Abstract
Background
Maternal perceived stress has been discussed to contribute to the development of childhood overweight. Our aim was to investigate the longitudinal relationship of early maternal perceived stress and BMI z-scores in preschool children (≤ five years).
Methods
A longitudinal analysis was conducted in 498 mother-child pairs of the German prospective birth cohort LINA with information on maternal perceived stress during pregnancy, one and two years after birth. BMI z-scores were based on annual measurements of children’s weight/height and calculated based on WHO reference data. General estimation equations were applied to evaluate the impact of maternal stress on children’s longitudinal BMI z-scores. Potential stressors contributing to the perceived stress of the mother were assessed by linear regression models. Using mediation analyses we evaluated the relationship between stressors, maternal perceived stress, and children’s BMI z-score development.
Results
Postnatal maternal stress during the first year after birth had a positive longitudinal relationship with children’s BMI z-scores up to the age of five years. Gender-stratified analyses revealed that only girls showed this positive association while boy’s BMI z-scores were unaffected by maternal stress. We identified three neighborhood strains and two socio-demographic factors, which contributed to the maternal perceived stress level. Stressors themselves did not directly affect girl’s BMI z-scores but rather mediated their effect through the perceived stress level.
Conclusions
While different stressors contribute to maternal stress, the perceived stress level - rather than the stressors themselves - is strongly positively associated with BMI z-score development in girls.
Electronic supplementary material
The online version of this article (10.1186/s12889-018-6110-5) contains supplementary material, which is available to authorized users.
Keywords: Stress dimensions, Perceived stress, Weight development, Stressor, Infant, Preschool children
Background
Overweight and obesity prevalence, especially among preschool children, has risen dramatically world-wide over the last decades affecting 6.1% of children under five years of age in 2016 [1]. Recent data from the KiGGS study shows that in Germany this fraction is even higher with 9.5% of children age two-six being overweight, of which 2.8% are classified as obese [2]. This is particularly concerning as most of these children will remain overweight in adolescence and adulthood, increasing their risk for co-morbidities like cardiovascular diseases or type 2 diabetes mellitus [3, 4].
Unhealthy diet and physical inactivity have been described as the main risk factors contributing to obesity development [5]. Consequently, child obesity intervention and prevention studies are mainly focusing on implementing changes in eating behavior and physical activity. However, most of these studies failed to reach long-term effects [6, 7], suggesting that other factors such as the living environment and parental behavior play an important role in the context of children’s overweight development. Among others, psychological aspects like early infant parental distress [8] as well as maternal depression [9] emerged as potential factors promoting children’s overweight.
Accumulating research shows the wide-ranging consequences of maternal stress on children’s health, which among others include an increased risk for behavioral problems, asthma, reduced birth weight, and an increased risk for becoming overweight [10–12]. Perceived stress is the individual perception about the stressfulness of life and the ability to handle such stress, which can be influenced by a variety of sources. These so-called stressors include socioeconomic disadvantages as well as recent life events like divorce/separation that can ultimately lead to stress-related physiological dysregulations [13, 14]. In this context maternal stress during pregnancy is known to alter signaling in the hypothalamic-pituitary-axis (HPA) exposing the developing fetus to an excess of glucocorticoids [15], one of the mechanisms discussed to contribute to prenatal growth restriction [16, 17] and an accelerated catch-up-growth increasing the risk for obesity in children’s later life [18–20]. While in the prenatal phase the physiological stress response of the mother can directly affect the child, in the postnatal period - as assessed here in the first two years after birth - the impact of maternal stress on parenting behavior and mother-child interactions become important. There is evidence that changes in feeding styles and practices [21] due to parental or maternal stress can have a significant impact on children’s food composition and energy intake [22]. Especially maternal stress becomes important as mothers often spend significantly more time in direct interaction with the child compared to the fathers. For example higher infant energy intake and increased consumption of breads and cereals during the first six month after birth have been described in association with maternal stress or depression [23], as has children’s reduced consumption of fruits and vegetables [24, 25]. Moreover, stress perceived by children themselves seems to alter their energy intake and food selection with a preference for sweet and high fat foods [25].
The majority of studies conducted so far focused on prenatal or postnatal stress exclusively, while longitudinal maternal stress assessments in relation to children’s weight development are rare. As recently reviewed by Tate et al. [12] and O’Connor et al. [26] most of these studies only used either longitudinal information on maternal stress or children’s overweight development. Therefore, our aim was to investigate the association between maternal stress and body mass index (BMI) trajectories in children in a longitudinal manner, including maternal stress evaluations from pregnancy until children’s age of two years and annual weight assessments of the children up to the age of five years. We complement our study by analyzing which stressors might contribute to the perceived maternal stress level and therefore might have a potential impact also on children’s weight development throughout the years. It has been discussed that the living environment including noise exposure and an unsecure living environment influence weight development in adults [27–29]. Since data on the effects of such stressors on children is sparse [30] we included not only the socioeconomic status of the study participants, but also factors characterizing their living environment such as traffic or residential noise in our analysis.
We hypothesize that with our longitudinal qualitative stress assessment we will be able to identify a time window, in which the child is particularly vulnerable to maternal stress and that such stress exposure experienced in this time window might have a long-lasting effect on the development of overweight in the child.
Methods
Study characteristics
The German prospective birth cohort LINA (Lifestyle and Environmental Factors and their Influence on Newborns Allergy risk) recruited 629 mother-child pairs at pregnancy (36th week of gestation) during May 2006 and December 2008, as has been described in more detail elsewhere[31–33]. Lifestyle, housing, and environmental factors were assessed by questionnaires during pregnancy and annually thereafter. Stress questionnaires from three time points (pregnancy, age 1, age 2) together with information on gender, gestational week at delivery, mode of delivery, breastfeeding and prenatal environmental tobacco smoke exposure (ETS) were available for 498 mother-child pairs, which we defined as our analyzed sub-cohort (Table 1). All questionnaires were self-administered by the parents and participation in the study was voluntary. Written informed consent was obtained from all individual participants included in the study. The study was approved by the Ethics Committee of the University of Leipzig (file ref # 046-2006, 160-2008, 160b/2008, 144-10-31052010, 113-11-18042011).
Table 1.
General study characteristics of the LINA cohort
| entire LINA cohort n (%), n = 629 a | analyzed sub-cohort n (%), n = 498 a | χ2-test | |
|---|---|---|---|
| Gender | 0.810 | ||
| Male | 330 (52.5) | 253 (50.8) | |
| Female | 299 (47.5) | 245 (49.2) | |
| Week of gestation at birth | 0.951 | ||
| <37 weeks | 25 (4.0) | 16 (3.2) | |
| 37-40 weeks | 389 (61.8) | 308 (61.8) | |
| > 40 weeks | 214 (34.0) | 174 (34.9) | |
| Mode of delivery | 0.979 | ||
| Spontaneous | 471 (74.9) | 387 (77.7) | |
| C-section | 132 (21.0) | 104 (20.9) | |
| Others | 7 (1.1) | 7 (1.4) | |
| Birth weight | 0.996 | ||
| < 3000g | 123 (19.6) | 92 (18.5) | |
| ≥ 3000g – 3500g | 242 (38.5) | 195 (39.2) | |
| ≥ 3500g – 4000g | 192 (30.5) | 151 (30.3) | |
| ≥ 4000g | 71 (11.3) | 60 (12.0) | |
| Household members | 0.887 | ||
| 2 | 33 (5.2) | 26 (5.2) | |
| 3 | 365 (56.6) | 300 (60.2) | |
| ≥4 | 203 (32.3) | 196 (39.4) | |
| Breastfeeding | 0.515 | ||
| 1.-3. month | 112 (17.8) | 87 (17.5) | |
| 1.-6. month | 268 (42.6) | 166 (33.3) | |
| 1.-12. month | 254 (40.4) | 226 (45.4) | |
| Parental education b | 0.697 | ||
| Low | 16 (2.5) | 6 (1.2) | |
| Medium | 144 (22.9) | 101 (20.3) | |
| High | 468 (74.4) | 391 (78.5) | |
| Household income / month | 0.648 | ||
| < 2000€ | 240 (38.2) | 172 (34.5) | |
| 2000€ - 4000€ | 308 (49.0) | 171 (34.3) | |
| > 4000€ | 42 (6.7) | 35 (7.0) | |
| Separation/divorce c | 0.973 | ||
| Yes | 25 (4.0) | 23 (4.6) | |
| No | 169 (26.9) | 158 (31.7) | |
| Prenatal ETS exposure d | 0.243e | ||
| Median [μg/g creatinine] | 2.0 | 1.85 | |
| < 25% , > 75% | 0.8,5.6 | 0.75,4.95 |
an may be different from total n due to missing data
bLow = 8 yrs of schooling (‘Hauptschulabschluss`); medium = 10 yrs of schooling (`Mittlere Reife`); high = 12 yrs of schooling or more (`(Fach-)hochschulreife’)
cParental separation/divorce in the last 3 years from children’s age 2 years
dETS environmental tobacco smoke (urinary cotinine level at pregnancy)
ep-value derived from Student’s T test between group means
Perceived maternal stress assessment
Maternal stress levels were assessed at 36th weeks of gestation and at the one- and two-year follow-up using the 20-item reduced Perceived Stress Questionnaire (PSQ), a validated instrument by Fliege et al. [34, 35]. The PSQ is comprised of 5 items for each of the different stress dimensions “demands”, “tension”, “worries”, and “lack of joy”. All items were scored on a four-point scale according to the frequency of perception with one (hardly ever) to four (usually). The total stress score was derived as the mean of all 20 scored questions. The scores for each dimension were derived accordingly from the 5 dimension-specific questions [36]. Higher scores indicate higher stress levels.
Anthropometric data
Children’s body weight and height up to the age of five years were obtained from annual clinical visits or from questionnaires of well-child exams (“U examination”). Child length was measured horizontally at birth and at the year 1 follow-up using an infantometer (“Dr. Keller II”). From year two onwards standing height was measured without shoes to the nearest 0.1 cm (“Dr. Keller I”). Body weight was measured to the nearest 0.1 kg, and BMI z-scores were calculated according to the WHO reference data [37] to adjust for child’s age and gender. The use of z-scores is recommended for several reasons. First, z-scores are calculated based on the distribution of the reference population (both the mean and the SD); thus, they reflect the reference distribution. Second, as standardized measures, BMI z-scores are comparable across age and sex. Third, a group of z-scores can be subject to summary statistics such as mean and SD and - even more importantly in our case - can be studied as a continuous variable. Children with BMI z-score < -1 were classified as underweight, children with a BMI z-score of -1 to <1 were classified as normal weight, and children with BMI z-scores ≥1 were classified as overweight.
Assessment of stressors
We assessed the impact of several stressors evaluated by questionnaires on maternal stress perceived during the first year after birth. The stressors evaluated considered the neighborhood quality (living conditions, exposure to traffic or residential noise) and the socio-demographic factors (household income, parental educational level, number of household members, the age of the mother at birth, divorce/separation).
Information about the household income, the parental educational level and the number of household members (all children and adults in the household), were recorded once during pregnancy. Neighborhood quality was assessed each year from pregnancy onwards using the questions summarized in Additional file 1: Table S1. The information on divorce/separation was based on a retrospective assessment at year two with respect to the preceding three years, the exact time point of divorce/separation was not inquired.
Statistical analysis
In all analyses except where explicitly stated otherwise, a p-values ≤ 0.05 was considered to be significant. Whenever Bonferroni-correction was applied the adjusted significant levels are indicated.
To test for potential differences in study characteristics of the entire cohort compared to the analyzed sub-cohort and to test for equal distribution of parameters in the gender-specific analyses chi-square tests were conducted. The maternal perceived stress scores were assessed by Spearman correlations and a repeated measurement analysis of variance (RANOVA) was performed to assess time-dependent changes.
Generalized estimating equation (GEE) models with unstructured correlation matrices were applied to assess the effect of maternal stress on children’s longitudinal BMI z-scores (birth to age five or age one to age five respectively). These models were calculated with BMI z-scores and maternal perceived stress scores as continuous variables and were adjusted for weight-related confounding parameters based on a literature review[38–40], namely: gestational week at delivery, mode of delivery, breastfeeding duration, exposure to environmental tobacco smoke (urinary cotinine level during pregnancy, as described in Table 1), and for gender if applicable. To test for gender differences in response to maternal perceived stress the GEE models were stratified accordingly.
A principal factor analysis with oblique rotation was conducted on 10 questionnaire items assessing neighborhood strains to extract potential underlying scales (Additional file 1: Table S1). Factors with an eigenvalue greater than 1 were chosen following Kaiser’s criterion. Scales were composed of variables with factor loadings greater 0.4. These extracted scales together with different socio-demographic-factors (household income, parental educational level, number of household members, age of the mother at birth, divorce/separation) were assessed by linear regression for their impact on maternal perceived stress and the different dimensions thereof.
Mediation analysis based on the PROCESS SPSS macro (release 2.16.3 [41]) was applied to test the hypothesis whether the stressors identified to contribute to the perceived maternal stress level affect children’s BMI z-score development directly or indirectly. For this purpose, children were categorized in being overweight (BMI z-score ≥ 1) ever in the first five years of life and compared to children never being overweight (BMI-z-scores <1) in this time window. Perceived stress scores and stressors were considered as continuous variables in these analyses. Confidence intervals were computed based on 5000 bootstrap samples.
General statistical analyses, regression- and GEE models were conducted using STATISTICA 12.0 for Windows (Dell Inc., USA) or IBM SPSS Statistics version 22 (IBM Corps., USA) respectively.
Results
Study characteristics
Our analyses were based on the sub-cohort of 498 mother-child pairs for which a complete PSQ assessment for pregnancy, year 1 and year 2 including all weight-related confounders were available. General study characteristics of the analyzed sub-cohort (n=498) and the entire LINA cohort (n=629) were equally distributed as shown in Table 1. In the analyzed sub-cohort the majority of children (68 %) started daycare in their second year of life.
Perceived maternal stress assessment
Pre-and postnatal maternal stress levels were highly correlated with each other (Spearman correlation, birth vs. age 1: R= 0.60, p<10-13; age 1 vs. age 2: R= 0.69, p<10-13; birth vs. age 2: R= 0. 58 p<10-13). There was a statistically significant increase in the maternal perceived stress scores over time as determined by RANOVA (F = 3772, p<0.0005, partial eta-squared = 0.94). An overview of median, minimum, maximum, lower and upper quartiles of maternal stress scores over time is given in Table 2A.
Table 2.
Descriptive statistics of (A) maternal perceived stress scores. Given are median, min, max, and quartile boundaries (n=498). (B) BMI z-score categories within the analyzed sub-cohorta
| A - Stress score | median | min | max | <25 % | >75% | |
| pregnancy | 1.9 | 1.0 | 3.6 | 1.6 | 2.3 | |
| year 1 | 2.0 | 1.1 | 3.9 | 1.7 | 2.4 | |
| year 2 | 2.2 | 1.0 | 3.9 | 1.9 | 2.5 | |
| B - BMI z-scorea | Year 1 n = 487(%) | Year 2 n = 456(%) | Year 3 n = 428(%) | Year 4 n = 394(%) | Year 5 n = 352(%) | |
| < 0 | Underweight | 112 (23.0) | 25 (5.5) | 43 (10.0) | 40 (10.2) | 45 (12.8) |
| 0 < 1 | Normal weight | 336 (69) | 307 (67.3) | 309 (72.2) | 303 (76.9) | 266 (75.6) |
| ≥ 1 | Overweight | 39 (8.0) | 124 (27.2) | 76 (17.8) | 51 (12.9) | 41 (11.6) |
aCategorization based on WHO-reference data
Anthropometric data
On average, about 15.5 % of the analyzed LINA children became overweight during the first five years of life, reaching highest percentages at year two and three. A summary of categorized BMI z-scores of preschool children up to the age of five years is given in Table 2B.
Longitudinal association of maternal perceived stress and children’s BMI z-scores
The adjusted GEE model showed a significant longitudinal effect on children’s BMI z-scores until the age of 5 years only for maternal perceived stress assessed at year one (adj. β: 0.23, 95% CI (0.08-0.37), p = 0.002) (Table 3A, Additional file 1: Table S2). There was no effect of maternal stress during pregnancy or year two on children’s BMI z-scores. Neither weight- nor height- z-scores were affected by maternal stress (data not shown).
Table 3.
Impact of maternal perceived stress levels on longitudinal BMI z-score development in preschool children (birth-age 5)
| β estimatea | 95% CI | p-value | ||
|---|---|---|---|---|
| A - Maternal perceived stress at | ||||
| Pregnancy | (n = 498) | 0.06 | -0.07 – 0.20 | 0.372 |
| Year 1 | (n = 491) | 0.23 | 0.08 – 0.37 | 0.002 |
| Year 2 | (n = 473) | 0.09 | -1.47 – 1.64 | 0.283 |
| Pregnancy to year 2 | (n = 473) | 0.06 | -0.01 – 0.12 | 0.078 |
| B - Sex-stratified effect of maternal perceived stress at year 1 | ||||
| Girls only | (n = 241) | 0.30 | 0.11 – 0.49 | 0.002 |
| Boys only | (n = 250) | 0.10 | -0.11 – 0.31 | 0.333 |
(A) Effects of maternal perceived stress during pregnancy, year 1 and year 2 (B) Sex disparity in susceptibility to maternal perceived stress at year 1. Significant associations are presented in bold (p ≤ 0.05)
aEstimates derived from general estimation equations GEE for BMI z-scores (birth to age 5) as dependent variable, adjusted for gestational week at delivery, mode of delivery, pregnancy cotinine levels and breastfeeding duration (not for pregnancy stress levels)
Stratifying the GEE model for gender revealed a positive association of BMI z-scores with maternal stress during the first year after birth only for of girl’s (adj. β: 0.30, 95% CI (0.11-0.49), p = 0.002; Table 3B, Additional file 1: Table S3), whereas no association was seen for boys. This gender-specific effect was not based on different study characteristics between boys and girls, as can be seen from Additional file 1: Table S4. As only maternal perceived stress during the first year after birth had an effect on BMI z-scores, we focused our further analyses on this early postnatal period.
Influence of different stress dimensions on BMI z-score development
Median, minimum, maximum, lower (<25%) and upper quartiles (>75%) of the four maternal stress dimensions assessed by the PSQ (“demands”, “tension”, “worries” and “lack of joy”) are given in Additional file 1: Table S5. GEE models were applied to evaluate their association with children’s longitudinal BMI z-scores. Results of the adjusted models are summarized in Table 4. After Bonferroni-correction, the stress dimensions “tension”, “lack of joy”, and “demands” showed a significantly positive association with children’s BMI z-scores. Similar to the total stress score the different dimensions of maternal stress only showed an association to girl’s BMI z-scores. “Worries”, “lack of joy”, and “demands” were significantly associated with higher BMI z-scores in girls, “lack of joy” showed the best model fit (QIC).
Table 4.
Effect of the different stress dimensions on the longitudinal BMI z-score development in preschool children
| β estimatea | 95% CI | p-valueb | QICc | |
|---|---|---|---|---|
| Entire cohort (n = 491) | ||||
| Worries | 0.14 | 0.01 – 0.27 | 0.039 | 2051.99 |
| Tension | 0.19 | 0.07 – 0.31 | 0.003 | 2041.97 |
| Lack of Joy | 0.15 | 0.04 – 0.27 | 0.009 | 2045.80 |
| Demands | 0.19 | 0.05 – 0.33 | 0.006 | 2048.45 |
| Girls only (n = 241) | ||||
| Worries | 0.24 | 0.06 – 0.41 | 0.009 | 1005.93 |
| Tension | 0.22 | 0.05 – 0.39 | 0.014 | 1013.26 |
| Lack of Joy | 0.23 | 0.08 – 0.38 | 0.002 | 1003.31 |
| Demands | 0.24 | 0.05 – 0.43 | 0.012 | 1012.31 |
| Boys only (n = 250) | ||||
| Worries | -0.01 | -0.18 – 0.18 | 0.963 | 1056.08 |
| Tension | 0.13 | -0.03 – 0.29 | 0.108 | 1048.00 |
| Lack of Joy | 0.06 | -0.11 – 0.23 | 0.503 | 1054.36 |
| Demands | 0.11 | -0.08 – 0.31 | 0.247 | 1053.93 |
aEstimates derived from general estimation equations (GEE) for BMI z-scores (birth to age 5) as dependent variable, adjusted for gestational week at delivery, mode of delivery, pregnancy cotinine levels and breastfeeding duration
bBonferroni adjusted significance level, p ≤ 0.0125
cQuasi- Akaike Information Criterion QIC for model selection
Influence of maternal stress on early maternal feeding behavior
The strong association of maternal perceived stress on BMI z-scores during the first year of life suggested a possible involvement of breastfeeding duration or the time of solid food introduction. However, both parameters, evaluated in three-month-intervals during the first year of life, were not related to pre- and postnatal maternal perceived stress in our analyses (Additional file 1: Table S6).
Multiple stressors contribute to maternal stress perception
Based on a 10-items questionnaire assessing the neighborhood quality (Additional file 1: Table S1), three factors, which in combination explained 58.1% of the variance (Additional file 1: Table S7), were extracted. The items that clustered on the same factor loadings suggest that the factors represent “poor living conditions”, burdens due to “traffic”, and exposure to “residential noise”, respectively (Additional file 1: Table S7). For each of these three factors corresponding variables were created including items with a factor loading >0.4. The variable “poor living conditions” considered the occurrence of vandalism, graffities, dirty streets and attempted break-ins in the living environment. The impairment due to “traffic” was summarized by items asking for disturbance due to traffic noise or related odors or exhausts. The variable “Noise” took into account noise from pedestrians and neighbors. All three of these variables showed a significant association to the maternal stress level at year one (Fig. 1).
Fig.1.
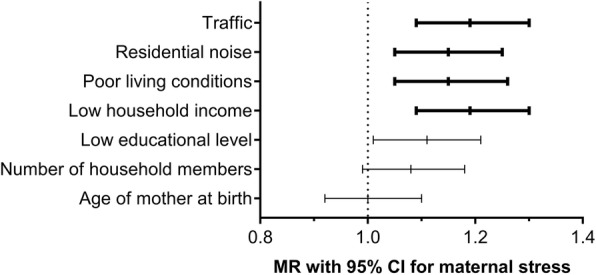
Effects of different stressors on maternal perceived stress. Mean ratios and 95 % confidence intervals for the effect of shown stressors on maternal perceived stress levels at year 1 were calculated from multiple regression models. Significant associations after Bonferroni correction are depicted in bold, p ≤ 0.007
Of the socio-demographic factors analyzed, only low household income contributed significantly to the overall stress perceived by the mothers (Fig. 1, Additional file 1: Table S8). Parental separation or divorce during the first three years (available from 191 participants) significantly increased the total maternal stress level at year 1 and three of the four different dimensions thereof (Additional file 1: Table S9).
Associations of different stressors on stress dimensions
While “worries”, “tension”, and “demands” were similarly positively associated with the factors of poor neighborhood quality, “lack of joy” was not affected (Additional file 1: Figure S1A). However, “lack of joy” was positively associated with a “low household income”, as was an increase in “worries”. A low educational level and the number of household members, which were not associated to the overall maternal stress level, were significantly associated with the stress dimensions “lack of joy” and “demands”.
Impact of stressors on BMI z-score development
To further elucidate how the stressors, which showed a significant association with the maternal perceived stress level at year 1 (see Additional file 1: Table S8), namely noise, traffic, poor living environment) and a low household income affect the BMI z-score development we assessed their relationship by mediation analyses. These stressors did not affect girl’s BMI z-score development directly. However, they had an indirect effect on BMI z-scores mediated by maternal perceived stress (Additional file 1: Figure S1B). Due to the small case number the effect of divorce/separation could not be further evaluated in the mediation analysis.
Discussion
Obesity is a multifactorial disease with an often protracted onset in childhood and adolescence. Despite the appreciation that high caloric intake and sedentary behavior contribute to overweight development, less attention has been given to the effects of pre- and postnatal perceived maternal stress on weight development in early childhood. Given the few longitudinal studies evaluating this relationship in children [8, 39, 42, 43] our aim was to investigate whether and how maternal stress assessed at different time points in the highly vulnerable early pre- and postnatal period is related to overweight development in preschool children.
Previous studies have suggested that dramatic life events during the prenatal phase, such as the occurrence of a natural disaster or the death of a close relative, can contribute to overweight development in preschool children or in young teenagers [44–46]. In our study we did not observe a longitudinal association of prenatal stress on BMI z-scores but rather identified the perceived stressfulness for the mother during the first year of life as an important factor for an increase in BMI z-scores. This observation is in line with a recent meta-analysis by Tate et al., suggesting that toddlers (1-3 years old) are more vulnerable to maternal stress than infants (<one year) [12]. In addition, as the type of stress evaluated in our study is most likely not comparable in its impact to a natural disaster, the missing association between prenatal perceived stress and BMI z-scores might also at least in part be related to the difference in the type of stress evaluated.
As only maternal stress during the first year after birth had an effect on BMI trajectories we hypothesized that duration of breast feeding, which can be negatively affected by maternal stress, might play a role [47]. However, we did not observe significant differences in breastfeeding duration or the time of solid food introduction in relation to the maternal stress level. This does not exclude the possibility that maternal feeding styles and attitudes may play a role [48, 49] as earlier studies indicate that the parent-created environment can foster obesity-promoting feeding styles and attitudes, which shape the child’s food preferences [50, 51]. In older children however, other social influences and food environments experienced in daycare or preschool may dilute the maternal stress effect. Although this is in line with our observation that only maternal perceived stress during the first year but not thereafter had a persistent effect on BMI z-scores, the design of our study did not allow further evaluation of parental feeding styles and attitudes.
Although high maternal stress levels were associated with higher BMI z-scores in the total LINA cohort, this effect was only present in girls, whereas boys were not affected. Similar observations were made by Suglia et al. [39], who described a higher risk of being obese for five-year-old girls, who had experienced high cumulative stress (including food insecurity, housing insecurity, maternal depressive symptoms, and maternal substance abuse) compared to girls without this experience, a similar effect was missing in boys [39].
In adults and adolescents gender disparity in stress perception and processing has previously been associated to differences in coping mechanisms [52–54]. Children seem to respond in a similar way as in particular girls have been described to respond by impulsive eating, emotional binge eating, and by requesting sweet and high fat foods [25, 39, 55]. In light of these previous findings, our results suggest that already at a very young age changes in eating behavior might play a role in the gender-disparity of BMI-development related to the experience of stress.
In accordance with what we saw for the overall maternal perceived stress, “demands”, “worries” and “lack of joy” had a strong positive association with BMI z-scores in girls only. There are several studies suggesting that maternal depression can promote overweight development in children [56, 57], with indications that this might also be a gender-specific effect [9], e.g. Hernandez et al. reported that maternal depression placed females but not males at a higher risk for obesity at age 18 [58].
On a last scale of our analyses we aimed to characterize potential stressors, which add to the perceived maternal stress level throughout the years. We were able to identify three potential stressors, which were all related to the quality of the living environment (burden due to traffic, residential noise, and poor living conditions) contributing to the overall maternal perceived stress.
We show in this study that different sources of noise including “Residential Noise” - noise from neighbors and pedestrians - and “Traffic” summarizing traffic noise and exhaust, can affect the maternal stress level. Noise exposure is a well-described example of a potentially obesogenic factor, which has already been studied for its impact on prenatal/postnatal growth [59–61] and in association to adiposity and metabolic outcomes in adults [27, 29, 62]. Noise during pregnancy and childhood increased the risk of overweight at age 7, although no association to BMI z-scores was found in this study [63]. Also, an unsecure living environment has been suggested to contribute to overweight development. While Mathis et al. observed that adults who perceive their neighborhood as unsecure were 12 times more likely to be overweight [64], in children neighborhood crime was associated with an increase in weight and a limited outdoor activity [65]. Next to the quality of the living environment the only other stressor with a significant impact on overall perceived maternal stress was a low household income, These stressors are likely related to a low socioeconomic status of the families, which is known to be strongly associated with obesity in the Western world [66, 67].
Interestingly, the stressors themselves did not have a direct effect on girl’s BMI z-scores but rather mediated their effect through their impact on the maternal overall perceived stress level. As the maternal perceived stress is most likely a cumulative account of these stressors and potentially also covers others stress-related factors, single stressors might not have a sufficient predictive power. This is in line with the observation that a combination of adverse effects within a family can increase the obesity risk, whereas unfavorable social factors in isolation often do not [68, 69].
Conclusion
In summary, with our longitudinal qualitative stress assessment we were able to provide evidence for the idea that childhood BMI trajectories develop early and that maternal stress during the first year after birth is a persistent positive predictor of BMI z-scores in girls up to the age of five years.
To reduce the risk for childhood obesity – in particular in girls - behavioral interventions to reduce the mental stress in mothers should be considered in the future.
Additional file
Table S1. Questionnaire for the assessment of the living environment. Table S2. Impact of maternal perceived stress levels during pregnancy, year 1 and year 2 on longitudinal BMI z-score development in preschool children (birth-age5). Table S3. Gender disparity in susceptibility to maternal stress-related BMI development in preschool children age 1-5 years. Table S4. Comparison of gender-related study characteristics of the analyzed sub-cohort. Table S5. Characteristics of maternal perceived stress scores of the four different stress dimensions at year 1. Table S6. Influence of maternal stress during the first year after birth on breastfeeding duration and introduction of solid food. Table S7. Summary of exploratory factor analysis results of questionnaire items assessing the living environment. Table S8. Association of different stressors with the maternal stress levels at year 1. Table S9. Contribution of separation or divorce on perceived maternal stress at year 1. Figure S1. (A) Associations of different stressors and the four different stress dimensions at year 1. (B) Summary of mediation analysis. (DOCX 241 kb)
Acknowledgements
We thank all LINA families for participation in the study, Melanie Bänsch for her excellent study organization and assistance, and Mick Wu for his statistical advice.
Funding
Beate Leppert was supported by the “Helmholtz Impulse and Networking Fund” through the “Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE)”. Irina Lehmann and Saskia Trump were supported by the Helmholtz Initiative for Personalized Medicine (iMed). Further support for Saskia Trump came from the e:Med initiative of the German Ministry of Education and Research (BMBF, grant 01ZX1402D). The LINA study was financed via Helmholtz institutional funding (Helmholtz Centre for Environmental Research – UFZ). Anja Hilbert was funded by grant 01EO1501 from the German Ministry of Education and Research (BMBF).
Availability of data and materials
The datasets regarding the LINA cohort generated and/or analyzed during the current study are not publicly available due to limited consent of the study participants but are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body mass index
- GEE
Generalized estimating equation
- PSQ
Perceived Stress Questionnaire
- WHO
World Health Organization
Authors’ contributions
BL and ST performed the statistical analyses. BL, ST and KJ wrote the initial manuscript. MBo, SR, KJ, and IL collected data and provided proband materials. IL, ST, GS, AH, RW provided project leadership. All authors contributed to and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from all individual participants included in the study and participation in the study was voluntary. The study was approved by the Ethics Committee of the University of Leipzig (file ref # 046-2006, 160-2008, 160b/2008, 144-10-31052010, 113-11-18042011). All administrative permissions were obtained to access and use the data obtained in the LINA cohort.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Beate Leppert, Email: beate.leppert@bristol.ac.uk.
Kristin M. Junge, Email: kristin.junge@ufz.de
Stefan Röder, Email: stefan.roeder@ufz.de.
Michael Borte, Email: michael.borte@sanktgeorg.de.
Gabriele I. Stangl, Email: gabriele.stangl@landw.uni-halle.de
Rosalind J. Wright, Email: rosalind.wright@mssm.edu
Anja Hilbert, Email: anja.hilbert@medizin.uni-leipzig.de.
Irina Lehmann, Phone: 0049 30 450 543081, Email: irina.lehmann@charite.de.
Saskia Trump, Phone: 0049 30 450 543089, Email: saskia.trump@charite.de.
References
- 1.International Food Policy Research Institute. 2016. Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2013. Washington, D.C. 10.2499/9780896295841. [DOI]
- 2.Kurth BM, Schaffrath Rosario A. The prevalence of overweight and obese children and adolescents living in Germany. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2007;50(5-6):736–743. doi: 10.1007/s00103-007-0235-5. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Kelly AS. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin Proc. 2017;92(2):251–265. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Kim Jieun, Lee Insook, Lim Sungwon. Overweight or obesity in children aged 0 to 6 and the risk of adult metabolic syndrome: A systematic review and meta-analysis. Journal of Clinical Nursing. 2017;26(23-24):3869–3880. doi: 10.1111/jocn.13802. [DOI] [PubMed] [Google Scholar]
- 5.Trandafir LM, Temneanu OR. Pre and post-natal risk and determination of factors for child obesity. J Med Life. 2016;9(4):386–391. [PMC free article] [PubMed] [Google Scholar]
- 6.Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD: Interventions for preventing obesity in children. Cochrane Database Syst Rev 2011(12):CD001871. 10.1002/14651858.CD001871.pub3 [DOI] [PubMed]
- 7.Flynn MA, McNeil DA, Maloff B, Mutasingwa D, Wu M, Ford C, Tough SC. Reducing obesity and related chronic disease risk in children and youth: a synthesis of evidence with 'best practice' recommendations. Obes Rev. 2006;7(Suppl 1):7–66. doi: 10.1111/j.1467-789X.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 8.Koch FS, Sepa A, Ludvigsson J. Psychological stress and obesity. J Pediatr. 2008;153(6):839–844. doi: 10.1016/j.jpeds.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Lampard AM, Franckle RL, Davison KK. Maternal depression and childhood obesity: a systematic review. Prev Med. 2014;59:60–67. doi: 10.1016/j.ypmed.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Raikkonen K, King S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2017;S0149-7634(16):30734–5. [DOI] [PubMed]
- 11.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Tate EB, Wood W, Liao Y, Dunton GF. Do stressed mothers have heavier children? A meta-analysis on the relationship between maternal stress and child body mass index. Obes Rev. 2015;16(5):351–361. doi: 10.1111/obr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22(1-2):6–19. doi: 10.1159/000362736. [DOI] [PubMed] [Google Scholar]
- 14.Stavrou S, Nicolaides NC, Critselis E, Darviri C, Charmandari E, Chrousos GP. Paediatric stress: from neuroendocrinology to contemporary disorders. Eur J Clin Invest. 2017;47(3):262–269. doi: 10.1111/eci.12724. [DOI] [PubMed] [Google Scholar]
- 15.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3(6):479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 16.Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol. 2004;181(2):291–296. doi: 10.1677/joe.0.1810291. [DOI] [PubMed] [Google Scholar]
- 17.Kanaka-Gantenbein C, Mastorakos G, Chrousos GP. Endocrine-related causes and consequences of intrauterine growth retardation. Ann N Y Acad Sci. 2003;997:150–157. doi: 10.1196/annals.1290.017. [DOI] [PubMed] [Google Scholar]
- 18.Mina TH, Raikkonen K, Riley SC, Norman JE, Reynolds RM. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–122. doi: 10.1016/j.psyneuen.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. Fetal programming of children's obesity risk. Psychoneuroendocrinology. 2015;53:29–39. doi: 10.1016/j.psyneuen.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev. 2008;6(2):241–247. [PubMed] [Google Scholar]
- 21.Shloim N, Edelson LR, Martin N, Hetherington MM. Parenting Styles, Feeding Styles, Feeding Practices, and Weight Status in 4-12 Year-Old Children: A Systematic Review of the Literature. Front Psychol. 2015;6:1849. doi: 10.3389/fpsyg.2015.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi SM. Maternal employment and time with children: Dramatic change or surprising continuity? Demography. 2000;37(4):401–414. doi: 10.1353/dem.2000.0001. [DOI] [PubMed] [Google Scholar]
- 23.Hurley KM, Black MM, Merry BC, Caulfield LE. Maternal mental health and infant dietary patterns in a statewide sample of Maryland WIC participants. Matern Child Nutr. 2015;11(2):229–239. doi: 10.1111/mcn.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Walton-Moss B. Parenting style, parenting stress, and children's health-related behaviors. J Dev Behav Pediatr. 2012;33(6):495–503. doi: 10.1097/DBP.0b013e318258bdb8. [DOI] [PubMed] [Google Scholar]
- 25.Michels N, Sioen I, Braet C, Eiben G, Hebestreit A, Huybrechts I, Vanaelst B, Vyncke K, De Henauw S. Stress, emotional eating behaviour and dietary patterns in children. Appetite. 2012;59(3):762–769. doi: 10.1016/j.appet.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor SG, Maher JP, Belcher BR, Leventhal AM, Margolin G, Shonkoff ET, Dunton GF. Associations of maternal stress with children's weight-related behaviours: a systematic literature review. Obes Rev. 2017;18(5):514–525. doi: 10.1111/obr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen Jeppe Schultz, Raaschou-Nielsen Ole, Tjønneland Anne, Overvad Kim, Nordsborg Rikke B., Ketzel Matthias, Sørensen Thorkild IA, Sørensen Mette. Road Traffic and Railway Noise Exposures and Adiposity in Adults: A Cross-Sectional Analysis of the Danish Diet, Cancer, and Health Cohort. Environmental Health Perspectives. 2016;124(3):329–335. doi: 10.1289/ehp.1409052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oftedal B, Krog NH, Pyko A, Eriksson C, Graff-Iversen S, Haugen M, Schwarze P, Pershagen G, Aasvang GM. Road traffic noise and markers of obesity - a population-based study. Environ Res. 2015;138:144–153. doi: 10.1016/j.envres.2015.1001.1011. [DOI] [PubMed] [Google Scholar]
- 29.Pyko Andrei, Eriksson Charlotta, Oftedal Bente, Hilding Agneta, Östenson Claes-Göran, Krog Norun Hjertager, Julin Bettina, Aasvang Gunn Marit, Pershagen Göran. Exposure to traffic noise and markers of obesity. Occupational and Environmental Medicine. 2015;72(8):594–601. doi: 10.1136/oemed-2014-102516. [DOI] [PubMed] [Google Scholar]
- 30.Weyde Kjell Vegard, Krog Norun Hjertager, Oftedal Bente, Magnus Per, White Richard, Stansfeld Stephen, Øverland Simon, Aasvang Gunn Marit. A Longitudinal Study of Road Traffic Noise and Body Mass Index Trajectories from Birth to 8 Years. Epidemiology. 2018;29(5):729–738. doi: 10.1097/EDE.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz D, Simon JC, Maier-Simon C, Milkova L, Roder S, Sack U, Borte M, Lehmann I, Herberth G. Reduced maternal regulatory T cell numbers and increased T helper type 2 cytokine production are associated with elevated levels of immunoglobulin E in cord blood. Clin Exp Allergy. 2010;40(3):419–426. doi: 10.1111/j.1365-2222.2009.03434.x. [DOI] [PubMed] [Google Scholar]
- 32.Herberth G, Hinz D, Roder S, Schlink U, Sack U, Diez U, Borte M, Lehmann I. Maternal immune status in pregnancy is related to offspring's immune responses and atopy risk. Allergy. 2011;66(8):1065–1074. doi: 10.1111/j.1398-9995.2011.02587.x. [DOI] [PubMed] [Google Scholar]
- 33.Weisse K, Lehmann I, Heroux D, Kohajda T, Herberth G, Roder S, von Bergen M, Borte M, Denburg J. The LINA cohort: indoor chemical exposure, circulating eosinophil/basophil (Eo/B) progenitors and early life skin manifestations. Clin Exp Allergy. 2012;42(9):1337–1346. doi: 10.1111/j.1365-2222.2012.04024.x. [DOI] [PubMed] [Google Scholar]
- 34.Fliege H, Rose M, Arck P, Levenstein S, Klapp BF. Validierung des “Perceived Stress Questionnaire” (PSQ) an einer deutschen Stichprobe. Diagnostica. 2001;47(3):142–152. doi: 10.1026//0012-1924.47.3.142. [DOI] [Google Scholar]
- 35.Fliege H, Rose M, Arck P, Walter OB, Kocalevent RD, Weber C, Klapp BF. The Perceived Stress Questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosom Med. 2005;67(1):78–88. doi: 10.1097/01.psy.0000151491.80178.78. [DOI] [PubMed] [Google Scholar]
- 36.Trump S, Bieg M, Gu Z, Thurmann L, Bauer T, Bauer M, Ishaque N, Roder S, Gu L, Herberth G, et al. Prenatal maternal stress and wheeze in children: novel insights into epigenetic regulation. Sci Rep. 2016;6:28616. doi: 10.1038/srep28616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Onis M, Onyango A, Borghi E, Siyam A, Blossner M, Lutter C, Group WHOMGRS Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012;15(9):1603–1610. doi: 10.1017/S136898001200105X. [DOI] [PubMed] [Google Scholar]
- 38.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. 2016;50(6):761–779. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R. Cumulative social risk and obesity in early childhood. Pediatrics. 2012;129(5):e1173–e1179. doi: 10.1542/peds.2011-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, Cooper C, Inskip HM. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101(2):368–375. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach: Guilford Publications. 2013. [Google Scholar]
- 42.Bergmeier H, Skouteris H, Horwood S, Hooley M, Richardson B. Child temperament and maternal predictors of preschool children's eating and body mass index. A prospective study. Appetite. 2014;74:125–132. doi: 10.1016/j.appet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Sowan NA, Stember ML. Parental risk factors for infant obesity. MCN Am J Matern Child Nurs. 2000;25(5):234–240; quiz 241. doi: 10.1097/00005721-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Dancause KN, Laplante DP, Fraser S, Brunet A, Ciampi A, Schmitz N, King S. Prenatal exposure to a natural disaster increases risk for obesity in 5(1/2)-year-old children. Pediatr Res. 2012;71(1):126–131. doi: 10.1038/pr.2011.18. [DOI] [PubMed] [Google Scholar]
- 45.Dancause KN, Laplante DP, Hart KJ, O'Hara MW, Elgbeili G, Brunet A, King S. Prenatal stress due to a natural disaster predicts adiposity in childhood: the Iowa Flood Study. J Obes. 2015;2015:570541. doi: 10.1155/2015/570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sorensen TI. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PloS one. 2010;5(7):e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dozier AM, Nelson A, Brownell E. The Relationship between Life Stress and Breastfeeding Outcomes among Low-Income Mothers. Adv Prev Med. 2012;2012:902487. doi: 10.1155/2012/902487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson SE, Gooze RA, Lemeshow S, Whitaker RC. Quality of early maternal-child relationship and risk of adolescent obesity. Pediatrics. 2012;129(1):132–140. doi: 10.1542/peds.2011-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holdsworth Elizabeth A., Schell Lawrence M. Maternal-infant interaction as an influence on infant adiposity. American Journal of Human Biology. 2017;29(5):e23023. doi: 10.1002/ajhb.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones RA, Okely AD, Caputi P, Cliff DP. Relationships between child, parent and community characteristics and weight status among young children. Int J Pediatr Obes. 2010;5(3):256–264. doi: 10.3109/17477160903271971. [DOI] [PubMed] [Google Scholar]
- 51.Scaglioni S, Salvioni M, Galimberti C. Influence of parental attitudes in the development of children eating behaviour. Br J Nutr. 2008;99(Suppl 1):S22–S25. doi: 10.1017/S0007114508892471. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez DC, Pressler E, Dorius C, Mitchell KS. Does Family Instability Make Girls Fat? Gender Differences Between Instability and Weight. J Marriage Fam. 2014;76(1):175–190. doi: 10.1111/jomf.12080. [DOI] [Google Scholar]
- 53.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Archives of pediatrics & adolescent medicine. 2006;160(3):285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 54.Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, Connell F. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157(8):739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- 55.Cartwright M, Wardle J, Steggles N, Simon AE, Croker H, Jarvis MJ. Stress and dietary practices in adolescents. Health Psychol. 2003;22(4):362–369. doi: 10.1037/0278-6133.22.4.362. [DOI] [PubMed] [Google Scholar]
- 56.Gross RS, Velazco NK, Briggs RD, Racine AD. Maternal depressive symptoms and child obesity in low-income urban families. Acad Pediatr. 2013;13(4):356–363. doi: 10.1016/j.acap.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Audelo J, Kogut K, Harley KG, Rosas LG, Stein L, Eskenazi B. Maternal Depression and Childhood Overweight in the CHAMACOS Study of Mexican-American Children. Matern Child Health J. 2016;20(7):1405–1414. doi: 10.1007/s10995-016-1937-9. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez DC, Pressler E. Gender disparities among the association between cumulative family-level stress & adolescent weight status. Prev Med. 2015;73:60–66. doi: 10.1016/j.ypmed.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Schell LM, Denham M. Environmental Pollution in Urban Environments and Human Biology. Ann Rev Anthropol. 2003;32(1):111–134. doi: 10.1146/annurev.anthro.32.061002.093218. [DOI] [Google Scholar]
- 60.Cohen S, Weinstein N. Nonauditory Effects of Noise on Behavior and Health. J Soc Issues. 1981;37(1):36–70. doi: 10.1111/j.1540-4560.1981.tb01057.x. [DOI] [Google Scholar]
- 61.Abrahamsson TR, Sandberg Abelius M, Forsberg A, Bjorksten B, Jenmalm MC. A Th1/Th2-associated chemokine imbalance during infancy in children developing eczema, wheeze and sensitization. Clin Exp Allergy. 2011;41(12):1729–1739. doi: 10.1111/j.1365-2222.2011.03827.x. [DOI] [PubMed] [Google Scholar]
- 62.Eriksson Charlotta, Hilding Agneta, Pyko Andrei, Bluhm Gösta, Pershagen Göran, Östenson Claes-Göran. Long-Term Aircraft Noise Exposure and Body Mass Index, Waist Circumference, and Type 2 Diabetes: A Prospective Study. Environmental Health Perspectives. 2014;122(7):687–694. doi: 10.1289/ehp.1307115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christensen JS, Hjortebjerg D, Raaschou-Nielsen O, Ketzel M, Sorensen TI, Sorensen M. Pregnancy and childhood exposure to residential traffic noise and overweight at 7years of age. Environ Int. 2016;94:170–176. doi: 10.1016/j.envint.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Mathis A, Rooks R, Kruger D. Improving the Neighborhood Environment for Urban Older Adults: Social Context and Self-Rated Health. Int J Environ Res Public Health. 2015;13(1):ijerph13010003. doi: 10.3390/ijerph13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandy R, Tchernis R, Wilson J, Liu G, Zhou X. Effects of the built environment on childhood obesity: The case of urban recreational trails and crime. Econ Hum Biol. 2013;11(1):18–29. doi: 10.1016/j.ehb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vieweg VR, Johnston CH, Lanier JO, Fernandez A, Pandurangi AK. Correlation between high risk obesity groups and low socioeconomic status in school children. South Med J. 2007;100(1):8–13. doi: 10.1097/01.smj.0000253479.03665.6f. [DOI] [PubMed] [Google Scholar]
- 67.Mech P, Hooley M, Skouteris H, Williams J. Parent-related mechanisms underlying the social gradient of childhood overweight and obesity: a systematic review. Child Care Health Dev. 2016;42(5):603–624. doi: 10.1111/cch.12356. [DOI] [PubMed] [Google Scholar]
- 68.Evans GW, Fuller-Rowell TE, Doan SN. Childhood cumulative risk and obesity: the mediating role of self-regulatory ability. Pediatrics. 2012;129(1):e68–e73. doi: 10.1542/peds.2010-3647.Epub2011Dec1545.. [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez A, Boyle MH, Georgiades K, Duncan L, Atkinson LR, MacMillan HL. Childhood and family influences on body mass index in early adulthood: findings from the Ontario Child Health Study. BMC Public Health. 2012;12:755. doi: 10.1186/1471-2458-1112-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Questionnaire for the assessment of the living environment. Table S2. Impact of maternal perceived stress levels during pregnancy, year 1 and year 2 on longitudinal BMI z-score development in preschool children (birth-age5). Table S3. Gender disparity in susceptibility to maternal stress-related BMI development in preschool children age 1-5 years. Table S4. Comparison of gender-related study characteristics of the analyzed sub-cohort. Table S5. Characteristics of maternal perceived stress scores of the four different stress dimensions at year 1. Table S6. Influence of maternal stress during the first year after birth on breastfeeding duration and introduction of solid food. Table S7. Summary of exploratory factor analysis results of questionnaire items assessing the living environment. Table S8. Association of different stressors with the maternal stress levels at year 1. Table S9. Contribution of separation or divorce on perceived maternal stress at year 1. Figure S1. (A) Associations of different stressors and the four different stress dimensions at year 1. (B) Summary of mediation analysis. (DOCX 241 kb)
Data Availability Statement
The datasets regarding the LINA cohort generated and/or analyzed during the current study are not publicly available due to limited consent of the study participants but are available from the corresponding author on reasonable request.


