Abstract
Between 28 June and 17 September 2018, 27 cases of human West Nile virus infections were recorded in Austria; four cases of West Nile neuroinvasive disease, 11 cases of West Nile fever, six infections detected by blood donation screening and six imported cases. In addition, 18 cases of human Usutu virus infections (all blood donors) were recorded. This is the highest number of annual infections recorded in Austria since the introduction of both viruses.
Keywords: West Nile virus, West Nile fever, West Nile neuroinvasive disease, Usutu virus, blood donor, Austria
West Nile virus (WNV) and Usutu virus (USUV) are closely related mosquito-borne viruses (genus Flavivirus; family Flaviviridae). WNV infection in humans may result in disease of varying severity, from West Nile fever (WNF) to possibly lethal West Nile neuroinvasive disease (WNND) [1]. Human infections with USUV are usually asymptomatic or occasionally associated with rash [2]; severe disease is rarely seen and occurs mainly in immunocompromised patients [3,4]. In 2018, the highest ever number of WNV and USUV infections were detected in Austria.
Human West Nile virus and Usutu virus infections in Austria, 2009–2018
The first three human West Nile disease (WND) cases were identified in eastern Austria in 2009 and 2010; two cases of WNND and one case of WNF [5]. Since then, autochthonous human WND cases have been diagnosed every year, with the exception of 2011–2013, when only imported WNV cases were detected (Table 1).
Table 1. Number of diagnosed human West Nile and Usutu virus infections, Austria, 2009–2018.
| Year | West Nile virus infections | Reference | Usutu virus infections | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Autochthonous | Imported | Total | Autochthonous | ||||||
| WNND | WNF | Asympt WNV | Blood donors | Blood donors | |||||
| 2009 | 1 | 1 | 0 | ND | 0 | 2 | [5] | ND | NA |
| 2010 | 1 | 0 | 0 | ND | 0 | 1 | ND | NA | |
| 2011 | 0 | 0 | 0 | ND | 0 | 0 | NA | ND | NA |
| 2012 | 0 | 0 | 0 | ND | 3 | 3 | NA | ND | NA |
| 2013 | 0 | 0 | 0 | ND | 0 | 0 | NA | ND | NA |
| 2014 | 1 | 0 | 0 | 1 | 0 | 2 | [7,8] | 0 | NA |
| 2015 | 1 | 0 | 1 | 5 | 0 | 7 | [6] [9] |
0 | NA |
| 2016 | 1 | 1 | 0 | 3 | 0 | 5 | 1 | [9] | |
| 2017 | 2 | 2 | 1 | 1 | 1 | 7 | 6 | ||
| 2018 | 4 | 11 | 0 | 6 | 6 | 27 | NA | 18 | NA |
| Total | 11 | 15 | 2 | 16 | 10 | 54 | NA | 25 | NA |
Asympt: asymptomatic; NA: not applicable; ND: not done; WNF: West Nile fever; WNND: West Nile neuroinvasive disease; WNV: Nest Nile virus.
Between 28 June and 17 September 2018, 27 cases of human WNV infections were recorded of which 21 were locally acquired. Four cases were WNND, 11 cases were WNF, six were WNV infections detected by blood donation screening and six cases were associated with travels to Serbia (n = 2), Italy (n = 1), Greece (n = 1), Hungary (n = 1), and Croatia (n = 1). USUV infections were identified in 18 of 31,598 blood donations tested (Table 1). All autochthonous WNV and USUV infections were acquired in eastern Austria (Figure 1).
Figure 1.
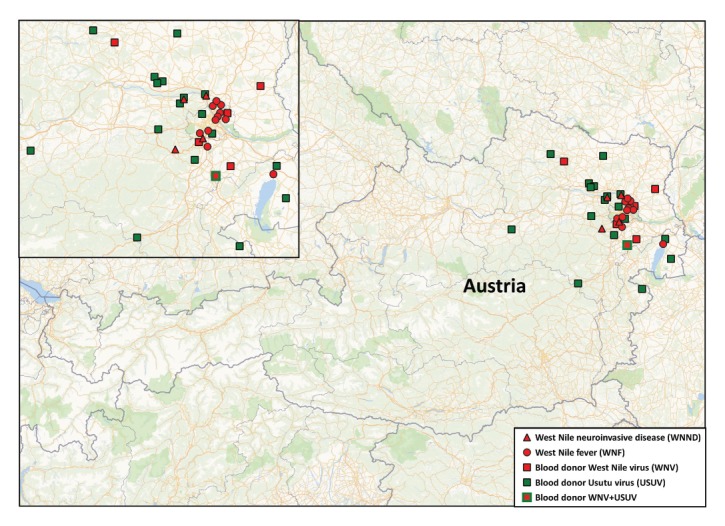
Geographic distribution of human West Nile and Usutu virus infections, Austria, 2018
In Austria, human, veterinary and entomological surveillances to detect WNV and USUV include seasonal testing of blood donations originating from endemic areas, detailed sample examination of humans and equids with neurological symptoms, passive ornithological monitoring as well as regular, nationwide mosquito surveillance (for details see [6]).
In 2014, the Blood Service for Vienna, Lower Austria and Burgenland of the Austrian Red Cross introduced an automated nucleic acid test (NAT) on the cobas 8800 system (cobas WNV assay; Roche, Rotkreuz, Switzerland) for all blood donated between 1 June and 30 November each year. Already in the first year of screening, one WNV-positive donation was identified [7,8] as well as one clinical WNND case (Table 1). In the 2015/16 [6] and 2017 seasons, further clinical WNV cases were diagnosed and positive blood donations were identified [9]. To date, no WND associated fatalities have occurred in Austria. Increasing USUV activity in birds in recent years in Austria was reflected in human infections, when one of four, and six of seven flavivirus NAT-positive blood donations turned out to be USUV positive and not WNV positive in 2016 and 2017, respectively (Table 1) [9].
In 2018, overall, 15 patients and three blood donors showed signs and symptoms compatible with WNV infection (Table 2). Three of four patients with WNND were male and the median age was 62 years (range 58–77). Of the 11 WNF cases, six were male and the median age was 51 years (range 33–87). The overall median age of the blood donors was 56 years (range 23–71) with no difference between WNV and USUV-positive persons. Five of six and 14 of 18 WNV and USUV-positive blood donors, respectively, were male. Of the 18 USUV infections identified from blood donations tested, 16 donors remained asymptomatic, one developed a rash and one donor did not disclose information. One blood donor had a dual infection with WNV and USUV (Table 2).
Table 2. Details of autochthonous human West Nile and Usutu virus infections, Austria, 2018.
| Cases | Main symptoms | Month of symptom onset/ blood donation | Cobas WNV assaya | WNV RT-PCRb |
USUV RT-PCRb |
WNV NT | USUV NT |
|---|---|---|---|---|---|---|---|
| WNND | |||||||
| 1 | Disorientation, aphasia | August | ND | Pos | Neg | 240 | 40 |
| 2 | Disorientation, coma | September | ND | Pos | Neg | 640 | 60 |
| 3 | Fever, stiff neck | August | ND | Pos | Neg | 160 | 20 |
| 4 | Dysarthria, limb weakness | September | ND | Neg | Neg | 240 | 60 |
| WNF | |||||||
| 1 | Fever, rash, fatigue, headache | August | ND | Pos | Neg | 960 | 160 |
| 2 | Fever, rash, diarrhoea | August | ND | Pos | ND | 320 | 80 |
| 3 | Fever, rash | August | ND | Pos | Neg | 640 | 60 |
| 4 | Rash | August | ND | Pos | ND | ND | ND |
| 5 | Fever, rash | August | ND | Pos | ND | 40 | 30 |
| 6 | Fever, rash | August | ND | Pos | ND | 320 | 120 |
| 7 | Rash, ocular pain | August | ND | Pos | ND | 320 | 40 |
| 8 | Fever, fatigue, muscle aches | August | ND | Pos | ND | 480 | 60 |
| 9 | Rash, fatigue | August | ND | Neg | Neg | 240 | 60 |
| 10 | Fever, rash | August | ND | Pos | ND | 120 | < 20 |
| 11 | Fever, fatigue | August | ND | Pos | Neg | 320 | < 20 |
| Blood donors | |||||||
| WNV | |||||||
| BD2 | Rash, fatigue, headachec | August | Pos (30.4) | Pos | Neg | 480 | 30 |
| BD4 | Rash, fatigued | August | Pos (40.0) | Pos | Neg | 1,920 | 160 |
| BD9 | Fatigue, joint achesc | August | Pos (30.8) | Pos | Neg | 240 | 80 |
| BD17 | Asympt | August | Pos (30.0) | Pos | Neg | 160 | 60 |
| BD18 | Asympt | August | Pos (39.4) | Pos | Neg | 240 | 160 |
| WNV/USUV | |||||||
| BD7 | Asympt | August | Pos (34.1) | Pos | Pos | 960 | 120 |
| USUV | |||||||
| BD1 | Asympt | June | Pos (38.7) | Neg | Pos | < 20 | 40 |
| BD3 | Rashc | August | Pos (37.6) | Neg | Pos | 60 | 160 |
| BD5 | Asympt | August | Pos (39.4) | Neg | Pos | ND | ND |
| BD6 | Asympt | August | Pos (37.1) | Neg | Pos | 80 | 240 |
| BD8 | Asympt | August | Pos (44.0) | Neg | Pos | < 20 | 80 |
| BD10 | Asympt | August | Pos (35.3) | Neg | Pos | ND | ND |
| BD11 | Asympt | August | Pos (38.8) | Neg | Pos | 30 | 120 |
| BD12 | Asympt | August | Pos (40.7) | Neg | Pos | < 20 | 160 |
| BD13 | Asympt | August | Pos (38.4) | Neg | Pos | < 20 | 40 |
| BD14 | No information | August | Pos (39.3) | Neg | Pos | ND | ND |
| BD15 | Asympt | August | Pos (36.5) | Neg | Pos | ND | ND |
| BD16 | Asympt | August | Pos (36.8) | Neg | Pos | 20 | 40 |
| BD19 | Asympt | August | Pos (36.6) | Neg | Pos | 20 | 120 |
| BD20 | Asympt | August | Pos (42.1) | Neg | Neg | < 20 | 80 |
| BD21 | Asympt | August | Pos (41.7) | Neg | Neg | < 20 | 80 |
| BD22 | Asympt | September | Pos (40.0) | Neg | Pos | ND | ND |
| BD23 | Asympt | Septenber | Pos (38.6) | Neg | Pos | 40 | 240 |
Asympt: asymptomatic; ND: not done; Neg: negative; NT: Neutralizing antibody titer in first or follow-up serum sample; Pos: positive; USUV: Usutu virus; WNF: West Nile fever; WNND: West Nile neuroinvasive disease; WNV: West Nile virus.
aNucleic acid test performed in plasma samples. Cq values obtained with pools of 19 donations are shown in brackets.
bWNV and USUV PCR performed in plasma, whole blood, serum, urine and/or cerebral spinal fluid.
cSymptoms occurred 1–3 days after blood donation. The donors were viremic but asymptomatic.
dMild symptoms 2 days before blood donation, but asymptomatic at blood donation.
All infections diagnosed up to 24 October 2018 are included.
WNV and USUV PCRs, as well as neutralisation tests, were performed as previously described [5,6,9].
Sequencing information
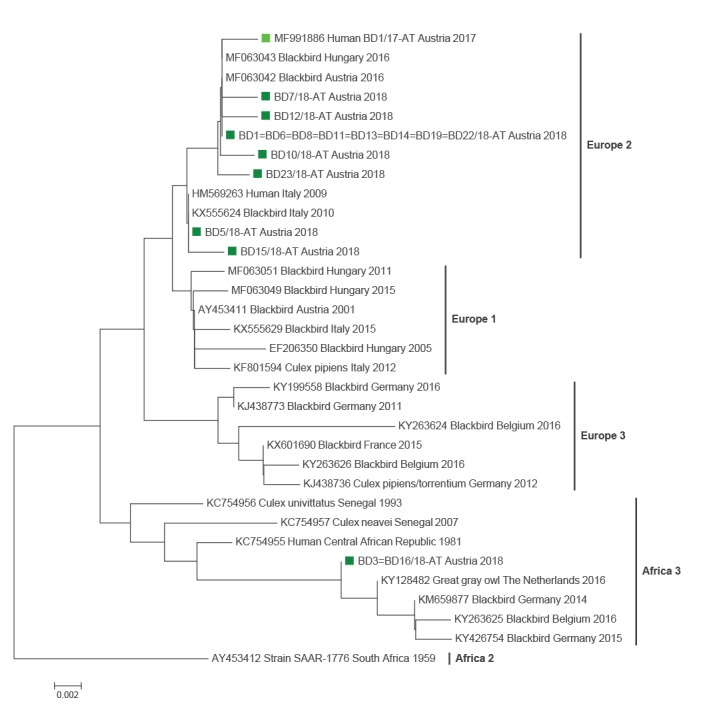
Sequencing of short fragments of the WNV C/prM (294nts) and USUV NS5 (396nts) genes enabled exact identification of the detected viruses. This analysis revealed that all WNV strains belonged to lineage 2 with a 98.98–100% identity to the Austrian strain BD2/2016 [6] (GenBank accession number MF984347). All but two USUV strains belonged to the currently in Austria most widespread ‘Europe 2’ lineage showing 99.84–100% identity to European 2 sequences of Hungarian blackbirds (e.g. GenBank accession number MF063048) and an Austrian blood donor sequence from 2017 (GenBank accession number MF991886)(Figure 2). The two USUV strains from BD3 and BD16, however, clustered within the ‘Africa 3’ lineage and were 99.52-99.84% identical to the African 3 sequences of birds from Germany and The Netherlands (e.g. GenBank accession numbers KY294723, KY128482)(Figure 2).
Figure 2.
Phylogram demonstrating the genetic relationships among Usutu viruses based on partial (396 nts) NS5 protein coding nucleotide sequences
BD: blood donor.
The phylogenetic tree was created by the neighbour-joining method with the MEGA7 program as described previously [6]. Usutu virus sequences determined in this study are marked with dark green squares and one sequence from 2017 [9] with a light green square. Sequences of eight samples in cluster Europe 2 and sequences of two samples in cluster Africa 3 are genetically identical to each other and cluster therefore together, respectively. GenBank accession numbers of USUV sequences derived from 16 blood donors were submitted on 22 October 2018 and will be added when available.
Discussion
With 21 locally-acquired and six imported human WNV infections the 2018 transmission season has been the most relevant since the introduction of the aetiological lineage 2 WNV to Austria in 2008 [10,11]. The number of autochthonous human infections identified so far in 2018 has almost reached the cumulative number diagnosed in the past 10 years (2008–2017) in eastern Austria (23 locally-acquired human infections). In addition, obligatory seasonal blood donation screening and subsequent testing by virus-specific RT-PCR assays revealed 18 USUV infections among blood donors, which is the highest number of human infections reported since the emergence of USUV in Austria in 2001 [12]. An early start of the WNV/USUV transmission season was observed in Austria in 2018, which (at least for WNV) was also seen in other European countries [13,14] and might have been associated with favourable environmental and climatic conditions for an early upsurge of the vector population [14].
One blood donor was found to have a double infection with both WNV and USUV for the first time. While the central European lineage 2 [15] was the causative WNV strain in all sequenced cases, two different USUV lineages were identified in the blood donors. The vast majority were infected with USUV lineage ‘Europe 2’, which is currently the dominating USUV strain in Austria [9,16]. Two donors, however, were infected with the USUV ‘Africa 3’ lineage, which is also circulating in Austria on a smaller scale (unpublished data). Follow-up investigations of WNV positive blood donors revealed mild symptoms e.g. rash and fatigue in three of the six cases a few days before or after donating blood, whereas 16 of 18 USUV-RNA positive donors did not report any symptoms; one donor reported a rash and for one case no information was available. Although USUV seems to be less pathogenic for humans than WNV, the virus might cause severe disease in immunocompromised patients [3,4] or might be involved in other neurologic disorders such as idiopathic facial paralysis, as recently reported from France [17].
USUV is spreading in Europe, which may lead to increased numbers of human infections. In countries with blood donation testing for flavivirus RNA, health authorities should be aware that positive WNV screening results could be due to USUV infections and have to be further differentiated. In addition, countries where blood donation testing is not performed for flaviviruses, USUV might be transmitted through contaminated blood units. Although no transfusion-associated USUV infection has been reported so far, it is of utmost importance to further investigate the clinical relevance of USUV infections.
Acknowledgements
The authors wish to acknowledge Dominika Kapuscinska for the excellent art work, and Tanja Amon, Barbara Dalmatiner, Jutta Hutecek, Silvia Schwödiauer, Thomas Urbanek and Pia Weidinger for excellent technical assistance.
Conflict of interest: None declared.
Authors’ contributions: NN initiated the study. SWA, CJ, JHA, AZ and LW were involved in data collection.
SWA, JK, KS and MKH performed laboratory analyses and interpreted results. SWA, JK, CJ and NN drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
References
- 1. Hubálek Z, Rudolf I, Nowotny N. Arboviruses pathogenic for domestic and wild animals. Adv Virus Res. 2014;89:201-75. 10.1016/B978-0-12-800172-1.00005-7 [DOI] [PubMed] [Google Scholar]
- 2.Weissenböck H, Chvala-Mannsberger S, Bakonyi T, Nowotny N. Emergence of Usutu virus in Central Europe: diagnosis, surveillance and epizootiology. In: Willem Takken and Bart GJ Knols, editors. Emerging pests and vector-borne diseases in Europe - Ecology and Control of Vector-borne diseases, Volume 1. Wageningen: Wageningen Academic Publishers; 2007. p 153-168. [Google Scholar]
- 3. Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. 2009;14(50):19448. [PubMed] [Google Scholar]
- 4. Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini A, Tagliazucchi S, et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. 2009;14(50):19446. [PubMed] [Google Scholar]
- 5. Stiasny K, Aberle SW, Heinz FX. Retrospective identification of human cases of West Nile virus infection in Austria (2009 to 2010) by serological differentiation from Usutu and other flavivirus infections. Euro Surveill. 2013;18(43):20614. 10.2807/1560-7917.ES2013.18.43.20614 [DOI] [PubMed] [Google Scholar]
- 6. Kolodziejek J, Jungbauer C, Aberle SW, Allerberger F, Bagó Z, Camp JV, et al. Integrated analysis of human-animal-vector surveillance: West Nile virus infections in Austria, 2015-2016. Emerg Microbes Infect. 2018;7(1):25. 10.1038/s41426-018-0021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jungbauer C, Hourfar MK, Stiasny K, Aberle SW, Cadar D, Schmidt-Chanasit J, et al. West Nile virus lineage 2 infection in a blood donor from Vienna, Austria, August 2014. J Clin Virol. 2015;64:16-9. 10.1016/j.jcv.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 8. Kolodziejek J, Seidel B, Jungbauer C, Dimmel K, Kolodziejek M, Rudolf I, et al. West Nile virus positive blood donation and subsequent entomological investigation, Austria, 2014. PLoS One. 2015;10(5):e0126381. 10.1371/journal.pone.0126381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bakonyi T, Jungbauer C, Aberle SW, Kolodziejek J, Dimmel K, Stiasny K, et al. Usutu virus infections among blood donors, Austria, July and August 2017 - Raising awareness for diagnostic challenges. Euro Surveill. 2017;22(41). 10.2807/1560-7917.ES.2017.22.41.17-00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakonyi T, Ferenczi E, Erdélyi K, Kutasi O, Csörgő T, Seidel B, et al. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165(1-2):61-70. 10.1016/j.vetmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 11. Wodak E, Richter S, Bagó Z, Revilla-Fernández S, Weissenböck H, Nowotny N, et al. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. 2011;149(3-4):358-66. 10.1016/j.vetmic.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 12. Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8(7):652-6. 10.3201/eid0807.020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riccardo F, Monaco F, Bella A, Savini G, Russo F, Cagarelli R, et al. An early start of West Nile virus seasonal transmission: the added value of One Heath surveillance in detecting early circulation and triggering timely response in Italy, June to July 2018. Euro Surveill. 2018;23(32). 10.2807/1560-7917.ES.2018.23.32.1800427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haussig JM, Young JJ, Gossner CM, Mezei E, Bella A, Sirbu A, et al. Early start of the West Nile fever transmission season 2018 in Europe. Euro Surveill. 2018;23(32). 10.2807/1560-7917.ES.2018.23.32.1800428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12(4):618-23. 10.3201/eid1204.051379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakonyi T, Erdélyi K, Brunthaler R, Dán Á, Weissenböck H, Nowotny N. Usutu virus, Austria and Hungary, 2010-2016. Emerg Microbes Infect. 2017;6(10):e85. 10.1038/emi.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonin Y, Sillam O, Carles MJ, Gutierrez S, Gil P, Constant O, et al. Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerg Infect Dis. 2018;24(5):875-8. 10.3201/eid2405.171122 [DOI] [PMC free article] [PubMed] [Google Scholar]


