. [DOI] [PubMed] [Google Scholar]
- [127].Liew SC, Gupta ED, Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases, Eur. J. Med. Genet 58 (2015) 1–10. [DOI] [PubMed] [Google Scholar]
- [128].Khandanpour N, Willis G, Meyer FJ, et al. , Peripheral arterial disease and methylenetetrahydrofolate reductase (MTHFR) C677T mutations: a casecontrol study and meta-analysis, J. Vasc. Surg 49 (2009) 711–718. [DOI] [PubMed] [Google Scholar]
- [129].Kumar A, Kumar P, Prasad M, et al. , Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene (MTHFR gene) with ischemic stroke: a meta-analysis, Neurol. Res 37 (2015) 568–577. [DOI] [PubMed] [Google Scholar]
- [130].Zhu XY, Hou RY, Pan XD, et al. , Association between the methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and ischemic stroke in the Chinese population: a meta-analysis, Int. J. Neurosci 125 (2015) 885–894. [DOI] [PubMed] [Google Scholar]
- [131].Gao S, Li H, Xiao H, et al. , Association of MTHFR 677T variant allele with risk of intracerebral haemorrhage: a meta-analysis, J. Neurol. Sci 323 (2012) 40–45. [DOI] [PubMed] [Google Scholar]
- [132].Kang S, Zhao X, Liu L, et al. , Association of the C677T polymorphism in the MTHFR gene with hemorrhagic stroke: a meta-analysis, Genet. Test. Mol. Biomarker 17 (2013) 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Thomas P, Fenech M, Methylenetetrahydrofolate reductase, common polymorphisms, and relation to disease, Vitam. Horm 79 (2008) 375–392. [DOI] [PubMed] [Google Scholar]
- [134].Montecucco F, Mach F, Statins ACE inhibitors and ARBs in cardiovascular disease, Best practice & research, Clin. Endocrinol. Metab 23 (2009) 389–400. [DOI] [PubMed] [Google Scholar]
- [135].Kavalipati N, Shah J, Ramakrishan A, et al. , Pleiotropic effects of statins, Indian J. Endocrinol. Metab 19 (2015) 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Bockorny B, Dasanu CA, HMG-CoA reductase inhibitors as adjuvant treatment for hematologic malignancies: what is the current evidence? Ann. Hematol 94 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Simon MS, Rosenberg CA, Rodabough RJ, et al. , Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk, Ann. Epidemiol 22 (2012) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Poynter JN, Gruber SB, Higgins PD, et al. , Statins and the risk of colorectal cancer, N. Engl. J. Med 352 (2005) 2184–2192. [DOI] [PubMed] [Google Scholar]
- [139].El-Serag HB, Johnson ML, Hachem C, et al. , Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes, Gastroenterology 136 (2009) 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Corcos L, Le Jossic-Corcos C, Statins: perspectives in cancer therapeutics, Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 45 (2013) 795–802. [DOI] [PubMed] [Google Scholar]
- [141].Shah NR, Mahmoudi M, The role of DNA damage and repair in atherosclerosis: a review, J. Mol. Cell. Cardiol 86 (2015) 147–157. [DOI] [PubMed] [Google Scholar]
- [142].Chogtu B, Magazine R, Bairy KL, Statin use and risk of diabetes mellitus, World J. Diabetes 6 (2015) 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sattar N, Preiss D, Murray HM, et al. , Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials, Lancet (Lond. Engl.) 375 (2010) 735–742. [DOI] [PubMed] [Google Scholar]
- [144].Sampson UK, Linton MF, Fazio S, Are statins diabetogenic? Curr. Opin. Cardiol 26 (2011) 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lever AF, Hole DJ, Gillis CR, et al. , Do inhibitors of angiotensin-Iconverting enzyme protect against risk of cancer? Lancet (Lond. Engl.) 352 (1998) 179–184. [DOI] [PubMed] [Google Scholar]
- [146].Arafat HA, Gong Q, Chipitsyna G, et al. , Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma, J. Am. Coll. Surg 204 (2007) 996–1005 discussion 1005–1006. [DOI] [PubMed] [Google Scholar]
- [147].Reddy MK, Baskaran K, Molteni A, Inhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cells, Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. (New York N.Y.) 210 (1995) 221–226. [DOI] [PubMed] [Google Scholar]
- [148].Prontera C, Mariani B, Rossi C, et al. , Inhibition of gelatinase A (MMP-2) by batimastat and captopril reduces tumor growth and lung metastases in mice bearing Lewis lung carcinoma, Int. J. Cancer J. Int. du Cancer 81 (1999) 761–766. [DOI] [PubMed] [Google Scholar]
- [149].Escobar E, Rodriguez-Reyna TS, Arrieta O, et al. , Angiotensin II, cell proliferation and angiogenesis regulator: biologic and therapeutic implications in cancer, Curr. Vasc. Pharmacol 2 (2004) 385–399. [DOI] [PubMed] [Google Scholar]
- [150].Fleming I, Kohlstedt K, Busse R, The tissue renin-angiotensin system and intracellular signalling, Curr. Opin. Nephrol. Hypertens 15 (2006) 8–13. [DOI] [PubMed] [Google Scholar]
- [151].Ino K, Shibata K, Kajiyama H, et al. , Manipulating the angiotensin systemenew approaches to the treatment of solid tumours, Expert Opin. Biol. Ther 6 (2006) 243–255. [DOI] [PubMed] [Google Scholar]
- [152].Taylor GM, Cook HT, Sheffield EA, et al. , Renin in blood vessels in human pulmonary tumors. An immunohistochemical and biochemical study, Am. J. Pathol 130 (1988) 543–551. [PMC free article] [PubMed] [Google Scholar]
- [153].Yoshiji H, Kuriyama S, Fukui H, Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor, Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med 23 (2002) 348–356. [DOI] [PubMed] [Google Scholar]
- [154].Parekh AK, Galloway JM, Hong Y, et al. , Aspirin in the secondary prevention of cardiovascular disease, N. Engl. J. Med 368 (2013) 204–205. [DOI] [PubMed] [Google Scholar]
- [155].Halvorsen S, Andreotti F, ten Berg JM, et al. , Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis, J. Am. Coll. Cardiol 64 (2014) 319–327. [DOI] [PubMed] [Google Scholar]
- [156].Baigent C, Blackwell L, Collins R, et al. , Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials, Lancet (Lond. Engl.) 373 (2009) 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Chan AT, Cook NR, Are we reaValidation Report: 2error(s)dy to recommend aspirin for cancer prevention? Lancet (Lond. Engl.) 379 (2012) 1569–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rothwell PM, Wilson M, Elwin CE, et al. , Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials, Lancet (Lond. Engl.) 376 (2010) 1741–1750. [DOI] [PubMed] [Google Scholar]
- [159].Rothwell PM, Fowkes FG, Belch JF, et al. , Effect of daily aspirin on longterm risk of death due to cancer: analysis of individual patient data from randomised trials, Lancet (Lond. Engl.) 377 (2011) 31–41. [DOI] [PubMed] [Google Scholar]
- [160].Alfieri O, Mayosi BM, Park SJ, et al. , Exploring unknowns in cardiology, Nature reviews, Cardiology 11 (2014) 664–670. [DOI] [PubMed] [Google Scholar]